Abstract
Atrial fibrillation is the most common heart‐rhythm disorder, affecting about 1.5% to 2% of the population with an increased risk of mortality and morbidity due to stroke, thromboembolism, and heart failure. If the conversion back to sinus rhythm does not happen spontaneously, pharmacological or electrical cardioversion (ECV) is the next available treatment options for some patients. However, the long‐term success following ECV is variable. This review describes the factors that are associated with maintenance of sinus rhythm following ECV and proposes a clinical strategy based on the available evidence.
Keywords: Atrial Fibrillation, Electrical Cardioversion, Sinus Rhythm
1. INTRODUCTION
Atrial fibrillation (AF) is a supraventricular arrhythmia characterized by extremely rapid and uncoordinated electrical activity in the atria with variable conduction through the atrioventricular node, resulting in irregular, and often rapid, ventricular contraction. AF is the most common sustained heart‐rhythm disorder in the developed world, found in 1% to 2% of the general population, with a higher prevalence in females and older patients.1 Because of an aging population, it is likely that AF patient numbers will continue to rise, along with associated increases in healthcare costs.2 Palpitations, breathlessness, fatigue, and reduced exercise tolerance are AF‐associated symptoms, and there is an increased risk of stroke with AF, with the need for anticoagulation.2
In some patients, AF will be permanent and therapy is aimed at controlling the ventricular rate (rate‐control strategy). In other patients, normal rhythm initially can be restored with pharmacological or electrical cardioversion (ECV) but often requires additional strategies to maintain sinus rhythm (SR; rhythm‐control strategy).
There is no convincing mortality benefit to achieving rhythm control over rate control; therefore, treatment decisions are usually based on the presence of symptoms and the perceived likelihood of successful cardioversion. Cardioversion can be achieved by pharmacological methods or ECV; however, maintenance of SR may only be temporary.3, 4, 5, 6, 7 The use of ECV may be indicated where pharmacological rhythm‐control strategies have failed and the patient remains symptomatic. Patient selection is important, as the maintenance of long‐term SR can be difficult. The advantages of ECV are that it is associated with a high initial success rate (68%–98%)8; however, it requires sedation or general anesthesia, and although initially successful, long‐term maintenance of SR is not reliably achieved.9 AF relapse following ECV is also associated with increased mortality,10 thus highlighting the importance of identifying the correct patient group for cardioversion and, where possible, addressing reversible factors associated with poorer outcomes. Furthermore, it is crucial at the time of ECV that patients are appropriately prepared with adequate hydration and correction of any electrolyte abnormalities. ECV should also be avoided in stable patients with concurrent infection or significant inflammation.
Many factors have been reported to be associated with the maintenance of SR following ECV, with the aim of this review article to discuss these risk factors in more detail to help better decide who might gain long‐term benefit from ECV. The review will consider patient factors, medication, comorbidities, coexistent cardiac disease, AF type, and ECV procedural details.
2. AF AND PATIENT FACTORS
2.1. AF vs atrial flutter
ECV for atrial flutter has a higher success rate and lower recurrence rate compared with ECV for AF,7, 11 and this appears also to be true in older patients (Table 1).10
Table 1.
Duration of AF, patient factors, and ECV procedural details
| Outcome | HR/OR (95% CI) | P Value | Reference | |
|---|---|---|---|---|
| AF vs atrial flutter | ECV of atrial flutter has a higher initial success rate and lower recurrence rate than those of AF | — | 0.009 | Ammash et al11 |
| AF duration | >6 months; risk for recurrence of AF | HR: 1.59 (1.22–2.07) | 0.001 | Toso et al3 |
| Shorter total duration of AF | OR: 0.92 (0.89–0.97) | <0.001 | Pisters et al4 | |
| AF duration (1‐day increase) | OR: 1.01 (1.00–1.03) | 0.04 | Marchese et al5 | |
| Duration of AF in weeks | OR: 0.9 (0.87–0.93) | <0.0001 | Soran et al12 | |
| Sex | Women have a higher risk of recurrence of AF after successful ECV | HR: 1.3 (1.0–1.6) | 0.04 | Gurevitz et al17 |
| Independent risk factor for AF recurrence within 30 days of ECV | OR: 1.23 (1.01–1.51) | 0.04 | Grönberg et al8 | |
| Age | >65 years | HR: 0.7 (0.5–0.99) | <0.05 | Boriani et al7 |
| Per year of advancing age | OR: 0.38 (0.97–0.99) | 0.004 | Pisters et al4 | |
| Per year of advancing age | OR: 1.03 (1.00–1.06) | 0.04 | Marchese et al5 | |
| Weight | Independent predictor for the success of cardioversion if <80 kg | OR: 2.3 (1.1–4.8) | <0.03 | Frick et al6 |
| Smoking status | Smoking women are significantly more likely to have AF recurrence than are nonsmoking women; no differences in smoking or nonsmoking males regarding recurrence of AF | HR: 1.71 (1.10–0.67) | 0.02 | Kinoshita et al19 |
| Number of shocks at ECV | ≥2 shocks for successful ECV have a higher long‐term recurrence of AF compared with those converting to SR with the first shock | OR: 2.05 (1.00–3.93) | 0.044 | Alla et al43 |
| Increasing number of ECVs associated with increased recurrence within 30 days | OR: 1.07 (1.05–1.09) | <0.001 | Grönberg et al8 | |
| ECV after ablation | High recurrence rates after ablation and cardioversion: success vs partial success vs failed ECV; time (<30 days vs >30 days) from atrial arrhythmia recurrence to cardioversion as independent predictor of maintenance of SR after a single ablation procedure | 16% vs 22% vs 62%, respectively | — | Chilukuri et al45 |
Abbreviations: AF, atrial fibrillation; CI, confidence interval; ECV, electrical cardioversion; HR, hazard ratio; OR, odds ratio; SR, sinus rhythm.
Figure 1.
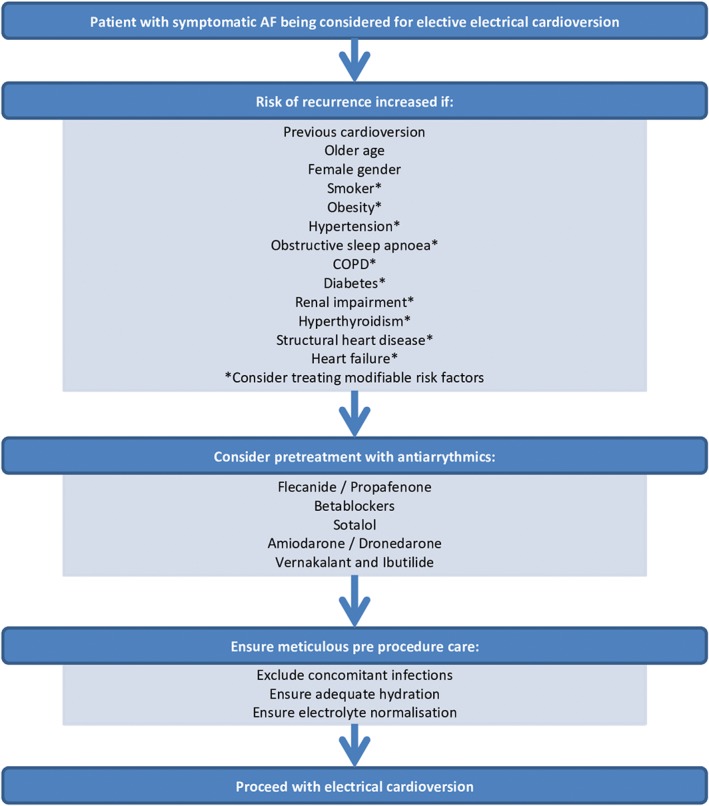
Suggested pathway for management of stable patients with AF undergoing ECV. Abbreviations: AF, atrial fibrillation; COPD, chronic obstructive pulmonary disease; ECV, electrical cardioversion
2.2. AF duration
AF can be described in terms of its duration as first diagnosed: paroxysmal, persistent (>7 days), long‐standing persistent (>1 year), and permanent (decision made that rate‐control strategy is appropriate). It is often difficult to determine how long patients have had AF, especially if they are asymptomatic. Multiple studies have suggested that a shorter duration of AF prior to ECV is associated with a better outcome from ECV and maintenance of SR.3, 4, 5, 12, 13 In addition, patients with their first episode of AF also have been shown to have increased ECV success rates within 30 days of cardioversion.8, 14 It is likely that chronic AF promotes electrical and structural remodeling, so that patients with chronic AF appear to have larger atria and are more resistant to ECV.6 The clinical implication is that patients with prolonged AF may be less likely to benefit from a rhythm‐control strategy. An AF duration >6 months has been shown to have a significant increase in AF recurrence, with no difference in recurrence between 6 months and 1 year, suggesting 6 months as a realistic cutoff for trial of ECV.3
2.3. Sex
The prevalence of AF is higher in males than in females, at any given age. However, given that women live longer, there is a higher number of female patients with AF.15 Women also tend to be more symptomatic with AF16 and have a higher risk of recurrence of AF after successful ECV.8, 17 Sex‐based differences are also apparent in treatment delivered, with women less likely to be treated with ECV, less likely to be referred for ablation, and, if referred, they tend to be treated later in the disease course.16 A possible explanation for this finding is that women often present with a higher number of risk factors, which may dissuade clinicians from referral.16 Female AF patients are generally older15, 17 with a higher prevalence of hypertension,17 thyroid disease, and valve regurgitation15; symptomatic vascular disease and systolic heart failure (HF) were more prevalent in male patients.15, 16 Further research is necessary regarding the sex differences in ECV treatment, as women are generally underrepresented in studies, although the literature suggests they suffer a higher symptom burden than men.15, 16
2.4. Age
In general, older patients are more likely to have AF.2 The incidence of AF in those ages 50 to 59 years is 0.5%, compared with 9% in those age > 80 years.18 Interestingly, although age had no effect on the immediate success of ECV, at 1‐year follow‐up younger patients were significantly more likely to be in SR.4 This has been shown in several studies with variable methodologies and definitions of age groups4, 5, 7, 14; therefore, in clinical practice, older patients are less likely to be offered ECV.
2.5. Weight
Body weight < 80 kg has been shown to be an independent predictor for the success of ECV.6 The reasoning for this finding may be that patients who have a higher body weight may have greater energy requirements for successful ECV. Therefore, patients who are scheduled for ECV should be encouraged to achieve a normal body mass index.
2.6. Smoking
Female smokers are significantly more likely to have AF recurrence than are nonsmoking females, but interestingly there appears to be no difference between smoking or nonsmoking males regarding recurrence of AF.19 This could be due to a higher mortality rate in men masking an increased recurrence rate and a possible increase of diastolic HF, atrial stretch, and atrial arrhythmias.19 The proposed theories of why smoking may increase AF recurrence include nicotine acting directly on atrial tissue, promoting fibrosis; nicotine interaction with ion channels and sympathetic effects, increasing the heart rate; and finally, hypoxia and pulmonary hypertension due to smoking, increasing right atrial hypertrophy and fibrosis.19 As a result, patients should be strongly encouraged to stop smoking prior to ECV.
2.7. Medication
There is a large body of research detailing medication for maintaining SR following ECV, with a Cochrane review outlining the current trends.20 Overall, amiodarone, sotalol, ibutilide, aprindine, vernakalant, flecainide, and propafenone are proven to improve outcomes following ECV (Table 2). β‐Blockers and rate‐limiting calcium channel blockers also appear to have a positive effect. The role of digoxin is less certain. With regard to how long treatment should be given before ECV, there is a lack of reliable data; however, when used, amiodarone should be taken for a few weeks prior to ECV, and other shorter‐acting drugs for 1 to 3 days as per European Society of Cardiology (ESC) guidelines.2
Table 2.
Medications
| Outcome | OR (95% CI) | P Value | Reference | |
|---|---|---|---|---|
| Class I | ||||
| Flecainide | Significantly superior compared with control at reducing AF recurrence in meta‐analysis | 0.31 (0.16–0.60) | <0.001 | Lafuente‐Lafuente et al20 |
| Propafenone | Significantly superior compared with control at reducing AF recurrence in meta‐analysis | 0.37 (0.28–0.48) | <0.001 | Lafuente‐Lafuente et al20 |
| Class II | ||||
| β‐Blocker | Significantly superior compared with control at reducing AF recurrence in meta‐analysis | 0.62 (0.44–0.88) | 0.008 | Lafuente‐Lafuente et al20 |
| Increased success of ECV and maintenance of SR | 7.0 (3.0–16.3) | <0.00001 | Frick et al6 | |
| Class III | ||||
| Sotalol | Significantly reduced AF recurrence in meta‐analysis | 0.43 (0.33–0.56) | <0.001 | Lafuente‐Lafuente et al20 |
| Amiodarone | Continuous use of amiodarone during follow‐up as independent predictor for SR at 1‐year follow‐up | 2.11 (1.56–2.86) | <0.001 | Pisters et al4 |
| Significantly superior compared with control at reducing AF recurrence in meta‐analysis | 0.19 (0.14–0.27) | <0.001 | Lafuente‐Lafuente et al20 | |
| Dronedarone | Significantly superior compared with control at reducing AF recurrence in meta‐analysis | 0.59 (0.46–0.75) | <0.001 | Lafuente‐Lafuente et al20 |
| Class IV | ||||
| Rate‐limiting CCBs | Use of verapamil significantly reduced AF recurrence when used in combination with amiodarone or flecainide | — | 0.03 | De Simone et al23 |
| Multivariate analysis predictor for success of ECV and maintenance of SR | 3.6 (1.1–12.1) | <0.04 | Frick et al6 | |
| Other | ||||
| RAS inhibitor | A significant reduction of recurrence of AF after ECV compared with no RAS‐inhibitor treatment; underutilization of ACEI is related to AF recurrence | 0.50 (0.37–0.69) | <0.01 | Li et al25 |
| MRAs | Lower plasma aldosterone levels associated with SR after cardioversion | Liu et al27 | ||
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; CCB, calcium channel blocker; CI, confidence interval; ECV, electrical cardioversion; HR, hazard ratio; MRA, mineralocorticoid receptor antagonist; OR, odds ratio; RAS, renin‐angiotensin system; SR, sinus rhythm.
2.7.1. Class I antiarrhythmics: flecainide and propafenone
Flecainide and propafenone inhibit fast Na+ channels and therefore reduce the fast Na+ influx in phase 0 of the cardiac action potential. Additionally, they may delay the inactivation of slow sodium channels and inhibit the rapid component in the repolarizing phase, which could be an explanation for the prolongation of the QRS complex and QT interval. Because of their very slow dissociation of the sodium channel, flecainide and propafenone produce some degree of a block in atria and ventricles, even at normal rates. Although the underlying mechanism is not fully understood, these drugs should not be used in patients with ischemic heart disease or other serious structural heart disease.2 Both drugs only show limited efficiency in persistent AF but are effective in preventing recurrence of AF after successful ECV.2, 20
2.7.2. Class II antiarrhythmics: β‐blockers
β‐Blockers achieve their antiarrhythmic effects (1) by directly blocking the sympathetic activity and (2) indirectly because of their antihypertensive and anti‐ischemic effect. β‐Blocker therapy given prior to ECV has shown to be an independent factor for maintenance of SR.6 Prior β‐blockade is likely beneficial through lowering the intracellular calcium from calcium handling instability that can occur in induced AF electrical remodeling,2 along with decreasing the atrial automaticity and blood pressure.20
2.7.3. Class III antiarrhythmics: sotalol
Sotalol is a β‐blocker with additional class III properties. It appears to be more effective than β‐blockers but less effective than amiodarone in the maintenance of SR after ECV.20 However, sotalol also has been found to confer a significant increase in mortality; therefore, its use is limited to only carefully selected patients.20
2.7.4. Amiodarone and dronedarone
Amiodarone and dronedarone are mixed ion channel blockers with additional antisympathetic nervous system effects and significantly reduce the rate of recurrence of paroxysmal and persistent AF.20 Amiodarone appears more efficient than class 1 antiarrhythmics, dronedarone, or sotalol.20 However, it is associated with a high rate of adverse effects leading to discontinuation of treatment.2 Because of the high rate of adverse effects, amiodarone is generally reserved for patients with congestive HF, or as second‐line therapy after failure of other antiarrhythmic drugs.2, 21 Dronedarone is an amiodarone analogue that lacks the iodine atoms; although, like amiodarone, it inhibits numerous ion channels and therefore slows the ventricular rate in patients with AF. Attention should also be paid to the fact that dronedarone increases mortality in decompensated HF and permanent AF,2 although patients treated with dronedarone prior to ECV are significantly more likely to be AF free.4, 20
2.7.5. Vernakalant and ibutilide
Vernakalant and ibutilide are both class III antiarrhythmics with action blocking potassium and sodium channels. Vernakalant has been shown to be superior to ibutilide in cardioversion; and although there are no data on maintenance of SR, due to a high early success rate with cardioversion, a reduction in AF recurrence is thought to be likely.22
2.7.6. Class IV antiarrhythmics: rate‐limiting calcium channel blockers
Rate‐limiting calcium channel blockers diltiazem and verapamil have direct effects on the heart by blocking L‐type calcium channels, and therefore they are used for ventricular rate control in both new‐onset and chronic AF. They reduce resting and exercise heart rate and can, in contrast to β‐blockers, improve exercise tolerance.2 However, the reduction of intracellular calcium also causes a negative inotropic effect; therefore, they should be avoided in patients with left ventricular systolic dysfunction.2 The addition of verapamil to flecainide or amiodarone after failed cardioversion appears to improve ECV success,23 although routine use is not standard clinical practice.
2.7.7. Other medications
Digitalis glycosides
Digoxin is an effective agent for controlling the heart rate at rest and can be a useful agent for rate control in both acute and chronic AF therapy, especially if patients have HF.2 However, digoxin has no role in the maintenance of SR after ECV.24
Renin‐angiotensin‐system (RAS) blockade (in HTN or HF)
Angiotensin‐converting enzyme inhibitors inhibit the conversion of angiotensin I to angiotensin II, and this protein plays a major role in cardiovascular hemostasis. As a contractile agonist, it induces signaling pathways in smooth muscular cells and cardiomyocytes, which results in contraction. Angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers are commonly used in all grades of HF and in hypertension. They increase the likelihood of long‐term SR after ECV.25 However, more research is required, as some studies show no benefit to RAS blockade26 and they are not recommended for routine use pre‐ECV.
Mineralocorticoid receptor antagonists
Mineralocorticoid receptor antagonists such as spironolactone and eplerenone may have some benefit in patients with AF undergoing ECV, although more evidence is required before these can be recommended for widespread use.27 This effect is thought to be due to lowering plasma aldosterone levels with higher levels in persistent AF.27
2.8. Comorbidities
2.8.1. Hypertension
High blood pressure is an independent risk factor for initial failure of ECV 9 to restore SR. β‐Blockers and RAS blockers appear to be the most effective agents to improve outcomes following ECV in hypertensive patients. Although β‐blockers have less effect on blood pressure than do RAS blockers, the positive influence of hypertension treated with β‐blockers has been shown.28 Patients should have hypertension effectively controlled with a pharmacological strategy that includes a β‐blocker, a renin‐angiotensin‐aldosterone system–blocking drug, and a diuretic (if needed) prior to ECV (Table 3).
Table 3.
Comorbidities
| Outcome | OR/HR (95% CI) | P Value | Reference | |
|---|---|---|---|---|
| Hypertension | High blood pressure is an independent risk factor for initial failure of ECV | OR: 1.73 (1.22–1.91) | 0.015 | Berry et al9 |
| DM | DM is an independent risk factor reducing the likelihood of maintenance of SR after successful ECV | OR: 0.34 (0.14–0.84) | 0.01 | Soran et al12 |
| DM shown to be a risk factor with multivariate analysis for failure of cardioversion within 30 days of ECV | OR: 1.36 (1.07–1.72) | 0.01 | Grönberg et al8 | |
| COPD | COPD absence as independent predictor of SR at follow‐up | OR: 0.23 (0.13–0.41) | 0.003 | Pisters et al4 |
| OSA | Higher recurrence risk after 1‐year follow‐up after catheter ablation and ECV in patients with OSA; appropriate treatment shows lower recurrence rate (without treatment vs treatment vs control) | OR: 3.04 (1.45–6.36) | 0.003 | Mazza et al31 |
| Inappropriately treated vs treated vs no OSA | 82% vs 42% vs 53%, respectively | 0.013 (treated group), 0.009 (control group) | Kanagala et al32 | |
| Renal impairment | Recurrence rates rise with the severity of renal impairment compared with patients with normal renal function: eGFR <60 mL/min vs >60 mL/min vs <30 mL/min | HR: 0.97 (0.95–0.99) | 0.004 | Schmidt et al33 |
| Multivariate analysis for failure of cardioversion within 30 days of ECV | OR: 1.65 (1.09–2.50) | 0.02 | Grönberg et al8 | |
| Hyperthyroidism | Patients with hyperthyroid‐induced persistent AF had a much lower AF recurrence rate compared with those with AF of nonthyroid origins after ECV (83% vs 59% after 1‐year follow‐up) | HR: 0.64 (0.39–0.97) | 0.04 | Siu et al35 |
Abbreviations: AF, atrial fibrillation; CI, confidence interval; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; ECV, electrical cardioversion; eGFR, estimated glomerular filtration rate; HR, hazard ratio; OR, odds ratio; OSA, obstructive sleep apnea; SR, sinus rhythm.
2.8.2. Diabetes mellitus (DM)
DM is associated with the development of AF 29 and progression from paroxysmal AF to persistent AF.30 Furthermore, DM is an independent risk factor reducing the likelihood of maintenance of SR after successful ECV.8, 12 Suggested mechanisms for this effect include increased hyperglycemia‐induced cardiac myocyte necrosis, macro and microvascular ischemia, autonomic neuropathy, and hypoglycemia‐induced adrenosympathetic axis activation with those on blood sugar–lowering medications.
2.8.3. Chronic obstructive pulmonary disease (COPD)
The absence of COPD was found to be an independent predictor of SR at 1‐year follow‐up.4 This effect may be due to increased transthoracic resistance due to increased chest size, along with pathophysiological changes, right atrial stretch, and remodeling due to raised pulmonary pressures.4 Identification of COPD before ECV is useful, as determining the relative contributions to breathlessness in patients with AF and COPD can be difficult.
2.8.4. Obstructive sleep apnea (OSA)
OSA is associated with AF through various proposed mechanisms such as hypoxia, increased afterload, left ventricular diastolic dysfunction, sympathovagal imbalance, and systemic inflammation.31 There is a higher recurrence risk after 1‐year follow‐up in patients with sleep apnea undergoing ECV.31 These findings are similar to those of other studies that both also found that patients appropriately treated for OSA had a lower rate of recurrence compared with untreated patients with OSA.32 The clinical implications are that patients who are at risk of OSA, especially obese patients, should be screened for OSA and appropriately treated prior to ECV to help reduce recurrence rates. However, the delay in organizing investigation and treatment for OSA will inevitably increase the duration of AF prior to ECV, and therefore further research is required to assess the clinical impact of such a strategy.
2.8.5. Renal impairment
Renal disease has been found to be a risk factor for AF recurrence within 30 days.8 Recurrence rates of AF were shown to increase with increasing severity of renal impairment.33 Interestingly, patients with an estimated glomerular filtration rate (eGFR) between 30 and 90 mL/min were shown to have an improvement in their eGFR after 1 month in SR, thought to be due to an improvement in renal hemodynamics caused by improved cardiac output.33 These findings may have an implication for treatment strategies for AF, with patients with mild to moderate renal impairment (eGFR 30–90 mL/min) to be treated with more aggressive approaches such as ablation, as they might have an improvement in their renal function as a result.
2.8.6. Hyperthyroidism
Hyperthyroidism is a well‐recognized but rare cause of AF. Approximately two‐thirds of patients will have spontaneous resolution of AF with normalization of thyroid levels, and the remaining third may require intervention.34 Once treated, however, patients with hyperthyroid‐induced persistent AF have a much lower AF recurrence rate after ECV compared with those with nonthyroid AF.35 An explanation for this effect could be the removal of the trigger factor, hyperthyroidism in this case, and also a difference in atrial electrophysiological properties in hyperthyroid AF with normalization of the atrial effective refractory period once thyroid levels have been corrected.35
2.9. Coexistent cardiac disease
2.9.1. Coronary artery disease (CAD)
There are conflicting data as to whether CAD is a clinically relevant parameter to predict recurrence of AF after ECV. The presence of vascular disease, including CAD, was also found to be a risk factor for recurrence of AF within 30 days.8 However, data are conflicting, and in the Atrial Fibrillation Follow‐up Investigation of Rhythm Management (AFFIRM) study, CAD was protective for AF recurrence at 2 months.36 Clearly, research is required to ascertain the interaction between CAD and AF recurrence and whether treating CAD improved AF outcomes. The presence of CAD should not be seen as a contraindication to ECV (Table 4).
Table 4.
Coexistent cardiac disease
| Outcome | OR/HR (95% CI) | P Value | Reference | |
|---|---|---|---|---|
| CAD | Vascular disease shown to be a risk factor with multivariate analysis for failure of cardioversion within 30 days of ECV | OR: 1.54 (1.27–1.85) | <0.001 | Grönberg et al8 |
| CHD | Treatment of atrial arrhythmias in CHD has lower success rate, although not statistically significant and increased likelihood of arrhythmia recurrence (CHD vs control) | 40% vs 54% | 0.13 | Ammash et al11 |
| Fontan physiology was a multivariate predictor of AF recurrence | HR: 2.16 (1.24–4.35) | <0.001 | Egbe et al37 | |
| Rheumatic heart disease | History of rheumatic heart disease significantly shortens arrhythmia‐free episodes after ECV | — | — | Abu‐El‐Haija et al38 |
| LV systolic dysfunction | Multivariate analysis for failure of cardioversion within 30 days of ECV | OR: 1.54 (1.10–2.17) | 0.01 | Grönberg et al8 |
| LV diastolic dysfunction | Independent predictor of early (2‐week) and longtime (1‐year) AF recurrence; LV filling pressure > 8 | 2 weeks, OR: 1.297 (1.099–1.531); 1 year, OR: 1.319 (1.134–1.536) | 0.002, 0.0003 | Caputo et al39 |
| LVH | Patients with AF recurrence had a higher degree of LVH than did those without recurrence | OR: 2.52 (1.26–5.01) | 0.01 | Marchese et al5 |
| Valvular heart disease | Significant association with the need for multiple (≥2) ECVs in the first year after the initial one | HR: 2.36 (1.46–3.80) | 0.00004 | Raitt et al36 |
| LA size | LA diameter > 4.5 cm (sens, 70%; spec, 50%; ≥2 ECV in the first year) | OR: 1.86 (1.20–2.88) | 0.005 | Raitt et al36 |
| Increased incidence of recurrence at long‐term follow‐up with enlarged atrial volume | HR: 1.39 (1.11–1.18) | 0.013 | Toso et al3 | |
| Smaller LA diameter as independent predictor for SR at follow‐up | OR: 0.92 (0.95–0.99) | 0.005 | Pisters et al4 | |
| Independent predictor for AF recurrence >4.4 cm | OR: 1.126 (1.015–1.249) | 0.025 | Efremidis et al42 | |
| LA enlargement; each mL/cm2 increase in indexed LA volume 21% increase of AF recurrence | OR: 1.21 (1.11–1.30) | <0.0001 | Marchese et al5 | |
| LA dimension <3.7 cm | OR: 5.9 (1.4–25.4) | 0.02 | Frick et al6 |
Abbreviations: AF, atrial fibrillation; CAD, coronary artery disease; CHD, congenital heart disease; CI, confidence interval; ECV, electrical cardioversion; HR, hazard ratio; LA, left atrial; LV, left ventricular; LVH, left ventricular hypertrophy; OR, odds ratio; sens, sensitivity; spec, specificity.
2.9.2. Congenital heart disease
Treatment of atrial arrhythmias can be more challenging in congenital heart disease, with data on ECV success limited. Two studies, a small pilot study in 201211 and a larger study in 2017,37 have shown that the recurrence rates were higher in congenital heart disease patients with spontaneous echo contrast in the left atrium, presumably due to advanced atrial dysfunction from chronic volume and pressure overload. A proportion of the congenital heart disease patients in the 2012 study went on to have radiofrequency ablation (22%), with varying success rates of 50% to 85%.11 The larger study found that recurrence rates were higher in those having undergone the Fontan procedure, but there were no differences between the control group and congenital heart disease group once those with the Fontan group were eliminated from analysis.37 Although patients were not matched for age in the studies, the results suggest that congenital heart disease patients should be considered for ECV in the same way as the general population, with special consideration reserved for those with the Fontan procedure or spontaneous echo contrast.
2.9.3. Rheumatic heart disease and chronic inflammation
Rheumatic heart disease is associated with AF recurrence following ECV,38 most likely caused by the impact of rheumatic fever on mitral valve function, leading to increased atrial strain and remodeling. However, there is also increasing evidence that chronic inflammation is associated with increased incidence of AF and poorer outcome, independent of the severity of the valvular disease.13
2.9.4. Left ventricular hypertrophy (LVH) and dysfunction
Patients with LVH and AF recurrence had a higher degree of LVH than did those without recurrence.5 This finding helps support the theory that LV dysfunction such as reduced LV compliance may contribute to AF through structural changes to left atria via increased LV filling pressures.
Left ventricular diastolic dysfunction, the main feature of LVH, is more common in older patients and patients with DM and HTN, and it may explain the higher failure rates of ECV in these patient groups even in the absence of overt LVH. Impairment of LV diastolic function is an independent predictor of early (2 weeks) and longtime (1 year) AF recurrence.39 At 1 year it is also found that left atrial volume index was a risk factor for recurrence at 1 year, along with the ratio of mitral inflow to mitral annulus velocity at end‐diastole. The diastolic impairment of the LV can lead to atrial pressure increase and hypertrophy leading to electrical instability manifesting itself as AF.
AF is associated with progression of LV systolic dysfunction along with increased mortality.40 The presence of symptomatic LV dysfunction has been shown to have a negative impact upon ECV success in the first 30 days of cardioversion,8 although whether any difference between severity or symptomatic HF on success rates has not been studied. However, conversion from AF to SR has not been shown to decrease cardiovascular mortality in comparison with a rate‐control strategy.41
2.9.5. Valvular heart disease
Mitral valve thickening was found, in the AFFIRM trial data, to be associated with the need for multiple (≥2) ECVs in the first year after the initial ECV.36 This is consistent with previous data showing AF recurrence being associated with a past history of rheumatic fever, one of the leading causes of mitral valve disease.38 Data from other studies, however, have been variable, with Pisters et al. finding that the absence of valvular disease was beneficial for recurrence rates with pharmacological cardioversion but not with ECV.4 Therefore, the presence of valvular heart disease per se is not a contraindication to ECV.
2.9.6. Left atrial (LA) size
Increased LA size is strongly associated with lower SR rates following ECV in the short and longer term.3, 4, 5, 6, 36, 42 Several studies have found an increased atrial diameter as a risk factor for AF recurrence, such as with left atrial diameter > 4.4 cm42 and 4.5 cm.36 Correcting for body size with indexed LA volume is a stronger predictor of AF than simple measurement of LA diameter.3, 5 This effect is most likely caused by atrial remodeling from stress on cardiac myocytes from volume/pressure overload or tachycardia leading to atrial fibrosis and electrophysiological predisposing to AF.5
However, there is no standard size or cutoff that defines an enlarged LA, and there is much variation in the literature. It has been suggested that moderate LA volume enlargement suggests a degree of irreversibility and a significant increase with a volume > 33.5 mL/m2,5 with similar results found with a LA volume > 34 mL/m2.3 Thus, although increased LA size is not a contraindication to ECV, patients with LA enlargement should be counseled that long‐term maintenance of SR is less likely.
3. ECV PROCEDURAL DETAILS
3.1. Number of shocks at ECV
It has been demonstrated that if achieving SR was initially difficult (≥2 shocks), then the risk of recurrent AF was higher.43 It may be that the need for higher energy and multiple attempts could indicate more extensive cardiac structural and electrical remodeling, or that comorbidities such as COPD, HF, and high body mass index often require a higher amount of energy, although they found no difference in prevalence of these conditions between patients with 1 and multiple shocks (Table 1).43
3.2. Serial ECV
One study demonstrated merit in an aggressive strategy of serial ECV (up to 2 further ECVs) in patients with early AF recurrence. This strategy resulted in a 53% success at 1 year, compared with 29% in the control group.44
3.3. ECV after ablation
Following atrial ablation therapy, a 3‐month “blanking period” is expected, with early recurrence of AF due to inflammatory reactions associated with the procedure.45 During this period, ECV has been used to treat persistent AF recurrence. Patients who require ECV after a prior AF ablation have a higher recurrence rates and the need for a further ablation.45
4. CONCLUSION
Many factors are associated with the maintenance of SR after ECV. This makes it difficult to identify patients most likely to have a successful long‐term outcome after ECV. Generally, the longer‐term outcomes following ECV are disappointing; however, as described above, certain factors appear to be associated with better outcomes, and knowledge of these may help clinicians better select the patients who are most likely to achieve long‐term maintenance of SR after ECV. To help aid the clinician, a scoring system has been designed and validated with a selection of factors as described earlier.14
Some of the risk factors described are potentially modifiable, and treatment of conditions such as hypertension, OSA, and hyperthyroidism should be considered before ECV. Smoking cessation and achieving a normal weight should be encouraged where needed, and pretreatment with β‐blockers or RAS blockade may help others. However, there remains a large number of nonmodifiable risk factors—such as age, sex, structural heart disease, and comorbidities. Greater knowledge of the impact of these risk factors will hopefully help cardiologists and patients decide wisely when choosing ECV as a treatment option.
Conflicts of interest
The authors declare no potential conflicts of interest.
Ecker V, Knoery C, Rushworth G, et al. A review of factors associated with maintenance of sinus rhythm after elective electrical cardioversion for atrial fibrillation. Clin Cardiol. 2018;41:862–870. 10.1002/clc.22931
REFERENCES
- 1. Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. [DOI] [PubMed] [Google Scholar]
- 2. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur J Cardiothorac Surg. 2016;50:e1–e88. [DOI] [PubMed] [Google Scholar]
- 3. Toso E, Blandino A, Sardi D, et al. Electrical cardioversion of persistent atrial fibrillation: acute and long‐term results stratified according to arrhythmia duration. Pacing Clin Electrophysiol. 2012;35:1126–1134. [DOI] [PubMed] [Google Scholar]
- 4. Pisters R, Nieuwlaat R, Prins MH, et al; Euro Heart Survey Investigators . Clinical correlates of immediate success and outcome at 1‐year follow‐up of real‐world cardioversion of atrial fibrillation: the Euro Heart Survey. Europace. 2012;14:666–674. [DOI] [PubMed] [Google Scholar]
- 5. Marchese P, Bursi F, Donne GD, et al. Indexed left atrial volume predicts the recurrence of non‐valvular atrial fibrillation after successful cardioversion. Eur J Echocardiogr. 2011;12:214–221. [DOI] [PubMed] [Google Scholar]
- 6. Frick M, Frykman V, Jensen‐Urstad M, et al. Factors predicting success rate and recurrence of atrial fibrillation after first electrical cardioversion in patients with persistent atrial fibrillation. Clin Cardiol. 2001;24:238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boriani G, Diemberger I, Biffi M, et al. Electrical cardioversion for persistent atrial fibrillation or atrial flutter in clinical practice: predictors of long‐term outcome. Int J Clin Pract. 2007;61:748–756. [DOI] [PubMed] [Google Scholar]
- 8. Grönberg T, Hartikainen JE, Nuotio I, et al. Can we predict the failure of electrical cardioversion of acute atrial fibrillation? The FinCV study. Pacing Clin Electrophysiol. 2015;38:368–375. [DOI] [PubMed] [Google Scholar]
- 9. Berry C, Stewart S, Payne EM, et al. Electrical cardioversion for atrial fibrillation: outcomes in “real‐life” clinical practice. Int J Cardiol. 2001;81:29–35. [DOI] [PubMed] [Google Scholar]
- 10. Elesber AA, Rosales AG, Herges RM, et al. Relapse and mortality following cardioversion of new‐onset vs. recurrent atrial fibrillation and atrial flutter in the elderly. Eur Heart J. 2006;27:854–860. [DOI] [PubMed] [Google Scholar]
- 11. Ammash NM, Phillips SD, Hodge DO, et al. Outcome of direct current cardioversion for atrial arrhythmias in adults with congenital heart disease. Int J Cardiol. 2012;154:270–274. [DOI] [PubMed] [Google Scholar]
- 12. Soran H, Younis N, Currie P, et al. Influence of diabetes on the maintenance of sinus rhythm after a successful direct current cardioversion in patients with atrial fibrillation. QJM Mon J Assoc Physicians. 2008;101:181–187. [DOI] [PubMed] [Google Scholar]
- 13. Harada M, Van Wagoner DR, Nattel S. Role of inflammation in atrial fibrillation pathophysiology and management. Circ J. 2015;79:495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jaakkola S, Lip GY, Biancari F, et al. Predicting unsuccessful electrical cardioversion for acute atrial fibrillation (from the AF‐CVS Score). Am J Cardiol. 2017;119:749–752. [DOI] [PubMed] [Google Scholar]
- 15. Volgman AS, Manankil MF, Mookherjee D, et al. Women with atrial fibrillation: greater risk, less attention. Gend Med. 2009;6:419–432. [DOI] [PubMed] [Google Scholar]
- 16. Michelena HI, Powell BD, Brady PA, et al. Gender in atrial fibrillation: ten years later. Gend Med. 2010;7:206–217. [DOI] [PubMed] [Google Scholar]
- 17. Gurevitz OT, Varadachari CJ, Ammash NM, et al. The effect of patient sex on recurrence of atrial fibrillation following successful direct current cardioversion. Am Heart J. 2006;152:155.e9–155.e13. [DOI] [PubMed] [Google Scholar]
- 18. Kannel WB, Wolf PA, Benjamin EJ, et al. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population‐based estimates. Am J Cardiol. 1998;82:2N–9N. [DOI] [PubMed] [Google Scholar]
- 19. Kinoshita M, Herges RM, Hodge DO, et al. Role of smoking in the recurrence of atrial arrhythmias after cardioversion. Am J Cardiol. 2009;104:678–682. [DOI] [PubMed] [Google Scholar]
- 20. Lafuente‐Lafuente C, Valembois L, Bergmann JF, et al. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst Rev. 2015;3:CD005049. [DOI] [PubMed] [Google Scholar]
- 21. Lip GY, Tse HF, Lane DA. Atrial fibrillation. Lancet. 2012;379:648–661. [DOI] [PubMed] [Google Scholar]
- 22. Simon A, Niederdoeckl J, Skyllouriotis E, et al. Vernakalant is superior to ibutilide for achieving sinus rhythm in patients with recent‐onset atrial fibrillation: a randomized controlled trial at the emergency department. Europace. 2017;19:233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. De Simone A, De Pasquale M, De Matteis C, et al. Verapamil plus antiarrhythmic drugs reduce atrial fibrillation recurrences after an electrical cardioversion (VEPARAF Study). Eur Heart J. 2003;24:1425–1429. [DOI] [PubMed] [Google Scholar]
- 24. Atarashi H, Inoue H, Fukunami M, et al; Sinus Rhythm Maintenance in Atrial Fibrillation Randomized Trial (SMART) Investigators . Double‐blind placebo‐controlled trial of aprindine and digoxin for the prevention of symptomatic atrial fibrillation. Circ J. 2002;66:553–556. [DOI] [PubMed] [Google Scholar]
- 25. Li TJ, Zang WD, Chen YL, et al. Renin‐angiotensin system inhibitors for prevention of recurrent atrial fibrillation: a meta‐analysis. Int J Clin Pract. 2013;67:536–543. [DOI] [PubMed] [Google Scholar]
- 26. Disertori M, Latini R, Barlera S, et al; GISSI‐AF Investigators . Valsartan for prevention of recurrent atrial fibrillation. N Engl J Med. 2009;360:1606–1617. [DOI] [PubMed] [Google Scholar]
- 27. Liu T, Korantzopoulos P, Shao Q, et al. Mineralocorticoid receptor antagonists and atrial fibrillation: a meta‐analysis. Europace. 2016;18:672–678. [DOI] [PubMed] [Google Scholar]
- 28. Van Noord TV, Tieleman RG, Bosker HA, et al. β‐Blockers prevent subacute recurrences of persistent atrial fibrillation only in patients with hypertension. Europace. 2004;6:343–350. [DOI] [PubMed] [Google Scholar]
- 29. Lip GY, Varughese GI. Diabetes mellitus and atrial fibrillation: perspectives on epidemiological and pathophysiological links. Int J Cardiol. 2005;105:319–321. [DOI] [PubMed] [Google Scholar]
- 30. Sakamoto H, Okamoto E, Imataka K, et al. Prediction of early development of chronic nonrheumatic atrial fibrillation. Jpn Heart J. 1995;36:191–199. [DOI] [PubMed] [Google Scholar]
- 31. Mazza A, Bendini MG, Cristofori M, et al. Baseline apnoea/hypopnoea index and high‐sensitivity C‐reactive protein for the risk of recurrence of atrial fibrillation after successful electrical cardioversion: a predictive model based upon the multiple effects of significant variables. Europace. 2009;11:902–909. [DOI] [PubMed] [Google Scholar]
- 32. Kanagala R, Murali NS, Friedman PA, et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. 2003;107:2589–2594. [DOI] [PubMed] [Google Scholar]
- 33. Schmidt M, Rieber J, Daccarett M, et al. Relation of recurrence of atrial fibrillation after successful cardioversion to renal function. Am J Cardiol. 2010;105:368–372. [DOI] [PubMed] [Google Scholar]
- 34. Shimizu T, Koide S, Noh JY, et al. Hyperthyroidism and the management of atrial fibrillation. Thyroid. 2002;12:489–493. [DOI] [PubMed] [Google Scholar]
- 35. Siu CW, Jim MH, Zhang X, et al. Comparison of atrial fibrillation recurrence rates after successful electrical cardioversion in patients with hyperthyroidism‐induced versus non–hyperthyroidism‐induced persistent atrial fibrillation. Am J Cardiol. 2009;103:540–543. [DOI] [PubMed] [Google Scholar]
- 36. Raitt MH, Volgman AS, Zoble RG, et al. Prediction of the recurrence of atrial fibrillation after cardioversion in the Atrial Fibrillation Follow‐up Investigation of Rhythm Management (AFFIRM) study. Am Heart J. 2006;151:390–396. [DOI] [PubMed] [Google Scholar]
- 37. Egbe AC, Asirvatham SJ, Connolly HM, et al. Outcomes of direct current cardioversion in adults with congenital heart disease. Am J Cardiol. 2017;119:1468–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Abu‐El‐Haija B, Giudici MC. Predictors of long‐term maintenance of normal sinus rhythm after successful electrical cardioversion. Clin Cardiol. 2014;37:381–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Caputo M, Urselli R, Capati E, et al. Usefulness of left ventricular diastolic dysfunction assessed by pulsed tissue Doppler imaging as a predictor of atrial fibrillation recurrence after successful electrical cardioversion. Am J Cardiol. 2011;108:698–704. [DOI] [PubMed] [Google Scholar]
- 40. Dries DL, Exner DV, Gersh BJ, et al. Atrial fibrillation is associated with an increased risk for mortality and heart failure progression in patients with asymptomatic and symptomatic left ventricular systolic dysfunction: a retrospective analysis of the SOLVD trials. Studies of Left Ventricular Dysfunction. J Am Coll Cardiol. 1998;32:695–703. [DOI] [PubMed] [Google Scholar]
- 41. Roy D, Talajic M, Nattel S, et al; Atrial Fibrillation and Congestive Heart Failure Investigators . Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358:2667–2677. [DOI] [PubMed] [Google Scholar]
- 42. Efremidis M, Alexanian IP, Oikonomou D, et al. Predictors of atrial fibrillation recurrence in patients with long‐lasting atrial fibrillation. Can J Cardiol. 2009;25:e119–e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Alla VM, Kanuri S, Bansal O, et al. Number of shocks during elective cardioversion predicts long‐term recurrence of atrial fibrillation. Int J Cardiol. 2012;158:451–453. [DOI] [PubMed] [Google Scholar]
- 44. Bertaglia E, D'Este D, Zerbo F, et al. Success of serial external electrical cardioversion of persistent atrial fibrillation in maintaining sinus rhythm: a randomized study. Eur Heart J. 2002;23:1522–1528. [DOI] [PubMed] [Google Scholar]
- 45. Chilukuri K, Dukes J, Dalal D, et al. Outcomes in patients requiring cardioversion following catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2010;21:27–32. [DOI] [PubMed] [Google Scholar]


