Abstract
Background
Patients with atrial myocardial infarction (ATMI) have frequent cardiac and noncardiac complications. However, ATMI is uncommonly diagnosed because of its nonspecific ECG changes. Our objective was to analyze the ECG characteristics of ATMI in patients with inferior STEMI.
Hypothesis
Electrocardiographic P wave parameters can help in diagnosis of ATMI.
Methods
We evaluated 932 patients who underwent coronary angiography and recruited 39 patients with ATMI and 33 patients without ATMI with inferior STEMI for a retrospective study. Twelve‐lead ECGs were obtained to measure P‐wave parameters in diagnosis of ATMI. P‐wave parameters and PR‐segment displacement were compared in patients with and without ATMI.
Results
In inferior leads, PWD and PWDisp were significantly longer in the ATMI group than in the non‐ATMI group (limb lead II, 109.79 ±15.51 ms and 86.65 ±5.02 ms, respectively; P < 0.001; limb lead III, 108.31 ±12.51 ms and 85.27 ±7.47 ms, P < 0.001; aVF, 106.49 ±13.68 ms and 83.01 ±7.89 ms, P < 0.001; PWDisp, 41.67 ±10.72 ms and 25.18 ±5.17 ms, P < 0.001). By contrast, PWA was significantly lower in the ATMI group than in the non‐ATMI group (limb lead II, 0.96 ±0.18 mV and 1.39 ±0.22 mV, respectively; P < 0.001; limb lead III, 0.90 ±0.11 and 1.21 ±0.23, P < 0.001; aVF, 0.88 ±0.17 and 1.26 ±0.28, P < 0.001). PR‐segment displacement was found in 8 (20.5%) patients with ATMI. A PWD ≥95.5 ms in lead DII diagnosed ATMI with a higher sensitivity and specificity (90%, 94%) than did PWA or PWDisp.
Conclusions
This study suggests P‐wave parameters might be considered ECG findings in diagnosis of ATMI in patients with inferior STEMI.
1. INTRODUCTION
Although ventricular myocardial infarction (MI) currently has a universally accepted definition, there is no consensus as to the definition of atrial myocardial infarction (ATMI).1 ATMI was first defined in 1925 and was first published as a case series in 1942.2, 3 Although it is commonly observed along with ventricular MI, isolated ATMI may also occur, albeit rarely.4 Based on the data, the vast majority of which were obtained from postmortem studies, ATMI incidence ranges between 0.7% and 42%.5, 6, 7 In the largest autopsy series reported, ATMI incidence accompanied with ventricular MI was reported as 15.8%, and isolated ATMI was 2.8%.8
Rhythm disturbances and thromboembolic events including pulmonary and systemic circulation are the most common complications of ATMI and are associated with increased morbidity and mortality. Also, atrial rupture, loss of atrial “kick,” and reduced secretion of atrial natriuretic peptide are less frequent complications.9, 10, 11 Consequently, a ventricular MI complicated by arrhythmia and hemodynamic deterioration may result in delayed diagnosis of ATMI. Diagnosis of ATMI is generally made by observing changes in a surface electrocardiogram (ECG). As commonly accepted today, these changes are PR‐segment depression or elevation, changes in P‐wave morphology, and supraventricular rhythm abnormalities.12 However, diagnosis of ATMI becomes difficult to make, both because of insufficient atrial voltage signal in surface ECG and because of clinicians who interpret the results frequently showing more interest in ventricular MI.11, 13
Although it is known that the atrial branch that supplies blood to the atrium originates from the right coronary artery (RCA) or the left circumflex coronary artery (LCX), studies that evaluate total occlusion or severe stenosis of the atrial branch along with these arteries on coronary angiography (CA) and of its reflection on surface ECG are lacking. The objective of the present study was to assess the changes of P‐wave parameters and PR‐segment displacement in the surface ECG in patients with inferior ST‐segment elevation myocardial infarction (STEMI) undergoing primary percutaneous coronary intervention (PCI) in which the atrial branch was involved. In addition, we aimed to highlight the contribution of ATMI in creating a complicated ventricular MI.
2. METHODS
2.1. Design, patient population, and study groups
This retrospective study included patients who received a diagnosis of inferior‐wall STEMI at 1 of 2 hospitals (Sisli Hamidiye Etfal and Bagcilar Training and Research hospitals). We excluded patients with other wall infarctions, multivessel disease, previous coronary artery bypass grafting, severe heart valve diseases, atrial fibrillation (AF)/flutter rhythm, known heart failure, thyroid function disorders, and renal failure. The diagnosis of STEMI was made according to the third universal definition of MI.1 Data on clinical history, physical examination, 12‐lead ECG, blood tests, and cardiac troponin T (cTnT) were collected from the hospital records and were stored in an electronic database. The study was approved by the ethics committee of both hospitals.
2.2. ECG analysis
Twelve‐lead surface ECG recordings were obtained for each patient at a paper speed of 25 mm/s and signal size of 10 mm/mV in the emergency department. P‐wave duration (PWD), P‐wave dispersion (PWDisp), P‐wave amplitude (PWA), and PR‐segment displacement were measured in all derivations in computer environment using an electronic calipers program (CardioCalipers version 3.3; Iconico.com, shareware) under adequate image magnification. All 12‐lead ECG interpretations were independently reviewed by 2 cardiologists. The onset of the P wave was defined as the junction between the isoelectric line and the first upward or downward wave. The junction between the end of the P‐wave deflection and the isoelectric line was defined as the end of the P wave. The distance between the onset and offset of the P wave was defined as PWD. The P‐wave amplitude was measured from the P‐wave peak or nadir to the isoelectric line (T–P interval).14 The isoelectric line (T–P interval) is defined as the isoelectric region beginning with the end of the T wave and ending with the onset of the P wave of the next cycle.15 Maximum P wave (Pmax) was defined as the longest P‐wave duration in any surface ECG lead. Minimum P wave (Pmin) was defined as the shortest P‐wave duration in any surface ECG lead. PWDisp was defined as the difference between the maximum and the minimum P‐wave duration (PWDisp = Pmax – Pmin). The displacement of the PR segment was measured as the distance to the isoelectric line. When PR‐segment depression or elevation was ≥0.5 mm, it was considered significant. The patients who had indiscernible P waves in >5 leads on a baseline 12‐lead ECG were not enrolled in the study.
P‐wave parameters were derived from ≥3 P‐wave measurements in all leads; PWD and PWDisp were recorded in ms, PWA in mV. Intra‐ and interobserver coefficients of variation were found to be 3.2% and 3.5% for Pmax, 3.7% and 4.1% for Pmin, and 4.2% and 4.7% for PWA, respectively.
2.3. CA and primary PCI
CA and primary PCI images of patients who met the inclusion criteria were observed by 2 independent, experienced invasive cardiologists. Atrial branch involvement (ABI) was defined as the occurrence of total occlusion of infarct‐related RCA or LCX arteries before the exit of the atrial branch; this leads to the inability of imaging the atrial branch in CA or the observation of an atherosclerotic plaque or thrombus that causes severe stenosis in the ostium of the atrial branch. Patients who had ABI were defined as the ATMI group. All patients with ABI were young and had single‐vessel disease (RCA or LCX), with no or only 1 cardiovascular risk factor without prior heart disease. However, patients without ABI had multivessel disease with many accompanying comorbidities. Therefore, the 2 groups were not similar. Based on this, we tried to match the 2 groups in terms of risk factors. Finally, 33 non‐ABI patients and 39 ABI patients with similar age, sex, and clinical and angiographic characteristics were enrolled in the study. If a patient's atrial branch was derived from both RCA and LCX, the one with larger calibration in the infarct‐related artery (IRA) was selected for this study. Coronary arterial blood flow was qualitatively evaluated using the Thrombolysis In Myocardial Infarction (TIMI) score.16 A successful angiographic result was defined as being associated with TIMI grade 3 flow.
2.4. Laboratory measurement
In all patients, serum fasting blood glucose, creatinine, blood urea nitrogen, triglycerides, total cholesterol, low‐density lipoprotein cholesterol, and high‐density lipoprotein cholesterol levels were measured with standard laboratory techniques (Hitachi 7600 Automatic Biochemical Analyzer; Hitachi Co., Japan). Serum TnI was measured using the ADVIA Centaur Immunoassay System (Bayer Diagnostics, Tarrytown, NY). All blood tests were performed in emergency department.
2.5. Statistical analysis
Data are reported as the mean ±SD for continuous variables and, if appropriate, were compared using independent samples t test or Mann–Whitney U test. Categorical variables were reported as percentages and numbers and, if appropriate, were compared using the χ2 or Fischer exact test. The normality assumption was evaluated by the Kolmogorov–Smirnov test. Continuous variables were compared between groups. Diagnostic accuracy of P‐wave parameters (PWD, PWA, and PWDisp) in ATMI was evaluated by the area under receiver operating characteristic (ROC) curve (AUC) along with its 95% confidence interval (95% CI). The AUC with 95% CI, sensitivity, and specificity were also estimated by selecting a probability threshold giving comparable sensitivity and specificity values. A pairwise comparison of ROC curves was assessed by the DeLong method (MedCalc Software, version 16; free trial). For all statistical tests, a P value <0.05 was considered statistically significant. Statistical analysis was performed using SPSS Statistics for Windows, version 21 (IBM Corp., Armonk, NY).
3. RESULTS
Angiographic imaging data of 932 patients were retrospectively analyzed. Of these patients, 450 had inferior‐wall STEMI. Thirty‐nine patients with ABI and 33 patients without ABI met the inclusion criteria. A flowchart of the study is shown in Figure 1. Baseline demographic, clinical, and biochemical characteristics of both groups are shown in Table 1. No significant differences were found among the groups with respect to age, sex, smoking history, hypertension, and ejection fraction, right and left atrium. The creatinine, blood glucose, and cholesterol levels were similar in both groups. Cardiac TnI was significantly higher in the ATMI group than in the non‐ATMI group. Of the ATMI patients, 6 patients (15.4%) were established as having AF during their in‐hospital follow‐ups; this was not found in any patient belonging to the non‐ATMI group (P < 0.005). All AF episodes in the ATMI group were paroxysmal. Because 5 patients did not have hemodynamic instability and chest pain, an antiarrhythmic agent was not administered. On the first day of hospitalization, their follow‐up ECGs spontaneously reversed from AF to sinus rhythm. Only 1 patient required synchronized electrical cardioversion due to acute hemodynamic instability. In that patient, sinus rhythm was restored successfully.
Figure 1.
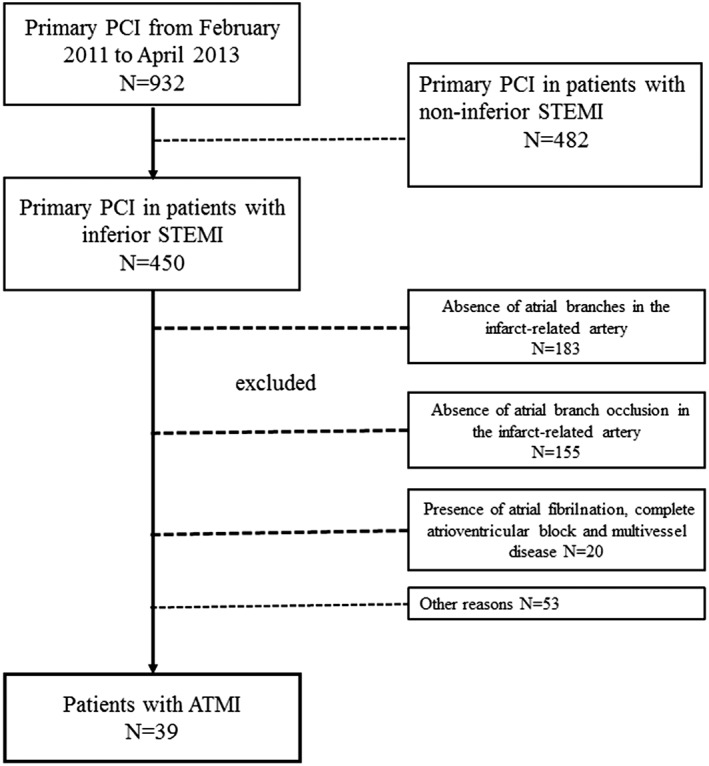
Flowchart of the study. Abbreviations: ATMI, atrial myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST‐segment elevation myocardial infarction
Table 1.
The clinical, demographic, and biochemical characteristics of patients with and without ATMI
| With ATMI, n = 39 | Without ATMI, n = 33 | P Value | |
|---|---|---|---|
| Mean age, y | 57.59 ± 12.56 | 58.77 ± 8.15 | 0.643 |
| Male sex | 22 (55) | 18 (45) | 0.531 |
| Heart rate, bpm | 81.51 ± 10.38 | 78.03 ± 9.39 | 0.153 |
| SBP, mm Hg | 118.56 ± 11.57 | 122.15 ± 11.03 | 0.185 |
| DBP, mm Hg | 71.89 ± 8.70 | 75.60 ± 8.13 | 0.068 |
| Glucose, mg/dL | 146.67 ± 72.66 | 139.15 ± 66.95 | 0.652 |
| eGFR, mL/min per 1.73 m2 | 98.2 ± 12.8 | 99.8 ± 11.9 | 0.777 |
| Cr, mg/dL | 0.93 ± 0.22 | 0.92 ± 0.25 | 0.879 |
| LDL‐C, mg/dL | 100.41 ± 36.32 | 108.05 ± 34.36 | 0.365 |
| HDL‐C, mg/dL | 41.10 ± 13.33 | 44.33 ± 12.18 | 0.290 |
| Total cholesterol, mg/dL | 167.34 ± 37.49 | 170.56 ± 39.78 | 0.726 |
| TnI, ng/mL | 47.5 ± 26.2 | 34.8 ± 22.0 | 0.01 |
| AF | 6 (15.4) | 0 (0.0) | 0.005 |
| LVEF, % | 52.5 ± 3.8 | 53.9 ± 3.5 | 0.538 |
| LA, mm | 35.3 ± 2.5 | 35.0 ± 2.2 | 0.963 |
| RA, mm | 36.1 ± 1.8 | 35.3 ± 2.1 | 0.718 |
Abbreviations: AF, atrial fibrillation; ATMI, atrial myocardial infarction; Cr, creatinine; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HDL‐C, high‐density lipoprotein cholesterol; LA, left atrium; LDL‐C, low density lipoprotein cholesterol; LVEF, left ventricular ejection fraction; RA, right atrium; SBP, systolic blood pressure; SD, standard deviation; TnI, troponin I.
Data are expressed as n (%) or mean ± SD.
3.1. Angiographic findings
TIMI grade 3 flow was verified in all patients of both groups who underwent primary PCI. The RCA was the IRA in 33 (84.6%) patients with ATMI and in 27 (81.8%) patients without it. Although total occlusion near the ostium in the main coronary artery was observed in 15 patients (38.5%), dissected lesion with thrombus that also included the atrial branch was observed in the remaining 24 patients (61.5%). Even though TIMI grade 3 flow was observed in the main coronary artery following primary PCI in all patients, severe stenosis in the ostium of the atrial branch due to shift of plaque or thrombus was seen only in the ATMI group. All patients in the ATMI group had severe stenosis in the ostium of the atrial branch. We only observed AF in 6 patients, so we speculated that AF development was not associated with severe stenosis in the ostium of the atrial branch.
3.2. ECG findings
In inferior leads, PWDs were significantly longer in the ATMI group than in the control group (limb lead II, 109.79 ± 15.51 and 86.65 ± 5.02, respectively; P < 0.001; limb lead III, 108.31 ± 12.51 and 85.27 ± 7.47, respectively; P < 0.001; aVF, 106.49 ± 13.68 and 83.01 ± 7.89, respectively; P < 0.001; Table 2). By contrast, PWA in inferior leads was significantly lower in patients with ATMI than in patients without it (limb lead II, 0.096 ± 0.018 and 0.139 ± 0.022, respectively; P < 0.001; limb lead III, 0.090 ± 0.011 and 0.121 ± 0.023, respectively; P < 0.001; aVF, 0.088 ± 0.017 and 0.126 ± 0.028, respectively; P < 0.001; Table 2). In addition, PWDisp measured from the 12‐lead ECG was increased in the ATMI group vs the no‐ATMI group (41.67 ± 10.72 vs 25.18 ± 5.17, respectively; P < 0.001). Although PR‐segment displacement was found in 8 (20.5%) patients with ATMI, it was not found in any patient without ATMI (odds ratio: 1.92, 95% CI: 1.53–2.40, 20.5% sensitivity, 100% specificity).
Table 2.
P‐wave parameters of the study population
| With ATMI, n = 39 | Without ATMI, n = 33 | P Value | |
|---|---|---|---|
| PWD‐DII, ms | 109.79 ± 15.51 | 86.65 ± 5.02 | <0.001 |
| PWD‐DIII, ms | 108.31 ± 12.51 | 85.27 ± 7.47 | <0.001 |
| PWD‐aVF, ms | 106.49 ± 13.68 | 83.01 ± 7.89 | <0.001 |
| PWDisp, ms | 41.67 ± 10.72 | 25.18 ± 5.17 | <0.001 |
| PWA‐DII, mV | 0.96 ± 0.18 | 1.39 ± 0.22 | <0.001 |
| PWA‐DIII, mV | 0.90 ± 0.11 | 1.21 ± 0.23 | <0.001 |
| PWA‐aVF, mV | 0.88 ± 0.17 | 1.26 ± 0.28 | <0.001 |
Abbreviations: ATMI, atrial myocardial infarction; PWA, P‐wave amplutide; PWD, P‐wave duration; PWDisp, P‐wave disperison.
3.3. Diagnostic value of ECG findings in ATMI
As seen in Table 3, ROC curve analysis for PWD and PWA in inferior leads and for PWDisp in all leads was created to make a diagnosis of ATMI. The analysis revealed that PWD in the DII lead had a cutoff value of 95.5 ms, allowing for a diagnosis of ATMI with higher sensitivity and specificity than other leads (AUC: 0.964, 95% CI: 0.925–1.000, sensitivity 90%, specificity 94%). The corresponding cutoff for PWA in DII lead was 0.122 mV (AUC: 0.929, 95% CI: 0.862–0.995, sensitivity 90%, specificity 91%). In the analysis for PWDisp in all leads, the cutoff value was 31.5 ms (AUC: 0.924, 95% CI: 0.864–0.984, sensitivity 85%, specificity 85%; Figure 2). P‐wave duration above the cutoff value in the DII lead was detected in 34 out of 39 ATMI patients, and the PWDisp value was detected in 30 out of 39 ATMI cases. However, PWA below the cutoff value in DII lead was detected in 33 out of 39 ATMI patients.
Table 3.
Cutoff values that allowed for a diagnosis of ATMI with sensitivity and specificity for PWD, PWA, and PWDisp in inferior leads
| Sensitivity, % | Specificity, % | AUC | 95% CI | |
|---|---|---|---|---|
| DII, PWD (95.5 ms) | 90 | 94 | 0.964 | 0.925–1.000 |
| DIII, PWD (90.0 ms) | 90 | 91 | 0.953 | 0.897–1.000 |
| aVF, PWD (93.5 ms) | 88 | 90 | 0.919 | 0.851–0.986 |
| DII, PWA (0.122 mV) | 90 | 91 | 0.929 | 0.862–0.995 |
| DIII, PWA (0.100 mV) | 90 | 88 | 0.908 | 0.823–0.993 |
| aVF, PWA (0.116 mV) | 85 | 85 | 0.865 | 0.775–0.955 |
| PWDisp (31.5 ms) | 85 | 85 | 0.924 | 0.864–0.984 |
Abbreviations: ATMI, atrial myocardial infarction; AUC, area under the curve; CI, confidence interval; PWA, P‐wave amplutide; PWD, P‐wave duration; PWDisp, P‐wave dispersion.
Figure 2.
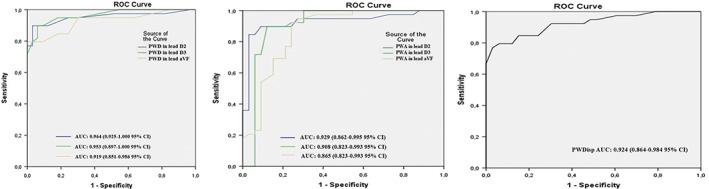
Predictive values of P‐wave parameters in diagnosis of ATMI. ROC curves illustrate the sensitivity and specificity of the 95.5‐ms cutoff value for PWD, 0.122‐mV cutoff value for PWA, and 31.5‐ms cutoff value for PWDisp predicting ATMI before primary PCI. Abbreviations: ATMI, atrial myocardial infarction; AUC, area under the curve; CI, confidence interval; PCI, percutaneous coronary intervention; PWA, P‐wave amplitude; PWD, P‐wave duration; PWDisp, P‐wave dispersion; ROC, receiver operating characteristic; STEMI, ST‐segment elevation myocardial infarction
4. DISCUSSION
4.1. Main findings
The main findings of the present study were as follows: (1) ATMI incidence in patients with inferior‐wall STEMI was 8.7%, and the infarct‐related coronary artery was the RCA in 85% of these patients; (2) Although PWD and PWDisp in inferior leads significantly increased in the surface ECG of ATMI patients, and PWA significantly decreased in the same derivations; (3) PR‐segment displacement was observed only in 20.5% of patients with ATMI, but was not seen in patients without ATMI; (4) PWD was >95.5 ms in 87% of patients with ATMI, and this cutoff value had a higher sensitivity and specificity than did the other cutoff values of ECG parameters; and (5) Compared with patients without ATMI, patients with ATMI had higher in‐hospital AF incidence. To our knowledge, the present study was the first to analyze P‐wave parameters and PR‐segment displacement in patients with inferior‐wall STEMI in whom occlusion of ventricular and atrial branches together was shown by CA.
4.2. ECG in diagnosis of ATMI
AN ECG is the most common noninvasive diagnostic tool used for the diagnosis of cardiac diseases. Diagnosis of atrial diseases by ECG is made by identifying abnormalities in P‐wave parameters and PR‐segment deviation.13 However, the atrial wall is thinner than is the ventricular wall, and the occurring voltage cannot be satisfactorily measured by ECG. Additionally, because ventricular muscle mass is greater, the atrial voltage is overshadowed by the ventricular voltage. Therefore, it becomes difficult to interpret atrial depolarization and repolarization waves on ECG recordings. However, even currently, ATMI diagnosis is made using surface ECG findings. Although the ECG criteria involve PR‐segment displacement, P‐wave changes, and/or supraventricular rhythm disturbances, there is no established consensus for diagnosis of ATMI. Furthermore, these changes are not yet validated in prospective studies.17, 18, 19, 20
The most commonly accepted ECG diagnosis of ATMI was defined by Liu et al.. in 1961.12 According to this definition, ATMI major and minor criteria were structured on the basis of PR‐segment elevation or depression, abnormal P‐wave morphology, and the presence of supraventricular arrhythmia. However, the major criteria defined by Liu et al.. were not established in any of the patients in the 2 studies that included cases with ventricular STEMI in all localizations.20, 21 In both studies, researchers reported that abnormal P‐wave morphology in surface ECG predicted AF and mortality. In another study, Alvarez et al reported that increased PWD, decreased PWA, and PR‐segment deviation were present in the first 10 minutes of the Holter monitoring test in patients confirmed as having accidental atrial‐branch occlusion following elective percutaneous transluminal coronary angioplasty.22 In the present study, we found that PWD and PWDisp increased, particularly in limb lead II compared with other leads, and that PWA decreased in the same derivation. Similarly, even we did not observe the major criteria suggested by Liu et al. in any patient with ATMI.
4.3. Possible mechanisms of P‐wave indices in lead DII and PR‐segment deviation in ATMI
As in the pathophysiology of ST‐segment deviation observed in ventricular MI, PR‐segment deviation is also expected in atrial ischemia or injury. Theoretically, it is possible to understand whether ATMI was biatrial or confined in a single atrium by specifically checking limb derivations.11 However, PR‐segment deviation was reported to not be a finding specific to atrial ischemia. Just like the ST‐segment deviation, which can be seen in ventricular MI, is not specific to infarction, the PR‐segment deviation is also not specific to atrial ischemia. Two studies suggested that PR‐segment deviation was caused by other cardiac diseases, including atrial dilatation, atrial hypertrophy, intra‐atrial block, and pericarditis, and noncardiac diseases including chronic obstructive pulmonary disease.19, 23 In the current study, secondary diseases that could cause PR‐segment deviation were excluded. In addition, we found that PR‐segment deviation occurred in only one‐fifth of the ATMI population. Further, we did not find PR‐segment deviation in any patient without ATMI.
In the literature, electrophysiological alterations occurring due to ATMI were investigated in a limited number of experimental studies in which the atrial branch was selectively ligated.24, 25 These preclinical studies showed that electrical conduction slowed down and spread nonhomogeneously across the atria, all of which stabilized the reentrant rotors that initiate and sustain AF in the ischemic atrial myocardium. In the surface ECG, PWA, PWD, and PWDisp provide information about the magnitude of depolarization, prolongation, and heterogeneity of conduction, respectively. On the other hand, apart from atrial ischemia, atrial dilatation and hypertrophy can also cause alterations in PWD and PWA, and therefore these changes should be evaluated cautiously.13
In our study, transthoracic echocardiography was recorded after primary PCI, and biatrial diameters of all patient populations were found to be normal. Therefore, pathologies that occur in atria other than ischemia were ruled out. The values of P‐wave parameters that were measured in all leads were different in the patient group and the non‐ATMI group. This difference was more significant in inferior leads than in other leads. Regardless of whether ATMI is isolated or not, it may cause complications, such as heart failure, arrhythmia, thromboembolic events, and even death as a result of atrial free‐wall rupture.11 We suggest that atrial ischemia occurring with inferior‐wall STEMI causes a slow conduction and a disruption of homogeneity in the atrial tissue. Such pathophysiological changes in the atrium result in changes in P‐wave parameters on surface ECG, rather than PR‐segment deviations not specific to primary ischemia in the atrium.
4.4. Study limitations
The most significant limitation of our study was that it was designed as a retrospective case–control study. The ECG parameters of patients were obtained only in the emergency department, so we could not measure the post‐reperfusion P waves and PR segments to see how they changed with reperfusion. Further, the sample size of our study was small; therefore, the results of our study should be confirmed by larger prospective trials.
5. CONCLUSION
Our study showed that PWD and PWDisp were prolonged and PWA was reduced on the surface ECG of patients with acute inferior‐wall MI whose atrial branch occlusion was established by CA during primary PCI. For ATMI diagnosis, PWD in lead II with a cutoff value of 95.5 ms had the highest sensitivity and specificity. In patients with inferior‐wall STEMI, the use of P‐wave parameters in ECG may help diagnose ATMI that causes cardiac complications.
Conflicts of interest
The authors declare no potential conflicts of interest.
Supporting information
Figure A. Analysis of P‐wave duration and P‐wave amplitude in a 12‐lead ECG recording in inferior STEMI. The onset and offset points of the P‐wave were determined as the intersection point of upward or downward deflection of the P‐wave and the isoelectrical line (A). The P‐ wave amplitude was measured as the height of the peak of positive deflection from the isoelectrical line (B).
Figure B. Coronary angiography illustrates atrial branch involvement in patients with inferior wall STEMI. Panel A shows an example coronary angiography of a patient with ATMI and acute inferior MI. Total occlusion in segment close to the ostial of right coronary artery (RCA) before primary PCI. No atrial branch. Panel B shows TIMI 3 flow in RCA after primary PCI in the same patient. Orange arrow indicates subtotal occlusion in atrial branch. Panel C shows total occlusion in the mid segment of RCA before primary PCI in the another patient. Panel D shows TIMI 3 flow in RCA after primary PCI. Yellow arrow indicates subtotal occlusion and thrombosis in atrial branch. Panel E shows total occlusion in the mid segment of RCA without atrial branch involvement before primary PCI. Panel F shows TIMI 3 flow both atrial branch and RCA after primaryPCI.
Yıldız SS, Keskin K, Avsar M, et al. Electrocardiographic diagnosis of atrial infarction in patients with acute inferior ST‐segment elevation myocardial infarction. Clin Cardiol. 2018;41:972–977. 10.1002/clc.22987
This paper was presented at the 33th International Participation Turkish Cardiology Congress, Antalya, Turkey, October 5–8, 2017, as an oral presentation.
REFERENCES
- 1. Thygesen K, Alpert JS, Jaffe AS, et al; Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction . Third universal definition of myocardial infarction. Circulation. 2012;126:2020–2035. [DOI] [PubMed] [Google Scholar]
- 2. Clerc A, Levy R. Infarctus auriculaire: tachyarrhythmia terminale [article in French]. Bull Mem Soc Med Hop Paris. 1925;41:1603–1607. [Google Scholar]
- 3. Cushing EH, Feil HS, Stanton EJ, et al. Infarction of the cardiac auricles (atria): clinical, pathological and experimental studies. Br Heart J. 1942;4:17–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lazar EJ, Goldberger J, Peled H, et al. Atrial infarction: diagnosis and management. Am Heart J. 1988;116:1058–1063. [DOI] [PubMed] [Google Scholar]
- 5. Chida K, Ohkawa S, Maeda S, et al. A clinicopathologic study of atrial infarction complicating left ventricular posterior myocardial infarction [article in Japanese]. J Cardiol. 1992;22:1–10. [PubMed] [Google Scholar]
- 6. Hilton TC, Pearson AC, Serota H, et al. Right atrial infarction and cardiogenic shock complicating acute myocardial infarction: diagnosis by transesophageal echocardiography. Am Heart J. 1990;120:427–430. [DOI] [PubMed] [Google Scholar]
- 7. Vargas‐Barrón J, Espinola‐Zavaleta N, Romero‐Chdenas A, et al. Clinical‐electrocardiographic correlation of myocardial infarction with extension to right chambers. Echocardiography. 1988;15:171–180. [DOI] [PubMed] [Google Scholar]
- 8. Ventura T, Colantonio D, Leocata P, et al. Isolated atrial myocardial infarction: pathological and clinical features in 10 cases. Cardiologia. 1991;36:345–350. [PubMed] [Google Scholar]
- 9. Nielsen FE, Andersen HH, Gram‐Hansen P, et al. The relationship between ECG signs of atrial infarction and the development of supraventricular arrhythmias in patients with acute myocardial infarction. Am Heart J. 1992;123:69–72. [DOI] [PubMed] [Google Scholar]
- 10. Yasuda S, Nonogi H, Miyazaki S, et al. Hyposecretion of atrial natriuretic peptide due to associated right atrial infarction in a patient with acute right ventricular infarction? Eur Heart J. 1994;15:718–719. [DOI] [PubMed] [Google Scholar]
- 11. Shakir DK, Arafa SO. Right atrial infarction, atrial arrhythmia and inferior myocardial infarction form a missed triad: a case report and review of the literature. Can J Cardiol. 2007;23:995–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu CK, Greenspan G, Piccirillo RT. Atrial infarction of the heart. Circulation. 1961;23:331–338. [DOI] [PubMed] [Google Scholar]
- 13. Lu ML, De Venecia T, Patnaik S, et al. Atrial myocardial infarction: a tale of the forgotten chamber. Int J Cardiol. 2016;202:904–909. [DOI] [PubMed] [Google Scholar]
- 14. Park JK, Park J, Uhm JS, et al. P‐wave amplitude (<0.1 mV) in lead I is associated with displaced inter‐atrial conduction and clinical recurrence of paroxysmal atrial fibrillation after radiofrequency catheter ablation. Europace. 2016;18:384–391. [DOI] [PubMed] [Google Scholar]
- 15. Mirvis DM, Goldberger AL. Electrocardiography In: Bonow RO, Mann LM, Zipes DP, et al. Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine. Philadelphia, PA: Elsevier Press; 2012:126–199. [Google Scholar]
- 16. TIMI Study Group . The Thrombolysis in Myocardial Infarction (TIMI) trial: phase I findings. N Engl J Med. 1985;312:932–936. [DOI] [PubMed] [Google Scholar]
- 17. Young EW, Koenig A. Auricular infarction. Am Heart J. 1944;28:287–294. [Google Scholar]
- 18. Hellerstein HK. Atrial infarction with diagnostic electrocardiographic findings. Am Heart J. 1948;36:422–430. [DOI] [PubMed] [Google Scholar]
- 19. Nagahama Y, Sugiura T, Takehana K, et al. Clinical significance of PQ segment depression in acute Q wave anterior wall myocardial infarction. J Am Coll Cardiol. 1994;23:885–900. [DOI] [PubMed] [Google Scholar]
- 20. Lu ML, Bhalla V, Figueredo VM. Prognostic value of abnormal P wave morphology and PR‐segment displacement for mortality in ST‐elevation myocardial infarction. Int J Cardiol. 2016;202;800. [DOI] [PubMed] [Google Scholar]
- 21. Van Diepen S, Siha Y, Fu Y, et al; APEX AMI Investigators . Do baseline atrial electrocardiographic and infarction patterns predict new onset atrial fibrillation after ST‐elevation myocardial infarction? Insights from the Assessment of Pexelizumab in Acute Myocardial Infarction Trial. J Electrocardiol. 2010;43:351–358. [DOI] [PubMed] [Google Scholar]
- 22. Álvarez‐García J, Vives‐Borrás M, Gomis P, et al. Electrophysiological effects of selective atrial coronary artery occlusion in humans. Circulation. 2016;133:2235–2242. [DOI] [PubMed] [Google Scholar]
- 23. Jim MH, Siu CW, Chan AO, et al. Prognostic implications of PR‐segment depression in inferior leads in acute inferior myocardial infarction. Clin Cardiol. 2006;29:363–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yamazaki M, Morgenstern S, Klos M, et al. Left atrial coronary perfusion territories in isolated sheep hearts: implications for atrial fibrillation maintenance. Heart Rhythm. 2010;7:1501–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nishida K, Qi XY, Wakili R, et al. Mechanisms of atrial tachyarrhythmias associated with coronary artery occlusion in a chronic canine model. Circulation. 2011;123:137–146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure A. Analysis of P‐wave duration and P‐wave amplitude in a 12‐lead ECG recording in inferior STEMI. The onset and offset points of the P‐wave were determined as the intersection point of upward or downward deflection of the P‐wave and the isoelectrical line (A). The P‐ wave amplitude was measured as the height of the peak of positive deflection from the isoelectrical line (B).
Figure B. Coronary angiography illustrates atrial branch involvement in patients with inferior wall STEMI. Panel A shows an example coronary angiography of a patient with ATMI and acute inferior MI. Total occlusion in segment close to the ostial of right coronary artery (RCA) before primary PCI. No atrial branch. Panel B shows TIMI 3 flow in RCA after primary PCI in the same patient. Orange arrow indicates subtotal occlusion in atrial branch. Panel C shows total occlusion in the mid segment of RCA before primary PCI in the another patient. Panel D shows TIMI 3 flow in RCA after primary PCI. Yellow arrow indicates subtotal occlusion and thrombosis in atrial branch. Panel E shows total occlusion in the mid segment of RCA without atrial branch involvement before primary PCI. Panel F shows TIMI 3 flow both atrial branch and RCA after primaryPCI.


