Abstract
Background
Right ventricular (RV) involvement in inferior myocardial infarction (MI) increases in‐hospital morbidity and mortality.
Hypothesis
RV systolic dysfunction assessed by 2‐dimensional speckle tracking echocardiography (STE) might be a predictor of early mortality in patients with acute inferior MI.
Methods
Eighty‐one consecutive patients with acute inferior MI (mean age, 60.8 ± 12.7 years; 18 females) were included. RV myocardial involvement was defined as an elevation >1 mm in V1 or V4R within 12 hours of symptom onset. RV function was assessed by STE. Patients were followed for 30 days for all‐cause mortality.
Results
Thirty‐eight patients had RV myocardial involvement, and they had significantly lower tricuspid annular plane systolic excursion (TAPSE), tricuspid annular systolic velocity (RVS), and left ventricular (LV) and RV global longitudinal strain (GLS). Nine patients (11%) died within 30 days. The mean age of mortality group was higher with more female frequency. They had significantly higher pro‐BNP, hs‐troponin T, and creatinine levels, but lower hemoglobin levels. TIMI 3 flow was significantly less achieved in mortality group. RV myocardial involvement was more frequent in the mortality group, and they had significantly lower TAPSE, RVS, and LV and RV GLS. Multivariate analysis revealed that age and RV GLS were independent predictors of early mortality. RV GLS ≤ –14% predicted early mortality in patients with acute inferior MI with a sensitivity of 88.9% and a specificity of 62.5% (AUC: 0.817, P = 0.002).
Conclusions
RV GLS may be useful in predicting early mortality in patients with acute inferior MI.
Keywords: Inferior Myocardial Infarction, Mortality, Right Ventricular Function, Speckle Tracking Echocardiography
1. INTRODUCTION
The mortality rate is high in patients with myocardial infarction (MI), despite current invasive treatment strategies and antiplatelet, anticoagulant, and anti‐ischemic treatment options.1 Although acute inferior MI is usually regarded as being low risk compared with acute anterior MI, right ventricular (RV) myocardial involvement (RVMI) carries an increased risk of cardiovascular (CV) morbidity and mortality in patients with inferior MI.2, 3 Postmortem studies demonstrated that approximately half of the patients with inferior MI had additional RVMI.4 The clinical presentation of RVMI in patients with acute inferior MI may range from no hemodynamic deterioration to severe hypotension and/or cardiogenic shock, depending on the extent of RV ischemia and infarction.5 Approximately 25% to 50% of RVMI cases are hemodynamically significant.6 As RVMI increases in‐hospital morbidity and mortality in patients with inferior MI,7 evaluation of the RV systolic function has an important role in the prognosis of these patients, especially in those with RVMI in electrocardiography (ECG).
Imaging of the RV is difficult owing to its complex crescent‐shaped structure, heavy trabeculations, and retrosternal location. Evaluation of RV function by 2‐dimensional (2D) speckle tracking echocardiography (STE) is commonly used because of its accuracy, feasibility, and reliability.8 Moreover, conventional 2DE measures, including velocity and displacement‐based analyses, can be affected by translational motion of the heart and respiratory variation. The new echocardiographic method of STE assesses myocardial deformation on grayscale (B‐mode) images and can be used to evaluate both global and regional myocardial strain without being limited by the Doppler beam angle.9
The aim of our study was to explore the impact of RV systolic function detected by 2D STE on early mortality in patients with acute inferior MI.
2. METHODS
Eighty‐one consecutive patients with acute inferior ST‐segment elevation MI (STEMI) were included in the study. Acute inferior STEMI was defined as presence of acute ST‐segment elevation on leads DII, DIII, and aVF. RVMI was defined as an elevation >1 mm in V1 or V4R within 12 hours of symptom onset. Patients with previous MI, severe valvular heart disease, history of heart failure or coronary artery bypass surgery, severe arrhythmia, permanent pacemaker, chronic liver or active autoimmune diseases, poor echogenicity, malignancy, and patients who refused percuteneous coronary intervention (PCI) were excluded. The investigation conformed to the principles outlined in the Declaration of Helsinki. The study was approved by local ethics committee. All participants gave written informed consent.
All patients underwent a complete cardiac evaluation including patient history, physical examination, and standard 12‐lead ECG. All patients underwent primary PCI within 30 minutes, as suggested by the current guidelines.10 Thrombolysis In Myocardial Infarction (TIMI) flow was assessed after PCI visually by an experienced interventional cardiologist.11 Optimal PCI outcome was defined as restoration of coronary blood flow with TIMI classification grade 3.
The study population was evaluated for the presence of CV risk factors, including hypertension, hyperlipidemia, diabetes mellitus, renal dysfunction, and smoking status. Blood samples for plasma glucose, high‐sensitivity troponin T (hs‐TnT), creatine kinase–MB, pro brain natriuretic peptide (pro‐BNP), creatinine, and hemoglobin levels were collected from all patients at admission. Patients were followed for 30 days for detection of early all‐cause mortality.
2.1. Echocardiographic evaluation
All study participants underwent a complete transthoracic echocardiographic study using a Philips iE33 echocardiography device (Philips Medical Systems, Andover, MA) by an experienced cardiologist. Data acquisition was performed with a 3.5‐MHz transducer at a depth of 16 cm in the parasternal and apical views (standard parasternal short‐axis from mid‐ventricular level; apical long‐axis; 2, 3, and 4‐chamber images). Standard M‐mode, 2D, and color‐coded tissue Doppler imaging images were obtained during breathhold, stored in cine loop format from 3 consecutive beats, and transferred to a workstation for further offline analysis. Gain settings, filters, and pulse repetition frequency were adjusted to optimize color saturation, and a color Doppler frame scanning rate of 100 to 140 Hz was used for color tissue Doppler imaging images and grayscale images at a frame rate of 44 to 82 frames/s. Conventional echocardiographic measurements were performed in accordance with the recommendations of the American Society of Echocardiography guidelines.12
All of the images were recorded with a frame rate of ≥50 frames/s to allow for reliable operation of the software. Multidirectional analysis of left ventricular (LV) and RV global longitudinal strain (GLS) was performed using 2D STE as previously described.13, 14 The speckles, which are natural acoustic markers equally distributed within the myocardium, can be detected and tracked on the standard grayscale 2D images. Myocardial strain can be calculated by measuring the change of the position of the speckles within a myocardial segment during the cardiac cycle. The assessment of LV GLS was performed by applying 2D STE to the apical 2, 3, and 4‐chamber views of the LV. The LV was divided into 6 segments in each apical view. The value of LV GLS was derived from the average of the 6 segmental peak systolic longitudinal strain values. The assessment of RV GLS was performed by applying 2D STE to the apical 4‐chamber view of the RV. The RV free wall (basal, mid, and apical segments) endocardial borders were manually traced at the end‐systolic frame by software. The value of RV GLS was derived from the average of the 3 segmental peak systolic longitudinal strain values.
2.2. Statistical analysis
All statistical tests were performed with a commercially available software program (SPSS version 16.0; SPSS Inc., Chicago, IL). All continuous‐variable results were checked for normal distribution by the Kolmogorov–Smirnov test and presented as mean ± SD or median (interquartile range), and categorical variables were expressed as n (%). The χ2 test was used for comparison of the categorical variables, whereas the Student t test or Mann–Whitney U test was used for comparison of quantitative data. Correlation analysis was performed to determine the relation between RV GLS and conventional RV parameters. Logistic regression analyses were performed to determine the predictors of mortality. Receiver operating characteristic (ROC) curve analysis was performed to determine the cutoff for RV GLS and RV conventional parameters in predicting early mortality. A P value <0.05 was considered statistically significant.
3. RESULTS
Eighty‐one patients with inferior STEMI were included into the study. The mean age of the study population was 60.8 ± 12.7 years, and 18 of 81 (22%) patients were female.
Thirty‐eight patients had RVMI. Comparison of the clinical characteristics and echocardiographic parameters of inferior MI patients with and without RVMI are shown in Table 1 and Table 2, respectively. The patients with RVMI had significantly higher pro‐BNP (median level, 1688 pg/mL vs 317 pg/mL; P < 0.001) and hs‐TnT levels (median level, 4073 ng/mL vs 2137 ng/mL; P = 0.012). Among the conventional echocardiographic parameters, tricuspid annular plane systolic excursion (TAPSE; 12.7 ± 2.9 mm vs 17.9 ± 3.3 mm, P < 0.001) and tricuspid annular systolic velocity (RVS; 9.4 ± 1.7 cm/s vs 12.3 ± 2.3 cm/s, P < 0.001) were significantly lower in patients with RVMI. 2D STE demonstrated that the patients with RVMI had significantly lower LV GLS (–14.3% ± 1.9% vs –16.6% ± 3.0%; P < 0.001) and RV GLS (–13.8% ± 3.4% vs –17.1% ± 4.3%; P < 0.001) compared with patients without RVMI.
Table 1.
Comparison of clinical and demographic characteristics between patients with and without RV involvement
| Patients With RV Involvement on ECG, n = 38 | Patients Without RV Involvement on ECG, n = 43 | P Value | |
|---|---|---|---|
| Age, y | 61.6 ± 12.5 | 60.1 ± 13.0 | 0.59 |
| Female sex | 11 (28.9) | 7 (16.3) | 0.17 |
| HTN | 16 (42.1) | 19 (44.2) | 0.85 |
| DM | 13 (34.2) | 8 (18.6) | 0.11 |
| Hyperlipidemia | 16 (42.1) | 12 (27.9) | 0.18 |
| Smoking | 25 (65.8) | 30 (69.8) | 0.70 |
| Pro‐BNP, ng/L | 1688 (459–3968) | 317 (102–865) | <0.00a |
| hs‐TnT, μg/L | 4073 (2062–7533) | 2137 (1191–4550) | 0.012a |
| CK‐MB, ng/mL | 140.3 ± 114.4 | 131.0 ± 102.4 | 0.684 |
| Glucose, mg/dL | 186 ± 90 | 125 ± 48 | 0.001a |
| Hb, g/dL | 12.6 ± 2.2 | 13.4 ± 1.7 | 0.063 |
| Cr, mg/dL | 1.38 ± 1.03 | 1.21 ± 1.4 | 0.063 |
| Mortality within 30 d | 8 (21.1) | 1 (2.3) | 0.01a |
Abbreviations: CK‐MB, creatine kinase MB; Cr, creatinine; DM, diabetes mellitus; ECG, electrocardiogram; Hb, hemoglobin; hs‐TnT, high‐sensitivity cardiac troponin T; HTN, hypertension; IQR, interquartile range; pro‐BNP, pro‐brain natriuretic peptide; RV, right ventricular; SD, standard deviation.
Data are presented as mean ± SD or median (IQR), and categorical variables are expressed as n (%).
Statistical significance at P < 0.05.
Table 2.
Comparison of echocardiographic characteristics between patients with and without RV involvement
| Patients With RV Involvement on ECG, n = 38 | Patients Without RV Involvement on ECG, n = 43 | P Value | |
|---|---|---|---|
| LVEDD, mm | 45.7 ± 4.0 | 46.9 ± 4.2 | 0.19 |
| LVESD, mm | 32.9 ± 4.1 | 30.9 ± 4.7 | 0.04a |
| LVEF, % | 54 ± 9.2 | 56 ± 10.1 | 0.09 |
| RV diameter, mm | 34.2 ± 5.1 | 35.1 ± 4.9 | 0.44 |
| RV FAC, % | 42 ± 11 | 38 ± 12 | 0.29 |
| TAPSE, mm | 12.7 ± 2.9 | 17.9 ± 3.3 | <0.001a |
| RV S′, cm/s | 9.4 ± 1.7 | 12.3 ± 2.3 | <0.001a |
| LV GLS, –% | 14.3 ± 1.9 | 16.6 ± 3.0 | <0.001a |
| RV GLS, –% | 13.8 ± 3.4 | 17.1 ± 4.3 | <0.001a |
Abbreviations: ECG, electrocardiography; FAC, fractional area change; GLS, global longitudinal strain; LV, left ventricular; LVEDD, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; LVESD, left ventricular end‐systolic diameter; RV, right ventricular; RV S′, right ventricular systolic velocity; SD, standard deviation; TAPSE, tricuspid annular plane systolic excursion.
Data are presented as mean ± SD.
Statistical significance at P < 0.05.
Nine patients (11%) died within 30 days. The characteristics of these patients are listed in Table 3. The mean age of the mortality group was higher, with more female patients. The mortality group had significantly higher pro‐BNP (median level, 5418 pg/mL vs 495 pg/mL; P < 0.001), hs‐TnT (median level, 9025 ng/mL vs 2555 ng/mL; P < 0.001), and creatinine (mean level, 1.6 ± 0.6 mg/dL vs 1.2 ± 1.3 mg/dL; P = 0.003), whereas they had lower hemoglobin levels (mean level, 11.3 ± 1.5 mg/dL vs 13.2 ± 2.0 mg/dL; P = 0.004). TIMI 3 flow was significantly less achieved in the mortality group (33.3% vs 72.2%; P = 0.027). RVMI was more frequent in the mortality group (88.9% vs 41.7%; P = 0.011) and they had significantly lower TAPSE (11.4 ± 4.0 mm vs 16.0 ± 3.8 mm; P = 0.001), RVS (8.6 ± 1.1 cm/s vs 11.2 ± 2.5 cm/s; P = 0.001), LV GLS (–12.6% ± 1.3% vs –15.9% ± 2.7%; P < 0.001), and RV GLS (–11.9% ± 3.1% vs –16.0% ± 4.1%; P = 0.002) indicating RV dysfunction.
Table 3.
Comparison of characteristics and echocardiographic parameters of the mortality group
| Early Mortality, n = 9 | Survived, n = 72 | P Value | |
|---|---|---|---|
| Age, y | 76.0 ± 5.9 | 58.9 ± 12.1 | <0.001a |
| Female sex | 5 (55.6) | 13 (18.1) | 0.023a |
| Pro‐BNP, ng/L | 5418 (322) | 495 (349) | <0.001a |
| hs‐TnT, μg/L | 9025 (971) | 2555 (995) | <0.001a |
| Cr, mg/dL | 1.6 ± 0.6 | 1.2 ± 1.3 | 0.003a |
| Hb, g/dL | 11.3 ± 1.5 | 13.2 ± 2.0 | 0.004a |
| TIMI‐3 flow | 3 (33.3) | 52 (72.2) | 0.027a |
| RVMI | 8 (88.9) | 30 (41.7) | 0.01a |
| RV diameter, mm | 32.8 ± 6.4 | 34.9 ± 4.7 | 0.366 |
| RV FAC, % | 41 ± 11 | 39 ± 12 | 0.662 |
| TAPSE, mm | 11.4 ± 4.0 | 16.0 ± 3.8 | 0.001a |
| RV S′, cm/s | 8.6 ± 1.1 | 11.2 ± 2.5 | 0.001a |
| LV GLS, –% | 12.6 ± 1.3 | –15.9 ± 2.7 | <0.001a |
| RV GLS, –% | 11.9 ± 3.1 | –16.0 ± 4.1 | 0.002a |
Abbreviations: Cr, creatinine; FAC, fractional area change; GLS, global longitudinal strain; Hb, hemoglobin; hs‐TnT, high‐sensitivity cardiac troponin T; IQR, interquartile range; LV, left ventricular; pro‐BNP, pro‐brain natriuretic peptide; RV, right ventricular; RVMI, right ventricular myocardial involvement; RV S′, right ventricular systolic velocity; SD, standard deviation; TAPSE, tricuspid annular plane systolic excursion; TIMI, Thrombolysis In Myocardial Infarction.
Data are presented as mean ± SD or median (IQR), and categorical variables are expressed as n (%).
Statistical significance at P < 0.05.
Univariate analysis showed that RV GLS was significantly correlated with TAPSE (r = 0.563, P < 0.001) and RVS (r = 0.332, P = 0.002), whereas TAPSE was significantly correlated with RVS (r = 0.541, P < 0.001). Multivariate logistic regression analysis was performed to determine the independent predictors of mortality in patients with acute inferior MI (Table 4). Age and RV GLS were found as independent predictors of early mortality in patients with acute inferior MI (r 2 of the model = 0.629; P < 0.001). Two additional logistic regression analysis were performed to determine whether RVs or TAPSE were independent predictors of early mortality like RV GLS including the same covariates in Table 4. Neither RVs (odds ratio: 0.521, 95% confidence interval: 0.263‐1.034, P = 0.062) nor TAPSE (odds ratio: 0.814, 95% confidence interval: 0.603‐1.098, P = 0.178) were independent predictors for mortality when adjusted by the same covariates.
Table 4.
Multivariate logistic regression analysis to determinate the predictors of mortality
| OR | 95% CI | P Value | |
|---|---|---|---|
| Age, y | 1.138 | 1.011‐1.281 | 0.032a |
| Female sex | 1.852 | 0.167‐20.600 | 0.616 |
| Pro‐BNP, ng/L | 1.009 | 0.993‐1.026 | 0.264 |
| TIMI 3 flow | 0.301 | 0.028‐3.244 | 0.323 |
| RV GLS, % | 0.588 | 0.225‐0.964 | 0.026a |
| LV GLS, % | 0.620 | 0.301‐1.276 | 0.194 |
Abbreviations: CI, confidence interval; GLS, global longitudinal strain; LV, left ventricular; OR, odds ratio; pro‐BNP, pro‐brain natriuretic peptide; RV, right ventricular; TIMI, Thrombolysis In Myocardial Infarction.
Statistical significance at P < 0.05.
ROC analysis revealed that a RV GLS ≤ –14% predicted early mortality in patients with acute inferior MI with a sensitivity of 88.9%, a specificity of 62.5%, positive predictive value of 22.9%, and negative predictive value of 97.8% (AUC: 0.817, P = 0.002; Figure 1). ROC analysis also revealed that a RVS ≤9.2 cm/s (sensitivity: 88.9%, specificity: 76.4%, positive predictive value: 32.0%, and negative predictive value: 98.2%) and TAPSE ≤12 mm (sensitivity: 88.9%, specificity: 83.3%, positive predictive value: 40.0%, and negative predictive value: 98.4%) predicted early mortality in patients with acute inferior MI.
Figure 1.
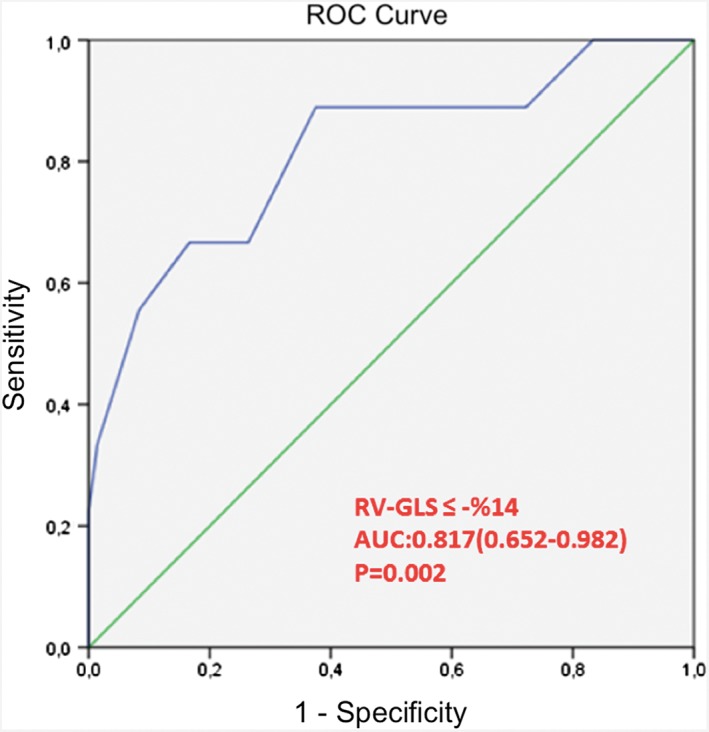
ROC analysis revealed that a RV GLS ≤ –14% predicted early mortality in patients with acute inferior MI with a sensitivity of 88.9%, specificity of 62.5%, positive predictive value of 22.9%, and negative predictive value 97.8%. AUC: 0.817, 95% CI: 0.652‐0.982, P = 0.002. Abbreviations: AUC, area under the curve; CI, confidence interval; MI, myocardial infarction; ROC, receiver operating characteristic; RV GLS, right ventricular global longitudinal strain
4. DISCUSSION
RVMI carries an increased risk of CV morbidity and mortality in patients with inferior MI.7, 15, 16 In this study, we demonstrated that RVMI results in a significant impairment in RV and LV systolic function in patients with inferior MI, and the mortality rate was higher in patients with RVMI (21.1% vs 2.3%; P = 0.011). Assali et al17 evaluated the prognostic importance of RV infarction in patients with acute MI treated with primary PCI. In that study, patients with RV involvement had much poorer prognosis compared with those without RV infarction or anterior‐wall AMI. The presence of RV involvement was a strong independent predictor of 30‐day mortality. Hamon et al16 demonstrated RV dysfunction to be a predictor of adverse prognosis in STEMI in a meta‐analysis of data from 22 studies. However, Mendes et al18 evaluated the prognostic value of RV function in a substudy from the Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock (SHOCK) trial. In this study, no difference was demonstrated between groups of LV shock patients with and without RV dysfunction. However, in patients with RV dysfunction, the presence of RV dilatation predicted an improved prognosis, with significantly greater 12‐month survival. Furthermore, Foussas et al19 reported that RVMI did not lead to an increased risk for long‐term mortality in patients who survived to discharge after hospitalization for acute inferior MI.
RV systolic function can be affected by LV systolic dysfunction. Because the RV is sensitive to changes in ventricular loading conditions, increased LV end‐diastolic pressure reflected backward to the RV will cause RV enlargement and decrease RV systolic function.20 Mehta et al21 showed that the involvement of the RV in inferior AMI was associated with increased adverse clinical events in a meta‐analysis. They found that RV involvement was not due to more extensive infarction of the LV. In addition, RV infarction could be related to an increased propensity to develop life‐threatening ventricular arrhythmias, as the RV might be more arrhythmogenic than the LV.
Novel diagnostic modalities have been used in the detection of RVMI in patients with inferior MI due to the unreliability of ECG.22 Even though RV dysfunction after a STEMI is better detected by cardiovascular magnetic resonance than conventional 2D‐echocardiography,23 transthoracic echocardiography remains the first‐line imaging modality because of being widely available and inexpensive, with no side effects and achievable at the bedside.24 Conventional echocardiographic parameters such as TAPSE and S′ are regional methods and study only longitudinal shortening compared with STE. Moreover, TAPSE and S′ showed weak correlations with cardiovascular magnetic resonance measurements.25 There is currently significant enthusiasm for STE, as it provides an objective quantification of myocardial mechanical function.
STE is a novel technique for assessing both regional and global myocardial deformation in multidirectional axes. A growing body of evidence has accumulated showing good feasibility, reproducibility, and accuracy of STE for RV analysis under various pathologic conditions.14, 26 RV strain has been shown to be lower in patients with RV infarctions defined by ST‐segment elevation in lead V4R.27 Our results also demonstrated that RV GLS was lower in patients with RVMI compared with patients without RVMI and was an independent predictor of early mortality in patients with acute inferior MI. Similarly, Antoni et al28 showed that RV strain <22.1% provided incremental value over clinical information and conventional RV function parameters to predict adverse outcome in post‐MI patients and all cases in‐hospital mortality group had RV GLS < –22.1%. In a velocity vector imaging study, Park et al29 showed that RV GLS <15.5% showed significantly lower 5‐year survival rate and lower major adverse cardiac events–free survival rate in patients with inferior STEMI. Accordingly, to the best of our knowledge, our study is the first clinical investigation that used STE‐derived strain to evaluate the RV function and its relation with mortality in patients with inferior STEMI.
Our study has important clinical implications. Early mortality rate was higher in patients with RVMI and persisting RV dysfunction, although all patients were given optimal medical treatment and underwent primary PCI.30 Therefore, closer follow‐up of patients with persisting RV dysfunction may be useful. RV GLS may be used to identify patients who are at higher risk for mortality. It is also worth noting that ROC analysis showed similar sensitivity and specificity values for RVS and TAPSE in predicting early mortality in our study. There is no consensus as to the best echocardiographic technique to evaluate the RV functions and a combination of approaches is still suggested for overall evaluation of RV function.31
4.1. Study limitations
The study was a single‐center study and the sample size was relatively small. The follow‐up period (30 days) was short, and patients were followed for all‐cause mortality. Validation of RVMI and RV dysfunction by cardiovascular magnetic resonance imaging and correlation with RV GLS might strengthen our results. The baseline echocardiographic measurements were performed within 24 hours after primary PCI. The time course of RV function before and after the primary PCI was unclear. The preexisting RV systolic dysfunction could not be excluded. Because RV systolic function can be affected by various conditions, there might be the possibility of the presence of RV systolic dysfunction from causes other than acute MI. Further prospective, large‐scale studies are required to determine the association between RV GLS and long‐term CV mortality.
5. CONCLUSION
RV and LV systolic function is impaired in inferior MI patients with RVMI. Early mortality rate is high in patients with RVMI. RV GLS may provide valuable information in predicting prognosis of the patients with acute inferior MI.
Author contributions
Dr. Batur G. Kanar was the primary investigator of the study, responsible for the study design, data collection, analysis of the data, and drafting of the article. Dr. Beste Ozben was responsible for analysis of the data and drafting of the article. Drs. Mustafa K. Tigen and Murat Sunbul were responsible for critical revision of the data. Drs. Altug Cincin, Alper Kepez, and Halil Atas were responsible for data collection.
Conflicts of interest
The authors declare no potential conflicts of interest.
Kanar BG, Tigen MK, Sunbul M, et al. The impact of right ventricular function assessed by 2‐dimensional speckle tracking echocardiography on early mortality in patients with inferior myocardial infarction. Clin Cardiol. 2018;41:413–418. 10.1002/clc.22890
REFERENCES
- 1. Wang JY, Goodman SG, Saltzman I, et al; Global Registry of Acute Coronary Events (GRACE/GRACE‐2); Canadian Registry of Acute Coronary Events (CANRACE) Investigators . Cardiovascular risk factors and in‐hospital mortality in acute coronary syndromes: insights from the Canadian Global Registry of Acute Coronary Events. Can J Cardiol. 2015;31:1455–1461. [DOI] [PubMed] [Google Scholar]
- 2. Khosoosi Niaki M, Abbaszade Marzbali N, Salehiomran M. Clinical manifestations of right ventricle involvement in inferior myocardial infarction. Caspian J Intern Med. 2014;5:13–16. [PMC free article] [PubMed] [Google Scholar]
- 3. Khan S, Kundi A, Sharieff S. Prevalence of right ventricular myocardial infarction in patients with acute inferior wall myocardial infarction. Int J Clin Pract. 2004;58:354–357. [DOI] [PubMed] [Google Scholar]
- 4. Isner JM, Roberts WC. Right ventricular infarction complicating left ventricular infarction secondary to coronary heart disease: frequency, location, associated findings and significance from analysis of 236 necropsy patients with acute or healed myocardial infarction. Am J Cardiol. 1978;42:885–894. [DOI] [PubMed] [Google Scholar]
- 5. Yoshino H, Udagawa H, Shimizu H, et al. ST‐segment elevation in right precordial leads implies depressed right ventricular function after acute inferior myocardial infarction [published correction appears in Am Heart J. 1998;136:5]. Am Heart J. 1998;135:689–695. [DOI] [PubMed] [Google Scholar]
- 6. Shah PK, Maddahi J, Berman DS, et al. Scintigraphically detected predominant right ventricular dysfunction in acute myocardial infarction: clinical and hemodynamic correlates and implications for therapy and prognosis. J Am Coll Cardiol. 1985;6:1264–1272. [DOI] [PubMed] [Google Scholar]
- 7. Ondrus T, Kanovsky J, Novotny T, et al. Right ventricular myocardial infarction: from pathophysiology to prognosis. Exp Clin Cardiol. 2013;18:27–30. [PMC free article] [PubMed] [Google Scholar]
- 8. Wang Y, Ma C, Zhang Y, et al. Assessment of left and right ventricular diastolic and systolic functions using two‐dimensional speckle‐tracking echocardiography in patients with coronary slow‐flow phenomenon. PLoS One. 2015;10:e0117979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vitarelli A, Mangieri E, Terzano C, et al. Three‐dimensional echocardiography and 2D‐3D speckle‐tracking imaging in chronic pulmonary hypertension: diagnostic accuracy in detecting hemodynamic signs of right ventricular (RV) failure. J Am Heart Assoc. 2015;4:e001584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Windecker S, Kolh P, Alfonso F, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio‐Thoracic Surgery (EACTS), developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2014;35:2541–2619. [DOI] [PubMed] [Google Scholar]
- 11. Kammler J, Kypta A, Hofmann R, et al. TIMI 3 flow after primary angioplasty is an important predictor for outcome in patients with acute myocardial infarction. Clin Res Cardiol. 2009;98:165–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cheitlin MD, Armstrong WF, Aurigemma GP, et al. ACC/AHA/ASE 2003 Guideline Update for the Clinical Application of Echocardiography: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography). J Am Soc Echocardiogr. 2003;16:1091–1110. [DOI] [PubMed] [Google Scholar]
- 13. Delgado V, Ypenburg C, van Bommel RJ, et al. Assessment of left ventricular dyssynchrony by speckle tracking strain imaging comparison between longitudinal, circumferential, and radial strain in cardiac resynchronization therapy. J Am Coll Cardiol. 2008;51:1944–1952. [DOI] [PubMed] [Google Scholar]
- 14. Donal E, Tournoux F, Leclercq C, et al. Assessment of longitudinal and radial ventricular dyssynchrony in ischemic and nonischemic chronic systolic heart failure: a two‐dimensional echocardiographic speckle‐tracking strain study. J Am Soc Echocardiogr. 2008;21:58–65. [DOI] [PubMed] [Google Scholar]
- 15. Malla RR, Sayami A. In hospital complications and mortality of patients of inferior wall myocardial infarction with right ventricular infarction. JNMA J Nepal Med Assoc. 2007;46:99–102. [PubMed] [Google Scholar]
- 16. Hamon M, Agostini D, Le Page O, et al. Prognostic impact of right ventricular involvement in patients with acute myocardial infarction: meta‐analysis. Crit Care Med. 2008;36:2023–2033. [DOI] [PubMed] [Google Scholar]
- 17. Assali AR, Teplitsky I, Ben‐Dor I, et al. Prognostic importance of right ventricular infarction in an acute myocardial infarction cohort referred for contemporary percutaneous reperfusion therapy. Am Heart J. 2007;153:231–237. [DOI] [PubMed] [Google Scholar]
- 18. Mendes LA, Picard MH, Sleeper LA, et al. Cardiogenic shock: predictors of outcome based on right and left ventricular size and function at presentation. Coron Artery Dis. 2005;16:209–215. [DOI] [PubMed] [Google Scholar]
- 19. Foussas SG, Zairis MN, Tsiaousis GZ, et al. The impact of right ventricular involvement on the postdischarge long‐term mortality in patients with acute inferior ST‐segment elevation myocardial infarction. Angiology. 2010;61:179–183. [DOI] [PubMed] [Google Scholar]
- 20. Anavekar NS, Skali H, Bourgoun M, et al. Usefulness of right ventricular fractional area change to predict death, heart failure, and stroke following myocardial infarction (from the VALIANT ECHO Study). Am J Cardiol. 2008;101:607–612. [DOI] [PubMed] [Google Scholar]
- 21. Mehta SR, Eikelboom JW, Natarajan MK, et al. Impact of right ventricular involvement on mortality and morbidity in patients with inferior myocardial infarction. J Am Coll Cardiol. 2001;37:37–43. [DOI] [PubMed] [Google Scholar]
- 22. Rambihar S, Dokainish H. Right ventricular involvement in patients with coronary artery disease. Curr Opin Cardiol. 2010;25:456–463. [DOI] [PubMed] [Google Scholar]
- 23. Jensen CJ, Jochims M, Hunold P, et al. Right ventricular involvement in acute left ventricular myocardial infarction: prognostic implications of MRI findings. AJR Am J Roentgenol. 2010;194:592–598. [DOI] [PubMed] [Google Scholar]
- 24. Rallidis LS, Makavos G, Nihoyannopoulos P. Right ventricular involvement in coronary artery disease: role of echocardiography for diagnosis and prognosis. J Am Soc Echocardiogr. 2014;27:223–229. [DOI] [PubMed] [Google Scholar]
- 25. Pavlicek M, Wahl A, Rutz T, et al. Right ventricular systolic function assessment: rank of echocardiographic methods vs. cardiac magnetic resonance imaging. Eur J Echocardiogr. 2011;12:871–880. [DOI] [PubMed] [Google Scholar]
- 26. Huttin O, Lemarié J, Di Meglio M, et al. Assessment of right ventricular functional recovery after acute myocardial infarction by 2D speckle‐tracking echocardiography. Int J Cardiovasc Imaging. 2015;31:537–545. [DOI] [PubMed] [Google Scholar]
- 27. Sevimli S, Gundogdu F, Aksakal E, et al. Right ventricular strain and strain rate properties in patients with right ventricular myocardial infarction. Echocardiography. 2007;24:732–738. [DOI] [PubMed] [Google Scholar]
- 28. Antoni ML, Scherptong RW, Atary JZ, et al. Prognostic value of right ventricular function in patients after acute myocardial infarction treated with primary percutaneous coronary intervention. Circ Cardiovasc Imaging. 2010;3:264–271. [DOI] [PubMed] [Google Scholar]
- 29. Park SJ, Park JH, Lee HS, et al. Impaired RV global longitudinal strain is associated with poor long‐term clinical outcomes in patients with acute inferior STEMI. JACC Cardiovasc Imaging. 2015;8:161–169. [DOI] [PubMed] [Google Scholar]
- 30. Goldstein JA. Pathophysiology and management of right heart ischemia. J Am Coll Cardiol. 2002;40:841–853. [DOI] [PubMed] [Google Scholar]
- 31. Dutta T, Aronow WS. Echocardiographic evaluation of the right ventricle: clinical implications. Clin Cardiol. 2017;40:542–548. [DOI] [PMC free article] [PubMed] [Google Scholar]


