Abstract
Background
Many recurrences occur after electrical cardioversion (ECV) of atrial fibrillation (AF). Assessment of extent of remodeling and continuous prolonged rhythm monitoring might reveal actionable recurrence mechanisms.
Hypothesis
After ECV of AF specific patterns of arrhythmia recurrence can be distinguished.
Methods
All patients who underwent successful ECV due to persistent AF were included. Tissue velocity echocardiography during AF was performed before ECV to study atrial fibrillatory cycle length and fibrillatory velocity. After ECV, the heart rhythm of all patients was monitored 3 times daily during 4 weeks, and timing of recurrence was noted.
Results
In total, 50 patients (68% male) were included; mean age was 68 ± 9 years. Median duration of the current AF episode was 102 (range, 74–152) days. Twenty‐one (42%) patients showed recurrence of persistent AF. No recurrences occurred during the first 24 hours. There were no differences in clinical characteristics between patients with or without recurrence of AF. However, patients with early recurrence of AF had significantly higher precardioversion wall‐motion velocity compared with patients who remained in sinus rhythm (2.8 [1.6–3.6] vs 1.4 [0.9–3.3] cm/s; P = 0.017), whereas atrial fibrillatory cycle length did not differ.
Conclusions
In this study on 50 patients successfully cardioverted for persistent AF, there was a relapse gap of ≥24 hours. This phenomenon has not been well appreciated before and offers an AF‐free window of opportunity for electrocardiographically triggered cardiac imaging or complex electrophysiological procedures. Echocardiographic tissue velocity imaging may visualize atrial remodeling relevant to AF recurrence.
Keywords: Atrial Fibrillation, Cardioversion, Recurrence, Relapse Gap, Tissue Velocity Imaging
1. INTRODUCTION
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia clinicians encounter. Electrical cardioversion (ECV) was introduced in clinical practice in 1962, and nowadays it is a frequently applied treatment to restore sinus rhythm in AF patients with success rates of 70% to 91% in patients with persistent AF.1 Unfortunately, only 30% to 35% of the patients are still in sinus rhythm after 1 year.2, 3 Though it has been reported that relapses are most frequent during the first 2 weeks after cardioversion,4 the exact timing of early recurrences of persistent AF is not well known. To improve long‐term outcome of cardioversion, it may be important to focus on the timing of these early relapses. Recurrence of AF may relate to extent of atrial remodeling. Using novel electro‐echocardiographic imaging, extent of remodeling may be assessed beyond simple atrial sizes measurement (Figure 1).5, 6 The goal of the present study was to meticulously investigate the incidence and timing of early recurrence of AF after ECV, in relation to pre‐cardioversion assessment of extent of atrial remodeling using novel electro‐echocardiographic tissue velocity imaging (TVI).
Figure 1.
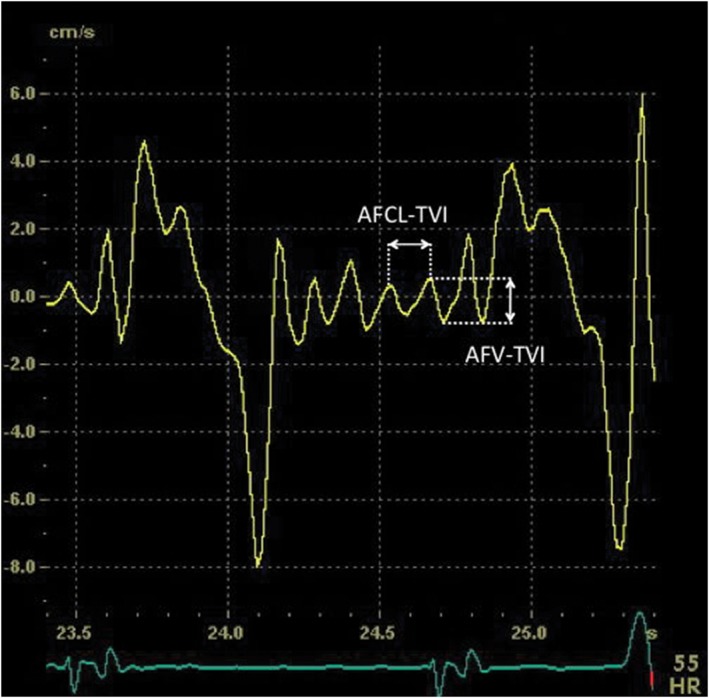
Echocardiographic measurement of AF cycle length (AFCL‐TVI) and AF wall‐motion velocity (AFV‐TVI) with TVI curves obtained from the lateral wall of the LA just above the mitral annulus. Abbreviations: AF, atrial fibrillation; LA, left atrium; TVI, tissue velocity imaging
2. METHODS
In this study we included all patients who underwent a successful ECV for persistent AF during 12 months. Patients age < 18 years and patients with atrial flutter, a pacemaker or implantable cardioverter‐defibrillator (ICD), as well as those on antiarrhythmic drugs were excluded. Baseline characteristics were extracted from the medical chart, including demographic characteristics, medical history, and electrocardiographic (ECG) as well as echocardiographic data. None of the patients was prescribed antiarrhythmic drugs post‐cardioversion. Written informed consent was obtained from all patients, and the institutional review board approved the study.
2.1. Rhythm monitoring
Heart rhythm was monitored during 4 weeks after cardioversion using a handheld telemetry device (MyDiagnostick; Applied Biomedical Systems BV, Maastricht, The Netherlands; Figure 2).7 Patients received the MyDiagnostick 1 hour after electrical cardioversion; this was instantly the first monitoring of heart rhythm post‐ECV. Patients were instructed to use the device 3× daily (morning, noon, and evening), and also in case of complaints. When the device showed a red light (indicating presence of AF), the patient was instructed to repeat the procedure every 15 minutes for the following hour. Only if the device showed a red light (AF) at each of 4 consecutive measurements over 1 hour, the patient was instructed to visit the hospital for a confirmatory ECG. All individual MyDiagnostick recordings were analyzed for presence of AF.
Figure 2.

MyDiagnostick telemetry device
2.2. Echocardiographic examination
Before cardioversion, a transthoracic echocardiography was performed with a Vivid 7 ultrasound system (GE Healthcare, Little Chalfont, United Kingdom). The AF cycle length (AFCL‐TVI) and the AF wall‐motion velocity (AFV‐TVI) were measured (from ≥3 heart cycles) using TVI as described previously.5, 8 Figure 1 shows a clear example of those measurements. In addition, standard left ventricular (LV) and left atrial (LA) dimensions were determined according to the recommendations as described in the European Echocardiography guidelines.
2.3. Definitions
AF was defined as any documented arrhythmia that has the ECG characteristics of AF and lasts sufficiently long to be recorded on 12‐lead ECG, or lasts ≥30 seconds on a rhythm strip. We defined successful cardioversion as restoration of sinus rhythm after delivery of a shock without immediate recurrence of AF within 60 seconds. Recurrence of persistent AF after ECV was defined as the combination of 5 consecutive MyDiagnostick recordings indicating AF recurrence (5 consecutive red lights) during 1 hour, followed by an in‐hospital 12‐lead ECG confirming presence of AF.
2.4. Statistical analysis
Data analysis was performed using SPSS statistical software version 20.0 (IBM Corp., Armonk, NY). Continuous variables were tested for normal distribution using the Kolmogorov–Smirnov test. Continuous variables are reported as mean ± SD when normally distributed and as median (interquartile range [IQR], 25th percentile–75th percentile) if they did not follow a normal distribution. Categorical variables were reported as observed number of patients (%). Differences between continuous variables were tested with an independent t test when normally distributed and with the Mann–Whitney U test if not normally distributed. Differences in categorical variables were tested for with the Fisher exact test.
3. RESULTS
In total, 50 patients with successful ECV were included. Patients were predominantly male (68%), with a mean age of 68 ± 9 years. At cardioversion, the median duration of the current AF episode was 102 days (IQR, 74–152 d). The patients' baseline characteristics are shown in the Table 1.
Table 1.
Baseline characteristics
| All, N = 50 | No Recurrence of AF, n = 29 (58) | Recurrence of AF, n = 21 (42) | P Value | |
|---|---|---|---|---|
| Age, y | 68 ± 9 | 68 ± 10 | 68 ± 7 | 0.79 |
| Male sex | 34 (68) | 20 (69) | 14 (67) | 1.00 |
| BSA, m2 (n = 40) | 2.0 ± 0.2 | 2.0 ± 0.2 | 2.0 ± 0.3 | 0.76 |
| Known with AF, m | 3 (2–6) | 3 (2–5) | 3 (2–29) | 0.43 |
| Duration current AF, d | 102 (74–152) | 103 (77–159) | 95 (71–134) | 0.49 |
| Medical history | ||||
| HTN | 28 (56) | 15 (52) | 13 (62) | 0.57 |
| CAD | 17 (34) | 12 (41) | 5 (24) | 0.23 |
| DM | 4 (8) | 1 (3) | 3 (14) | 0.30 |
| Stroke | 2 (4) | 2 (7) | 0 (0) | 0.50 |
| TIA | 3 (6) | 0 | 3 (14) | 0.07 |
| HF | 13 (26) | 8 (28) | 5 (24) | 1.00 |
| COPD | 0 (0) | 0 (0) | 0 (0) | 1.00 |
| Medications | ||||
| β‐Blocker | 44 (88) | 27 (93) | 16 (81) | 1.00 |
| Verapamil | 1 (2) | 1 (3) | 0 (0) | 1.00 |
| Digoxin | 11 (22) | 5 (17) | 6 (29) | 0.49 |
| ACEI/ARB | 33 (66) | 16 (66) | 14 (67) | 1.00 |
| Statin | 27 (54) | 15 (52) | 12 (57) | 0.78 |
| Echocardiographic parameters | ||||
| LVEF, % | 55 (40–59) | 54 (37–63) | 55 (50–59) | 0.76 |
| LA diameter, mm | 45 (43–49) | 45 (42–51) | 45 (43–49) | 0.98 |
| LA volume, mL (n = 46) | 99 ± 25 | 102 ± 27 | 94 ± 20 | 0.28 |
| LA indexed volume, mL/m2 | 51 ± 15 | 53 ± 16 | 49 ± 13 | 0.48 |
| RA volume, mL (n = 40) | 78 ± 24 | 78 ± 26 | 79 ± 18 | 0.90 |
| AFCL‐TVI LA, ms (n = 35) | 157 (132–173) | 154 (123–172) | 162 (141–175) | 0.35 |
| AFCL‐TVI RA, ms (n = 18) | 162 ± 25 | 160 ± 25 | 165 ± 28 | 0.73 |
| AFV‐TVI LA, cm/s | 1.6 (1.1–3.3) | 1.4 (0.9–2.3) | 2.8 (1.6–3.6) | 0.017 |
| AFV‐TVI RA, cm/s | 3.6 ± 2.6 | 3.6 ± 2.3 | 3.6 ± 3.3 | 0.99 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; AFCL, atrial fibrillation cycle length; AFV, atrial fibrillatory velocity; ARB, angiotensin II receptor blocker; BSA, body surface area; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; HF, heart failure; HTN, hypertension; IQR, interquartile range; LA, left atrium; LVEF, left ventricular ejection fraction; RA, right atrium; SD, standard deviation; TIA, transient ischemic attack; TVI, tissue velocity imaging.
Data are presented as n (%), mean ± SD, or median (IQR).
3.1. Recurrence of persistent AF
Twenty‐one (42%) patients showed recurrence of persistent AF within 1 month after cardioversion. Of note, during the first 24 hours there was no recurrence (Figure 3). On the second day after cardioversion there were 3 patients with recurrence of persistent AF; 15 other patients followed over the next 6 days; and 3 more patients had AF recurrence during weeks 2 and 3. Thus, almost all persistent AF recurrences (86%) occurred during the first week post‐cardioversion. Eight patients who showed ≥1 MyDiagnostick episodes of self‐terminating (<60 min) AF or short atrial runs before developing recurrence of persistent AF.
Figure 3.
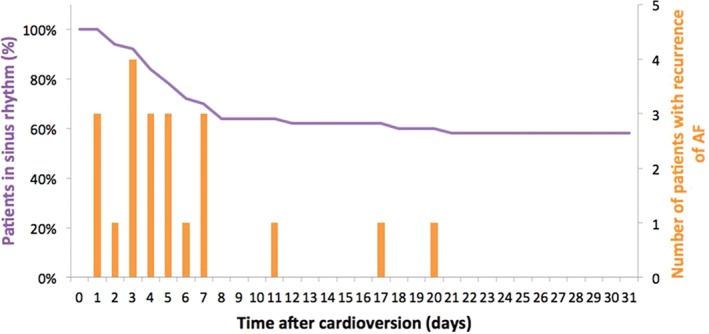
Time to relapse of AF. Abbreviations: AF, atrial fibrillation
Age, total duration of AF history, and duration of the current AF episode were similar in patients who remained in sinus rhythm compared with patients with recurrence of persistent AF (Table). There was also no association between recurrence of AF and underlying disease or baseline medication of the study population. However, patients with a recurrence had a significantly higher AFV‐TVI compared with patients who remained in sinus rhythm (2.8 [1.6–3.6] vs 1.4 [0.9–3.3] cm/s; P = 0.017). The LA size was not different in the respective groups (LA diameter: 45 [43–49] mm vs 45 [42–51] mm; P = 0.98; LA volume: 94 ± 20 mL vs 102 ± 27 mL).
4. DISCUSSION
In this study, we investigated the incidence and timing of recurrences of AF after successful ECV for persistent AF, using intensive rhythm monitoring. To our knowledge, this is the first study that shows the inability of the atria to harbor a sustained recurrence of AF during the initial 24 hours following successful electrical cardioversion despite having been in AF continuously during several previous months. Although this 24‐hour relapse gap has been suggested before,9 it has never been formally investigated nor demonstrated. Further, we found higher LA wall‐motion velocities in those patients with early recurrence of persistent AF, suggesting that stretch or higher metabolic demand may trigger early recurrences during the phase of reversed remodeling after restoration of sinus rhythm.
4.1. Relapse gap after cardioversion of persistent AF
Directly after successful cardioversion, immediate recurrence of AF can occur within 1 to 2 minutes. Duytschaever et al. showed that these hyperacute recurrences could be explained by the supervulnerable status of the atrial substrate directly after termination of AF, which is associated with a short‐lasting, transient (<1 min) extreme shortening of atrial refractoriness. During this period, the atria are susceptible to re‐initiation of AF triggered by premature beats.10, 11 Lown (1962) and Rossi (1967) were the first to describe that the initial ultrashort phase of immediate recurrences lasted <1 minute, whereas all other patients—who initially converted after the shock—showed sustained sinus rhythm until discharge from the hospital. Using Holter monitors immediately after cardioversion of persistent AF, Varounis et al12 did not explicitly mention the 24‐hour relapse gap, yet such gap can be appreciated from the arrhythmia‐free survival curves in their article. The same applies to the Verapamil Versus Digoxin Cardioversion (VERDICT) trial, where none of the patients experienced a relapse of the arrhythmia within the first 24 hours after electrical cardioversion.13 Schwartzman et al. reported on recurrences after ambulatory atrial cardioversion by an implanted device and saw that recurrences within 1 day were associated with paroxysmal rather than persistent AF.14 These observations are in line with our finding that the 1‐minute post‐shock phase with immediate recurrences is followed by a significant relapse gap during which immediate recurrences do not occur. The present study using detailed arrhythmia event monitoring shows that this period lasts ≥24 hours. In accordance with existing literature, we also found that the majority of subacute relapses of AF (86%) happen within 1 week after this relapse gap.
The observation of this so‐called 24‐hour relapse gap is unexpected considering that, until the ECV, the atria were fibrillating continuously for months. One may conjecture that after cardioversion the atria are in a “metabolic shock” condition for ≥1 day, because the switch from intrinsic tachycardia to the relative bradycardia of sinus rhythm may lead to various metabolic alterations, such as changes in intracellular calcium concentrations and signaling, and intracellular acidosis.10, 15, 16 It may be surmised that the metabolic derangements in the initial minutes after ECV that lead to extreme shortening of refractoriness with recurrences in the hypervulnerable phase also set the stage for subsequent atrial cellular stunning with inability to sustain AF within a window of ≥24 hours. This is in line with the well‐known pattern of occurrence of AF after open‐heart surgery, which relates to transient atrial ischemia and atrial stunning. Classically, postoperative AF also virtually skips the first day, with most episodes happening from day 2 onward.17 It may be hypothesized that the metabolic atrial changes during open‐heart surgery—not always effectively prevented by cardioplegia, which protects the ventricles but less so the atria, which then suffer from normothermic ischemia—lead to stunning and the electrophysiologic inability of the atria to fibrillate within ≥1 day after surgery. Only after sufficient resolving of atrial stunning, the shock effect (stunning) disappears and the atria return to their initial preexisting vulnerable status.
It has been suggested that the absence of atrial trigger beats during the first 24 hours might be another explanation for the relapse gap. However, previous studies have clearly shown an abundance of atrial ectopy during the 24‐hour relapse gap, strongly supporting the notion that, temporarily, the substrate is not fit to carry AF.4, 12
Tieleman et al. suggested that progressive development of heterogeneity of refractoriness—related to reverse remodeling after ECV—is a prerequisite for atrial ectopy to induce a recurrence. However, due to chronic electrical remodeling during AF before ECV, all atrial cells have an equally short refractory period preventing the development of heterogeneity of refractoriness initially after ECV, whereas lengthening associated with reversed remodeling may happen with an uneven spatial distribution.4 The initiation of reversed electrical remodeling will certainly take some time, presumably ≥24 hours, and may be spatially nonuniform. It has been shown that reversed electrical remodeling after ECV in terms of normalization of the action potential duration or atrial refractory period takes ≥4 days.18, 19 After the first week, beneficial electrical remodeling of the atria may have become more uniformly distributed, resulting in a decreasing incidence of recurrences from then on.4
4.2. Atrial stunning after cardioversion
As mentioned earlier, atrial stunning may relate to the occurrence of a relapse gap after cardioversion of AF. Manning et al. described the restoration of atrial function as measured by transmitral echo‐Doppler peak A‐wave velocity and found initially a strong depression of atrial functioning that lasted up to 1 week or longer after the cardioversion.20 This is compatible with post‐shock atrial stunning, which in turn may set the stage for absence of recurrences during the relapse gap. Similarly, in the present study fibrillatory velocity of the atrial wall during AF before cardioversion was reduced compared with control patients with acute AF as reported previously (left atrial TV 1.6 [1.1–3.3] cm/s and 3.6 [2.7–5.8] cm/s, respectively).8 This indicates that also in our patients a reduced wall motion as an expression of chronic atrial remodeling (not present so much in acute AF) was present that may turn the atria susceptible to post‐shock stunning. Interestingly and unexpectedly, the wall‐motion velocity was highest in patients with recurrences. We conjecture that patients with a relatively preserved atrial wall‐motion velocity before cardioversion would have an earlier and faster (regional) recovery, setting the stage for early recurrences within 1 week; whereas concurrently a higher contractility may effectively have generated stretch‐induced atrial premature beats, eliciting the recurrence.
4.3. Predictors of early recurrence
Data concerning predictors of early recurrence of AF are scarce. Bertaglia et al. and Varounis et al found no differences between patients with or without an early recurrence (ie, within 7 days or 1 month, respectively).12, 21 Tieleman et al found hypertension and chronic use of intracellular calcium‐lowering medication (β‐blockers and non‐dihydropyridin calcium channel blockers) before ECV as independent predictors of maintained sinus rhythm within 1 month after ECV in 2 different cohorts.4, 18 Apart from the well‐known efficacy of Vaughan‐Williams class I and III antiarrhythmic drugs, several studies reported use of renin‐angiotensin inhibitors or verapamil combined with amiodarone or propafenone associated with fewer early recurrences of persistent AF. Echocardiographic parameters also have been related to recurrence. Decreased mechanical atrial function immediately after cardioversion, as characterized by a lower peak A‐wave velocity, is associated with AF recurrence within 1 month after ECV. In contrast, in our study, patients with recurrence of AF had a significantly higher echocardiographic AFV‐TVI compared with patients in maintaining sinus rhythm. Further studies in larger patient populations are needed to elucidate the relationship between pre‐cardioversion atrial mechanical function and ECV outcome.
4.4. Implications
The relapse gap, as demonstrated in this study, offers a window of opportunity to image AF patients more accurately with ECG‐triggered computed tomography (CT) scanning or cardiac magnetic resonance imaging (CMR), because machine triggering may be much more effective when patients are in sinus rhythm. In this respect, pre‐CT or pre‐CMR cardioversion to enhance imaging during the relapse gap may be adopted in clinical practice. Moreover, prescription of antiarrhythmic drugs, especially in patients with a high—potentially arrhythmogenic—wall‐motion velocity before cardioversion, might modify and prolong this 24‐hour AF‐free window of opportunity. Also, AF ablationists already make use of the fact that AF recurrences do not occur during complex ablation procedures once cardioversion has been applied during or before the ablation procedure. Conversely, absence of AF recurrence until discharge after ablation of persistent AF (which is frequently combined with electrical cardioversion during the intervention) may not be taken as an acute success of the ablation, although the patient left the hospital in sinus rhythm due to the relapse gap. To assess success of ablation procedures for persistent AF, the phenomenon of the relapse gap should clearly be taken into account.
4.5. Study limitations
In this study, we have consciously excluded patients with current use of antiarrhythmic drugs because our goal was to study the natural course of incidence and timing of early AF recurrences after cardioversion. As a result, the population we studied may not be representative of the general AF population. In our view, the studied population comprises exactly those patients in whom clinical advantage of a relapse gap might be useful in daily clinical practice. Moreover, we could not analyze atrial premature beats properly, because the telemetry device used was not developed to diagnose premature beats. However, the main goal of this study was to report the incidence and timing of early recurrences within 1 month post‐cardioversion. The MyDiagnostick is a user‐friendly device to instantly detect AF paroxysms noninvasively. Although we used a comprehensive follow‐up protocol (3× daily during 4 weeks), overall AF recurrence rate, in particular of nonsustained self‐terminating episodes, might have been underestimated. However, persistent non–self‐terminating recurrences (ie, the main analysis parameter in the present study) cannot escape recording by the MyDiagnostick.
In addition, it appeared that electro‐echocardiography, although potentially valuable in assessing extent of atrial remodeling, is not feasible in all patients before cardioversion due to absence of a sufficiently long diastole. Finding predictors of recurrence was not the primary goal of this study, and more patients are needed to reliably study predictors of early recurrence of AF after cardioversion, including the role that TVI representing atrial refractoriness and contractility may have.
5. CONCLUSION
In this study on 50 patients successfully cardioverted for persistent AF, we found a relapse gap of 24 hours after cardioversion during which no recurrences occurred. This relapse gap offers the clinicians an AF‐free window of opportunity, for instance for the purpose of ECG‐triggered imaging of AF patients or during complex electrophysiological procedures. This study also confirms that early recurrences occur, especially during the first week after cardioversion. In addition, extent of atrial remodeling may relate to AF recurrence. These findings may be important for further insights in preventing recurrence of AF.
Author contributions
Bob Weijs, MD, PhD, and Ione Limantoro, MD, PhD, contributed equally to this work. All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Conflicts of interest
The authors declare no potential conflicts of interest.
Weijs B, Limantoro I, Delhaas T, et al. Cardioversion of persistent atrial fibrillation is associated with a 24‐hour relapse gap: Observations from prolonged postcardioversion rhythm monitoring. Clin Cardiol. 2018;41:366–371. 10.1002/clc.22877
Funding information This work was supported by the Netherlands Heart Foundation (NHF) grant number 2010B275.
REFERENCES
- 1. Crijns HJ, Weijs B, Fairley AM, et al. Contemporary real‐life cardioversion of atrial fibrillation: Results from the multinational RHYTHM‐AF study. Int J Cardiol. 2014;172:588–594. [DOI] [PubMed] [Google Scholar]
- 2. Kim SK, Pak HN, Park JH, et al. Clinical and serological predictors for the recurrence of atrial fibrillation after electrical cardioversion. Europace. 2009;11:1632–1638. [DOI] [PubMed] [Google Scholar]
- 3. Van Gelder IC, Crijns HJ, Van Gilst WH, et al. Prediction of uneventful cardioversion and maintenance of sinus rhythm from direct‐current electrical cardioversion of chronic atrial fibrillation and flutter. Am J Cardiol. 1991;68:41–46. [DOI] [PubMed] [Google Scholar]
- 4. Tieleman RG, Van Gelder IC, Crijns HJ, et al. Early recurrences of atrial fibrillation after electrical cardioversion: a result of fibrillation‐induced electrical remodeling of the atria? J Am Coll Cardiol. 1998;31:167–173. [DOI] [PubMed] [Google Scholar]
- 5. De Vos CB, Pison L, Pisters R, et al. Atrial fibrillatory wall motion and degree of atrial remodeling in patients with atrial fibrillation: a tissue velocity imaging study. J Cardiovasc Electrophysiol. 2009;20:1374–1381. [DOI] [PubMed] [Google Scholar]
- 6. Limantoro I, de Vos CB, Delhaas T, et al. Clinical correlates of echocardiographic tissue velocity imaging abnormalities of the left atrial wall during atrial fibrillation. Europace. 2014;16:1546–1553. [DOI] [PubMed] [Google Scholar]
- 7. Tieleman RG, Plantinga Y, Rinkes D, et al. Validation and clinical use of a novel diagnostic device for screening of atrial fibrillation. Europace. 2014;16:1291–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Limantoro I, De Vos CB, Delhaas T, et al. Tissue velocity imaging of the left atrium predicts response to flecainide in patients with acute atrial fibrillation. Heart Rhythm. 2014;11:478–484. [DOI] [PubMed] [Google Scholar]
- 9. Van Gelder IC, Tuinenburg AE, Schoonderwoerd BS, et al. Pharmacologic versus direct‐current electrical cardioversion of atrial flutter and fibrillation. Am J Cardiol. 1999;84:147R–151R. [DOI] [PubMed] [Google Scholar]
- 10. Duytschaever M, Danse P, Allessie M. Supervulnerable phase immediately after termination of atrial fibrillation. J Cardiovasc Electrophysiol. 2002;13:267–275. [DOI] [PubMed] [Google Scholar]
- 11. Burashnikov A, Antzelevitch C. Reinduction of atrial fibrillation immediately after termination of the arrhythmia is mediated by late phase 3 early after depolarization‐induced triggered activity. Circulation. 2003;107:2355–2360. [DOI] [PubMed] [Google Scholar]
- 12. Varounis C, Dagres N, Maounis T, et al. Atrial premature complexes and heart rate have prognostic significance in 1‐month atrial fibrillation recurrence after electrical cardioversion. Europace. 2007;9:633–637. [DOI] [PubMed] [Google Scholar]
- 13. Hemels ME, Van Noord T, Crijns HJ, et al. Verapamil versus digoxin and acute versus routine serial cardioversion for the improvement of rhythm control for persistent atrial fibrillation. J Am Coll Cardiol. 2006;48:1001–1009. [DOI] [PubMed] [Google Scholar]
- 14. Schwartzman D, Musley SK, Swerdlow C, et al. Early recurrence of atrial fibrillation after ambulatory shock conversion. J Am Coll Cardiol. 2002;40:93–99. [DOI] [PubMed] [Google Scholar]
- 15. Janse MJ, van der Steen AB, van Dam RT. Refractory period of the dog's ventricular myocardium following sudden changes in frequency. Circ Res. 1969;24:251–262. [DOI] [PubMed] [Google Scholar]
- 16. Greiser M, Schotten U. Dynamic remodeling of intracellular Ca2+ signaling during atrial fibrillation. J Mol Cell Cardiol. 2013;58:134–142. [DOI] [PubMed] [Google Scholar]
- 17. Maesen B, Nijs J, Maessen J, et al. Postoperative atrial fibrillation: a maze of mechanisms. Europace. 2012;14:159–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tieleman RG, Van Gelder IC, Bosker HA, et al. Does flecainide regain its antiarrhythmic activity after electrical cardioversion of persistent atrial fibrillation? Heart Rhythm. 2005;2:223–230. [DOI] [PubMed] [Google Scholar]
- 19. Yu WC, Lee SH, Tai CT, et al. Reversal of atrial electrical remodeling following cardioversion of long‐standing atrial fibrillation in man. Cardiovasc Res. 1999;42:470–476. [DOI] [PubMed] [Google Scholar]
- 20. Manning WJ, Silverman DI, Katz SE, et al. Impaired left atrial mechanical function after cardioversion: relation to the duration of atrial fibrillation. J Am Coll Cardiol. 1994;23:1535–1540. [DOI] [PubMed] [Google Scholar]
- 21. Bertaglia E, Zoppo F, Pellizzari N, et al. Variables correlated with early relapses after external electrical cardioversion of persistent atrial fibrillation. Ital Heart J. 2003;4:532–536. [PubMed] [Google Scholar]


