Summary
Aim
Spinal cord injury (SCI) leads to severe neural damage for which there is currently no effective treatment. Exploration of the neuroprotective effect among clinically approved drugs will speed up clinical translation of SCI. Nafamostat mesilate (NM) as a synthetic serine protease inhibitor has been used clinically in pancreatitis treatments. However, its effectiveness in SCI is unknown. The aim of this study was to confirm the efficacy of NM in ameliorating SCI.
Methods
Intraperitoneal administration of NM was performed on a contusion SCI model in Wistar rat. Hematoxylin and eosin staining (H&E staining) and Luxol fast blue (LFB) staining were used to observe the histological lesions. Apoptosis was examined by TUNEL staining, Annexin V‐FITC/PI, caspase‐3, and Bcl‐2. Cytokines and neurotrophins were tested by Western blot. Locomotion recovery assessed by hindlimb BBB score and the inclined plane test.
Results
Nafamostat mesilate treatment significantly improved locomotion recovery as assessed by hindlimb BBB scores and the inclined plane test. H&E staining and LFB staining showed a significant increase in spared tissue in both gray matter and white matter. NM decreased the expression of the proinflammatory cytokines TNF‐α and IL‐6. In addition, apoptosis was also significantly decreased, as shown by TUNEL staining and Annexin V‐FITC/PI and by Western blotting for caspase‐3 and Bcl‐2 expression. Due to the mechanism of action of NM as a serine protease inhibitor, the drug decreased thrombin expression in the damaged spinal cord. Furthermore, NM increased the expression of neurotrophins (NT‐3, BDNF, and NGF).
Conclusions
Upon NM treatment, the functional and histological outcomes were improved, and microenvironment upon SCI was modulated. As a clinically approved drug, NM holds promise for clinical use after spinal cord injury.
Keywords: apoptosis, nafamostat mesilate, neuroinflammation, spinal cord injury, thrombin
1. INTRODUCTION
Spinal cord injury (SCI) is a devastating type of trauma that has a high incidence rate and causes neurological deficits, paralysis, or death.1, 2 The only drug currently available as a treatment option to curtail acute trauma following SCI is methylprednisolone, which can inhibit the inflammatory cascades. However, its clinical utility remains controversial,3, 4, 5, 6 and it has not been recommended by the guidelines of the American Association of Neurological Surgeons (AANS) and the Congress of Neurological Surgeons (CNS) since 2013. Thus, to identify a novel and effective medicine to repair SCI and decrease the morbidity and mortality of SCI is an urgent priority.
Serine proteases, such as thrombin, trypsin, and tissue kallikreins, can regulate cell signaling through cleaving and activating a novel family of G protein‐coupled proteinase‐activated receptors (PARs), which are also confirmed to be widely distributed in the nervous system.7, 8 The specific sites of PARs are cleaved by proteases, and the ligand domains are exposed, which can then bind to and activate the cleaved receptors. Then, PARs could activate a series of downstream signaling pathways that mediate inflammatory responses. The proteases that activate PARs are generated and released in increased amounts during injury and inflammation, and they mediate a series of responses to tissue damage.9, 10 Recent studies showed that serine proteases play important roles in inflammatory as well as degenerative central nervous system (CNS) disorders such as stroke, intracerebral hemorrhage, and multiple sclerosis.11, 12, 13, 14 Also, there were studies showed that serine proteases were involved in the neuroinflammation and neurodegeneration in SCI.15, 16
Nafamostat mesilate (NM) as a synthetic serine protease inhibitor has been applied in the treatment of acute pancreatitis, disseminated intravascular coagulopathy, and continuous renal replacement therapy.17, 18, 19 Recent researches have demonstrated that NM can attenuate neuronal damage and improve functional recovery from CNS injuries such as shock and transient focal ischemia/reperfusion‐induced brain injury in rats by decreasing infarct volume of brain edema, inhibiting neuroinflammation and other effects.20, 21, 22 As serine protease activation occurs in SCI and triggers the inflammatory cascade, we hypothesized that NM treatment could attenuate inflammation and promote the repair of SCI.
Therefore, a contusion SCI model of rats was used to investigate the effect of NM for SCI. Intraperitoneal NM treatment indeed showed significant improvement in function and tissue morphology, and also the apoptosis of cells after injury was decreased. We further investigated the potential mechanism and showed that NM reduced the expression of thrombin, a serine protease, and inhibited inflammatory cytokine expression.
2. MATERIALS AND METHODS
2.1. Experimental animals
Female Wistar rats (240 ± 10 g) were purchased from the Laboratory Animal Center of the Academy of Military Medical Sciences (Beijing, China). All animals were kept in a humidity‐ and temperature‐controlled environment with a 12‐hour light‐dark cycle and were allowed free access to water and food. The experiments were approved by the Ethics Committee of Tianjin Medical University (Approval No. TMUAMEC2017025).
2.2. Experimental groups
Animals were randomly divided into three groups:
Group I: sham (laminectomy only without SCI), (N = 30).
Group II: injury + saline, (N = 30).
Group III: injury + NM 10 mg/kg, (N = 30).
The animals were sacrificed at 24 hours, 3 days, and 8 weeks after SCI. After intracardial perfusion, spinal cords were collected and processed for molecular and biochemical analysis.
2.3. Spinal cord injury
An SCI model was established using a modification of Allen's method. The animals were weighed, deeply anesthetized with 4% chloral hydrate (3 mL/kg) via intraperitoneal injection at 25°C, and fixed in place. A skin incision approximately 1 cm long was made along the dorsal midline, and the T10 vertebral laminae were exposed. Subsequently, dorsal laminectomy of the T10 vertebra was performed using a fine rongeur. The impact bar was placed on the spinal cord, and the 10 g node was allowed to fall freely from a height of 25 mm, causing spinal cord contusion injury. Then, the muscles and skin were sutured. Cefuroxime sodium was used for 3 days after surgery to prevent incision infection. Additionally, the bladder of each rat was manual emptying twice per day, and a rigorous care of the perineum was conducted to prevent urinary infection.
2.4. Drug treatment
Nafamostat mesilate was provided by Hong Rui Kang Co., Wuhan (CAS: 82956‐11‐4). NM was administered by intraperitoneal injection immediately and 8 hours after surgery and was injected twice per day until day 7. As controls, animals in the sham and injury groups were injected with 0.9% NaCl.
2.5. Enzyme‐linked immunosorbent assay (ELISA)
The TNF‐α and IL‐6 enzyme‐linked immunosorbent assay kits (Beijing Equation Biotechnology Co) were used in ELISA. The rats were sacrificed under chloral hydrate anesthesia at the intended time points, and sections of spinal cord (1 cm long) were harvested from the epicenter of the injury. The spinal cord samples were homogenized and then centrifuged at 1000 g for 20 minutes. The liquid supernatant was collected and then the liquid supernatant and cerebrospinal fluid were tested at a wavelength of 450 nm according to the manufacturer's instructions of ELISA kits.
2.6. Immunofluorescence staining
For immunofluorescence staining, after the rats were sacrificed, their spinal cords were collected and embedded in paraffin. Spinal cord sections (5 μm thick) from each specimen were deparaffinized with xylene (2 × 20 minutes), incubated in graded concentrations of ethanol (2 × anhydrous ethanol, 95%, 90%, 80%, and 70%), and then washed with phosphate‐buffered saline (PBS) for 3 × 3 minutes. The sections were incubated in H2O2 for 15 minutes to block endogenous peroxidase and subsequently to block the sections with a blocking solution (0.1% Triton X‐100 in PBS and 10% normal goat serum) at room temperature (RT) for 1 hour. Next, the samples were incubated with the primary antibodies overnight at 4°C. The primary antibodies included mouse anti‐CD68 antibody (1:200, Abcam), anti‐TNF‐αantibody (1:100, Abcam), and goat anti‐rat GFAP antibody (1:400, Abcam). The next day, the sections were washed for 3 × 3 minutes with PBS, and then, the sections were incubated with the secondary antibodies for 60 minutes at RT. After 3 rinses with PBS, the nuclei were counterstained with 1 g/mL Hoechst (Biosharp, China) for 5 minutes. Finally, a drop of antifade mounting medium (Solarbio, China) was placed on each slide, and a coverslip was placed on top of each sample. The immunofluorescence imaging was conducted using an Olympus fluorescence microscope.
2.7. Western blotting (WB)
Injured spinal cord epicenters 0.5 cm in size were collected and lysed by homogenization in 300 μL of lysis buffer containing 20 m mol L−1 Tris pH 7.4, 50 m mol L−1 NaCl, 1% Triton X‐100, and protease inhibitor. The debris was separated out by centrifugation, and the supernatants were aliquoted and stored at −80°C before use. The protein samples were placed in ice when taken out from −80°C storage to reduce protein degradation. Then, the samples were placed in 2× sodium dodecyl sulfate gel loading buffer, boiled for 5 minutes, and centrifuged at 6000 rpm at 4°C for 3 minutes to collect the supernatant. After electrophoresis, the proteins in the gels were transferred to polyvinylidene difluoride (PDVF) membranes using a transfer apparatus at 65 V for 2 hour at 4°C. The membranes were blocked at RT for 2 hours on a shaker. Subsequently, the membranes were incubated with monoclonal antibodies against GAPDH (1:3000, Abcam), thrombin (1:1000, Abcam), neurotrophins (BDNF, 1:200; NGF, 1:500; NT‐3, 1:200, Abcam), caspase‐3 (1:250, Proteintech) and Bcl‐2 (1:500, Proteintech), p38 (1:1000, Abcam), and JNK (1:1000, Abcam) at 4°C overnight. After 3 washes with TBST for 10 minutes each, goat anti‐rabbit IgG conjugated to horseradish peroxidase (1:2000, MDL, China) was added for an additional 1.5 hours. Then, the blots were washed again in TBST and visualized using an enhanced chemiluminescence (ECL) system.
2.8. Flow cytometric analysis of Annexin V‐FITC and Propidium Iodide (PI)
Annexin V‐FITC/PI kit (Sanjian Biotechnology, Tianjin, China) was used to determine whether cells are viable, apoptotic, or necrotic. The rats were sacrificed at the intended time points, and injured spinal cord epicenters 1 cm in size were collected and disrupted with a tissue grinder. The samples were added with 1 mL of Hank's Balanced Salt Solution (Biyun Biotechnology, Shanghai, China) and mixed with a shaker. After a 70‐μm cell strainer, the cell concentration was adjusted to 2x106/mL with Hank's Balanced Salt Solution and then added into 5 uL of Annexin V and 5 uL of propidium iodide mixed for 15 minutes at room temperature in the dark. The samples were assessed on a flow cytometer (Accuri C6, BD Biosciences, USA). The Annexin V‐FITC+/PI‐ cell populations were considered to represent apoptotic cells.
2.9. TUNEL staining
About 4% chloral hydrate via intraperitoneal injection was used to anesthetize animals, and 4% paraformaldehyde was used for perfuse. According to the manufacturer's recommended instructions of In Situ Cell Death Detection Kit (Roche, US), the sections were rehydrated as previously described under “Immunofluorescence staining.” Then, the samples were incubated with proteinase K working solution for 30 minutes at 37°C. After 2 rinses with PBS, samples were incubated with Converter‐POD solution for 30 minutes at 37°C and washed twice with PBS. The above operation was performed in a humidified chamber. Then, DAB substrate was added, and the samples were incubated for 10 minutes. Finally, after rinses with PBS 3 times, the sections were treated with glycerol before the coverslips were mounted.
2.10. Histological Staining
H&E staining and LFB staining were used to assess contusion area, cavity formation, and demyelination in different groups. At two time points, 3 days, and 8 weeks after SCI, the rats were anesthetized and perfused transcardially with 200 mL of 0.9% NaCl, followed by 300 mL 4% paraformaldehyde. A 1‐cm‐long piece of tissue, centered on the injury epicenter, was excised from the spinal cord. Then, it was immersed in 4% paraformaldehyde for 24 hours. Samples were embedded in paraffin and sliced into 5‐μm‐thick sections. Finally, the sections were subjected to H&E staining and LFB staining according to the standard procedures.
2.11. Behavioral analysis
To evaluate the functional recovery, we administered a battery of behavioral tests from day 1 to week 8 following surgery. Two observers, blinded to all group assignments, recorded the behavioral tests. Functional restoration was assessed according to BBB locomotor scores.23 The BBB test score evaluated hindlimb locomotor function on a scale from 0 to 21, with 0 representing no observable movement and 21 representing normal movement. Before evaluation, the rats were allowed to move freely. Scoring was based on spontaneous hindlimb movement during a 5‐minute observation period in the open field. The progress of hindlimb function and trunk control was assessed by the inclined plane test. The test was conducted using a board secured in place at one end. The free edge of the board was gradually raised to increase the angle of the incline. The maximum angle at which the rats could stay stable for 5 seconds was recorded as the inclined plane test angle. The test was performed three times, and the mean value was the final angle for the individual rat.
2.12. Statistical analysis
The statistical analyses were conducted through GraphPad Prism 5 software (San Diego, CA). Pairwise comparisons were made using Student's t‐test. The comparisons among multiple groups were carried out by one‐way ANOVA followed by a Bonferroni correction. Significant differences in behavioral analysis were used by repeated measures two‐way ANOVA with Tukey's post hoc test. All data were presented as the means ± SEM. P < 0.05 was considered to indicate a statistically significant difference.
3. RESULTS
3.1. Nafamostat mesilate (NM) improved the functional recovery of rats after SCI
To investigate the effect of NM on functional restoration, BBB locomotor scores and the inclined plane test were used during 8‐week postoperative period (Figure 1). Immediately following SCI, rats showed no observable movement of their hindlimbs, indicating that a severe SCI model was established successfully. There was no statistically significant difference in the BBB locomotor scores between the injury group and the NM group within 1 week, although the injury group showed a more limited degree of recovery and lesser functional improvement than NM group. However, compared with the injury group, the BBB locomotor scores exhibited a significant improvement in the NM treatment group from the 2nd week after surgery until the time of euthanasia (Figure 1A).
Figure 1.
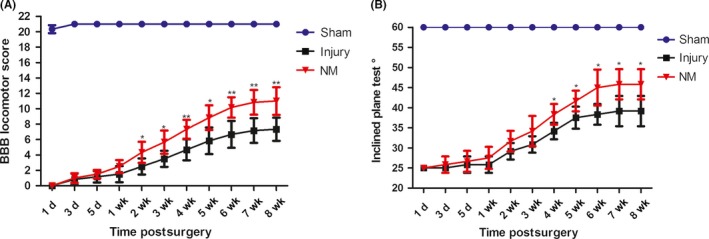
NM treatment improved behavioral functional recovery after SCI. Functional restoration was measured in terms of BBB locomotor scores and the inclined plane test. (A) BBB locomotor scores for the hindlimbs were assessed from day 1 to week 8 after surgery. (B) The inclined plane test was performed from day 1 to week 8 postsurgery. (*P < 0.05, **P < 0.01 vs. the injury group, n = 6)
The animals subjected to SCI could stay stable for 5 s at an angle of 25° at day 1 after surgery. Before the 4th week, the NM treatment group showed a more favorable result than the injury group on the inclined plane test, but the result was not statistically significant. However, NM treatment exhibited a significant improvement in the inclined plane test compared with the injury group after the 4th week (Figure 1B).
3.2. Nafamostat mesilate decreased the damaged area and alleviated cavity formation and demyelination in the lesion site after SCI
To determine whether NM treatment could repair SCI, histological analysis of spinal cord tissue was conducted by H&E staining and LFB staining. Spinal cord segments were derived from the injury group, the NM treatment group, and the sham group (Figure 2A‐D). The volumes of the damaged areas were observed at the 3rd day and the 8th week after injury. As illustrated in Figure 2A, H&E staining indicated that the areas or cavity of damaged regions in the injury group were larger than those in the NM treatment group at day 3 and week 8 after SCI (Figure 2B). Furthermore, to investigate the effect of NM on the demyelination after SCI, we performed LFB staining of spinal cord tissues from the injury epicenter at 3rd postinjury (Figure 2C). The NM treatment group showed larger LFB‐positive staining volumes than the injury group, indicating that NM can alleviate demyelination after SCI (Figure 2D). These data demonstrated that NM treatment promotes tissue preservation after SCI.
Figure 2.
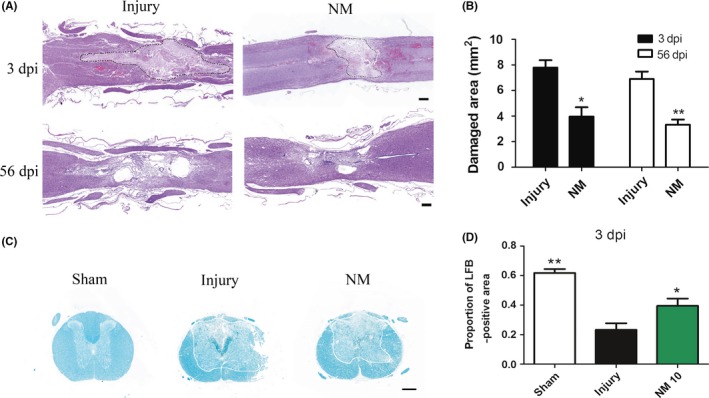
Damaged areas, cavity formation, and demyelination after SCI. (A) Images of representative H&E‐stained sagittal sections of spinal cords from the injury and NM groups at 3 days and 56 days after surgery. The damaged areas are enclosed by dashed lines. (B) Quantification of the damaged areas. (C) Representative images of LFB‐stained sections of the spinal cord at 3 days after surgery; the area outside the line represents the LFB‐positive tissue. (D) The quantitative results of LFB staining. Scale bars, 500 μm. (*P < 0.05, **P < 0.01 vs. the injury group, n = 6)
3.3. Nafamostat mesilate treatment attenuated apoptosis by p38 pathway in the spinal cord after SCI
TUNEL staining and flow cytometry of Annexin V‐FITC/PI were conducted to investigate cell apoptosis. As the results showed, amounts of apoptotic cells were increased at the spinal cord lesion site in the injury group compared with the sham group (Figure 3A). However, NM treatment markedly decreased the number of apoptotic cells in comparison with the injury group (Figure 3B). At the same time, the result of Annexin V‐FITC/PI flow cytometry showed that NM significantly decreased the cells that were induced to undergo apoptosis (Figure 3C‐F). Apparently, these results indicated that NM attenuates apoptosis after SCI.
Figure 3.
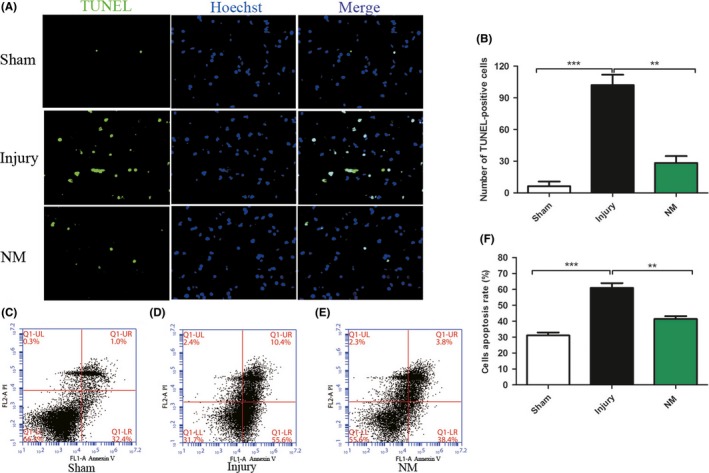
Cell apoptosis was detected by TUNEL staining and Annexin V‐FITC/PI flow cytometry. (A) TUNEL staining at day 3 after SCI. (B‐D) The results of Annexin V‐FITC/PI flow cytometry in sham, injury, and NM groups. (E) Quantification of TUNEL‐positive cells. (F) Quantification of flow cytometry. All data were expressed as the mean ± SEM. Scale bars, 100 μm. (*P < 0.05, **P < 0.01 vs. the injury group, n = 6)
Apoptosis‐related proteins caspase‐3 and Bcl‐2 were detected by Western blotting. Figure 4A shows the Western blots for caspase‐3, Bcl‐2, and GAPDH (an internal control) in each group. The quantitative results demonstrated that caspase‐3 protein was increased in the injury group (Figure 4B), indicating a higher rate of cell death, while NM decreased the level of the apoptosis‐related protein caspase‐3 in the injured spinal cord. Bcl‐2 inhibits apoptosis through preventing mitochondrial cytochrome c release and safeguarding mitochondrial membrane integrity.24 Compared with the sham group, the expression of Bcl‐2 was decreased in the injury group after SCI. NM treatment significantly increased the level of Bcl‐2 protein (Figure 4C). To study whether NM can attenuate apoptosis by modulating MAP kinase activity, we analyzed JNK and p38 by Western blot analysis (Figure 4D). As shown in Figure 4E and F, NM modulated p38 but not JNK signaling pathway.
Figure 4.
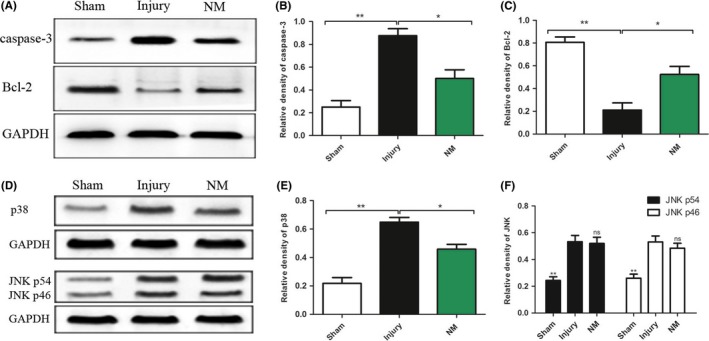
Apoptosis‐related proteins and signaling pathways were detected by Western blotting. (A) Western blots for caspase‐3 and Bcl‐2 in each group at 3 days after surgery. Quantification of the protein levels of caspase‐3 (B) and Bcl‐2 (C) in spinal cord tissues in each group. (D) Western blots for JNK and p38 in each group at 3 days after surgery. Quantification of the protein levels of p38 (E) and JNK (F) in spinal cord tissues in each group. All data were expressed as the mean ± SEM. (*P < 0.05, **P < 0.01 vs. the injury group, n = 3)
3.4. Nafamostat mesilate treatment suppressed the accumulation of microglia/macrophages after SCI
Microglia/macrophages are important elements in neuroinflammation, which could release inflammatory mediators, aggravate the secondary injury phase that occurs after SCI and further worsen tissue damage.25 CD68 is a specific marker of activated microglia/macrophages.26, 27 To investigate the inflammatory process in the experimental spinal cord lesions, CD68 cells were quantified by immunofluorescence staining. The results showed that there were more CD68‐positive cells in lesion site of spinal cord in injury group at day 3 after SCI (Figure 5A). Compared with the injury group, the NM treatment group had a significantly lower incidence of CD68‐positive cells (Figure 5B), indicating that NM can suppress the accumulation of microglia/macrophages.
Figure 5.
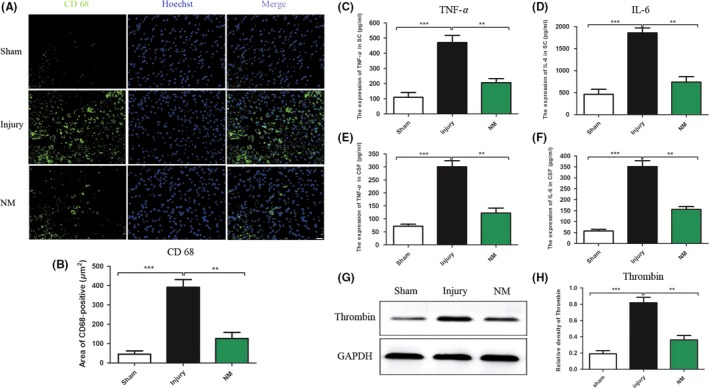
Accumulation of microglia/macrophages after SCI (A‐B). Expression of inflammatory cytokines and thrombin in spinal cord tissues and cerebrospinal fluid at 24 hours after surgery (C‐H). (A) Representative images of CD68‐stained sections from the epicenter of the spinal cord injury in the sham, injury, and NM groups 3 days after surgery. (B) Quantification of CD68 + cells. (C‐F) The effects of NM on the expression of TNF‐α and IL‐6 in spinal cord tissues (SC) and cerebrospinal fluid (CSF) in rats after SCI. (G) Western blotting of thrombin. (H) Quantitative analysis of thrombin expression, and data are expressed as the mean ± SEM. Scale bars, 100 μm. (*P < 0.05, **P < 0.01, ***P < 0.001 vs. the injury group, n = 11)
3.5. Nafamostat mesilate decreased the level of proinflammatory cytokines in spinal cord tissues and cerebrospinal fluid after SCI
To detect whether administration of NM decreases the level of inflammatory cytokines in spinal cord tissues as well as cerebrospinal fluid in the acute phase of injury, the proinflammatory cytokines were detected by ELISA28(Figure 5C‐F). TNF‐α and IL‐6 in the injury group were increased significantly in spinal cord tissues after SCI in comparison with the sham group. Compared with the injury group, the level of TNF‐α was significantly reduced in NM group (Figure 5C). As shown in Figure 5D, the concentration of IL‐6 was significantly decreased in the NM treatment group compared with the injury group at 24 hours after surgery. Similarly, NM treatment significantly decreased the expression of TNF‐α and IL‐6 in cerebrospinal fluid (Figure 5E and F). These results indicate that NM decreased the levels of proinflammatory cytokines in spinal cord tissues and cerebrospinal fluid after SCI. We also detected the effect of different dose NM on the expression of proinflammatory cytokines, and the results showed that the dose (10 or 20 mg/kg) was better than low dose group (5 mg/kg) (Figure S1).
Furthermore, the relationship of inflammation cell and proinflammatory cytokines was detected by immunofluorescence staining. The result showed that NM treatment significantly decreased the number of CD68+/TNF‐α+ cells. It indicated that NM can at least partially suppress the accumulation of microglia/macrophages to decrease the levels of proinflammatory cytokines (Figure S2).
3.6. Nafamostat mesilate decreased the expression of thrombin in rats after SCI
Thrombin has the ability to induce a neuroinflammatory effect.29, 30, 31 Under treatment with the serine protease inhibitor NM, the expression of thrombin was detected by WB (Figure 5G). As the results showed, the expression of thrombin was significantly higher after SCI than after the sham procedure. NM treatment significantly reduced thrombin compared with the injury group (Figure 5H).
3.7. Nafamostat mesilate increased neurotrophin expression after SCI in rats
BDNF, NGF, and NT‐3 were measured by WB at 3rd day after surgery (Figure 6A). After SCI, the expression of BDNF and NGF increased; however, the level of NT‐3 had no apparent change. NM markedly upregulated the expression of BDNF, NGF, and NT‐3 (Figure 6B). These data indicated that NM might enhance the expression of neurotrophic factors in spinal cord tissues to promote favorable neurological outcomes after SCI.
Figure 6.
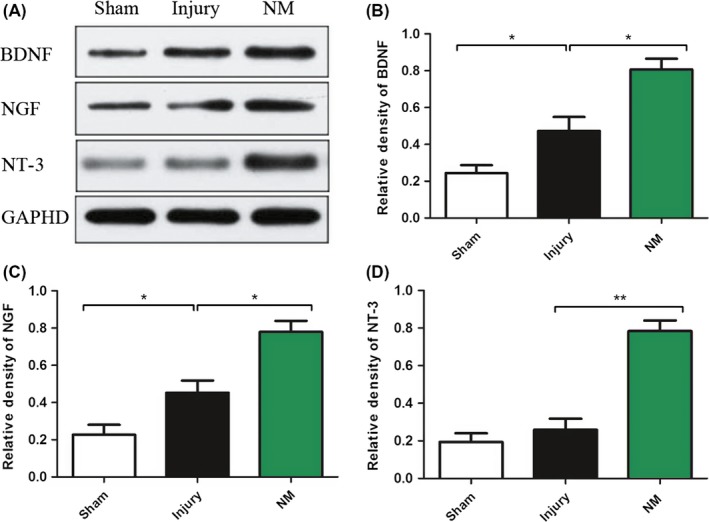
Expression of neurotrophins in rats at 3 days after surgery. (A) Quantification of BDNF (B), NGF (C), and NT‐3 (D). Data are expressed as the mean ± SEM. (*P < 0.05, **P < 0.01 vs. the injury group, n = 4)
4. DISCUSSION
Nafamostat mesilate (NM),a synthetic serine protease inhibitor,has been an effective medicine to cure acute pancreatitis, disseminated intravascular coagulopathy, and continuous renal replacement therapy. The present study first demonstrated that NM could attenuate the accumulation of microglia/macrophages,the expression of proinflammatory cytokine, and the expression of thrombin after SCI, and the apoptosis of cells was inhibited, finally the functional recovery of SCI rats was improved.
The posttraumatic inflammatory response to spinal cord injury plays a crucial role in tissue damage.32 Our results showed that the volumes of the damaged area and the cavity were reduced with the intervention of NM after SCI. Considering that the potential mechanisms of NM‐ameliorating SCI are antiinflammatory, thus we evaluated the accumulation of inflammation cells, microglia/macrophages. Microglia/macrophages, which can release inflammatory mediators, contributed to the development of the secondary injury phase that occurs after SCI and in the worsening of damage.25 Activated microglia/macrophages could express CD68. In the first 1‐3 days after spinal cord trauma, CD68 + microglia cells localize in the lesion area.25, 33 In our study, the number of CD68‐positive cells in the NM treatment group was reduced. Our findings suggest that NM can at least partially suppress the accumulation of microglia/macrophages.
Furthermore, NM could reduce the expression of proinflammatory cytokine. This study showed that the expression of TNF‐α was increased markedly after SCI. These overexpressed mRNA and protein intensify the processes of inflammation and neurodegeneration.34 IL‐6, a proinflammatory cytokine, has been indicated to play a crucial role in inflammatory response of SCI. Besides, it can also promote differentiation of astrocytes selectively. All above effects of IL‐6 are believed to coordinate to hinder nerve repair after SCI.35 The present study showed that NM treatment significantly decreased the expression of TNF‐α and IL‐6 at 24 hours after SCI. On the one hand, NM may reduce TNF‐α and IL‐6 expression through suppressing the accumulation of microglia/macrophages, which could produce TNF‐α and IL‐6. On the other hand, our results showed that NM also decreased astrocytes (Figure S3), which are known as immune effector cells to produce proinflammatory cytokines.
The mechanism of action of NM is inhibiting serine protease, and there is increasing evidence that serine proteases, particularly thrombin, are expressed locally in the CNS, and clear evidence is emerging that numerous CNS diseases, including Alzheimer's, multiple sclerosis, cerebral ischemia, and hemorrhage, are related to higher concentrations of thrombin, which causes neuroinflammation and apoptosis.11 Thus, the expression of thrombin was investigated with or without NM administration in SCI rats. Recently, studies of NM demonstrated its effectiveness in treating cerebral ischemia by inhibiting thrombin. These studies demonstrated that thrombin could activate microglia and astrocytes to produce proinflammatory cytokines.15, 36 Our results showed that the level of thrombin was upregulated as well as the microglia/macrophages were accumulated after SCI. Otherwise, NM could reduce the expression of thrombin and reduce the CD68 + cell, which indicated that NM may attenuate the inflammation after SCI through inhibiting the expression of thrombin, and NM treatment decreases the extent of histological damage, including cavity formation and demyelination, and improves locomotor function recovery after SCI.
The activation of microglia/macrophages and the upregulated expression of proinflammatory cytokines such as TNF‐α could cause oligodendrocyte necrosis.37 The death of neurons and oligodendrocytes that occurs after SCI makes a significant contribution to locomotor impairment and poor histopathological outcomes. In our study, NM inhibited caspase‐3 and the number of TUNEL‐positive cells and promoted the expression of Bcl‐2, demonstrating that NM attenuates cell apoptosis in the spinal cord after SCI. Further, neurotrophins, which contains BDNF, NGF, and NT3, could promote neuronal growth, synaptogenesis, cell survival, and neurogenesis.38 Our results showed that NM significantly upregulated the expression of BDNF, NGF, and NT3, consistent with the less cell death, the reduction in cavity volume, and the observed improvement in locomotor function.
This study also has the limitation. Radulovic M showed that PAR1 and thrombin were colocalized in microglia and astrocytes, which indicated thrombin may activate PAR1 to aggravate the inflammatory reaction. While we demonstrated that NM could attenuate the inflammatory reaction, the potential mechanism is that NM could inhibit thrombin. In the present study, the PAR‐1‐activated signaling pathway was not investigated; thus, further experiments are needed to figure out whether NM could impact the PAR‐1‐activated signaling pathway to attenuate SCI.
NM was demonstrated for the first time to be effective in treating acute SCI in rats. A series of pathophysiology changes occurred upon NM treatment, especially in the inflammatory response and apoptosis. Locomotion recovery also may have resulted from the increased levels of neurotrophins. Thrombin is critical in the development of pathophysiology after SCI. As a synthetic serine protease inhibitor, NM may exert the above effects by inhibiting thrombin, and further study is required to demonstrate it. Given that NM is already used clinically to treat pancreatitis, the translational advantage of NM is obvious.
5. CONCLUSIONS
Nafamostat mesilate (NM), a synthetic serine protease inhibitor, attenuates inflammation and apoptosis and promotes locomotion recovery after SCI. NM could be a promising drug for the treatment of SCI.
CONFLICT OF INTEREST
None.
Supporting information
ACKNOWLEDGMENTS
We thank the support of the following funding sources: the NSFC programme [grant number 81672171, 81330042, 81620108018] and the Tianjin Medical University General Hospital Youth Foundation [grant number ZYYFY2015008].
Duan H‐Q, Wu Q‐L, Yao X, et al. Nafamostat mesilate attenuates inflammation and apoptosis and promotes locomotor recovery after spinal cord injury. CNS Neurosci Ther. 2018;24:429–438. 10.1111/cns.12801
The first two authors contributed equally to this research.
REFERENCES
- 1. Ropper AE, Ropper AH. Acute spinal cord compression. N Engl J Med. 2017;376:1358‐1369. [DOI] [PubMed] [Google Scholar]
- 2. Yang EZ, Zhang GW, Xu JG, et al. Multichannel polymer scaffold seeded with activated Schwann cells and bone mesenchymal stem cells improves axonal regeneration and functional recovery after rat spinal cord injury. Acta Pharmacol Sin. 2017;38:623‐637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Evaniew N, Noonan VK, Fallah N, et al., RHSCIR Network . Methylprednisolone for the treatment of patients with acute spinal cord injuries: a propensity score‐matched cohort study from a Canadian multi‐center spinal cord injury registry. J Neurotrauma. 2015;32:1674‐1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ito Y, Sugimoto Y, Tomioka M, Kai N, Tanaka M. Does high dose methylprednisolone sodium succinate really improve neurological status in patient with acute cervical cord injury?: a prospective study about neurological recovery and early complications. Spine (Phila Pa 1976). 2009;34:2121‐2124. [DOI] [PubMed] [Google Scholar]
- 5. Qian T, Guo X, Levi AD, Vanni S, Shebert RT, Sipski ML. High‐dose methylprednisolone may cause myopathy in acute spinal cord injury patients. Spinal Cord. 2005;43:199‐203. [DOI] [PubMed] [Google Scholar]
- 6. Hurlbert RJ, Hadley MN, Walters BC, et al. Pharmacological therapy for acute spinal cord injury. Neurosurgery. 2015;76(Suppl 1):S71‐S83. [DOI] [PubMed] [Google Scholar]
- 7. Noorbakhsh F, Vergnolle N, Hollenberg MD, Power C. Proteinase‐activated receptors in the nervous system. Nat Rev Neurosci. 2003;4:981‐990. [DOI] [PubMed] [Google Scholar]
- 8. Ramachandran R, Hollenberg MD. Proteinases and signalling: pathophysiological and therapeutic implications via PARs and more. Br J Pharmacol. 2008;153(Suppl 1):S263‐S282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Coughlin SR. Thrombin signalling and protease‐activated receptors. Nature. 2000;407:258‐264. [DOI] [PubMed] [Google Scholar]
- 10. Saito T, Bunnett NW. Protease‐activated receptors: regulation of neuronal function. Neuromolecular Med. 2005;7:79‐99. [DOI] [PubMed] [Google Scholar]
- 11. Krenzlin H, Lorenz V, Danckwardt S, Kempski O, Alessandri B. The importance of thrombin in cerebral injury and disease. Int J Mol Sci. 2016;17:pii: E84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Radulovic M, Yoon H, Larson N, et al. Kallikrein cascades in traumatic spinal cord injury: in vitro evidence for roles in axonopathy and neuron degeneration. J Neuropathol Exp Neurol. 2013;72:1072‐1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stein ES, Itsekson‐Hayosh Z, Aronovich A, et al. Thrombin induces ischemic LTP (iLTP): implications for synaptic plasticity in the acute phase of ischemic stroke. Sci Rep. 2015;5:7912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Evans NR, Besser MW, Khadjooi K. Activated prothrombin complex in the management of direct thrombin inhibitor‐associated intracerebral haemorrhage. QJM. 2016;109:415‐416. [DOI] [PubMed] [Google Scholar]
- 15. Radulovic M, Yoon H, Wu J, Mustafa K, Scarisbrick IA. Targeting the thrombin receptor modulates inflammation and astrogliosis to improve recovery after spinal cord injury. Neurobiol Dis. 2016;93:226‐242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Festoff BW, Ameenuddin S, Santacruz K, et al. Neuroprotective effects of recombinant thrombomodulin in controlled contusion spinal cord injury implicates thrombin signaling. J Neurotrauma. 2004;21:907‐922. [DOI] [PubMed] [Google Scholar]
- 17. Makino S, Egi M, Kita H, Miyatake Y, Kubota K, Mizobuchi S. Comparison of nafamostat mesilate and unfractionated heparin as anticoagulants during continuous renal replacement therapy. Int J Artif Organs. 2016;39:16‐21. [DOI] [PubMed] [Google Scholar]
- 18. Hwang SD, Hyun YK, Moon SJ, Lee SC, Yoon SY. Nafamostat mesilate for anticoagulation in continuous renal replacement therapy. Int J Artif Organs. 2013;36:208‐216. [DOI] [PubMed] [Google Scholar]
- 19. Yang JW, Han BG, Kim BR, et al. Superior outcome of nafamostat mesilate as an anticoagulant in patients undergoing maintenance hemodialysis with intracerebral hemorrhage. Ren Fail. 2009;31:668‐675. [DOI] [PubMed] [Google Scholar]
- 20. Li C, Wang J, Fang Y, et al. Nafamostat mesilate improves function recovery after stroke by inhibiting neuroinflammation in rats. Brain Behav Immun. 2016;56:230‐245. [DOI] [PubMed] [Google Scholar]
- 21. Kwon SK, Ahn M, Song HJ, et al. Nafamostat mesilate attenuates transient focal ischemia/reperfusion‐induced brain injury via the inhibition of endoplasmic reticulum stress. Brain Res. 2015;1627:12‐20. [DOI] [PubMed] [Google Scholar]
- 22. Chen T, Wang J, Li C, et al. Nafamostat mesilate attenuates neuronal damage in a rat model of transient focal cerebral ischemia through thrombin inhibition. Sci Rep. 2014;4:5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1‐21. [DOI] [PubMed] [Google Scholar]
- 24. Marsden VS, O'Connor L, O'Reilly LA, et al. Apoptosis initiated by Bcl‐2‐regulated caspase activation independently of the cytochrome c/Apaf‐1/caspase‐9 apoptosome. Nature. 2002;419:634‐637. [DOI] [PubMed] [Google Scholar]
- 25. Papa S, Rossi F, Ferrari R, et al. Selective nanovector mediated treatment of activated proinflammatory microglia/macrophages in spinal cord injury. ACS Nano. 2013;7:9881‐9895. [DOI] [PubMed] [Google Scholar]
- 26. Fleming JC, Norenberg MD, Ramsay DA, et al. The cellular inflammatory response in human spinal cords after injury. Brain. 2006;129:3249‐3269. [DOI] [PubMed] [Google Scholar]
- 27. David S, Lopez‐Vales R, Wee Yong V. Harmful and beneficial effects of inflammation after spinal cord injury: potential therapeutic implications. Handb Clin Neurol. 2012;109:485‐502. [DOI] [PubMed] [Google Scholar]
- 28. Pineau I, Lacroix S. Proinflammatory cytokine synthesis in the injured mouse spinal cord: multiphasic expression pattern and identification of the cell types involved. J Comp Neurol. 2007;500:267‐285. [DOI] [PubMed] [Google Scholar]
- 29. Debeir T, Gueugnon J, Vige X, Benavides J. Transduction mechanisms involved in thrombin receptor‐induced nerve growth factor secretion and cell division in primary cultures of astrocytes. J Neurochem. 1996;66:2320‐2328. [DOI] [PubMed] [Google Scholar]
- 30. Suo Z, Wu M, Ameenuddin S, et al. Participation of protease‐activated receptor‐1 in thrombin‐induced microglial activation. J Neurochem. 2002;80:655‐666. [DOI] [PubMed] [Google Scholar]
- 31. Grabham P, Cunningham DD. Thrombin receptor activation stimulates astrocyte proliferation and reversal of stellation by distinct pathways: involvement of tyrosine phosphorylation. J Neurochem. 1995;64:583‐591. [DOI] [PubMed] [Google Scholar]
- 32. Lopez‐Vales R, García‐Alías G, Guzmán‐Lenis MS, et al. Effects of COX‐2 and iNOS inhibitors alone or in combination with olfactory ensheathing cell grafts after spinal cord injury. Spine (Phila Pa 1976). 2006;31:1100‐1106. [DOI] [PubMed] [Google Scholar]
- 33. Li G, Che MT, Zhang K, et al. Graft of the NT‐3 persistent delivery gelatin sponge scaffold promotes axon regeneration, attenuates inflammation, and induces cell migration in rat and canine with spinal cord injury. Biomaterials. 2016;83:233‐248. [DOI] [PubMed] [Google Scholar]
- 34. Hao J, Li B, Duan HQ, et al. Deferoxamine promotes functional recovery in a rat contusion spinal cord injury model by inhibiting expression of TNF‐α and IL‐1β. Neural Regen Res. 2017;17:1191‐1203. [Google Scholar]
- 35. Mukaino M, Nakamura M, Okada S, Toyama Y, Liu M, Okano H. Role of IL‐6 in regulation of inflammation and stem cell differentiation in CNS trauma. Nihon Rinsho Meneki Gakkai Kaishi. 2008;31:93‐98. [DOI] [PubMed] [Google Scholar]
- 36. Yang Y, Zhang M, Kang X, et al. Thrombin‐induced microglial activation impairs hippocampal neurogenesis and spatial memory ability in mice. Behav Brain Funct. 2015;11:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Selmaj KW, Raine CS. Tumor necrosis factor mediates myelin and oligodendrocyte damage in vitro. Ann Neurol. 1988;23:339‐346. [DOI] [PubMed] [Google Scholar]
- 38. Weishaupt N, Blesch A, Fouad K. BDNF: the career of a multifaceted neurotrophin in spinal cord injury. Exp Neurol. 2012;238:254‐264. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


