Abstract
Background
The role of catheter ablation (CA) is increasingly recognized as a reasonable therapeutic option in patients with atrial fibrillation (AF) and heart failure (HF).
Hypothesis
We aimed to compare CA to medical therapy in AF patients with HF with reduced ejection fraction (HFrEF).
Methods
We searched the literature for randomized clinical trials comparing CA to medical therapy in this population.
Results
Six trials with a total of 775 patients were included. AF was persistent in 95% of patients with a mean duration of 18.5 ± 23 months prior enrollment. The mean age was 62.2 ± 7.8 years, mostly males (83%) with mean left ventricular ejection fraction (LVEF) of 31.2 ± 6.7%. Compared to medical therapy, CA has significantly improved LVEF by 5.9% (Mean difference [MD] 5.93, confidence interval [CI] 3.59‐8.27, P < 0.00001, I 2 = 87%), quality of life, (MD −9.01, CI −15.56, −2.45, P = 0.007, I 2 = 47%), and functional capacity (MD 25.82, CI 5.46‐46.18, P = 0.01, I 2 = 90%). CA has less HF hospital readmissions (odds ratio [OR] 0.5, CI 0.32‐0.78, P = 0.002, I 2 = 0%) and death from any cause (OR 0.46, CI 0.29‐0.73, P = 0.0009, I 2 = 0%). Freedom from AF during follow‐up was higher in patients who had CA (OR 24.2, CI 6.94‐84.41, P < 0.00001, I 2 = 81%.
Conclusion
CA was superior to medical therapy in patients with AF and HFrEF in terms of symptoms, hemodynamic response, and clinical outcomes by reducing AF burden. However, these findings are applicable to the very specific patients enrolled in these trials.
Keywords: ablation, atrial fibrillation, heart failure
1. INTRODUCTION
Atrial fibrillation (AF) and heart failure (HF) are common medical conditions that often coexist. Up to 50% of patients with HF have AF while AF can also lead to HF and tachycardia induced cardiomyomathy.1 The presence of AF and HF is associated with increased morbidity, mortality, and healthcare costs.2, 3 Restoration and maintenance of sinus rhythm in patients with AF and HF are often attempted to improve symptoms.4 However, this approach is limited by suboptimal efficacy and side effects of antiarrhythmic drugs.5, 6 Catheter ablation (CA) has emerged as an effective therapeutic option over the last two decades for treatment of AF.7 Several studies have evaluated the role of AF ablation in patients with HF with reduced ejection fraction (HFrEF) and demonstrated that sinus rhythm could be often restored with a significant improvement in left ventricular ejection function (LVEF) and symptoms.8, 9 However, most of these studies were small and observational. Recently, several randomized control trials (RCTs) compared the efficacy and outcomes of CA vs medications in patients with AF and HFrEF and reported new findings. Therefore, we performed an updated meta‐analysis of RCTs comparing CA to medical therapy in patients with AF and HFrEF, not only to evaluate the directionality of the trends but also to refine measures and confidence intervals of odds ratios. Furthermore, this meta‐analysis might help mitigate the selection bias, which is a major limitation of these trials.
2. METHODS
A systematic literature review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement. Two authors (T.M.H., R.G.) separately searched PubMed, Web of Science, CINAHL (Cumulative Index of Nursing and Allied Health Literature), and Cochrane Library databases for studies comparing CA to medical therapy in patients with AF and HF from January 1966 through February 2018 (Figure 1). We used the following keywords in various combinations: atrial fibrillation (AF), ablation, and heart failure. Additional details of search terms and strategy are provided in the Supporting Information, Appendix S1. The bibliography of selected manuscripts and review articles were also manually searched for additional studies which were not identified in the original search. Titles and abstracts were then screened to identify studies for full text review.
Figure 1.
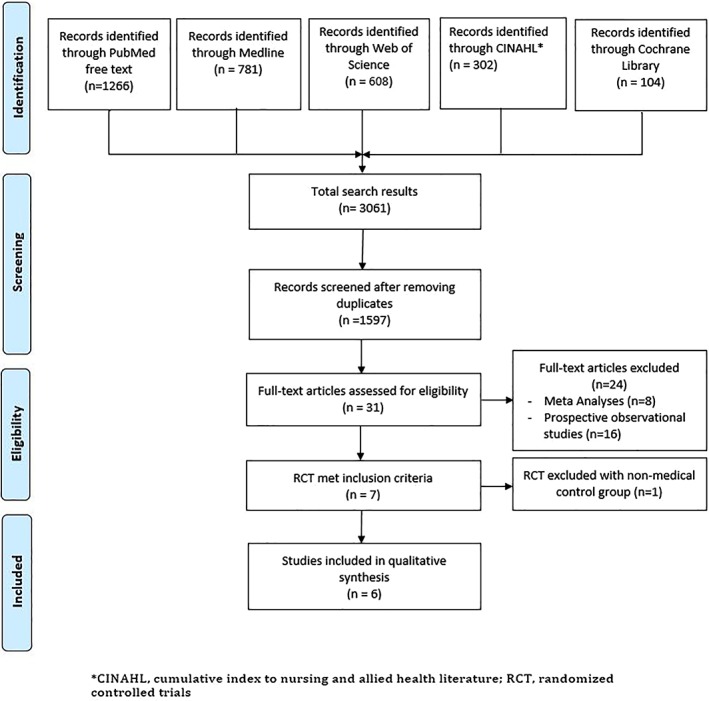
Flow diagram of the literature search
RCTs reporting AF treatment with CA vs medical therapy in patients with HF and fulfilling the predefined inclusion and exclusion criteria were selected for inclusion in the systematic review and quantitative analysis. Studies were included in the meta‐analysis if they included the following criteria: (a) history of AF with a diagnosis of HF, (b) it was a RCT to treat AF with CA vs medical therapy (rate or rhythm control), (c) reported mean differences and/or number of events for LVEF, HF related hospital admission, 6‐minute walk test distance (6MWTD), and overall mortality, and (d) adult subjects (> 18 years of age). Exclusion criteria included (a) published abstract without full text publication, (b) studies assessing the impact of CA on AF without medical treatment group, and (c) studies lacking endpoint measures. We excluded the study by Khan et al10 because it compared rate control using atrioventricular nodal ablation rather than medical therapy. Echocardiography was the primary imaging method to estimate the LVEF. However, given the difficulties in measuring the LVEF during AF, some studies used radionuclide ventriculography and cardiac magnetic resonance imaging to measure the LVEF. Adverse events were defined as death, stroke, bleeding, pericardial effusion, cardiac tamponade, pulmonary vein stenosis, and worsening HF. Two authors independently performed the study selection and data extraction using a standardized data extraction form (T.M.H., R.G.). Differences were resolved by consensus. Quality assessment was performed using the modified Jadad scale (Table S2) and Cochrane Collaboration tool (Table S3) for assessing risk of bias.
Data from selected studies were extracted and used to estimate the mean difference (MD) and its 95% confidence interval (CI) for continuous variables and odds ratio (OR) and 95% CI for dichotomous variables. Cochrane Review Manager (RevMan) 5.3 (The Nordic Cochrane Center, Copenhagen, Denmark) was used for meta‐analysis. The meta‐analysis was conducted with the random effects model using Mantel‐Haenszel weighting for dichotomous variables, and random inverse variance method for continuous variables. Heterogeneity was tested using χ2, Tau‐square and I‐square (I 2) statistics. I 2 index values of 25% to 50%, 51% to 75% and >75% were considered as low, moderate, and high heterogeneity, respectively. Publication bias was analyzed by visually inspecting the funnel plot. Subgroup analyses were performed on the type of medical therapy (rate control only vs rate and/or rhythm control). Sensitivity analysis based on quality score was performed by removing one study at a time and repeating analysis to identify any particularly influential study. Excel 2013 (Microsoft, Redmond, WA) was used to estimate means and SD for continuous variables in pooled groups of CA and medical therapy patients, after which Z tests were conducted to compare pooled groups of CA and medical therapy patients for any continuous variables. We calculated the mean and SD for all the variables that were reported as median and interquartile range (IQR) using specific formula.11 A two sided P value of <0.05 was considered statistically significant for all analyses.
3. RESULTS
A total of 1597 articles were screened, only six trials with a total of 775 patients met the inclusion criteria (CA 388 and medical therapy 387).12, 13, 14, 15, 16, 17 The basic characteristics of the included studies and patients are summarized in Tables 1 and 2. The mean age was 62.2 ± 7.8 years, and 83% were males. The median follow‐up is 9 months (IQR 6‐24) with an average duration of follow‐up ranging from 6 to 37 months. The mean LVEF was 31.2 ± 6.7% and the left atrial diameter was 48.3 ± 6.2 mm. Most patients (96.7%) had New York Heart Association (NYHA) class of II or III. Persistent AF accounted for 95% of the population. The mean duration of AF prior enrollment was 18.5 ± 23 months, with most patients in AF for 1 to 2 years, except for one trial in which AF duration was about 4 years. Postablation blanking period ranged from 1 to 3 months. CA procedural characteristics and complications are reported in Table S1.
Table 1.
Trials baseline characteristics
| Trials | MacDonald et al 201012 | Jones et al 201313 | Hunter et al 201414 | Di Biase et al 201615 | Prabhu et al 201716 | Marrouche et al 201817 | All trials | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Groups | Ablation | Medical | Ablation | Medical | Ablation | Medical | Ablation | Medical | Ablation | Medical | Ablation | Medical | Ablation | Medical | Total | P |
| Patient numbers | 22 | 19 | 26 | 26 | 26 | 24 | 102 | 101 | 33 | 33 | 179 | 184 | 388 | 387 | 775 | |
| Mean follow‐up mo |
6 | 12 | 6 | 24 | 6 | 37.6 | 15 | |||||||||
| Male | 17 (77%) | 15 (79%) | 21 (81%) | 24 (92%) | 25 (96%) | 23 (96%) | 77 (75%) | 74 (73%) | 31 (94%) | 29 (88%) | 156 (87%) | 155 (84%) | 327 (84%) | 320 (83%) | 647 (83%) | |
| Age, y M ± SD |
62.3 ± 6.7 | 64.4 ± 8.3 | 64 ± 10 | 62 ± 9 | 55 ± 12 | 60 ± 10 | 62 ± 10 | 60 ± 11 | 59 ± 11 | 62 ± 9.4 | 64 ± 2.5* | 64 ± 2.9* | 62.1 ± 7.9 | 62.4 ± 7.7 | 62.2 ± 7.8 | 0.54 |
| LVEF, % M ± SD |
36.1 ± 11.9 | 42.9 ± 9.6 | 22 ± 8 | 25 ± 7 | 32 ± 8 | 34 ± 12 | 29 ± 5 | 30 ± 8 | 32 ± 9.4 | 34 ± 7.8 | 31.5 ± 1.7* | 32.5 ± 2.2* | 30.5 ± 6.2 | 32.1 ± 7.1 | 31.2 ± 6.7 | 0.0008 |
| LAD, mm M ± SD |
NA | NA | 50 ± 6 | 46 ± 7 | 52 ± 11 | 50 ± 10 | 47 ± 4.2 | 48 ± 4.9 | 48 ± 5.5 | 47 ± 8.2 | 48 ± 1.5 | 49.5 ± 8.3* | 48.1 ± 4.6 | 48.6 ± 7.6 | 48.3 ± 6.2 | 0.28 |
| 6MWTD, m M ± SD |
278 ± 131 | 331 ± 117 | 416 ± 78 | 411 ± 109 | NA | NA | 348 ± 111 | 350 ± 130 | 491 ± 147 | 489 ± 132 | NA | NA | 375 ± 133 | 382 ± 137 | 378.5 ± 135 | 0.62 |
| MLWHF M ± SD |
64.3 ± 15 | 54.9 ± 22.3 | 42 ± 23 | 49 ± 21 | NA | NA | 52 ± 24 | 50 ± 27 | NA | NA | NA | NA | 52 ± 23.4 | 50 ± 25.3 | 51 ± 24.3 | 0.48 |
| BNP, pn/mL M ± SD |
2550 ± 2150 | 1846 ± 1687 | 412 ± 324 | 283 ± 285 | NA | NA | NA | NA | 266 ± 210 | 256 ± 208 | NA | NA | 933 ± 1501 | 652 ± 1083 | 792 ± 1315 | 0.17 |
| AF duration before enrollment, mo M ± SD |
44 ± 36.5 | 64 ± 47.6 | 23 ± 22 | 24 ± 29 | 24 ± 4* | 27 ± 9* | 8.6 ± 3.2 | 8.4 ± 4.1 | 23 ± 18 | 21 ± 15 | NA | NA | 18 ± 21.1 | 19 ± 24.8 | 18.5 ± 23 | 0.45 |
| AF monitoring method during follow‐up | ECG and 24 h Holter monitor | ECG, 48 h Holter monitor and existing implanted devices | ECG and 48 h Holter monitor | Remote monitoring using existing implanted devices | ECG and 24 h Holter monitor and existing implanted devices | Remote monitoring using existing implanted devices | ||||||||||
| AF freedom during follow‐up, % | 10 (50%) | 0 (0%) | 22 (84%) | 0 (0%) | 19 (73%) | 0 (0%) | 73 (71%) | 37(101%) | 33 (100%) | 0 (0%) | 113 (63%) | 41 (22%) | 270 (80%) | 78 (20%) | 348 (45%) | 0.0001 |
Abbreviations: 6MTD, 6‐minute walk test distance; AF, atrial fibrillation; BNP, Beta natriuretic peptide; ECG, Electrocardiogram; LAD, Left atrial diameter; LVEH, left ventricular ejection fraction; MLWHF, Minnesota living with heart failure.* indicates that any value with * sign in table 1 is calculated from the main manuscript data.
Table 2.
Baseline characteristics of the patients
| Authors, year | MacDonald et al 201012 | Jones et al 201313 | Hunter et al 201414 | Di Biase et al 201615 | Prabhu et al 201716 | Marrouche et al 201817 |
|---|---|---|---|---|---|---|
| Age Mean ± SD |
63.3 ± 7.5 | 63 ± 9.5 | 57.4 ± 11 | 62 ± 10 | 59 ± 11 | 64 (median) |
| Persistent AF, % | 100 | 100 | 100 | 100 | 100 | 70 |
| BMI | 30 ± 5.6 | 29 ± 4.6 | NA | 30 ± 8 | 30 ± 7.5 | 29.1 |
| Hypertension, % | 61 | 33 | NA | 45 | 39 | 72 |
| Diabetes, % | 26 | 23 | NA | 22 | 12 | 28 |
| Prior TIA/stroke, % | 9.8 | 11.8 | NA | NA | 6.1 | 12 |
| COPD, % | 22.0 | 13.7 | NA | NA | NA | NA |
| OSA, % | NA | NA | NA | 45 | 36 | NA |
| NYHA class, % | ||||||
| NYHA class I | 0 | 0 | 0 | NA | NA | 11 |
| NYHA class II | 10 | 52 | 45 | NA | NA | 59 |
| NYHA class III | 90 | 48 | 55 | NA | NA | 28 |
| NYHA class IV | 0 | 0 | 0 | NA | NA | 2 |
| NICM, % | 51.4 | 67.5 | 74 | 38 | 100 | 60 |
| Amiodarone/antiarrhythmic, % | NA | 34 | 53.8 | 0 | 24 | 28 |
| ACEI/ARB, % | 95 | 98 | NA | 92 | 94 | 94 |
| Beta blocker, % | 88 | 92 | NA | 76 | 97 | 92 |
| Aldosterone antagonist, % | 31.6 | 36.5 | NA | 45 | 33 | NA |
| Digoxin, % | 51.3 | 54 | NA | NA | NA | 20 |
Abbreviations: ACEI/ARB, Angiotensin converting enzyme inhibitor/angiotensin receptor blocker; BMI, Body mass index; COPD, Chronic obstructive pulmonary disease; NICM, Nonischemic cardiomyopathy; NYHA, New York Heart Association; OSA, Obstructive sleep apnea; TIA, Transient ischemic attack.
Compared to medical therapy, CA significantly improved LVEF by 5.9% (mean difference [MD] 5.93, CI 3.59‐8.27, P < 0.00001, I 2 = 87%) (Figure S1). CA is associated with significant improvement in quality of life, measured by the Minnesota living with HF (MLWHF) questionnaire (MD −9.01, CI −15.56, −2.45, P = 0.007, I 2 = 47%) (Figure 2A). There was also a significant improvement in functional capacity measured by 6MWTD (MD 25.82, CI 5.46‐46.18, P = 0.01, I 2 = 90%) (Figure 2B), and peak oxygen consumption (VO2) (MD 3.16, CI 1.04‐5.29, P = 0.004, I 2 = 0%) (Figure 2C). Patients in the CA group had less HF hospital readmissions (OR 0.5, CI 0.32‐0.78, P = 0.002, I 2 = 0%) (Figure 3B), and death from any cause (OR 0.46, CI 0.29‐0.73, P = 0.0009, I 2 = 0%) (Figure 3A). Freedom from AF during follow‐up was higher in patients who had CA (OR 24.2, CI 6.94‐84.41, P < 0.00001, I 2 = 81%) (Figure 3C).
Figure 2.
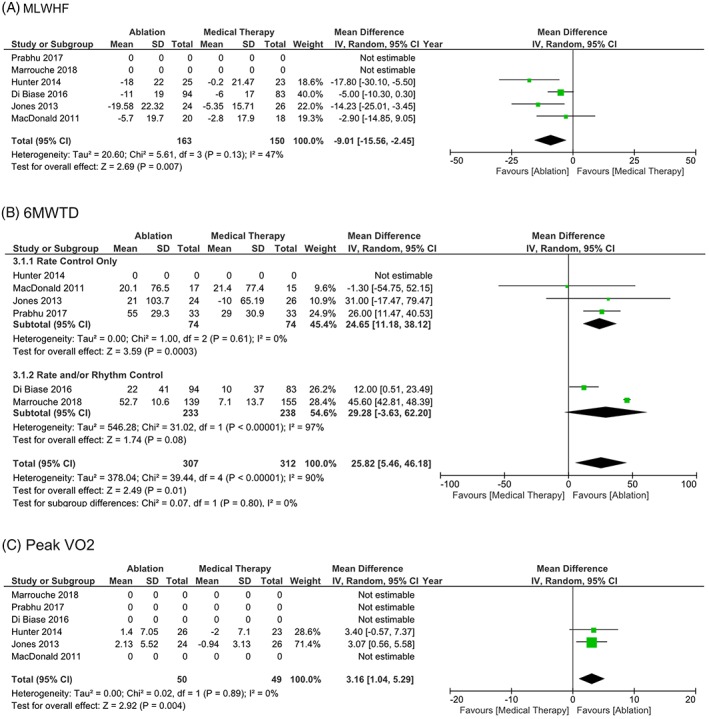
A, Health related quality of life measured by Minnesota living with heart failure (MLWHF) in patients with heart failure with reduced ejection fraction and AF randomized to CA vs medical therapy. B, Functional capacity measured by 6‐minute walk test distance (6MWTD) in patients with heart failure with reduced ejection fraction and AF randomized to CA vs medical therapy. C, Peak VO2 in patients with heart failure with reduced ejection fraction and AF randomized to CA vs medical therapy
Figure 3.
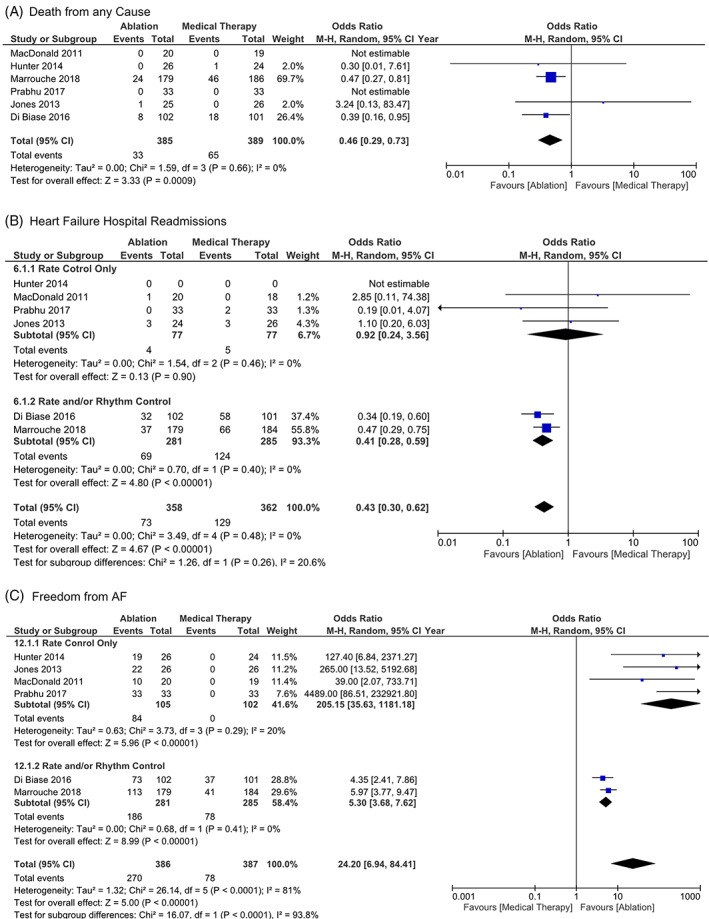
A, Death from any cause in patients with heart failure with reduced ejection fraction and AF randomized to CA vs medical therapy. B, Heart failure hospital readmissions in patients with heart failure with reduced ejection fraction and AF randomized to CA vs medical therapy. C, Freedom from AF in patients with heart failure with reduced ejection fraction and atrial fibrillation (AF) randomized to catheter ablation (CA) vs medical therapy
However, there was no difference between both groups in regards to; cardiovascular death (OR 0.62, CI 0.14‐2.68, P 0.52, I 2 = 29%) (Figure S1, all causes of hospital admissions (OR 0.49, CI 0.19‐1.22, P = 0.12, I 2 = 76%) (Figure S3), cerebrovascular accidents (OR 0.49, CI 0.18‐1.35, P = 0.17, I 2 = 0%) (Figure S4), or all adverse events (OR 1.18, CI 0.44‐3.15, P = 0.75, I 2 = 29%) (Figure S5). Sensitivity analysis showed that removal of the Prabhu et al trial had minor impact on the results of the 6MWTD but not on other endpoints (Figure S6). Removing other trials did not impact the effect measure significantly for the other outcomes. Visual evaluation of the funnel plot shows no evidence of publication bias (Figure S7). There was a statistically significant difference in the LVEF and freedom from AF during follow‐up between the subgroup of the rate control only vs rate and/or rhythm control.
The magnitude of LVEF improvement associated with CA was higher when compared to rate control only strategy (MD 8.40, CI 6.21‐10.58, P = 0.00001, I 2 = 0%), than the rate and/or rhythm control strategy (MD 3.72, CI 0.29‐7.14, P = 0.03, I 2 = 96%), Figure S1. CA was also associated with improvement in 6MWTD when compared to rate control only (MD 24.65, CI 11.18‐38.12, P = 0.0003, I 2 = 0%); however, there was no difference between CA vs rate and/or rhythm control (MD 29.28, CI −3.63 to 62.20, P = 0.08, I 2 = 97%), Figure 2B. CA was associated with fewer HF hospital readmissions when compared to rate and/or rhythm control (OR 0.41, CI 0.28‐0.59, P = 0.00001, I 2 = 0%); however, there was no difference when compared to rate control only (OR 0.92, CI 0.24‐3.56, P = 0.90, I 2 = 0%), Figure 3B. CA was associated with higher rate of freedom from AF when compared to rate control only vs rate and/or rhythm control (OR 205.15, CI 35.63‐1181.18, P = 0.00001, I 2 = 20%), (OR 5.30, CI 3.68‐7.62, P = 0.00001, I 2 = 0%), Figure 3C.
4. DISCUSSION
This meta‐analysis of six RCTs showed that CA in a highly selected population of patients with AF and HFrEF significantly improved LVEF, quality of life and functional capacity, as measured by MLWHF, 6MWTD, and peak VO2 compared to medical therapy. Furthermore, CA is associated with a significant reduction in HF related admission and total mortality without significant reduction in cardiovascular death or stroke in comparison to medications. There is a strong correlation between LVEF and peak VO2 level in predicting cardiovascular events.18, 19 Several studies have shown that even modest improvement in peak VO2 is associated with improved survival in patients with AF and HF.20, 21 These findings are consistent with our results as these improvements in LVEF and peak VO2 were associated with favorable clinical outcomes (eg, less HF readmissions).
CA is a well‐established therapy for patients with AF refractory to antiarrhythmic drugs.22, 23 This meta‐analysis addresses the rapidly evolving role of CA in patients with AF and HFrEF.24 Traditionally, CA in patients with AF and HFrEF has been deemed less desirable due to a potential lower success rate and higher procedural complications. However, the available efficacy and safety data on CA in patients with HFrEF is based mainly on small observational studies.9, 25, 26, 27 Previous meta‐analysis by Al Halabi et al28 included four RCTs showed a favorable symptom and hemodynamic response to CA in this population.10, 12, 13, 14, 28 Since that meta‐analysis was published, three additional RCTs (AATAC (Ablation Versus Amiodarone for Treatment of Persistent Atrial Fibrillation in Patients With Congestive Heart Failure and an Implanted Device), CAMERA‐MRI (Catheter Ablation Versus Medical Rate Control in Atrial Fibrillation and Systolic Dysfunction), and CASTLE‐AF (Catheter Ablation versus Standard Conventional Therapy in Patients with Left Ventricular Dysfunction and Atrial Fibrillation)) comparing CA to medical therapy have reported new findings, such as a mortality benefit with CA and the use of magnetic resonance imaging to define ventricular scar burden to predict a better response to CA.15, 16, 17 These new findings prompted this new meta‐analysis. We only retained RCTs in our meta‐analysis to minimize selection bias and the impact of unmeasured confounding factors. All available published data from these trials were utilized including some additional data provided in the aforementioned meta‐analysis by Al Halabi et al.28
The included studies compared rhythm control with CA to a strategy of using medications either for rate or rhythm control, provided that there was no mortality difference between rate and rhythm control with medications in this population.29 Four studies used rate control with medications as the control group while two studies used a rhythm control approach, mostly with Amiodarone (all AATAC patients and about 30% of patients in CASTLE‐AF).12, 13, 14, 15, 16, 17 Sensitivity analyses excluding these two alternative options used in the control group did not significantly affect the overall results described in this meta‐analysis. However, the high degree of heterogeneity noted in LVEF improvement, quality of life measures, functional capacity, and freedom from AF was improved after removal of AATAC and CASTLE‐AF. However, there is a low level of statistical heterogeneity for hard clinical end point outcomes, all‐cause mortality, and HF readmission (I 2 0%). The overall rate of major complications associated with CA was 5.2% and includes stroke, pericardial effusion, and vascular complications. Based on the present analysis and previous meta‐analysis, the rate of CA complications in patients with HFrEF was similar to that reported in general population, Table S1.28, 30, 31 This is likely because the procedures were performed by experienced operators in high volume medical centers.
There was a significant reduction in total mortality from any cause with CA compared to medical therapy, although this has not been demonstrated so far with CA of AF in non‐HF patients.32 The current indication for CA in AF aims to alleviate patients' symptoms and improve their quality of life. This significant finding of mortality benefit, however, needs to be interpreted very cautiously. The decreased total mortality without a difference in cardiovascular mortality, adverse events or stroke is puzzling, as one would expect total mortality to be driven by cardiovascular events. However, we must acknowledge that only two studies with a relatively small number of patients reported cardiovascular deaths, making it unlikely to reach statistical significance for this outcome. Furthermore, the CASTLE‐AF study, which is driving the reduction in total mortality in our meta‐analysis, has some significant flaws such as a substantial number of patients lost to follow up, which questions the accuracy of mortality outcomes.17
There is a complex relationship between AF and HFrEF with HF leading to AF through neurohormonal activation and atrial remodeling. At the same time, AF predisposes to HF via rapid and irregular ventricular rates and loss of atrioventricular synchrony.1, 33, 34 An important observation from our analysis is that in all the included studies, the use of CA was consistently associated with improvement in LVEF during follow‐up. This finding highlights the important role of sinus rhythm in preserving normal hemodynamic function of the left ventricle. The potential mechanisms for an improved LVEF after CA include better rate control and rhythm regularity achieved with sinus rhythm, restoration of atrial emptying, and improved diastolic filling, all of which can lead to an augmented cardiac output. It is notable that CA did not completely eliminate AF in all patients. However, there was a substantial reduction in AF burden, Table S1. This decrease in AF burden was enough to show clinical benefits.
This study has some limitations. In addition to a relatively small number of patients and shorter follow‐up duration in the majority of the included studies, there is an obvious selection bias due to the type of patients offered to participate in these RCTs. Patients with advanced HF such as those with NYHA class IV, mostly excluded from these studies, might not have had such favorable outcomes. For instance, MacDonald et al12 included patients with more advanced HFrEF as evidenced by a lower mean LVEF, higher baseline brain natriuretic peptide level, longer duration of AF in the CA arm (44 months), and more patients with NYHA class III, Table 1.12 In this trial, only 50% of the patients successfully maintained sinus rhythm with no improvement in the LVEF. There has been significant heterogeneity within and between the studies regarding the use of rate control medication with and without antiarrhythmic agents in the control group. Certain outcomes such as 6MWTD, MLWHF, and cardiovascular mortality were not included in all the studies. The typical patients included in this meta‐analysis were younger males with moderately depressed LVEF, shorter duration of persistent AF (mean 18.5 months) and less likely to have coronary artery disease, Table 2. Therefore, the results of our analysis may not be applicable to older patients with severely depressed LVEF and/or longer duration of AF (> 2 years).
We were not able to separate ischemic from nonischemic cardiomyopathy in our analysis. Patients with nonischemic cardiomyopathy may have an under‐recognized tachycardia mediated cardiomyopathy, which is associated with considerable improvement in LVEF after restoration of sinus rhythm as demonstrated in the CAMERA‐MRI study.16, 35 Another limitation of the included studies is that the ablation strategy was not uniform, Table S1. Although pulmonary vein isolation was the main ablation strategy, variable additional ablation lesions were performed. Finally, although CA procedures were done on oral anticoagulants, no anticoagulant details such as the type of anticoagulant or the dose were available in most studies.
Supporting information
Appendix S1.
Figure S1. Left ventricular ejection fraction (LVEF) improvement in patients with heart failure with reduced ejection fraction and atrial fibrillation (AF) randomized to catheter ablation (CA) vs medical therapy.
Figure S2. Cardiovascular death in patients with heart failure with reduced ejection fraction and atrial fibrillation (AF) randomized to catheter ablation (CA) vs medical therapy.
Figure S3. All causes of hospital admissions in patients with heart failure with reduced ejection fraction and atrial fibrillation (AF) randomized to catheter ablation (CA) vs medical therapy.
Figure S4. Cerebrovascular accidents in patients with heart failure with reduced ejection fraction and atrial fibrillation (AF) randomized to catheter ablation (CA) vs medical therapy.
Figure S5. All adverse events in patients with heart failure with reduced ejection fraction and atrial fibrillation (AF) randomized to catheter ablation (CA) vs medical therapy.
Figure S6. Sensitivity analysis.
Figure S7. Funnel plot.
Figure S8. Sensitivity analysis based on quality score.
Table S1. Procedural characteristics.
Table S2. Modified Jadad scores of the included studies.
Table S3. Cochrane Collaboration's tool for assessing risk of bias of the included studies.
ACKNOWLEDGMENTS
None.
Conflicts of interest
The authors declare no potential conflict of interests.
Smer A, Salih M, Darrat YH, et al. Meta‐analysis of randomized controlled trials on atrial fibrillation ablation in patients with heart failure with reduced ejection fraction. Clin Cardiol. 2018;41:1430–1438. 10.1002/clc.23068
REFERENCES
- 1. Maisel WH, Stevenson LW. Atrial fibrillation in heart failure: epidemiology, pathophysiology, and rationale for therapy. Am J Cardiol. 2003;91(6A):2D‐8D. [DOI] [PubMed] [Google Scholar]
- 2. Wang TJ, Larson MG, Levy D, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107(23):2920‐2925. [DOI] [PubMed] [Google Scholar]
- 3. Silva‐Cardoso J, Zharinov OJ, Ponikowski P, et al. Heart failure in patients with atrial fibrillation is associated with a high symptom and hospitalization burden: the RealiseAF survey. Clin Cardiol. 2013;36(12):766‐774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pedersen OD, Bagger H, Keller N, Marchant B, Kober L, Torp‐Pedersen C. Efficacy of dofetilide in the treatment of atrial fibrillation‐flutter in patients with reduced left ventricular function: a Danish investigations of arrhythmia and mortality on dofetilide (diamond) substudy. Circulation. 2001;104(3):292‐296. [DOI] [PubMed] [Google Scholar]
- 5. Corley SD, Epstein AE, DiMarco JP, et al. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow‐Up Investigation of Rhythm Management (AFFIRM) Study. Circulation. 2004;109(12):1509‐1513. [DOI] [PubMed] [Google Scholar]
- 6. Kober L, Torp‐Pedersen C, McMurray JJ, et al. Increased mortality after dronedarone therapy for severe heart failure. N Engl J Med. 2008;358(25):2678‐2687. [DOI] [PubMed] [Google Scholar]
- 7. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):e1‐e76. [DOI] [PubMed] [Google Scholar]
- 8. Dagres N, Varounis C, Gaspar T, et al. Catheter ablation for atrial fibrillation in patients with left ventricular systolic dysfunction. A systematic review and meta‐analysis. J Card Fail. 2011;17(11):964‐970. [DOI] [PubMed] [Google Scholar]
- 9. Bazoukis G, Letsas KP, Tse G, et al. Predictors of arrhythmia recurrence in patients with heart failure undergoing left atrial ablation for atrial fibrillation. Clin Cardiol. 2018;41(1):63‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Khan MN, Jais P, Cummings J, et al. Pulmonary‐vein isolation for atrial fibrillation in patients with heart failure. N Engl J Med. 2008;359(17):1778‐1785. [DOI] [PubMed] [Google Scholar]
- 11. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. MacDonald MR, Connelly DT, Hawkins NM, et al. Radiofrequency ablation for persistent atrial fibrillation in patients with advanced heart failure and severe left ventricular systolic dysfunction: a randomised controlled trial. Heart. 2011;97(9):740‐747. [DOI] [PubMed] [Google Scholar]
- 13. Jones DG, Haldar SK, Hussain W, et al. A randomized trial to assess catheter ablation versus rate control in the management of persistent atrial fibrillation in heart failure. J Am Coll Cardiol. 2013;61(18):1894‐1903. [DOI] [PubMed] [Google Scholar]
- 14. Hunter RJ, Berriman TJ, Diab I, et al. A randomized controlled trial of catheter ablation versus medical treatment of atrial fibrillation in heart failure (the CAMTAF trial). Circ Arrhythm Electrophysiol. 2014;7(1):31‐38. [DOI] [PubMed] [Google Scholar]
- 15. Di Biase L, Mohanty P, Mohanty S, et al. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC multicenter randomized trial. Circulation. 2016;133(17):1637‐1644. [DOI] [PubMed] [Google Scholar]
- 16. Prabhu S, Taylor AJ, Costello BT, et al. Catheter ablation versus medical rate control in atrial fibrillation and systolic dysfunction: the CAMERA‐MRI study. J Am Coll Cardiol. 2017;70(16):1949‐1961. [DOI] [PubMed] [Google Scholar]
- 17. Marrouche NF, Brachmann J, Andresen D, et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;378(5):417‐427. [DOI] [PubMed] [Google Scholar]
- 18. O'Neill JO, Young JB, Pothier CE, Lauer MS. Peak oxygen consumption as a predictor of death in patients with heart failure receiving beta‐blockers. Circulation. 2005;111(18):2313‐2318. [DOI] [PubMed] [Google Scholar]
- 19. Hussain N, Gersh BJ, Gonzalez Carta K, et al. Impact of cardiorespiratory fitness on frequency of atrial fibrillation, stroke, and all‐cause mortality. Am J Cardiol. 2018;121(1):41‐49. [DOI] [PubMed] [Google Scholar]
- 20. Myers J, McAuley P, Lavie CJ, Despres JP, Arena R, Kokkinos P. Physical activity and cardiorespiratory fitness as major markers of cardiovascular risk: their independent and interwoven importance to health status. Prog Cardiovasc Dis. 2015;57(4):306‐314. [DOI] [PubMed] [Google Scholar]
- 21. Luo N, Merrill P, Parikh KS, et al. Exercise training in patients with chronic heart failure and atrial fibrillation. J Am Coll Cardiol. 2017;69(13):1683‐1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130(23):2071‐2104. [DOI] [PubMed] [Google Scholar]
- 23. Georgiopoulos G, Tsiachris D, Manolis AS. Cryoballoon ablation of atrial fibrillation: a practical and effective approach. Clin Cardiol. 2017;40(5):333‐342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Joy PS, Gopinathannair R, Olshansky B. Effect of ablation for atrial fibrillation on heart failure readmission rates. Am J Cardiol. 2017;120(9):1572‐1577. [DOI] [PubMed] [Google Scholar]
- 25. Hsu LF, Jais P, Sanders P, et al. Catheter ablation for atrial fibrillation in congestive heart failure. N Engl J Med. 2004;351(23):2373‐2383. [DOI] [PubMed] [Google Scholar]
- 26. Sacher F, Corcuff JB, Schraub P, et al. Chronic atrial fibrillation ablation impact on endocrine and mechanical cardiac functions. Eur Heart J. 2008;29(10):1290‐1295. [DOI] [PubMed] [Google Scholar]
- 27. Wilton SB, Fundytus A, Ghali WA, et al. Meta‐analysis of the effectiveness and safety of catheter ablation of atrial fibrillation in patients with versus without left ventricular systolic dysfunction. Am J Cardiol. 2010;106(9):1284‐1291. [DOI] [PubMed] [Google Scholar]
- 28. Al Halabi S, Qintar M, Hussein A, et al. Catheter ablation for atrial fibrillation in heart failure patients: a meta‐analysis of randomized controlled trials. JACC Clin Electrophysiol. 2015;1(3):200‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Roy D, Talajic M, Nattel S, et al. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358(25):2667‐2677. [DOI] [PubMed] [Google Scholar]
- 30. Deshmukh A, Patel NJ, Pant S, et al. In‐hospital complications associated with catheter ablation of atrial fibrillation in the United States between 2000 and 2010: analysis of 93 801 procedures. Circulation. 2013;128(19):2104‐2112. [DOI] [PubMed] [Google Scholar]
- 31. Malhi N, Hawkins NM, Andrade JG, Krahn AD, Deyell MW. Catheter ablation of atrial fibrillation in heart failure with reduced ejection fraction. J Cardiovasc Electrophysiol. 2018;29:1049–1058. [DOI] [PubMed] [Google Scholar]
- 32. d'Avila A, Ruskin JN. Nonpharmacologic strategies: the evolving story of ablation and hybrid therapy. Am J Cardiol. 2008;102(6A):20H‐24H. [DOI] [PubMed] [Google Scholar]
- 33. Daoud EG, Weiss R, Bahu M, et al. Effect of an irregular ventricular rhythm on cardiac output. Am J Cardiol. 1996;78(12):1433‐1436. [DOI] [PubMed] [Google Scholar]
- 34. Naccarelli GV, Hynes BJ, Wolbrette DL, et al. Atrial fibrillation in heart failure: prognostic significance and management. J Cardiovasc Electrophysiol. 2003;14(12 Suppl):S281‐S286. [DOI] [PubMed] [Google Scholar]
- 35. Benjamin MM, Chaddha A, Sampene E, Field ME, Rahko PS. Comparison of outcomes of atrial fibrillation in patients with reduced versus preserved left ventricular ejection fraction. Am J Cardiol. 2016;118(12):1831‐1835. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1.
Figure S1. Left ventricular ejection fraction (LVEF) improvement in patients with heart failure with reduced ejection fraction and atrial fibrillation (AF) randomized to catheter ablation (CA) vs medical therapy.
Figure S2. Cardiovascular death in patients with heart failure with reduced ejection fraction and atrial fibrillation (AF) randomized to catheter ablation (CA) vs medical therapy.
Figure S3. All causes of hospital admissions in patients with heart failure with reduced ejection fraction and atrial fibrillation (AF) randomized to catheter ablation (CA) vs medical therapy.
Figure S4. Cerebrovascular accidents in patients with heart failure with reduced ejection fraction and atrial fibrillation (AF) randomized to catheter ablation (CA) vs medical therapy.
Figure S5. All adverse events in patients with heart failure with reduced ejection fraction and atrial fibrillation (AF) randomized to catheter ablation (CA) vs medical therapy.
Figure S6. Sensitivity analysis.
Figure S7. Funnel plot.
Figure S8. Sensitivity analysis based on quality score.
Table S1. Procedural characteristics.
Table S2. Modified Jadad scores of the included studies.
Table S3. Cochrane Collaboration's tool for assessing risk of bias of the included studies.


