Abstract
Background
Cocaine use has been associated with adverse cardiovascular outcomes in patients with coronary artery disease (CAD). It is unclear whether this is due to direct effects of cocaine or other factors.
Hypothesis
Cocaine use is associated with worse outcomes in patients undergoing cardiac catheterization
Methods
We used the Veterans Affairs database to identify veterans undergoing coronary catheterization between 2007 and 2014. We analyzed association between cocaine use and 1‐year all‐cause mortality, myocardial infarction (MI), and cerebrovascular accident (CVA) among veterans with obstructive CAD (N = 122 035). To explore factors contributing to these associations, we sequentially adjusted for cardiac risk factors, risky behaviors, and clinical conditions directly affected by cocaine.
Results
3082 (2.5%) veterans were cocaine users. Cocaine users were younger (median 58.2 vs 65.3 years; P < 0.001), more likely to be African American (58.9% vs 10.6%; P < 0.001), and had fewer traditional cardiac risk factors. After adjustment for cardiac risk factors, cocaine was associated with increased risk of mortality (HR: 1.23, 95% CI: 1.08‐1.39), MI (HR: 1.40, 95% CI: 1.07‐1.83), and CVA (HR: 1.88, 95% CI: 1.38‐2.57). With continued adjustment, increased CVA risk remained significantly associated with cocaine use, whereas MI risk was mediated by risky behaviors and mortality was fully explained by conditions directly affected by cocaine.
Conclusions
Cocaine use is associated with adverse cardiac events in veterans with CAD. Contributors to this association are multifaceted and specific to individual cardiovascular outcomes, including associated risky behaviors and direct effects of cocaine. Effective intervention programs to reduce cardiac events in this population will require multiple components addressing these factors.
Keywords: Cardiac Catheterization, Cocaine, Percutaneous Coronary Intervention
1. INTRODUCTION
Cocaine has been associated with numerous toxic effects on the cardiovascular (CV) system, notably myocardial infarction (MI), arrhythmias, congestive heart failure (CHF), cerebrovascular accidents (CVA), and sudden cardiac death.1, 2 Several mechanisms of these effects have been proposed, including increased myocardial oxygen demand, coronary‐artery vasoconstriction, enhanced platelet aggregation, and thrombus formation.3 In addition, cocaine use has been associated with unhealthy lifestyle choices,4 comorbid mental and substance‐abuse disorders,5, 6 poor socioeconomic status,7 and medication noncompliance8—all of which also can lead to adverse CV events.
Currently, it is unclear how these potential mechanisms, individually and in aggregate, contribute to adverse CV outcomes. Better understanding of these mechanisms would help guide more effective prevention and treatment.
To address this gap, we described the association of cocaine use with the 1‐year risk of all‐cause mortality, MI, and CVA in a Veterans Affairs (VA) population of coronary artery disease (CAD) patients undergoing cardiac catheterization. We then performed sequential, multivariable adjustment to assess the relationships with important confounders such as basic cardiac risk factors, risky behaviors (eg, alcohol and other substance abuse), and conditions directly affected by cocaine (eg, psychological disorders, CHF, and end‐stage renal disease). Insights from this analysis would be expected to inform more effective prevention and treatment options in this population.
2. METHODS
2.1. Study population source
The VA Clinical Assessment Reporting and Tracking (CART) program is a national quality initiative launched in 2005 focusing on coronary revascularization procedures conducted in all VA cardiac catheterization laboratories.9 A key feature of the CART program is a clinical software application designed to collect standardized data on all coronary angiograms and percutaneous coronary interventions (PCI).10 The software is embedded in the VA electronic health record and allows providers to enter patient and procedural information (preprocedure assessment, coronary angiography, and PCI) as part of routine clinical workflow. The CART software was designed using standardized definitions that conform to the definitions and standards of the American College of Cardiology (ACC) National Cardiovascular Data Registry (NCDR)11 and incorporates features such as pull‐down menus and automated clinical report generation to ensure uniformity of data entry by different providers and in different cardiac catheterization laboratories.12 Participation in CART has been mandatory and universal in all VA cardiac catheterization laboratories since 2009. Quality checks of the data are periodically conducted for completeness and accuracy.13 CART data are combined with other VA data sources to create a longitudinal clinical database in support of the quality assessment, quality improvement, and clinical research missions of the CART program.
2.2. Study population
A total of 218 387 veterans underwent cardiac catheterization during the study period between October 1, 2007, and September 31, 2014. We excluded all veterans without obstructive CAD, defined as 1‐, 2‐, or 3‐vessel/left main obstructive disease by coronary angiography (n = 81 053, 37.1%); those with indeterminate cocaine use (n = 8725, 4.0%); those missing procedural indication (n = 6537, 3.0%); and those without ≥1 year of administrative follow‐up (n = 37, 0.02%) in the VA healthcare system. Our final study cohort consisted of 122 035 veterans.
2.3. Cocaine use definition
Cocaine use was defined using International Classification of Diseases, Ninth Revision (ICD‐9) codes for “cocaine abuse” and “cocaine dependence” (305.6x and 304.2x, respectively) associated with any visit to a VA facility in the year prior to cardiac catheterization. ICD‐9 codes for “cocaine use in remission” (305.63 or 304.23) were excluded from the study to select for active cocaine users. Veterans were also excluded from the study population if drug use was indicated but no further ICD‐9 codes specified the type of drug use.
2.4. Outcomes
The outcomes of interest were rates of all‐cause mortality, rehospitalization for MI, and CVA events during the year following the index angiogram. All‐cause mortality was determined by the VA Vital Status file. MI and CVA events were assessed from the VA medical and administrative records, as well as the fee‐based data files, using the ICD‐9 codes associated with each condition.14
2.5. Statistical analysis
We compared baseline demographic and clinical characteristics between veterans with and without cocaine use using χ2 tests for categorical variables and Mann‐Whitney Wilcoxon tests for continuous variables. Cox proportional hazards models were then used to determine the unadjusted associations between cocaine use and 1‐year all‐cause mortality, MI, and CVA.
To determine the relative impact of cardiac risk factors, risky behaviors, and the physiological effects of cocaine, we modeled the hypothesized relationships using directed acyclic graphs (DAGs; Figure 1).15. DAGs are graphical representations of relationships between the variables of interest and can assist in constructing models to test these relationships. Both basic cardiac risk factors and risky behaviors were modeled as potential confounders, with effects on both the exposure and the outcome, as shown in Figure 1. Basic risk factors included age, sex, race, diabetes, hypertension, hyperlipidemia, peripheral arterial disease,16 cerebrovascular disease, obesity/overweight, chronic obstructive pulmonary disease, prior MI, prior PCI, and prior coronary artery bypass grafting. Risky behaviors included alcohol abuse, tobacco use, and other drug use.6 It should be emphasized that our differentiation between “basic” risk factors and “risky” behaviors is derived from the aforementioned literature; to address this dichotomy, we analyzed all outcomes as unadjusted and adjusted for basic risks, basic risks and risky behaviors, and basic risks, risky behaviors with the DAG pathway (Figure 2).
Figure 1.
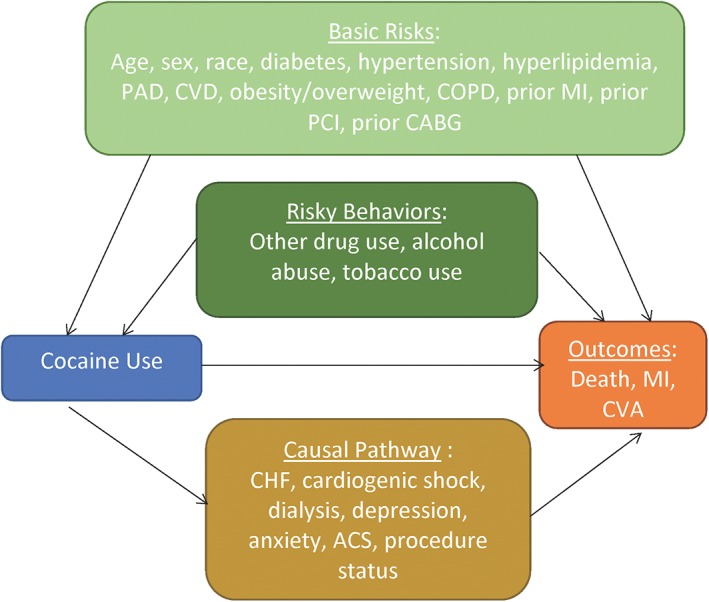
DAG of hypothesized confounders and mediators of cocaine use and adverse CV outcomes in veterans with obstructive CAD, based on prior research. Abbreviations: ACS, acute coronary syndrome; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CV, cardiovascular; CVA, cerebrovascular accident; CVD, cerebrovascular disease; DAG, directed acyclic graph; MI, myocardial infarction; PAD, peripheral arterial disease; PCI, percutaneous coronary intervention
Figure 2.
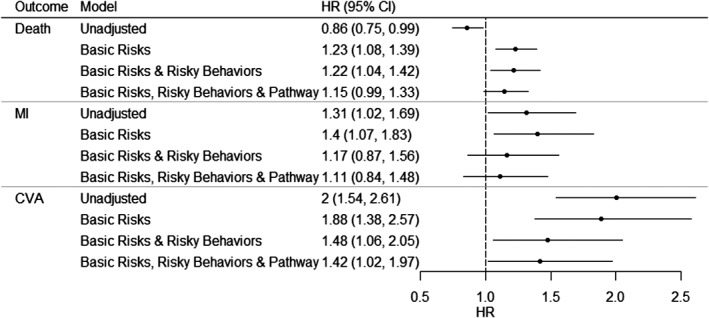
Sequential Cox proportional hazard models for death, MI, and CVA in veterans with obstructive CAD by cocaine use. Unadjusted: cocaine. Additional adjustment for basic risks: age, sex, race, DM, HTN, hyperlipidemia, PAD, CVD, obesity/overweight, COPD, prior MI, prior PCI, and prior CABG. Additional adjustment for risky behaviors: alcohol abuse, tobacco use, and other drug use. Additional adjustment for comorbid conditions affected by cocaine use (“causal pathway”): CHF, cardiogenic shock, dialysis, depression, anxiety, ACS, and clinical status (elective, emergent, urgent, or salvage). Abbreviations: ACS, acute coronary syndrome; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CHF, congestive heart failure; CI, confidence interval; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; CVD, cerebrovascular disease; DM, diabetes mellitus; HR, hazard ratio; HTN, hypertension; MI, myocardial infarction; PAD, peripheral arterial disease; PCI, percutaneous coronary intervention
Cocaine use may also have physiological effects that affect other major risk factors for adverse cardiac outcomes. Thus, we also examined “causal pathway” factors that may explain the connection between cocaine use and CV outcomes (ie, they are affected by cocaine use and have effects on the outcomes of interest, as shown in Figure 1). The potential “causal pathway” conditions that we examined included CHF, cardiogenic shock, dialysis, depression, anxiety, acute coronary syndrome (ACS) status, and clinical status at the time of the index catheterization (elective, emergent, urgent, or salvage).5, 6
The Cox models included a robust estimator of the covariance matrix to account for clustering by site. All tests for statistical significance were evaluated at a significance level of P < 0.05. Statistical analyses were performed by the CART Coordinating Center at the Denver VA Medical Center using SAS software, version 9.4 (SAS Institute Inc., Cary, NC). The study was approved by the Colorado Multiple Institutional Review Board.
3. RESULTS
3.1. Baseline patient characteristics
Among all veterans undergoing cardiac catheterization, the prevalence of cocaine use was 3.5%. In our cohort of veterans with obstructive CAD, 3082 (2.5%) had evidence of cocaine use. Cocaine users were younger (median age, 58.2 vs 65.3 years; P < 0.001) and more frequently African American (58.9% vs 10.6%; P < 0.001; Table 1). There was a lower incidence of traditional cardiac risk factors among cocaine users, including diabetes, hyperlipidemia, peripheral arterial disease, CVA, and obesity (P < 0.001 for all). In contrast, psychological comorbidities and substance abuse were substantially higher among cocaine users (P < 0.001 for all). Cardiac catheterization was performed more frequently as an emergent (7.5% vs 3.7%) or urgent (35.4% vs 25.6%) procedure, rather than elective (57.1% vs 70.6%), in cocaine users (Table 2). ACS (36.3% vs 25.2%) was noted more frequently as the indication for cardiac catheterization in cocaine users. Cocaine users had lower rates of medical therapy for CAD prior to cardiac catheterization, including the use of β‐blockers, nitrates, and statins. The degree of obstructive CAD was less severe in cocaine users than in non–cocaine users.
Table 1.
Baseline characteristics of veterans with obstructive CAD
| Total, N = 122 035 | No Cocaine, n = 118 953 | Cocaine, n = 3082 | P Value | |
|---|---|---|---|---|
| Demographics | ||||
| Age, y, median (IQR) | 65.1 (60.9–71.9) | 65.3 (61.1–72.2) | 58.2 (53.7–62.1) | <0.001 |
| Male sex | 98.6 | 98.6 | 98.6 | 0.96 |
| Race | <0.001 | |||
| White | 80.8 | 82 | 37.2 | |
| Black | 11.8 | 10.6 | 58.9 | |
| Other | 7.3 | 7.4 | 4 | |
| CV risk factors | ||||
| DM | 49.5 | 49.7 | 40.1 | <0.001 |
| HTN | 90.3 | 90.3 | 89.8 | 0.43 |
| Hyperlipidemia | 89.5 | 89.8 | 78.1 | <0.001 |
| PAD | 22.6 | 22.7 | 18.1 | <0.001 |
| CVD | 18.4 | 18.5 | 14.5 | <0.001 |
| CHF | 27.4 | 27.3 | 28.8 | 0.07 |
| COPD | 20.9 | 20.8 | 23.9 | <0.001 |
| Obesity | 46.9 | 47.2 | 35.3 | <0.001 |
| Overweight | 35.9 | 35.9 | 36.1 | 0.8 |
| eGFR, mL/min/1.73 m2, median (IQR) | 72.4 (59.6–88.3) | 72.2 (59.5–88.0) | 80.0 (61.3–98.0) | <0.001 |
| CKD | 21 | 21 | 20.3 | 0.39 |
| Dialysis | 2.8 | 2.8 | 3.3 | 0.07 |
| Psychological risk factors | ||||
| Chronic depression | 27 | 26.4 | 51.7 | <0.001 |
| Anxiety | 8.7 | 8.5 | 14.3 | <0.001 |
| Other substance use | ||||
| Tobacco use | 61.9 | 61.3 | 84.6 | <0.001 |
| Alcohol abuse | 7.2 | 6.1 | 47.3 | <0.001 |
| Any illicit drug other than cocaine | 4.1 | 2.8 | 55.2 | <0.001 |
| Cannabis | 2.6 | 1.7 | 35.1 | <0.001 |
| Sedatives | 0.3 | 0.2 | 5 | <0.001 |
| Hallucinogens | 0 | 0 | 0.6 | <0.001 |
| Amphetamines | 0.3 | 0.2 | 5 | <0.001 |
| Opioids | 1.4 | 0.9 | 21.3 | <0.001 |
| Unspecified type of drug use | 0.2 | 0 | 6.6 | <0.001 |
| Prior procedures | ||||
| PCI | 31 | 31.1 | 27.7 | <0.0001 |
| MI | 36.3 | 36.3 | 37.4 | 0.21 |
| CABG | 26.8 | 27.1 | 15.1 | <0.0001 |
| FRS category | ||||
| Low | 13.4 | 13.2 | 20 | <0.0001 |
| Medium | 46.6 | 46.5 | 49.7 | |
| High | 40 | 40.3 | 30.3 |
Abbreviations: CABG, coronary artery bypass grafting; CAD, coronary artery disease; CHF, congestive heart failure; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CV, cardiovascular; CVD, cerebrovascular disease; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; FRS, Framingham Risk Score; HTN, hypertension; IQR, interquartile range; MI, myocardial infarction; PAD, peripheral arterial disease; PCI, percutaneous coronary intervention; SD, standard deviation.
Data are presented as percentages (%) unless otherwise noted.
Table 2.
Procedural characteristics of veterans with obstructive CAD
| Presentation | Total, N = 122 035 | No Cocaine, n = 118 953 | Cocaine, n = 3082 | P Value |
|---|---|---|---|---|
| Indication for cardiac catheterization | ||||
| ACS | 25.5 | 25.2 | 36.3 | <0.001 |
| Chest pain | 44.4 | 44.4 | 41.4 | |
| IHD | 9.1 | 9.1 | 6.4 | |
| Positive functional study | 10.7 | 10.7 | 7.2 | |
| Cardiomyopathy | 3.8 | 3.8 | 4.7 | |
| Valvular disease | 2.8 | 2.9 | 1.2 | |
| Other | 3.8 | 3.8 | 2.8 | |
| Cardiogenic shock | 0.4 | 0.4 | 0.8 | <0.001 |
| Procedural status | ||||
| Elective | 70.3 | 70.6 | 57.1 | <0.001 |
| Emergent | 3.8 | 3.7 | 7.5 | |
| Urgent | 25.9 | 25.6 | 35.4 | |
| Salvage | 0.1 | 0.1 | 0 | |
| Preprocedural stress testing | 45 | 45.2 | 37.9 | <0.001 |
| Results | ||||
| Positive | 42.1 | 42.1 | 42.6 | 0.83 |
| Negative | 2.8 | 2.8 | 3.3 | |
| Equivocal | 1.8 | 1.8 | 1.5 | |
| Dysrhythmia | 0 | 0 | 0 | |
| Indeterminate | 0.4 | 0.4 | 0.4 | |
| Unknown | 52.9 | 53 | 52.1 | |
| Medications at presentation | ||||
| β‐Blockers | 60 | 60.3 | 50.4 | <0.001 |
| Nitrates | 38 | 38.1 | 33.1 | <0.001 |
| CCBs | 23.8 | 23.8 | 24.1 | 0.62 |
| Statins | 62.9 | 63.2 | 50.6 | <0.001 |
| Angiographic findings | ||||
| 1‐vessel obstructive | 35.6 | 35.3 | 45.8 | <0.001 |
| 2‐vessel obstructive | 26.3 | 26.3 | 26 | |
| 3‐vessel/LM obstructive | 38.1 | 38.3 | 28.2 |
Abbreviations: ACS, acute coronary syndrome; CAD, coronary artery disease; CCB, calcium channel blocker; IHD, ischemic heart disease; LM, left main artery.
Data are presented as percentages (%).
3.2. Cocaine use and outcomes
In unadjusted analyses, cocaine use was associated with an increased risk of MI (hazard ratio [HR]: 1.31, 95% confidence interval [CI]: 1.02‐1.69) and CVA (HR: 2.00, 95% CI: 1.54‐2.61), but a decreased risk of mortality (HR: 0.86, 95% CI: 0.75‐0.99; Figure 2). With adjustment for basic cardiac risk factors, cocaine was significantly associated with all 3 outcomes: mortality (HR: 1.23, 95% CI: 1.08‐1.39), MI (HR: 1.40, 95% CI: 1.07‐1.83), and CVA (HR: 1.88, 95% CI: 1.38‐2.57). After adjustment for risky behaviors, mortality and CVA remained significantly associated with cocaine use (HR for mortality: 1.22, 95% CI: 1.04‐1.42; HR for CVA: 1.48, 95% CI: 1.06‐2.05), but MI was no longer significant (HR: 1.17, 95% CI: 0.87‐1.56). After adjustment for causal pathway conditions, CVA remained significantly associated with cocaine use (HR: 1.42, 95% CI: 1.02‐1.97), but mortality was no longer significant (HR: 1.15, 95% CI: 0.99‐1.33).
3.3. Overall mortality, MI, and stroke
In unadjusted Kaplan‐Meier analyses, the overall mortality (P = 0.024) and stroke‐free survival curves (P < 0.001) appear to separate gradually over the 1‐year follow‐up period. In contrast, the MI‐free survival curve appears to separate at 75 days after initial cardiac catheterization and then remain parallel (P = 0.005; Figure 3).
Figure 3.
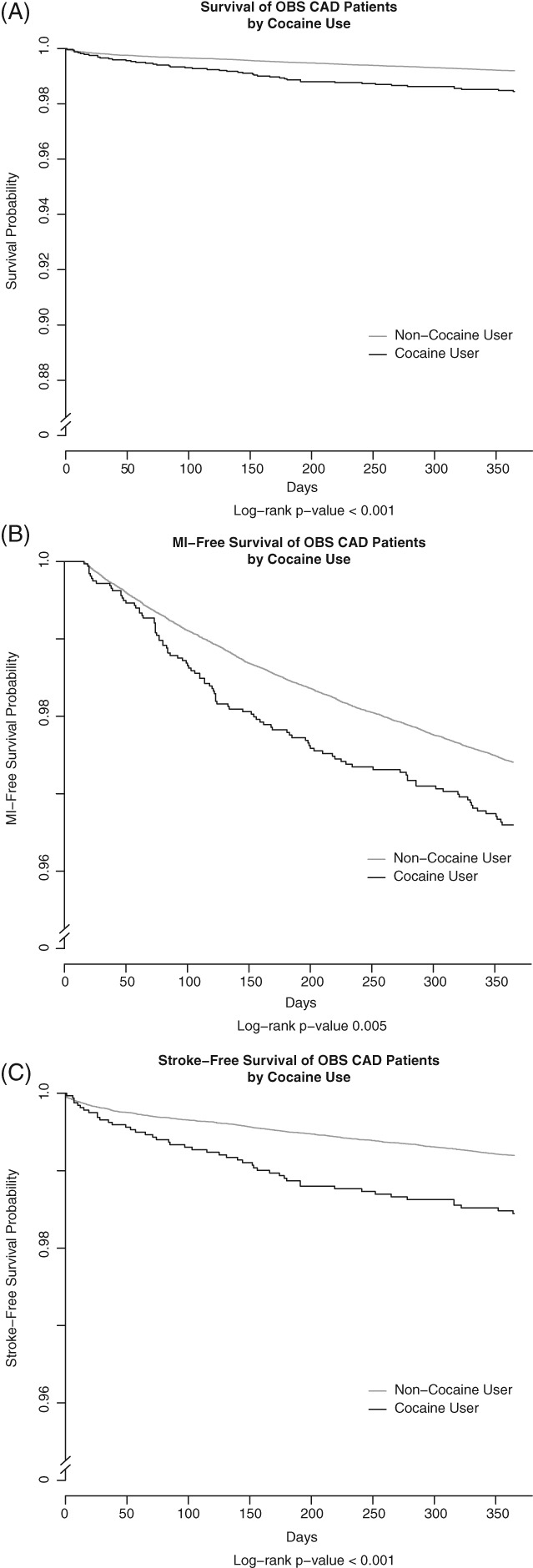
Unadjusted Kaplan‐Meier survival curves for cocaine users vs non–cocaine users. (A) Overall survival, (B) MI‐free survival, (C) stroke‐free survival. Abbreviations: MI, myocardial infarction
4. DISCUSSION
In this retrospective observational study of cocaine use among veterans with obstructive CAD undergoing cardiac catheterization, we confirmed the association between cocaine use and adverse CV outcomes and found that the contributors to this association varied by outcomes. Specifically, the association between cocaine and increased mortality risk was no longer apparent after adjustment for “causal pathway” conditions directly affected by cocaine, which included CHF, cardiogenic shock, dialysis, depression, anxiety, ACS, and clinical status at the time of index catheterization. The association with increased risk of MI was no longer apparent after adjustment for risky behaviors associated with cocaine use, which included alcohol abuse, tobacco use, and other drug use. Finally, increased risk of CVA remained significantly associated with cocaine, even after adjustment for basic cardiac risk factors, risky behaviors, and causal pathway conditions.
This study extends our knowledge about the effects of cocaine on adverse CV outcomes. It is the largest study, to our knowledge, of veterans with CAD undergoing cardiac catheterization, which enables us to more fully explore contributors to this association. It reveals a concerning prevalence of cocaine use (3.5%) among all veterans undergoing catheterization, which is congruent with a recent Congressional report (2013) that estimated a prevalence of substance dependence and abuse of 3% to 5% among all veterans.17 It also indicates that most of these veterans seek care in the VA healthcare system, because the ACC ACTION Registry–Get With the Guidelines (GWTG) program, which collects information from non‐VA hospitals, found that only 0.9% of veterans with acute MI had active cocaine use.18 Accordingly, it is important for the VA to understand the prevalence of cocaine use and how it contributes to CV outcomes, as this can inform its prevention and management.
A recent National (Nationwide) Inpatient Sample study retrospectively analyzed hospital admissions for chest pain associated with cocaine use.19 The study investigated an all‐comer chest‐pain population. In this overall lower‐risk population, the observed in‐hospital mortality was substantially lower (0.09%) than in our cohort, where we report 1‐year mortality. Additionally, all patients in our study underwent cardiac catheterization, compared with 6.7% in the paper by Singh et al. Comparison with this study is important as it underlines the profound significance in understanding the population being studied. When comparing veteran populations with non‐veteran populations, it is important to understand that veterans are mostly male and are considerably older than non‐veterans (veteran median age 64 years, vs 44 years for non‐veterans). Hence, any characteristics associated with age (such as income, occupation, or disabilities) may be impacted by the age difference. Lastly, individuals who enlist in the US Armed Forces may be selected based on physical or mental abilities, which likely differentiates them from those who elect not to enlist.20
Our study also begins to elucidate how cocaine use contributes to adverse cardiac events. Although multiple physiologic studies have characterized the impact of cocaine on the CV system, our approach also takes into account the variety of behavioral and environmental influences that often impact cocaine users. By characterizing these influences using DAGs and sequentially modeling them in our analyses, we are able to provide some additional insight into real‐world influences of cocaine use.
Although there are a variety of ways to model the mediators of adverse effects of cocaine, our selection and grouping of variables was guided by the likely interventions that could mitigate adverse outcomes. For example, risky behaviors, such as tobacco and alcohol use, can often exacerbate cardiac outcomes, both via direct effects of the substances on the CV system and through associated lifestyle factors such as poor diet, decreased exercise, and poor medication adherence. Our analysis demonstrates that these factors were particularly influential in MI rates, and interventions to reduce the harmful effects on these behaviors may be especially beneficial in MI patients. We observed a gradual separation in the overall mortality and stroke‐free survival curves over the study period, whereas the myocardial infarction‐free survival indicates a stepwise increase in myocardial infarctions 75 days after cardiac catheterization. While a number of biologically plausible mechanisms could be evoked for these observed differences, the cause for the stepwise increase in MIs is uncertain.
Similarly, medical conditions directly impacted by the vasoactive and other properties of cocaine, such as ACS, heart failure, and psychological conditions, were influential in our analysis, most notably in mortality rates. Mitigation strategies suggested by this association, in addition to cocaine cessation, include optimal guideline‐suggested therapies for the associated condition and optimal control of risk factors that could lead to these conditions. This is particularly germane for depression and anxiety, which have an inordinately high prevalence among the veteran population.17 Finally, our study is only 1 additional step in understanding the complex relationship between cocaine and CV disease. The persistent association between cocaine and CVA, even after adjusting for risky behavior and causal pathway variables, underscores the limitations of our current understanding.
4.1. Study limitations
Our findings should be interpreted in light of several limitations. First, as an observational study, causation between cocaine use and the outcomes cannot be conclusively determined. Although we constructed a coherent rationale for identifying variables that could be contributing to the association between cocaine and outcomes, no model can completely capture all potential associations and variables. Nonetheless, we collected as many associated confounders and potential mediators as was available in our dataset, and we incorporated them into our modeling to partially mitigate against this limitation. Second, data for our study were collected through the electronic health record and were dependent upon the accuracy and completeness of documentation. In particular, we utilized ICD‐9 codes to identify veterans with cocaine use within 1 year of coronary angiography. Thus, we were unable to distinguish between acute and recent cocaine ingestion at the time of outcome occurrence. Although we restricted our analyses to 1 year as a partial offset for this limitation, precise timing of exposure and outcome is not possible. It is also likely that veterans underreport their cocaine use,21, 22, 23 thus causing some potential misclassification of our exposure. Furthermore, we were only able to determine all‐cause mortality, rather than cardiac‐specific causes of death. Although our mortality findings have specific implications for this population, future studies with access to cause‐specific mortality data should be conducted. Therefore, it should be strongly emphasized that we cannot determine the exact causes or mechanisms of increased overall mortality observed in our cohort. Finally, as indicated previously, our patient population was limited to US veterans, which may not be representative of the general population.
5. CONCLUSION
Among veterans with CAD undergoing cardiac catheterization, cocaine use was associated with a significant increase in adjusted risk for 1‐year mortality, MI, and CVA. Contributors to this association are multifaceted and specific to individual CV outcomes; the toxic effects of cocaine on specific medical conditions augmented the risk of mortality, risky behaviors augmented the risk of MI, and factors unaccounted for in our analysis contributed to the risk of CVA. As a result, effective intervention programs to reduce cardiac events in this population will require multiple components addressing these factors.
Conflicts of interest
The authors declare no potential conflicts of interest.
Author contributions
Study concept and design, AG, MIV, TMM, and MAS. Drafting of the manuscript, AG, TMM, and MIV. Statistical analysis, MAS and AEB. Obtained funding, MIV. All authors were involved in acquisition, analysis, or interpretation of the data; provided critical revision of the manuscript for important intellectual content; and provided administrative, technical, or material support.
Gunja A, Stanislawski MA, Barón AE, Maddox TM, Bradley SM, Vidovich MI. The implications of cocaine use and associated behaviors on adverse cardiovascular outcomes among veterans: Insights from the VA Clinical Assessment, Reporting, and Tracking (CART) Program. Clin Cardiol. 2018;41:809–816. 10.1002/clc.22961
Funding information The study was funded by the US Department of Veterans Affairs. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs of the US government. The authors are solely responsible for the design and conduct of this study, all study analyses, and drafting and editing the article. All authors have read and agreed to the manuscript as written.
REFERENCES
- 1. Phillips K, Luk A, Soor GS, et al. Cocaine cardiotoxicity: a review of the pathophysiology, pathology, and treatment options. Am J Cardiovasc Drugs. 2009;9:177–196. [DOI] [PubMed] [Google Scholar]
- 2. Havakuk O, Rezkalla SH, Kloner RA. The cardiovascular effects of cocaine. J Am Coll Cardiol. 2017;70:101–113. [DOI] [PubMed] [Google Scholar]
- 3. Hollander JE, Hoffman RS, Burstein JL, et al; Cocaine‐Associated Myocardial Infarction Study Group . Cocaine‐associated myocardial infarction: mortality and complications. Arch Intern Med. 1995;155:1081–1086. [PubMed] [Google Scholar]
- 4. Kim TW, Samet JH, Cheng DM, et al. The spectrum of unhealthy drug use and quality of care for hypertension and diabetes: a longitudinal cohort study. BMJ Open. 2015;5:e008508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Falck RS, Wang J, Siegal HA, et al. The prevalence of psychiatric disorder among a community sample of crack cocaine users: an exploratory study with practical implications. J Nerv Ment Dis. 2004;192:503–507. [DOI] [PubMed] [Google Scholar]
- 6. Whiteford HA, Degenhardt L, Rehm J, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382:1575–1586. [DOI] [PubMed] [Google Scholar]
- 7. Williams CT, Latkin CA. Neighborhood socioeconomic status, personal network attributes, and use of heroin and cocaine. Am J Prev Med. 2007;32(6 suppl):S203–S210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Singh S, Arora R, Khraisat A, et al. Increased incidence of in‐stent thrombosis related to cocaine use: case series and review of literature. J Cardiovasc Pharmacol Ther. 2007;12:298–303. [DOI] [PubMed] [Google Scholar]
- 9. Maddox TM, Plomondon ME, Petrich M, et al. A national clinical quality program for Veterans Affairs catheterization laboratories (from the Veterans Affairs clinical assessment, reporting, and tracking program). Am J Cardiol. 2014;114:1750–1757. [DOI] [PubMed] [Google Scholar]
- 10. Endorsed by the Latin American Society of Interventional Cardiology; PCI Writing Committee , Levine GN, Bates ER, Blankenship JC, et al. 2015 ACC/AHA/SCAI focused update on primary percutaneous coronary intervention for patients with ST‐elevation myocardial infarction: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention and the 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Catheter Cardiovasc Interv. 2016;87:1001–1019. [DOI] [PubMed] [Google Scholar]
- 11. Klein LW, Block P, Brindis RG, et al. Percutaneous coronary interventions in octogenarians in the American College of Cardiology–National Cardiovascular Data Registry: development of a nomogram predictive of in‐hospital mortality. J Am Coll Cardiol. 2002;40:394–402. [DOI] [PubMed] [Google Scholar]
- 12. Brindis RG, Fitzgerald S, Anderson HV, et al. The American College of Cardiology–National Cardiovascular Data Registry (ACC‐NCDR): building a national clinical data repository. J Am Coll Cardiol. 2001;37:2240–2245. [DOI] [PubMed] [Google Scholar]
- 13. Byrd JB, Vigen R, Plomondon ME, et al. Data quality of an electronic health record tool to support VA cardiac catheterization laboratory quality improvement: the VA Clinical Assessment, Reporting, and Tracking System for Cath Labs (CART) program. Am Heart J. 2013;165:434–440. [DOI] [PubMed] [Google Scholar]
- 14. Farmer MM, Stanislawski MA, Plomondon ME, et al. Sex differences in 1‐year outcomes after percutaneous coronary intervention in the Veterans Health Administration. J Womens Health (Larchmt). 2017;26:1062–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Suttorp MM, Siegerink B, Jager KJ, et al. Graphical presentation of confounding in directed acyclic graphs. Nephrol Dial Transplant. 2015;30:1418–1423. [DOI] [PubMed] [Google Scholar]
- 16. Laynez Cerdeña I, Lacalzada Almeida J, Marrero Rodríguez F, et al. Acute myocardial infarct in a cocaine‐addicted young man [article in Spanish]. Rev Esp Cardiol. 1990;43:198–200. [PubMed] [Google Scholar]
- 17. Bagalman E. Mental Disorders Among OEF/OIF Veterans Using VA Health Care: Facts and Figures. Washington, DC: Congressional Research Service; February 4, 2013:7‐5700(R41921):1–8. [Google Scholar]
- 18. Gupta N, Washam JB, Mountantonakis SE, et al. Characteristics, management, and outcomes of cocaine‐positive patients with acute coronary syndrome (from the National Cardiovascular Data Registry). Am J Cardiol. 2014;113:749–756. [DOI] [PubMed] [Google Scholar]
- 19. Singh V, Rodriguez AP, Thakkar B, et al. Hospital admissions for chest pain associated with cocaine use in the United States. Am J Med. 2017;130:688–698. [DOI] [PubMed] [Google Scholar]
- 20.US Census Bureau. https://www.census.gov/topics/population/veterans.html. Last accessed January 5, 2018
- 21. Lee MO, Vivier PM, Diercks DB. Is the self‐report of recent cocaine or methamphetamine use reliable in illicit stimulant drug users who present to the emergency department with chest pain? J Emerg Med. 2009;37:237–241. [DOI] [PubMed] [Google Scholar]
- 22. Hollander JE, Todd KH, Green G, et al. Chest pain associated with cocaine: an assessment of prevalence in suburban and urban emergency departments. Ann Emerg Med. 1995;26:671–676. [DOI] [PubMed] [Google Scholar]
- 23. Bishop CR, Dargan PI, Greene SL, et al. Emergency department presentations with suspected acute coronary syndrome—frequency of self‐reported cocaine use. Eur J Emerg Med. 2010;17:164–166. [DOI] [PubMed] [Google Scholar]


