Abstract
Background
Digoxin use has been associated with a lower risk of 30‐day all‐cause admission and readmission in patients with heart failure and reduced ejection fraction (HFrEF).
Hypothesis
Digoxin use will be associated with improved outcomes in patients with HFrEF receiving β‐blockers.
Methods
Of the 3076 hospitalized Medicare beneficiaries with HFrEF (EF <45%), 1046 received a discharge prescription for β‐blockers, of which 634 were not on digoxin. Of the 634, 204 received a new discharge prescription for digoxin. Propensity scores for digoxin use, estimated for each of the 634 patients, were used to assemble a matched cohort of 167 pairs of patients receiving and not receiving digoxin, balanced on 30 baseline characteristics. Matched patients (n = 334) had a mean age of 74 years and were 46% female and 30% African American.
Results
30‐day all‐cause readmission occurred in 15% and 27% of those receiving and not receiving digoxin, respectively (hazard ratio [HR]: 0.51, 95% confidence interval [CI]: 0.31‐0.83, P = 0.007). This beneficial association persisted during 4 years of follow‐up (HR: 0.72, 95% CI: 0.57‐0.92, P = 0.008). Digoxin use was also associated with a lower risk of the combined endpoint of all‐cause readmission or all‐cause mortality at 30 days (HR: 0.54, 95% CI: 0.34‐0.86, P = 0.009) and at 4 years (HR: 0.76, 95% CI: 0.61‐0.96, P = 0.020).
Conclusions
In hospitalized patients with HFrEF receiving β‐blockers, digoxin use was associated with a lower risk of 30‐day all‐cause readmission but not mortality, which persisted during longer follow‐up.
Keywords: Digoxin, Heart Failure, Hospital Readmission, β‐Blockers
1. INTRODUCTION
Heart failure (HF) is the leading cause for 30‐day all‐cause readmission for older Medicare beneficiaries, the reduction of which is a target in the Affordable Care Act.1 In the Digitalis Investigation Group (DIG) main trial, digoxin significantly reduced the risk of all‐cause hospital admission during 37 months of average follow‐up in patients with heart failure (HF) and reduced left ventricular ejection fraction (HFrEF).2 We have demonstrated that among older patients in the DIG main trial, the beneficial effect of digoxin on all‐cause hospital admission was evident during the first 30 days after randomization.3 We have also demonstrated that digoxin use is associated with a lower risk of 30‐day all‐cause readmission in real‐world hospitalized patients with HF.4 However, in that study, HF patients were not restricted to those with HFrEF, and about two‐thirds of the patients were not receiving β‐blockers.
There remains a lingering concern about the role of digoxin in contemporary patients with HFrEF receiving β‐blockers. Most patients in clinical trials of β‐blockers in HFrEF were receiving digoxin,5, 6, 7, 8 suggesting that β‐blockers are effective when used concomitantly with digoxin. However, very few patients in the DIG main trial were on β‐blockers.2 Although β‐blockers would be expected to attenuate the proarrhythmic effects of digoxin, concerns remain for potential effects such as symptomatic bradycardia, which may in turn lead to hospitalization. The objective of the current study is to examine the association of digoxin use with hospital readmission with a focus on 30‐day all‐cause readmission in a propensity score–matched cohort of older adults with HFrEF, all of whom were receiving β‐blockers.
2. METHODS
2.1. Source of data
The Alabama Heart Failure Project was a Medicare quality‐improvement project, the details of which have been described elsewhere.9, 10, 11 Briefly, 9649 medical records of 8555 unique fee‐for‐service Medicare beneficiaries discharged with a primary diagnosis of HF from 106 Alabama hospitals between 1998 and 2001 were abstracted by professional abstractors.9 Of the 8555 patients, 8049 patients were discharged alive. If a patient had multiple hospitalizations, the first hospitalization was included in the analysis. HF was diagnosed using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) codes.9 Of the 8049 patients discharged alive, 5479 had data on left ventricular ejection fraction (LVEF), of which 3067 had LVEF <45%. We used the LVEF cutoff of 45% to be consistent with the cutoffs used in the DIG trial and the prior Alabama Heart Failure Project publications.2, 4 Of these, 1046 were discharged on a β‐blocker. However, we also conducted a sensitivity analysis using the LVEF cutoff of ≤40%, as recommended by the current HF guidelines.12, 13 Of the 2871 patients with LVEF ≤40%, 979 were discharged on a β‐blocker.
2.2. Assembly of an inception cohort
To minimize potential bias associated with prevalent drug use,14, 15 we assembled an inception cohort by excluding 412 patients who were receiving digoxin on admission. Thus, our inception cohort consisted of 634 patients with HFrEF, none on digoxin, and all on β‐blockers, of which 204 (32%) received a new discharge prescription for digoxin (Figure 1). Data on admission and discharge digoxin use and other baseline characteristics were collected by chart abstraction.9 Of the 979 patients with an LVEF ≤40% who received a discharge prescription for a β‐blocker, 441 were not receiving digoxin before admission, of which 193 (44%) received a new discharge prescription for digoxin.
Figure 1.
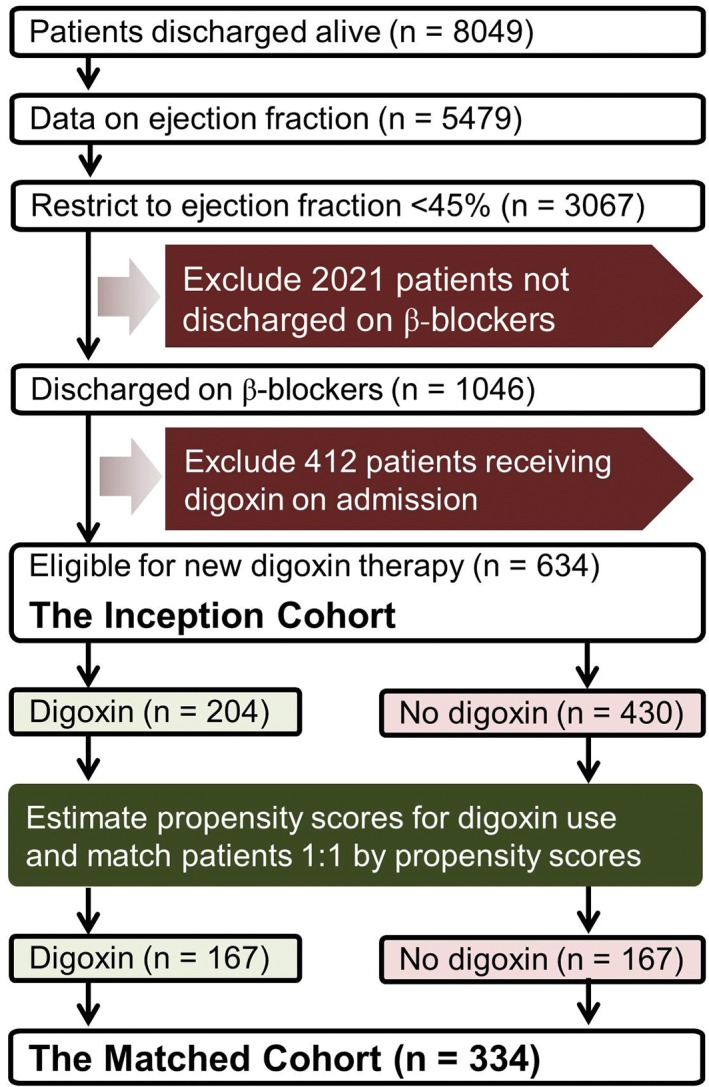
Flowchart displaying assembly of matched cohort of patients with HFrEF on β‐blockers eligible for new digoxin therapy. Abbreviations: HFrEF, heart failure with reduced ejection fraction
2.3. Propensity score matching: assembly of a balanced cohort
In a randomized controlled trial, patients receiving and not receiving digoxin would have a 50% probability of receiving the drug and would also be expected to be balanced on all measured and unmeasured baseline characteristics. In an observational study, because digoxin therapy would not be randomized, the probability of receiving digoxin would vary between 0% and 100%, and imbalances in baseline characteristics are possible. To reduce this selection bias, we used propensity scores for the receipt of a new discharge prescription of digoxin to assemble a matched cohort in which patients receiving and not receiving digoxin would be well balanced on all measured baseline covariates.16, 17
Propensity scores for the receipt of digoxin use were estimated for each of the 634 patients using a nonparsimonious multivariable logistic regression model in which the receipt of digoxin was the dependent variable and 30 baseline characteristics displayed in Figure 2 were used as covariates.4, 10, 18, 19 Using a greedy matching protocol described elsewhere,20, 21 we were able to match 167 (82% of the 204) patients receiving digoxin with 167 patients who were not receiving digoxin but had similar propensity scores (Figure 1). Repeating the above process, from the pre‐match cohort of 441 patients with LVEF ≤40%, we assembled a sensitivity cohort of 154 pairs of patients receiving and not receiving digoxin. Absolute standardized differences (0% indicates no residual bias and <10% indicates inconsequential bias) were estimated to assess balances in baseline characteristics before and after matching and were presented as a Love plot (Figure 2).22
Figure 2.
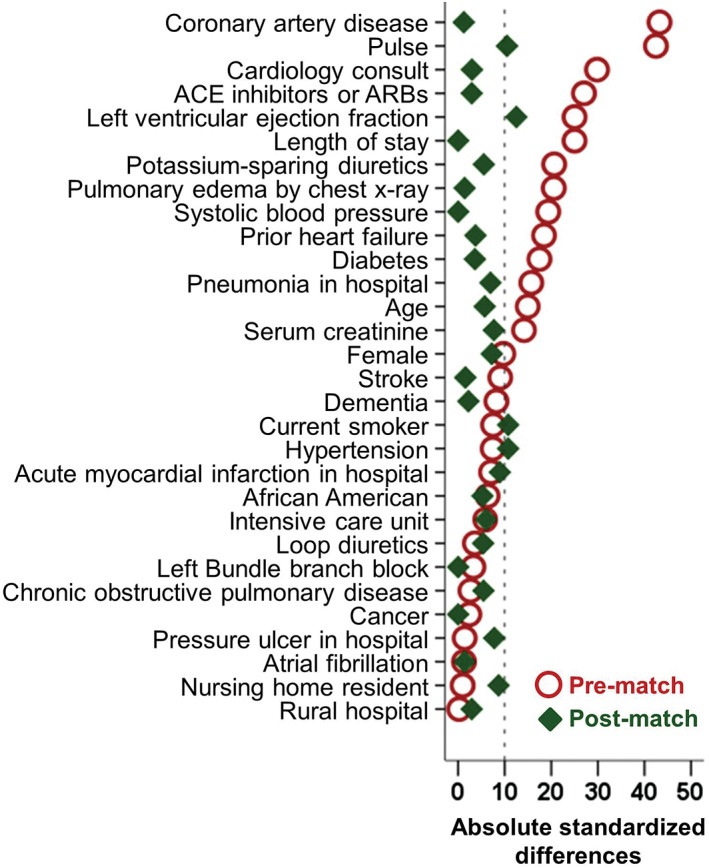
Love plot displaying absolute standardized differences comparing 30 baseline characteristics between hospitalized patients with HFrEF on β‐blockers receiving and not receiving a new discharge prescription for digoxin, before and after propensity‐score matching. Abbreviations: ACE, angiotensin‐converting enzyme; ARBs, angiotensin II receptor blockers; HFrEF, heart failure with reduced ejection fraction
2.4. Hospitalization and mortality data
The primary outcome of the current analysis was 30‐day all‐cause readmission. Secondary outcomes included HF readmission, all‐cause mortality, and the composite endpoint of all‐cause readmission or all‐cause mortality during the 30 days post‐discharge. All outcomes were also examined at 1 and 4 years post‐discharge. Data on outcomes and time to first occurrence of each outcome were obtained from Medicare data.9, 10, 11 For readmission outcomes, patients who did not have an event were censored if they died or were lost to follow‐up, whichever occurred first.
2.5. Statistical analysis
Pearson χ2 and Wilcoxon rank‐sum tests were used for descriptive analyses to compare baseline characteristics between the 2 treatment groups, as appropriate. Kaplan–Meier survival analysis was used to plot combined endpoint of 30‐day all‐cause readmission or 30‐day all‐cause mortality by digoxin use. Cox regression analyses were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for each outcome associated with a new discharge prescription of digoxin. We estimated the degree of a hidden bias associated with an unmeasured covariate that could explain away our observed associations by conducting formal sensitivity analyses.23 All statistical tests were 2‐tailed, with a P value <0.05 considered significant. SPSS version 24 (IBM Corp., Armonk, NY) was used for data analyses.
3. RESULTS
3.1. Baseline characteristics
Matched patients had a mean age (± SD) of 74 (±11) years and a mean LVEF of 29%; 46% were women, and 30% were African American. Before matching, more patients in the digoxin group had a lower mean LVEF, a lower mean serum creatinine (sCr), a higher mean heart rate, and a higher prevalence of atrial fibrillation and angiotensin‐converting enzyme inhibitor (ACEI) use (Table 1). These and other imbalances in baseline characteristics were attenuated after matching and absolute standardized differences for almost all measured variables were <10% (Figure 2).
Table 1.
Baseline characteristics of patients hospitalized for HF and LVEF <45% on β‐blockers, by the receipt of a new discharge prescription for digoxin, before and after propensity‐score matching
| Pre‐match, n = 634 | Post‐match, n = 334 | |||||
|---|---|---|---|---|---|---|
| Digoxin on Discharge | P Value | Digoxin on Discharge | P Value | |||
| No, n = 430 | Yes, n = 204 | No, n = 167 | Yes, n = 167 | |||
| Age, y | 72.5 ± 11.1 | 74.0 ± 9.9 | 0.084 | 73.2 ± 10.9 | 73.8 ± 9.8 | 0.605 |
| Female sex | 202 (47) | 86 (42) | 0.255 | 79 (47) | 73 (44) | 0.510 |
| African American ethnicity | 126 (29) | 54 (27) | 0.460 | 52 (31) | 48 (29) | 0.633 |
| Current smoker | 64 (15) | 36 (18) | 0.373 | 35 (21) | 28 (17) | 0.328 |
| Nursing home resident | 14 (3) | 7 (3) | 0.908 | 9 (5) | 6 (4) | 0.428 |
| LVEF, % | 30 ± 8 | 28 ± 8 | 0.003 | 28 ± 8 | 29 ± 8 | 0.226 |
| Prior HF | 297 (69) | 123 (60) | 0.029 | 107 (64) | 104 (62) | 0.734 |
| Comorbidities | ||||||
| HTN | 336 (78) | 153 (75) | 0.379 | 139 (83) | 132 (79) | 0.328 |
| DM | 210 (49) | 82 (40) | 0.041 | 77 (46) | 74 (44) | 0.742 |
| CAD | 294 (68) | 120 (59) | 0.018 | 102 (61) | 101 (61) | 0.911 |
| LBBB | 77 (18) | 34 (17) | 0.701 | 28 (17) | 28 (17) | 1.000 |
| AF | 60 (14) | 66 (32) | <0.001 | 40 (24) | 41 (25) | 0.898 |
| Stroke | 89 (21) | 35 (17) | 0.294 | 30 (18) | 31 (19) | 0.887 |
| COPD | 121 (28) | 55 (27) | 0.757 | 42 (25) | 46 (28) | 0.619 |
| Dementia | 23 (5) | 15 (7) | 0.321 | 14 (8) | 13 (8) | 0.841 |
| Cancer | 9 (2) | 5 (3) | 0.774 | 4 (2) | 4 (2) | 1.000 |
| Clinical and laboratory findings | ||||||
| Pulse, bpm | 89 ± 22 | 99 ± 25 | <0.001 | 97 ± 22 | 94 ± 23 | 0.335 |
| SBP, mm Hg | 152 ± 32 | 146 ± 30 | 0.019 | 149 ± 34 | 149 ± 30 | 0.980 |
| DBP, mm Hg | 84 ± 21 | 84 ± 20 | 1.000 | 87 ± 22 | 83 ± 20 | 0.118 |
| Respiration, breaths/min | 24 ± 7 | 24 ± 6 | 0.602 | 25 ± 7 | 24 ± 6 | 0.369 |
| Pulmonary edema by chest x‐ray | 287 (67) | 155 (76) | 0.018 | 127 (76) | 126 (75) | 0.898 |
| sCr, mg/dL | 1.8 ± 1.6 | 1.6 ± 1.2 | 0.042 | 1.7 ± 1.4 | 1.6 ± 1.2 | 0.496 |
| In‐hospital events | ||||||
| Pneumonia | 95 (22) | 59 (29) | 0.061 | 39 (23) | 44 (26) | 0.527 |
| Acute MI | 26 (6) | 16 (8) | 0.395 | 15 (9) | 11 (7) | 0.414 |
| Pressure ulcer | 28 (7) | 14 (7) | 0.868 | 8 (5) | 11 (7) | 0.479 |
| Hospital and care characteristics | ||||||
| Rural hospital | 91 (21) | 43 (21) | 0.981 | 35 (21) | 37 (22) | 0.790 |
| Cardiology consult | 300 (70) | 168 (82) | 0.001 | 132 (79) | 134 (80) | 0.786 |
| ICU | 16 (4) | 10 (5) | 0.484 | 6 (4) | 8 (5) | 0.585 |
| Length of stay, d | 6 ± 4 | 7 ± 4 | 0.001 | 7 ± 4 | 7 ± 4 | 0.292 |
| Discharge medications | ||||||
| ACEI/ARBs | 298 (69) | 165 (81) | 0.002 | 130 (78) | 132 (79) | 0.790 |
| β‐Blockersa | 430 (100) | 204 (100) | 1.00 | 167 (100) | 167 (100) | 1.00 |
| Loop diuretics | 359 (84) | 173 (85) | 0.674 | 147 (88) | 144 (86) | 0.624 |
| Potassium‐sparing diuretics | 79 (18) | 55 (27) | 0.013 | 44 (26) | 40 (24) | 0.614 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin II receptor blocker; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; DBP, diastolic blood pressure; DM, diabetes mellitus; HF, heart failure; HTN, hypertension; ICU, intensive care unit; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; MI, myocardial infarction; SBP, systolic blood pressure; sCr, serum creatinine; SD, standard deviation.
Values are presented as n (%) or mean ± SD.
All patients are on β‐blockers by study design.
3.2. Digoxin use and 30‐day outcomes
All‐cause readmission occurred in 15% and 27% of matched patients receiving and not receiving a new discharge prescription for digoxin, respectively (HR when digoxin use is compared with no digoxin use: 0.51, 95% CI: 0.31‐0.83, P = 0.007; Table 2). Findings from our sensitivity analysis demonstrate that of the 167 matched pairs, 57 patients clearly had longer 30‐day all‐cause readmission‐free survival compared with their matched counterparts. In the absence of a hidden bias, a sign‐score test for matched data with censoring demonstrated that in 38 (67%) of the 57 pairs, patients receiving digoxin outlived those not receiving digoxin (P = 0.012).
Table 2.
Association between a new discharge prescription for digoxin and post‐discharge outcomes in a propensity‐matched cohort of Medicare beneficiaries hospitalized for HF receiving β‐blockers
| Events | Absolute Risk Differencea | HRb (95% CI) | P Value | ||
|---|---|---|---|---|---|
| New Discharge Prescription for Digoxin | |||||
| No, n = 167 | Yes, n = 167 | ||||
| 30‐day outcomes | |||||
| All‐cause readmission | 27 (45) | 15 (25) | –12 | 0.51 (0.31‐0.83) | 0.007 |
| HF readmission | 11 (18) | 5 (9) | –6 | 0.48 (0.22‐1.07) | 0.071 |
| All‐cause mortality | 3 (5) | 2 (4) | –1 | 0.80 (0.22‐2.99) | 0.742 |
| All‐cause readmission or all‐cause mortality | 29 (49) | 17 (29) | –12 | 0.54 (0.34‐0.86) | 0.009 |
| 1‐year outcomes | |||||
| All‐cause readmission | 70 (117) | 59 (98) | –11 | 0.71 (0.55‐0.93) | 0.014 |
| HF readmission | 34 (56) | 24 (40) | –10 | 0.66 (0.44‐0.99) | 0.046 |
| All‐cause mortality | 27 (45) | 32 (53) | +5 | 1.18 (0.80‐1.76) | 0.405 |
| All‐cause readmission or all‐cause mortality | 76 (127) | 68 (113) | –8 | 0.76 (0.59‐0.98) | 0.032 |
| 4‐year outcomes | |||||
| All‐cause readmission | 86 (144) | 76 (127) | –10 | 0.72 (0.57‐0.92) | 0.008 |
| HF readmission | 52 (87) | 44 (73) | –8 | 0.77 (0.56‐1.05) | 0.092 |
| All‐cause mortality | 50 (84) | 47 (79) | –3 | 0.94 (0.69‐1.28) | 0.685 |
| All‐cause readmission or all‐cause mortality | 92 (154) | 86 (143) | –6 | 0.76 (0.61‐0.96) | 0.020 |
Abbreviations: CI, confidence interval; HF, heart failure; HR, hazard ratio.
Absolute risk differences were calculated by subtracting percent events in patients receiving digoxin from those not receiving digoxin.
The HRs compared patients receiving digoxin vs those not receiving digoxin.
Digoxin use was also associated with a significantly lower risk of the combined endpoint of all‐cause readmission or all‐cause mortality (HR: 0.54, 95% CI: 0.34‐0.86, P = 0.009; Table 2 and Figure 3). HRs (95% CIs) for HF readmission and all‐cause mortality were 0.48 (0.22‐1.07) and 0.80 (0.22‐2.99), respectively (Table 2). When we repeated our analysis using an LVEF cutoff of ≤40%, a similar association was observed among the 308 matched patients: all‐cause readmission occurred in 15% and 26% of patients receiving and not receiving digoxin, respectively (HR: 0.54, 95% CI: 0.32‐0.90, P = 0.017). Other 30‐day outcomes are displayed in Table 2.
Figure 3.
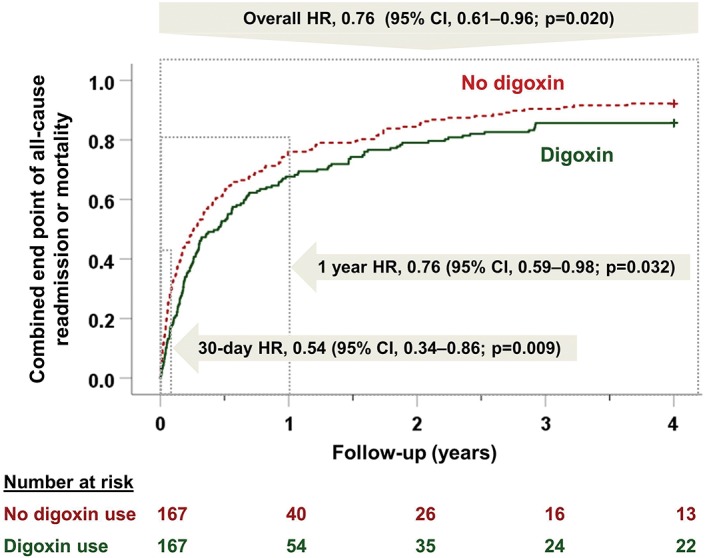
Kaplan–Meier plots for combined endpoint of all‐cause readmission or all‐cause mortality by a new discharge prescription for digoxin in a propensity score–matched cohort of hospitalized patients with HFrEF on β‐blockers. Abbreviations: CI, confidence interval; HFrEF, heart failure with reduced ejection fraction; HR, hazard ratio
3.3. Digoxin use and long‐term outcomes
Early beneficial association of digoxin with all‐cause readmission was observed both at 1‐year and 4‐year follow‐up (Table 2). Digoxin use was associated with a significantly lower risk of the combined endpoint of all‐cause readmission or all‐cause mortality at 1 year (HR: 0.76, 95% CI: 0.59‐0.98, P = 0.032) and 4 years (HR: 0.66, 95% CI: 0.61‐0.96, P = 0.020; Table 2 and Figure 3). Other long‐term associations are displayed in Table 2.
4. DISCUSSION
Findings from the current study demonstrate that in hospitalized older patients with HFrEF receiving β‐blockers, a discharge initiation of digoxin was associated with a significantly lower risk of 30‐day all‐cause readmission that persisted during longer follow‐up. This beneficial association was not at the expense of higher mortality. There was no difference in all‐cause mortality at any point, and as such there was a significantly lower risk of the combined endpoint of all‐cause readmission or all‐cause mortality. These findings complement the growing body of evidence about the important role of digoxin in contemporary older patients with HFrEF receiving β‐blockers. These findings are important, as in addition to financial consequences related to the Patient Protection and Affordable Care Act, 30‐day all‐cause readmission is associated with higher mortality.24
Digoxin is the only positive inotrope that does not increase the risk of death in patients with HF. This has been attributed in part to the neurohormonal‐inhibiting properties of digoxin, especially in lower doses,25, 26, 27, 28, 29, 30 and lack of down‐regulation of the digoxin receptor concentration and tolerance.31 Efficacy and clinical effectiveness of digoxin in reducing the risk of 30‐day all‐cause hospital admission and readmission have been well established.3, 4 However, most patients in these studies were not receiving β‐blockers, which remains a lingering concern.32 We have previously demonstrated that in 1842 propensity score–matched patients with HF (30% on β‐blockers), a new digoxin prescription was associated with a lower risk of 30‐day all‐cause readmission (17% vs 22%; HR: 0.77, 95% CI: 0.63‐0.95) and this association appeared to be stronger in the subset with an LVEF <45% (15% vs 23%; HR: 0.63, 95% CI: 0.47‐0.83).4 This association appeared stronger in the current analysis, in which all patients with an LVEF <45% were also receiving β‐blockers (15% vs 27%; HR: 0.51, 95% CI: 0.31‐0.83).
There are several mechanisms to explain the improved efficacy and effectiveness of digoxin in HF patients with reduced ejection fraction receiving β‐blockers. Digoxin use is associated with various arrhythmias, including fatal ventricular arrhythmias. In the DIG trial in HFrEF patients not receiving β‐blockers, although digoxin did not increase the risk of total or cardiovascular deaths, it increased the risk of cardiac deaths not due to worsening HF, which was presumed to be due to fatal arrhythmias.2 It is possible that any pro‐arrhythmic effects of digoxin would be expected to be attenuated by β‐blockers.33, 34 Β‐blockers reduce the risk of arrhythmias and sudden death in patients with HFrEF.5, 6, 7, 8 Through parasympathetic modulation of the adrenergic system and increase in vagal tone, digoxin may also augment the antiadrenergic effect of β‐blockers and improve outcomes in patients with HF.27, 30 The magnitude of the beneficial association of digoxin use and HF readmission is consistent with our prior findings.19 However, this association did not reach statistical significance due to the small sample size of our propensity score–matched cohort.
A post hoc analysis of 5 randomized controlled trials of carvedilol demonstrated that in patients with HFrEF, digoxin use was associated with a 28% lower risk of all‐cause hospital admission in the placebo group and a 38% reduction in the carvedilol group.35 Findings from our study now suggest that these beneficial associations of digoxin use also extend to hospitalized patients with HFrEF receiving β‐blockers. Although our study is based on fee‐for‐service Medicare beneficiaries from a single state during 1999–2001, nearly 80% were on ACEIs or ARBs, and by design, all were on β‐blockers. The role of aldosterone antagonists in real‐world older patients with HFrEF remains unclear.36, 37
HF remains a leading cause for hospital readmission. Few interventions appear to be effective in lowering the risk of 30‐day all‐cause readmission in HF.38 Among evidence‐based therapy, the use of renin‐angiotensin system inhibitors appears to be associated with a lower risk of 30‐day all‐cause readmission.39 However, β‐blockers and spironolactone have not been shown to be associated with 30‐day all‐cause readmission.37, 40 Digoxin is a relatively inexpensive and generally safe drug that is approved by the US Food and Drug Administration for use in patients with chronic HF.41 Findings from the current study suggest that digoxin may be useful in lowering the risk of readmission in contemporary patients with HFrEF receiving β‐blockers.
4.1. Study limitations
Our study has several limitations. Despite our use of an inception cohort and propensity score–matching design, bias due to unmeasured confounders is possible. However, findings from our sensitivity analysis suggest that associations observed in our study are rather insensitive to a hidden bias. An unmeasured confounder could potentially explain away the 49% risk reduction observed in our matched data if it would increase the odds of receiving digoxin by 16%. However, that missing confounder would need to be a near‐perfect predictor of 30‐day all‐cause readmission and also could not be strongly correlated with any of the 30 measured baseline characteristics displayed in Figure 2, which is an unlikely possibility. Post‐discharge crossover of therapy is possible and may have attenuated some of our associations.42 Lack of data on dose or serum digoxin concentration is another limitation. However, digoxin has been shown to reduce the risk of hospital admission in both low and high serum digoxin concentrations.19 Finally, we had no data on post‐discharge symptomatic bradycardia. However, the findings of a lower risk of all‐cause readmission suggest that the role of symptomatic bradycardia on overall readmission would be small.
5. CONCLUSION
Digoxin use is associated with a lower risk of 30‐day all‐cause readmission in hospitalized patients with HFrEF receiving ACEIs and β‐blockers. This benefit persisted during longer follow‐up and was not at the expense of mortality. These findings provide evidence to support the use of digoxin for contemporary HF patients at a high risk for readmission.
Author contributions
Phillip H. Lam, MD, and Poonam Bhyan, MD, contributed equally to this article.
Conflicts of interest
The authors declare no potential conflicts of interest.
Lam PH, Bhyan P, Arundel C, et al. Digoxin use and lower risk of 30‐day all‐cause readmission in older patients with heart failure and reduced ejection fraction receiving β‐blockers. Clin Cardiol. 2018;41:406–412. 10.1002/clc.22889
Funding information Dr. Ahmed was supported in part by the National Institutes of Health through grants R01‐HL085561, R01‐HL085561‐S, and R01‐HL097047 from the National Heart, Lung, and Blood Institute.
REFERENCES
- 1. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee‐for‐service program [published correction appears in N Engl J Med. 2011;364:1582]. N Engl J Med. 2009;360:1418–1428. [DOI] [PubMed] [Google Scholar]
- 2. Digitalis Investigation Group Investigators . The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997;336:525–533. [DOI] [PubMed] [Google Scholar]
- 3. Bourge RC, Fleg JL, Fonarow GC, et al. Digoxin reduces 30‐day all‐cause hospital admission in older patients with chronic systolic heart failure. Am J Med. 2013;126:701–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ahmed A, Bourge RC, Fonarow GC, et al. Digoxin use and lower 30‐day all‐cause readmission for Medicare beneficiaries hospitalized for heart failure. Am J Med. 2014;127:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Packer M, Bristow MR, Cohn JN, et al; US Carvedilol Heart Failure Study Group . The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. N Engl J Med. 1996;334:1349–1355. [DOI] [PubMed] [Google Scholar]
- 6. Packer M, Coats AJ, Fowler MB, et al; Carvedilol Prospective Randomized Cumulative Survival Study Group. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med. 2001;344:1651–1658. [DOI] [PubMed] [Google Scholar]
- 7. CIBIS‐II Investigators . The Cardiac Insufficiency Bisoprolol Study II (CIBIS‐II): a randomised trial. Lancet. 1999;353:9–13. [PubMed] [Google Scholar]
- 8. MERIT‐HF Investigators . Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT‐HF). Lancet. 1999;353:2001–2007. [PubMed] [Google Scholar]
- 9. Feller MA, Mujib M, Zhang Y, et al. Baseline characteristics, quality of care, and outcomes of younger and older Medicare beneficiaries hospitalized with heart failure: findings from the Alabama Heart Failure Project. Int J Cardiol. 2012;162:39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ahmed A, Fonarow GC, Zhang Y, et al. Renin‐angiotensin inhibition in systolic heart failure and chronic kidney disease. Am J Med. 2012;125:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ahmed A, Rich MW, Zile M, et al. Renin‐angiotensin inhibition in diastolic heart failure and chronic kidney disease. Am J Med. 2013;126:150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137–e161. [DOI] [PubMed] [Google Scholar]
- 13. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. [DOI] [PubMed] [Google Scholar]
- 14. Danaei G, Tavakkoli M, Hernan MA. Bias in observational studies of prevalent users: lessons for comparative effectiveness research from a meta‐analysis of statins. Am J Epidemiol. 2012;175:250–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Patel K, Fonarow GC, Kitzman DW, et al. Angiotensin receptor blockers and outcomes in real‐world older patients with heart failure and preserved ejection fraction: a propensity‐matched inception cohort clinical effectiveness study. Eur J Heart Fail. 2012;14:1179–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 17. Rubin DB. Using propensity scores to help design observational studies: application to the tobacco litigation. Health Serv Outcomes Res Methodol. 2001;2:169–188. [Google Scholar]
- 18. Ahmed A, Husain A, Love TE, et al. Heart failure, chronic diuretic use, and increase in mortality and hospitalization: an observational study using propensity score methods. Eur Heart J. 2006;27:1431–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ahmed A, Rich MW, Love TE, et al. Digoxin and reduction in mortality and hospitalization in heart failure: a comprehensive post hoc analysis of the DIG trial. Eur Heart J. 2006;27:178–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ahmed MI, White M, Ekundayo OJ, et al. A history of atrial fibrillation and outcomes in chronic advanced systolic heart failure: a propensity‐matched study. Eur Heart J. 2009;30:2029–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mujib M, Patel K, Fonarow GC, et al. Angiotensin‐converting enzyme inhibitors and outcomes in heart failure and preserved ejection fraction. Am J Med. 2013;126:401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wahle C, Adamopoulos C, Ekundayo OJ, et al. A propensity‐matched study of outcomes of chronic heart failure (HF) in younger and older adults. Arch Gerontol Geriatr. 2009;49:165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rosenbaum PR. Sensitivity to hidden bias In: Rosenbaum PR, ed. Observational Studies. New York, NY: Springer‐Verlag; 2002:105–170. [Google Scholar]
- 24. Arundel C, Lam PH, Khosla R, et al. Association of 30‐day all‐cause readmission with long‐term outcomes in hospitalized older Medicare beneficiaries with heart failure. Am J Med. 2016;129:1178–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Slatton ML, Irani WN, Hall SA, et al. Does digoxin provide additional hemodynamic and autonomic benefit at higher doses in patients with mild to moderate heart failure and normal sinus rhythm? J Am Coll Cardiol. 1997;29:1206–1213. [DOI] [PubMed] [Google Scholar]
- 26. Newton GE, Tong JH, Schofield AM, et al. Digoxin reduces cardiac sympathetic activity in severe congestive heart failure. J Am Coll Cardiol. 1996;28:155–161. [DOI] [PubMed] [Google Scholar]
- 27. Watanabe AM. Digitalis and the autonomic nervous system. J Am Coll Cardiol. 1985;5(5 suppl A):35A–42A. [DOI] [PubMed] [Google Scholar]
- 28. Gheorghiade M, Hall VB, Jacobsen G, et al. Effects of increasing maintenance dose of digoxin on left ventricular function and neurohormones in patients with chronic heart failure treated with diuretics and angiotensin‐converting enzyme inhibitors. Circulation. 1995;92:1801–1807. [DOI] [PubMed] [Google Scholar]
- 29. Gheorghiade M, Ferguson D. Digoxin: a neurohormonal modulator in heart failure? Circulation. 1991;84:2181–2186. [DOI] [PubMed] [Google Scholar]
- 30. Ferguson DW, Berg WJ, Sanders JS, et al. Sympathoinhibitory responses to digitalis glycosides in heart failure patients: direct evidence from sympathetic neural recordings. Circulation. 1989;80:65–77. [DOI] [PubMed] [Google Scholar]
- 31. Schmidt TA, Allen PD, Colucci WS, et al. No adaptation to digitalization as evaluated by digitalis receptor (Na,K‐ATPase) quantification in explanted hearts from donors without heart disease and from digitalized recipients with end‐stage heart failure. Am J Cardiol. 1993;71:110–114. [DOI] [PubMed] [Google Scholar]
- 32. Tariq S, Aronow WS. Use of inotropic agents in treatment of systolic heart failure. Int J Mol Sci. 2015;16:29060–29068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Echt DS, Liebson PR, Mitchell LB, et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo: the Cardiac Arrhythmia Suppression Trial. N Engl J Med. 1991;324:781–788. [DOI] [PubMed] [Google Scholar]
- 34. Kennedy HL, Brooks MM, Barker AH, et al; CAST Investigators. β‐Blocker therapy in the Cardiac Arrhythmia Suppression Trial. Am J Cardiol. 1994;74:674–680. [DOI] [PubMed] [Google Scholar]
- 35. Eichhorn EJ, Lukas MA, Wu B, et al. Effect of concomitant digoxin and carvedilol therapy on mortality and morbidity in patients with chronic heart failure. Am J Cardiol. 2000;86:1032–1035, A10–A11. [DOI] [PubMed] [Google Scholar]
- 36. Hernandez AF, Mi X, Hammill BG, et al. Associations between aldosterone antagonist therapy and risks of mortality and readmission among patients with heart failure and reduced ejection fraction. JAMA. 2012;308:2097–2107. [DOI] [PubMed] [Google Scholar]
- 37. Lam PH, Dooley DJ, Inampudi C, et al. Lack of evidence of lower 30‐day all‐cause readmission in Medicare beneficiaries with heart failure and reduced ejection fraction discharged on spironolactone. Int J Cardiol. 2017;227:462–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Feltner C, Jones CD, Cené CW, et al. Transitional care interventions to prevent readmissions for persons with heart failure: a systematic review and meta‐analysis. Ann Intern Med. 2014;160:774–784. [DOI] [PubMed] [Google Scholar]
- 39. Sanam K, Bhatia V, Bajaj NS, et al. Renin‐angiotensin system inhibition and lower 30‐day all‐cause readmission in Medicare beneficiaries with heart failure. Am J Med. 2016;129:1067–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bhatia V, Bajaj NS, Sanam K, et al. Beta‐blocker use and 30‐day all‐cause readmission in Medicare beneficiaries with systolic heart failure. Am J Med. 2015;128:715–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gheorghiade M, van Veldhuisen DJ, Colucci WS. Contemporary use of digoxin in the management of cardiovascular disorders. Circulation. 2006;113:2556–2564. [DOI] [PubMed] [Google Scholar]
- 42. Clarke R, Shipley M, Lewington S, et al. Underestimation of risk associations due to regression dilution in long‐term follow‐up of prospective studies. Am J Epidemiol. 1999;150:341–353. [DOI] [PubMed] [Google Scholar]


