Abstract
Atrial fibrillation and chronic kidney disease (CKD) commonly occur together, which poses a therapeutic dilemma due to increased risk of both systemic thromboembolism and bleeding. Chronic kidney disease also has implications for medication selection. The objective of this review is to evaluate the options for anticoagulation for thromboembolism prevention in patients with atrial fibrillation and chronic kidney disease. We searched PubMed for studies of patients with atrial fibrillation and CKD on warfarin or a direct oral anticoagulant (DOAC) for thromboembolism prevention through January 1 2018, in addition to evaluating major trials evaluating DOACs and warfarin use as well as society guidelines. For patients with mild to moderate chronic kidney disease, primarily observational data supports the use of warfarin, and high quality trial data and meta‐analyses support the use and possible superiority of DOACs. For patients with severe chronic kidney disease, there are limited data on warfarin which supports its use, and data for DOACs is limited primarily to pharmacologic studies which support dose reductions but lack information on patient outcomes. For patients with end‐stage renal disease, studies on warfarin are conflicting, but the majority suggest a lack of benefit and possible harm; studies in DOACs are very limited, but apixaban is the least renally cleared and may be both safe and effective. In conclusion, warfarin or DOACs may be used based on the degree of severity of chronic kidney disease, but further study in needed in patients with end‐stage renal disease.
Keywords: arrhythmia/all, atrial fibrillation, general clinical cardiology/adult, kidney disease, pharmacology, stroke prevention
1. INTRODUCTION
Atrial fibrillation (AF) is more common in patients with chronic kidney disease (CKD),1 especially dialysis patients.2 This leads to important therapeutic challenges as patients with CKD and AF are at increased risk for both systemic thromboembolism and bleeding.3, 4, 5 In particular, the combination of AF and dialysis has been associated with increased risk of death and bleeding.5 Increased risk of bleeding in dialysis patients is thought to be due to several mechanisms leading to impaired hemostasis.6 Increased thromboembolism risk in dialysis patients is thought to be due to the presence of traditional risk factors,7 increased platelet activation during dialysis,6 systemic inflammation and endothelial damage,7 and coagulation derangements.8
Society guidelines recommend anticoagulation for patients with AF and elevated stroke risk, such as prior stroke, transient ischemic attack (TIA), or a CHA2DS2‐VASc (congestive heart failure, hypertension, age 64‐74 [1 point], age over 75 [2 points], diabetes, stroke/TIA/thromboembolism [2 points], vascular disease, female sex) score over 1,9 but questions remain about the choice of anticoagulation in CKD patients. Warfarin had been the mainstay of oral anticoagulant treatment for many years until the development of direct oral anticoagulants (DOACs). However, there is limited data regarding warfarin use in CKD as prior studies did not quantify CKD patients or only included low numbers of them.10, 11, 12, 13, 14, 15 Warfarin use in end‐stage renal disease (ESRD) is especially controversial due to conflicting evidence. Finally, major trials supporting the use of DOACs excluded patients with severe CKD or ESRD. In general, for thromboembolism prevention, the combined American Heart Association (AHA), American College of Cardiology (ACC), and Heart Rhythm Society (HRS) guidelines recommend warfarin or DOACs with similar strength (class I recommendation),9 while the European Society of Cardiology (ESC)16 and Canadian Cardiovascular Society (CCS)17 guidelines recommend DOACs over warfarin (Table 1). In addition, patients with CKD have been shown to be at especially increased risk of off‐label dosing of DOACs, with overdosing associated with increased mortality, and underdosing associated with increased cardiovascular hospitalizations.18
Table 1.
Society guidelines for anticoagulation in AF by CKD stage
| CKD stage | AHA/ACC/HRS | ESC | CCS |
|---|---|---|---|
| Mild to moderate Stages 2‐3 (eGFR 30‐90 mL/min/1.73 m2) |
Warfarin (class 1, LOE A) DOACs (class 1, LOE B) with dose adjustment for moderate CKD (class Iib, LOE C) |
DOACs recommended in general (mild to moderate CKD not mentioned) | DOACs recommended in general (mild to moderate CKD not mentioned) |
| Severe Stage 4 (eGFR 15‐29 mL/min/1.73 m2) |
Warfarin recommended, DOACs may be considered (class Iib, LOE C) | Anticoagulation may safely be given (specific drugs not mentioned) | Warfarin recommended |
| End stage renal disease Stage 5 (eGFR <15 mL/min/1.73 m2 or on hemodialysis) |
Warfarin recommended (class IIa, LOE B), recommend against dabigatran and rivaroxaban (class III, LOE C) | No specific recommendation given | Cannot recommend routine anticoagulation for dialysis patients due to lack of data |
Abbreviations: ACC, American College of Cardiology; AF, atrial fibrillation; AHA, American Heart Associated; CCS, Canadian Cardiovascular Society; CKD, chronic kidney disease; DOAC, direct oral anticoagulant; eGFR, estimated glomerular filtration rate; ESC, European Society of Cardiology; HRS, Heart Rhythm Society; LOE, level of evidence.
The purpose of this review is to evaluate the available data regarding the options by CKD stage for anticoagulation for thromboembolism prevention in patients with AF. In addition to kidney function, other patient factors should be considered when choosing anticoagulation, such as age, weight, liver function, drug interactions, comorbid medical conditions, and ability to take daily or twice daily medications consistently.19
2. MANAGEMENT BY CKD STAGE
2.1. Mild to moderate CKD (glomerular filtration rate [GFR] 30‐90 mL/min/1.73 m2)
AHA/ACC/HRS guidelines recommend both warfarin (class I, level of evidence [LOE] A) and DOACs (class 1, LOE B) as options for thromboembolism prevention overall, with dose adjustments for patients with moderate to severe CKD (class IIb/C for DOACs).9 The ESC16 and CCS17 guidelines recommend DOACs over warfarin in general, without specific mention of mild to moderate CKD (Table 1).
2.1.1. Warfarin
In general, studies on warfarin in CKD patients are retrospective and observational, but the majority support its use in patients with mild to moderate CKD. Stroke Prevention in Atrial Fibrillation III was a randomized controlled trial comparing dose‐adjusted warfarin with aspirin plus fixed low dose warfarin. In patients with stage 3 CKD, dose‐adjusted warfarin resulted in a 76% relative risk reduction of ischemic stroke and systemic embolism.15 A large, observational, multi‐center study in Sweden of over 24 000 patients with CKD demonstrated a lower rate of the composite of death, myocardial infarction (MI), and ischemic stroke in each CKD category, without elevated bleeding risk with warfarin. A major limitation of this study was that time in the therapeutic range was over 75%, which is much better than what is generally achieved in the United States.20 Several other small studies have shown reductions in stroke with warfarin compared to nonuse.4, 21, 22 A meta‐analysis comprising 11 cohorts of CKD patients with AF totaling over 48 000 patients, including over 11 000 on warfarin, found a 30% lower risk of ischemic stroke and thromboembolism among non‐end‐stage CKD patients on warfarin.23 Another meta‐analysis found similar results with reduction in thromboembolic events in non‐end‐stage CKD, but also showed DOACs to be superior to warfarin.3
2.1.2. DOACs
The four major DOACs (dabigatran, rivaroxaban, apixaban, and edoxaban) were each studied in large, double‐blinded, and randomized controlled trials (Table 2) which included patients with mild to moderate CKD and demonstrated either noninferiority or superiority of DOACs over warfarin for stroke and thromboembolism prevention. Patients with creatinine clearance (CrCl) of at least 25 mL/min were included in ARISTOTLE, and at least 30 mL/min in the rest. In the RE‐LY Trial, the 110 mg twice daily (BID) dosing of dabigatran was noninferior to warfarin for stroke and systemic embolism prevention, with a lower risk of major hemorrhage. The 150 mg BID dosing (FDA‐approved dosing) carried a lower thromboembolic risk with similar overall hemorrhage, but higher rates of gastrointestinal and life threatening bleeding.24 Of note, there have been case reports of dabigatran‐related kidney injury, and this would be of particular concern in patients with CKD.25 ROCKET‐AF compared two doses of rivaroxaban (20 mg daily for CrCl of 50 mL/min or greater, and 15 mg daily for CrCl of 30‐49 mL/min) to warfarin. Rivaroxaban was noninferior to warfarin for stroke prevention, with no change in major bleeding.26 ARISTOTLE compared two doses of apixaban (5 mg BID for most patients, with a reduction to 2.5 mg BID in patients with two of the following: age ≥ 80, weight ≤ 60 kg, Cr ≥ 1.5 mg/dL) to warfarin. Overall, apixaban was superior to warfarin for stroke and embolism prevention, with less bleeding risk overall. The 2.5 mg BID dosing was noninferior to warfarin, but the overall number of patients who received this dosing was small.27 ENGAGE compared edoxaban (high dose of 60 mg and low dose of 30 mg) to warfarin. The dose of edoxaban in either group was decreased by half for CrCl of 30 to 50 mL/min, weight of 60 kg or less, or use of interacting medications based on pharmacokinetic modeling. Edoxaban was noninferior to warfarin for thromboembolism prevention, with lower rates of bleeding and cardiovascular death.28
Table 2.
Major trials for DOACs in AF
| DOAC | Trial | CrCl included (mL/min) | Efficacy | Safety |
|---|---|---|---|---|
| Dabigatran | RE‐LY | At least 30 | Dabigatran noninferior to warfarin | Similar overall hemorrhage, increased gastrointestinal and life threatening hemorrhage with 150 mg BID dosing |
| Rivaroxaban | ROCKET‐AF | At least 30 | Rivaroxaban noninferior to warfarin | No difference in major bleeding |
| Apixaban | ARISTOTLE | At least 25 | Apixaban superior to warfarin | Lower bleeding overall |
| Edoxaban | ENGAGE | At least 30 | Edoxaban noninferior to warfarin | Lower bleeding overall |
Abbreviations: AF, atrial fibrillation; CrCl, creatinine clearance; DOAC, direct oral anticoagulant.
Several further studies of DOACs in patients with mild and moderate CKD have confirmed the results observed in clinical trials. A 2014 prespecified analysis of RE‐LY demonstrated that rates of stroke or systemic embolism, major bleeding, and all‐cause mortality increased as renal function decreased. The rates of stroke or systemic embolism were lower with dabigatran 150 mg, and similar with 110 mg twice daily when compared with warfarin, without significant difference in subgroups defined by renal function. The study, however, grouped all patients with GFR < 50 mL/min together, and did not include patients with GFR < 30 mL/min.29 Another study comparing patients on dabigatran 110 mg twice daily with CrCl of 50 mL/min or greater to patients with CrCl of 30 to 49 mL/min showed no difference in total bleeding events between the two groups, demonstrating the safety of the low dose in moderate CKD with respect to bleeding risk.30
Apixaban 5 mg BID was compared to aspirin in stage 3 CKD patients from the AVERROES trial, and significantly reduced stroke risk without increase in major bleeding.31 When compared with warfarin in an analysis of ARISTOTLE, apixaban remained more effective and safe regardless of renal function. In fact, the relative risk reduction in major bleeding was greatest in patients with an estimated glomerular filtration rate (eGFR) ≤ 50 mL/min. Patients with eGFR < 50 mL/min were included in one group, and patients with eGFR < 30 mL/min were not included.32 Another 2016 analysis of ARISTOTLE also demonstrated that the benefit of apixaban was preserved regardless of renal function. Again, patients with eGFR <30 mL/min were not included, and eGFR <50 mL/min was treated as one group.33
An analysis of the ENGAGE trial comparing edoxaban to warfarin demonstrated that the reduction in risk of stroke and systemic embolism with edoxaban was preserved across renal function groups in patients with CrCl of at least 30 mL/min. The analysis treated CrCl < 50 mL/min as one group.34
In addition, multiple meta‐analyses have demonstrated the overall superiority of DOACs to warfarin. A 2014 meta‐analysis of the four major DOAC trials mentioned previously demonstrated that, as a class, DOACs were superior to warfarin for stroke prevention, especially for hemorrhagic stroke with reduced mortality and intracranial hemorrhage, but increased gastrointestinal bleeding. However, the increase in gastrointestinal bleeding may be driven primarily by dabigatran and rivaroxaban, rather than apixaban or edoxaban.35 As mentioned previously, another meta‐analysis of major DOAC trials also demonstrated the superiority of DOACs to warfarin in patients with non‐end‐stage CKD in thromboembolic event reduction.3 A meta‐analysis of patients with moderate CKD in the major DOAC trials again showed the superiority of DOACs as a class compared with warfarin overall with dabigatran 150 mg BID demonstrating the greatest efficacy, and apixaban and edoxaban demonstrating reductions in major bleeding compared with warfarin.36
In summary, DOACs have been shown to be at least noninferior, if not superior, to warfarin in large clinical trials as well as meta‐analyses, with these results confirmed in several studies of patients with mild and moderate CKD.
2.2. Severe CKD (GFR 15‐29 mL/min/1.73 m2)
In patients with severe CKD, guidelines favor warfarin, or lack specific recommendations. The AHA/ACC/HRS guidelines note that reduced dosing of DOACs may be considered, but are lacking information on safety and efficacy (class IIb, LOE C), and thus recommend warfarin as the anticoagulant of choice.9 The CCS guidelines recommend warfarin rather than DOACs for patients with eGFR of 15 to 30 mL/min.17 The ESC guidelines note that anticoagulation can safely be given for moderate to severe CKD, but do not make specific mention of DOACs (Table 1).16
Data for patients with severe CKD receiving warfarin is limited, but appears to favor its use. Warfarin had lower risk for a composite of death, ischemic stroke or TIA without an increased risk of bleeding compared to nonuse in 532 patients with eGFR <30 mL/min/1.73 m2. 22 As mentioned earlier, lower risk of a composite of death, readmission due to MI or ischemic stroke was demonstrated across each CKD category without higher risk of bleeding, including the 8.1% (n = 1966) of patients with CrCl of 15 to 30 mL/min, in the Carrero study which included patients with a very high time in the therapeutic range.20 Another study including 67 patients with severe CKD demonstrated a much lower rate of thromboembolic stroke for patients treated with warfarin compared with nonuse.21 As mentioned above, the large Dahal meta‐analysis demonstrated lower risk of ischemic stroke and thromboembolism as well as mortality with warfarin, without increased major bleeding in non‐end‐stage CKD patients.23
Evidence for the use of DOACs is limited, and dosing recommendations are based on small pharmacologic studies which lack hard clinical endpoints. RE‐LY excluded patients with CrCl less than 30 mL/min; however, prescribing recommendations allow for 75 mg twice daily dosing for patients with CrCl 15 to 30 mL/min,24 based on pharmacological modeling showing that 75 mg BID in patients with CrCl of 15 to 30 mL/min achieved similar plasma levels as 150 mg BID in patients with CrCl greater than 30 mL/min.37 In ROCKET‐AF, patients with CrCl 15 to 30 mL/min were not studied, but FDA labeling indicates that rivaroxaban 15 mg daily is expected to produce similar effects as 20 mg daily in patients with normal renal function based on pharmacodynamic study.38 Apixaban 2.5 mg twice a day is recommended if patients have two of the following (age of at least 80 years, body weight less than or equal to 60 kg, or serum creatinine of at least 1.5 mg/dL), but patients with CrCl less than 25 mL/min were not studied in ARISTOTLE.27
In summary, data for both warfarin and DOACs in severe CKD is limited. However, studies of warfarin support its use, while studies of DOACs are limited to pharmacologic modeling which lack clinical endpoints.
2.3. End‐stage CKD (GFR < 15 mL/min/1.73 m2 or dialysis)
In patients with ESRD, the AHA/ACC/HRS guidelines recommend warfarin as the drug of choice, noting it is reasonable to prescribe it with a class IIa recommendation (LOE B). The AHA/ACC/HRS guidelines do not recommend dabigatran or rivaroxaban because of lack of evidence (class III recommendation, LOE C).9 The CCS guidelines state they cannot recommend routine anticoagulation for dialysis patients due to the lack of data.17 The ESC does not give a specific recommendation, but notes that controlled studies for anticoagulation are needed in dialysis patients (Table 1).16 Warfarin has been the standard treatment for thromboembolism prevention related to atrial fibrillation in this group, but studies on its efficacy have been conflicting.
2.3.1. Studies supporting warfarin use
Positive studies supporting warfarin use in this population are generally observational and retrospective. An observational study which included 132 patients with ESRD, 93 of whom were on dialysis, demonstrated greatly reduced thromboembolic stroke risk with warfarin compared with nonuse without increased risk of major bleeding.21 In another large observational study of 1728 patients on renal replacement therapy, warfarin was significantly associated with a lower risk of all‐cause death, with a nonsignificant trend toward reduced cardiovascular death and composite of death, stroke, thromboembolism, and bleeding. This study took place in Denmark and included a high risk population of hospitalized patients.4 In the previously mentioned observational Swedish study, there was a lower risk of the aggregate outcome in each CKD category without increased bleeding risk, but only 2% (478) of patients had CrCl less than 15 mL/min, dialysis patients were not subdivided, and international normalized ratio (INR) control was very good.20 In 1324 patients on dialysis, warfarin was associated with significantly increased 90‐day and 6‐year survival with evidence for stronger early benefit, which the authors suggest is because more time is required for side effects of warfarin to develop. The results were influenced by age and history of stroke.39 A retrospective Danish study demonstrated a 56% decreased in stroke/death risk among 901 hemodialysis (HD) patients with warfarin use.40 It has been noted that the patients in this study were healthier than those in other studies.41
An observational study of over 12 000 patients on hemodialysis provides possible explanations for some of this positive data, and demonstrates the limitations of these observational studies. The study found that only 15% of patients initiated warfarin, with a reduced risk of ischemic stroke among these 1838 patients in the intention to treat analysis, but did not find a statistically significant difference in the as treated analysis. In addition, almost 70% of the patients discontinued warfarin within 1 year.42 This shows how observational data may be subject to bias due to low initiation rates and high discontinuation rates of warfarin, especially in intention to treat analyses.
2.3.2. Studies against warfarin use
In contrast to the relatively small number of studies supporting warfarin use in ESRD, there are numerous studies which question its efficacy. Of the 20 studies reviewed, 14 demonstrated lack of benefit or even harm. The strongest evidence comes from large retrospective studies41, 43 and multiple meta‐analyses.23, 44, 45
Three large meta‐analyses, each including 13 to 20 studies and 37 000 to over 50 000 patients, failed to show benefit to the use of warfarin among ESRD or dialysis patients in either thromboembolism prevention or mortality reduction.23, 44, 45 They did, however, demonstrate increased risk of either all‐cause bleeding by 21%44 or major bleeding by 30% to 35%.23, 45 Of note, one of the studies did demonstrate thromboembolism and mortality benefit without effect on major bleeding among non‐end‐stage CKD patients, providing internal validation for their methodology.23 A meta‐analysis with over 9000 patients actually demonstrated increased risk of stroke with warfarin, driven by a more than 2‐fold increased hemorrhagic stroke risk, in addition to increased bleeding risk.46 Another study demonstrated greater than 2‐fold increased risk of stroke in patients 75 or older.47 Several other small studies between 2011 and 2016 demonstrated no benefit in terms of mortality2, 5, 48, 49, 50, 51, 52 or stroke or thromboembolism prevention.5, 47, 48, 49, 50, 51, 52
A large retrospective study in Canada, comparing over 1600 hemodialysis patients, and over 200 000 nondialysis patients demonstrated that not only was warfarin not beneficial for stroke prevention in patients on dialysis, but was also associated with a 44% increased risk of bleeding. Warfarin was associated with lower stroke risk in nondialysis patients, and elevated but lower bleeding risk in nondialysis vs dialysis patients.41 Another retrospective cohort of over 1600 patients on hemodialysis actually demonstrated a 1.9‐fold increased risk of stroke.43 Several explanations have been proposed for the lack of effectiveness of warfarin in ESRD including impaired hemostasis, comorbidities, use of heparin during dialysis,41 accelerated vascular calcification with warfarin in dialysis patients (including calciphylaxis), decreased vitamin K‐dependent inhibitors of calcification,1 and that patients on dialysis tend to have lower times in the therapeutic range.53
In summary, the majority of studies, including those with larger populations, fail to show a benefit for warfarin use in ESRD patients, and some studies even suggest harm due to increased bleeding risk as well as increased stroke risk.
2.3.3. Dabigatran and rivaroxaban
Rivaroxaban is approximately 33% renally cleared (Figure 1),54 and not eliminated by hemodialysis.55, 56 A 10 mg dose of rivaroxaban has been shown to produce similar drug levels in dialysis patients as a 20 mg dose in healthy volunteers.55 A 15 mg dose in dialysis patients has also been shown to have similar pharmacokinetics and pharmacodynamics as in people with moderate to severe renal impairment not on dialysis.56 These pharmacologic studies raise the possibility of rivaroxaban's use in this population, but it has not been studied in terms of stroke prevention, and one study raised concern for excess bleeding and mortality risk in dialysis patients taking rivaroxaban compared with warfarin.57 Dabigatran was also shown to increase risk of death and bleeding in the same study, and has been shown to be effectively removed by dialysis.58, 59 As mentioned before, case reports of kidney injury due to dabigatran should also be noted.25 Thus, dabigatran is less likely to be of use in dialysis patients, and may be safe but more patient level data is needed given concerns over bleeding risk.
Figure 1.
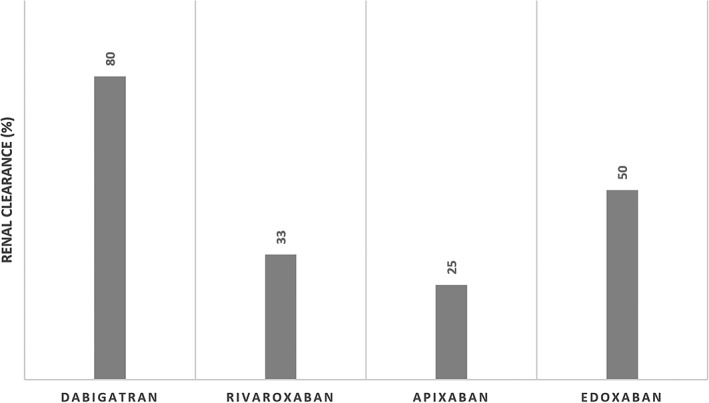
Degree of renal clearance of direct oral anticoagulants (DOACs)
2.3.4. Apixaban
Apixaban is the least renally excreted of all the DOACs (estimated 25%, Figure 1).27 A recent review supported its use in dialysis patients with reduced dosing of 2.5 mg twice daily.60 A small pharmacologic study comparing eight patients with ESRD on dialysis and eight patients with normal renal function demonstrated that 5 mg of apixaban resulted in only a small increase in apixaban exposure when off hemodialysis (compared with normal patients) and was minimally removed by hemodialysis, suggesting that apixaban can be used in ESRD patients on dialysis.61 However, a case report of gastrointestinal bleeding in a single patient with ESRD on HD on apixaban 2.5 mg BID advised caution as the patient had elevated apixaban levels.62 As mentioned above, patients with ESRD on warfarin are at increased risk for vascular calcification. A study of 20 patients with ESRD on dialysis and calciphylaxis demonstrated the successful use of apixaban as an alternative, with improvement in calciphylaxis, lower mortality than published rates for calciphylaxis, and no thrombosis.63
A few recent studies have sought primarily to evaluate the safety of apixaban in real patients with ESRD. A retrospective study of 146 patients (40 patients with ESRD on dialysis) on apixaban or warfarin demonstrated a nonsignificant decrease in major bleeding, the primary outcome, and similar stroke outcomes, suggesting that apixaban may be safe in these patients. However, the study grouped patients with severe renal impairment, ESRD not on dialysis and ESRD on dialysis together, and was not restricted to AF patients.64 Another recent study compared 74 dialysis patients on apixaban to 50 dialysis patients on warfarin, and demonstrated a lower incidence of overall bleeding with apixaban. However, the primary outcome was bleeding, most patients on apixaban did not have AF, and there were no ischemic strokes during the study, making conclusions about efficacy difficult.65 Finally, a recent large retrospective study of AF and ESRD patients using a national dialysis database, including 2351 patients on apixaban, and 23 172 patients on warfarin, found no difference in stroke or systemic embolism overall, but lower risk of major bleeding with apixaban compared with warfarin. Patients on apixaban 5 mg twice daily were found to have a lower risk of stroke or systemic embolism and death compared with warfarin and reduced dose apixaban. This study was the first to assess the efficacy of apixaban in ESRD patients with AF specifically.66 Apixaban appears to be safe in dialysis patients, with recent data also supporting its efficacy, but more prospective data is needed.
3. ALTERNATIVES TO ANTICOAGULATION
Although outside the scope of this review, it is important to note alternatives to anticoagulation for patients with AF and CKD who cannot tolerate anticoagulation. In particular, left atrial appendage closure has been developed as a viable alternative to anticoagulation.60 A recent observational study of AF patients demonstrated similar safety and efficacy of left atrial appendage closure in CKD patients compared with non‐CKD patients.67
4. CONCLUSIONS/FUTURE DIRECTIONS
The combination of AF and CKD poses a therapeutic dilemma given increased risks of both thromboembolism and bleeding. With the introduction of DOACs, the therapeutic options have increased, and can be chosen based on degree of CKD (Table 3). For mild to moderate CKD, warfarin and DOACs are both options, with DOACs demonstrating better efficacy and safety. In severe CKD, limited data has shown warfarin to be effective. There are FDA‐approved dose reductions for DOACs which are based on pharmacologic studies, but they lack clinical patient data. In ESRD and dialysis patients, the preponderance of evidence suggests a lack of benefit to warfarin with an increased risk of bleeding. There is limited data on DOACs, but recent retrospective studies of apixaban support both its safety and efficacy. Further study is needed for ESRD and dialysis patients in the form of well designed, prospective, controlled studies to settle the questions regarding efficacy, and safety of both warfarin and apixaban.
Table 3.
Summary of evidence for warfarin and DOACs in AF by CKD stage
| CKD stage | Warfarin | DOACs |
|---|---|---|
| Mild to moderate Stages 2‐3 (eGFR 30‐90 mL/min/1.73 m2) |
Primarily observational data supporting use | High quality data support use, may be superior to warfarin |
| Severe Stage 4 (eGFR 15‐29 mL/min/1.73 m2) |
Limited data supports use | Pharmacologic studies allow for use with dose reductions, lack patient data |
| End stage renal disease Stage 5 (eGFR <15 mL/min/1.73 m2 or on hemodialysis) |
Majority of studies suggest lack of benefit and possible harm |
Dabigatran removed by dialysis Rivaroxaban has safe drug levels based on modeling, but lacks patient data Apixaban safe and effective based on modeling and retrospective data, prospective data needed |
Abbreviations: AF, atrial fibrillation; CKD, chronic kidney disease; DOAC, direct oral anticoagulant; eGFR, estimated glomerular filtration rate.
ACKNOWLEDGMENTS
The authors contributed to the literature review and preparation and writing of the manuscript.
Dr. Jonathan C. Hsu has received honoraria from Medtronic, Abbott, Boston Scientific, and Biotronik and research grants from Biosense‐Webster and Biotronik.
Bhatia HS, Hsu JC, Kim RJ. Atrial fibrillation and chronic kidney disease: A review of options for therapeutic anticoagulation to reduce thromboembolism risk. Clin Cardiol. 2018;41:1395–1402. 10.1002/clc.23085
REFERENCES
- 1. Marinigh R, Lane DA, Lip GYH. Severe renal impairment and stroke prevention in atrial fibrillation: implications for thromboprophylaxis and bleeding risk. J Am Coll Cardiol. 2011;57(12):1339‐1348. 10.1016/j.jacc.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 2. Winkelmayer WC, Liu J, Setoguchi S, Choudhry NK. Effectiveness and safety of warfarin initiation in older hemodialysis patients with incident atrial fibrillation. Clin J Am Soc Nephrol. 2011;6(11):2662‐2668. 10.2215/CJN.04550511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Providência R, Marijon E, Boveda S, et al. Meta‐analysis of the influence of chronic kidney disease on the risk of thromboembolism among patients with nonvalvular atrial fibrillation. Am J Cardiol. 2014;114(4):646‐653. 10.1016/j.amjcard.2014.05.048. [DOI] [PubMed] [Google Scholar]
- 4. Bonde AN, Lip GYH, Kamper A‐L, et al. Renal function and the risk of stroke and bleeding in patients with atrial fibrillation: an observational cohort study. Stroke. 2016;47(11):2707‐2713. 10.1161/STROKEAHA.116.014422. [DOI] [PubMed] [Google Scholar]
- 5. Mitsuma W, Matsubara T, Hatada K, et al. Clinical characteristics of hemodialysis patients with atrial fibrillation: the RAKUEN (registry of atrial fibrillation in chronic kidney disease under hemodialysis from Niigata) study. J Cardiol. 2016;68(2):148‐155. 10.1016/j.jjcc.2015.08.023. [DOI] [PubMed] [Google Scholar]
- 6. Kaw D, Malhotra D. Platelet dysfunction and end‐stage renal disease. Semin Dial. 2006;19(4):317‐322. 10.1111/j.1525-139X.2006.00179.x. [DOI] [PubMed] [Google Scholar]
- 7. Tveit DP, Hypolite IO, Hshieh P, et al. Chronic dialysis patients have high risk for pulmonary embolism. Am J Kidney Dis. 2002;39(5):1011‐1017. 10.1053/ajkd.2002.32774. [DOI] [PubMed] [Google Scholar]
- 8. Boccardo P, Remuzzi G, Galbusera M. Platelet dysfunction in renal failure. Semin Thromb Hemost. 2004;30(5):579‐589. 10.1055/s-2004-835678. [DOI] [PubMed] [Google Scholar]
- 9. January CT, Wann LS, Alpert JS, et al.; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):e1‐e76. 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 10. Stroke prevention in atrial fibrillation study. Final results. Circulation. 1991;84(2):527‐539. [DOI] [PubMed] [Google Scholar]
- 11. Warfarin versus aspirin for prevention of thromboembolism in atrial fibrillation: stroke prevention in atrial fibrillation II study. Lancet. 1994;343(8899):687‐691. [PubMed] [Google Scholar]
- 12. Adjusted‐dose warfarin versus low‐intensity, fixed‐dose warfarin plus aspirin for high‐risk patients with atrial fibrillation: stroke prevention in atrial fibrillation iII randomised clinical trial. Lancet. 1996;348(9028):633‐638. [PubMed] [Google Scholar]
- 13. Hart RG, Benavente O, McBride R, Pearce LA. Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta‐analysis. Ann Intern Med. 1999;131(7):492‐501. [DOI] [PubMed] [Google Scholar]
- 14. Hart RG, Pearce LA, Aguilar MI. Meta‐analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146(12):857‐867. 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- 15. Hart RG, Pearce LA, Asinger RW, Herzog CA. Warfarin in atrial fibrillation patients with moderate chronic kidney disease. Clin J Am Soc Nephrol. 2011;6(11):2599‐2604. 10.2215/CJN.02400311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893‐2962. 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 17. Verma A, Cairns JA, Mitchell LB, et al.; CCS Atrial Fibrillation Guidelines Committee. 2014 focused update of the Canadian cardiovascular society guidelines for the management of atrial fibrillation. Can J Cardiol. 2014;30(10):1114‐1130. 10.1016/j.cjca.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 18. Steinberg BA, Shrader P, Thomas L, et al.; ORBIT‐AF Investigators and Patients. Off‐label dosing of non‐vitamin K antagonist oral anticoagulants and adverse outcomes: the ORBIT‐AF II registry. J Am Coll Cardiol. 2016;68(24):2597‐2604. 10.1016/j.jacc.2016.09.966. [DOI] [PubMed] [Google Scholar]
- 19. Shroff GR, Stoecker R, Hart A. Non‐vitamin K‐dependent oral anticoagulants for nonvalvular atrial fibrillation in patients with CKD: pragmatic considerations for the clinician. Am J Kidney Dis. 2018. 10.1053/j.ajkd.2018.02.360. Epub 2018 May 2. [DOI] [PubMed] [Google Scholar]
- 20. Carrero JJ, Evans M, Szummer K, et al. Warfarin, kidney dysfunction, and outcomes following acute myocardial infarction in patients with atrial fibrillation. JAMA. 2014;311(9):919‐928. 10.1001/jama.2014.1334. [DOI] [PubMed] [Google Scholar]
- 21. Lai HM, Aronow WS, Kalen P, et al. Incidence of thromboembolic stroke and of major bleeding in patients with atrial fibrillation and chronic kidney disease treated with and without warfarin. Int J Nephrol Renovasc Dis. 2009;2:33‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jun M, James MT, Ma Z, et al.; Alberta Kidney Disease Network. Warfarin initiation, atrial fibrillation, and kidney function: comparative effectiveness and safety of warfarin in older adults with newly diagnosed atrial fibrillation. Am J Kidney Dis. 2017;69(6):734‐743. 10.1053/j.ajkd.2016.10.018. [DOI] [PubMed] [Google Scholar]
- 23. Dahal K, Kunwar S, Rijal J, Schulman P, Lee J. Stroke, major bleeding, and mortality outcomes in warfarin users with atrial fibrillation and chronic kidney disease: a meta‐analysis of observational studies. Chest. 2016;149(4):951‐959. 10.1378/chest.15-1719. [DOI] [PubMed] [Google Scholar]
- 24. Connolly SJ, Ezekowitz MD, Yusuf S, et al.; RE‐LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139‐1151. 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 25. Awesat J, Sagy I, Haviv YS, et al. Dabigatran‐induced nephropathy and its successful treatment with idarucizumab—case report and literature review. Thromb Res. 2018;169:120‐122. 10.1016/j.thromres.2018.07.019. [DOI] [PubMed] [Google Scholar]
- 26. Patel MR, Mahaffey KW, Garg J, et al.; the ROCKET AF Steering Committee. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883‐891. 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 27. Granger CB, Alexander JH, McMurray JJV, et al.; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981‐992. 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 28. Giugliano RP, Ruff CT, Braunwald E, et al.; ENGAGE AF‐TIMI 48 Investigators.Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093‐2104. 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 29. Hijazi Z, Hohnloser SH, Oldgren J, et al. Efficacy and safety of dabigatran compared with warfarin in relation to baseline renal function in patients with atrial fibrillation: a RE‐LY (randomized evaluation of long‐term anticoagulation therapy) trial analysis. Circulation. 2014;129(9):961‐970. 10.1161/CIRCULATIONAHA.113.003628. [DOI] [PubMed] [Google Scholar]
- 30. Fukaya H, Niwano S, Oikawa J, et al. Safety of low‐dose dabigatran in patients with atrial fibrillation and mild renal insufficiency. J Cardiol. 2017;69(3):591‐595. 10.1016/j.jjcc.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 31. Eikelboom JW, Connolly SJ, Gao P, et al. Stroke risk and efficacy of apixaban in atrial fibrillation patients with moderate chronic kidney disease. J Stroke Cerebrovasc Dis. 2012;21(6):429‐435. 10.1016/j.jstrokecerebrovasdis.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 32. Hohnloser SH, Hijazi Z, Thomas L, et al. Efficacy of apixaban when compared with warfarin in relation to renal function in patients with atrial fibrillation: insights from the ARISTOTLE trial. Eur Heart J. 2012;33(22):2821‐2830. 10.1093/eurheartj/ehs274. [DOI] [PubMed] [Google Scholar]
- 33. Hijazi Z, Hohnloser SH, Andersson U, et al. Efficacy and safety of apixaban compared with warfarin in patients with atrial fibrillation in relation to renal function over time: insights from the ARISTOTLE randomized clinical trial. JAMA Cardiol. 2016;1(4):451‐460. 10.1001/jamacardio.2016.1170. [DOI] [PubMed] [Google Scholar]
- 34. Bohula EA, Giugliano RP, Ruff CT, et al. Impact of renal function on outcomes with edoxaban in the ENGAGE AF‐TIMI 48 trial. Circulation. 2016;134(1):24‐36. 10.1161/CIRCULATIONAHA.116.022361. [DOI] [PubMed] [Google Scholar]
- 35. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta‐analysis of randomised trials. Lancet. 2014;383(9921):955‐962. 10.1016/S0140-6736(13)62343-0. [DOI] [PubMed] [Google Scholar]
- 36. Andò G, Capranzano P. Non‐vitamin K antagonist oral anticoagulants in atrial fibrillation patients with chronic kidney disease: a systematic review and network meta‐analysis. Int J Cardiol. 2017;231:162‐169. 10.1016/j.ijcard.2016.11.303. [DOI] [PubMed] [Google Scholar]
- 37. Lehr T, Haertter S, Liesenfeld K‐H, et al. Dabigatran etexilate in atrial fibrillation patients with severe renal impairment: dose identification using pharmacokinetic modeling and simulation. J Clin Pharmacol. 2012;52(9):1373‐1378. 10.1177/0091270011417716. [DOI] [PubMed] [Google Scholar]
- 38.Xarelto (rivaroxaban) [prescribing information]. Titusville, NJ: Janssen Pharmaceuticals Inc; August 2018. www.accessdata.fda.gov/drugsatfda_docs/label/2011/202439s001lbl.pdf.
- 39. Brancaccio D, Neri L, Bellocchio F, et al. Patients' characteristics affect the survival benefit of warfarin treatment for hemodialysis patients with atrial fibrillation. A historical cohort study. Am J Nephrol. 2016;44(4):258‐267. 10.1159/000448898. [DOI] [PubMed] [Google Scholar]
- 40. Olesen JB, Lip GYH, Kamper A‐L, et al. Stroke and bleeding in atrial fibrillation with chronic kidney disease. N Engl J Med. 2012;367(7):625‐635. 10.1056/NEJMoa1105594. [DOI] [PubMed] [Google Scholar]
- 41. Shah M, Avgil Tsadok M, Jackevicius CA, et al. Warfarin use and the risk for stroke and bleeding in patients with atrial fibrillation undergoing dialysis. Circulation. 2014;129(11):1196‐1203. 10.1161/CIRCULATIONAHA.113.004777. [DOI] [PubMed] [Google Scholar]
- 42. Shen JI, Montez‐Rath ME, Lenihan CR, Turakhia MP, Chang TI, Winkelmayer WC. Outcomes after warfarin initiation in a cohort of hemodialysis patients with newly diagnosed atrial fibrillation. Am J Kidney Dis. 2015;66(4):677‐688. 10.1053/j.ajkd.2015.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chan KE, Lazarus JM, Thadhani R, Hakim RM. Warfarin use associates with increased risk for stroke in hemodialysis patients with atrial fibrillation. J Am Soc Nephrol. 2009;20(10):2223‐2233. 10.1681/ASN.2009030319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tan J, Liu S, Segal JB, Alexander GC, McAdams‐DeMarco M. Warfarin use and stroke, bleeding and mortality risk in patients with end stage renal disease and atrial fibrillation: a systematic review and meta‐analysis. BMC Nephrol. 2016;17(1):157 10.1186/s12882-016-0368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nochaiwong S, Ruengorn C, Awiphan R, Dandecha P, Noppakun K, Phrommintikul A. Efficacy and safety of warfarin in dialysis patients with atrial fibrillation: a systematic review and meta‐analysis. Open Heart. 2016;3(1):e000441 10.1136/openhrt-2016-000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lee M, Saver JL, Hong K‐S, et al. Warfarin use and risk of stroke in patients with atrial fibrillation undergoing hemodialysis: a meta‐analysis. Medicine. 2016;95(6):e2741 10.1097/MD.0000000000002741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wizemann V, Tong L, Satayathum S, et al. Atrial fibrillation in hemodialysis patients: clinical features and associations with anticoagulant therapy. Kidney Int. 2010;77(12):1098‐1106. 10.1038/ki.2009.477. [DOI] [PubMed] [Google Scholar]
- 48. Garg L, Chen C, Haines DE. Atrial fibrillation and chronic kidney disease requiring hemodialysis—does warfarin therapy improve the risks of this lethal combination? Int J Cardiol. 2016;222:47‐50. 10.1016/j.ijcard.2016.07.118. [DOI] [PubMed] [Google Scholar]
- 49. Yodogawa K, Mii A, Fukui M, et al. Warfarin use and incidence of stroke in Japanese hemodialysis patients with atrial fibrillation. Heart Vessels. 2016;31(10):1676‐1680. 10.1007/s00380-015-0777-7. [DOI] [PubMed] [Google Scholar]
- 50. Yamashita Y, Takagi D, Hamatani Y, et al. Clinical characteristics and outcomes of dialysis patients with atrial fibrillation: the Fushimi AF registry. Heart Vessels. 2016;31(12):2025‐2034. 10.1007/s00380-016-0818-x. [DOI] [PubMed] [Google Scholar]
- 51. Wakasugi M, Kazama JJ, Tokumoto A, et al. Association between warfarin use and incidence of ischemic stroke in Japanese hemodialysis patients with chronic sustained atrial fibrillation: a prospective cohort study. Clin Exp Nephrol. 2014;18(4):662‐669. 10.1007/s10157-013-0885-6. [DOI] [PubMed] [Google Scholar]
- 52. Hayashi M, Abe T, Iwai M, et al.; Warfarin Study Group. Safety of warfarin therapy in chronic hemodialysis patients: a prospective cohort study. Clin Exp Nephrol. 2016;20(5):787‐794. 10.1007/s10157-015-1205-0. [DOI] [PubMed] [Google Scholar]
- 53. Yang F, Hellyer JA, Than C, et al. Warfarin utilisation and anticoagulation control in patients with atrial fibrillation and chronic kidney disease. Heart. 2017;103(11):818‐826. 10.1136/heartjnl-2016-309266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rocket AF, Study Investigators . Rivaroxaban‐once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation: rationale and design of the ROCKET AF study. Am Heart J. 2010;159(3):340‐347.e1. 10.1016/j.ahj.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 55. De Vriese AS, Caluwé R, Bailleul E, et al. Dose‐finding study of rivaroxaban in hemodialysis patients. Am J Kidney Dis. 2015;66(1):91‐98. 10.1053/j.ajkd.2015.01.022. [DOI] [PubMed] [Google Scholar]
- 56. Dias C, Moore KT, Murphy J, et al. Pharmacokinetics, pharmacodynamics, and safety of single‐dose rivaroxaban in chronic hemodialysis. Am J Nephrol. 2016;43(4):229‐236. 10.1159/000445328. [DOI] [PubMed] [Google Scholar]
- 57. Chan KE, Edelman ER, Wenger JB, Thadhani RI, Maddux FW. Dabigatran and rivaroxaban use in atrial fibrillation patients on hemodialysis. Circulation. 2015;131(11):972‐979. 10.1161/CIRCULATIONAHA.114.014113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chai‐Adisaksopha C, Hillis C, Lim W, Boonyawat K, Moffat K, Crowther M. Hemodialysis for the treatment of dabigatran‐associated bleeding: a case report and systematic review. J Thromb Haemost. 2015;13(10):1790‐1798. 10.1111/jth.13117. [DOI] [PubMed] [Google Scholar]
- 59. Wilson J‐AS, Goralski KB, Soroka SD, et al. An evaluation of oral dabigatran etexilate pharmacokinetics and pharmacodynamics in hemodialysis. J Clin Pharmacol. 2014;54(8):901‐909. 10.1002/jcph.335. [DOI] [PubMed] [Google Scholar]
- 60. Kalra PA, Burlacu A, Ferro CJ, Covic A. Which anticoagulants should be used for stroke prevention in non‐valvular atrial fibrillation and severe chronic kidney disease? Curr Opin Nephrol Hypertens. 2018;1 10.1097/MNH.0000000000000443. [DOI] [PubMed] [Google Scholar]
- 61. Wang X, Tirucherai G, Marbury TC, et al. Pharmacokinetics, pharmacodynamics, and safety of apixaban in subjects with end‐stage renal disease on hemodialysis. J Clin Pharmacol. 2016;56(5):628‐636. 10.1002/jcph.628. [DOI] [PubMed] [Google Scholar]
- 62. Kufel WD, Zayac AS, Lehmann DF, Miller CD. Clinical application and pharmacodynamic monitoring of apixaban in a patient with end‐stage renal disease requiring chronic hemodialysis. Pharmacotherapy. 2016;36(11):e166‐e171. 10.1002/phar.1836. [DOI] [PubMed] [Google Scholar]
- 63. Garza‐Mayers AC, Shah R, Sykes DB, Nigwekar SU, Kroshinsky D. The successful use of apixaban in dialysis patients with calciphylaxis who require anticoagulation: a retrospective analysis. Am J Nephrol. 2018;48(3):168‐171. 10.1159/000491881. [DOI] [PubMed] [Google Scholar]
- 64. Stanton BE, Barasch NS, Tellor KB. Comparison of the safety and effectiveness of apixaban versus warfarin in patients with severe renal impairment. Pharmacotherapy. 2017;37(4):412‐419. 10.1002/phar.1905. [DOI] [PubMed] [Google Scholar]
- 65. Reed D, Palkimas S, Hockman R, Abraham S, Le T, Maitland H. Safety and effectiveness of apixaban compared to warfarin in dialysis patients. Res Pract Thromb Haemost. 2018;2(2):291‐298. 10.1002/rth2.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Siontis KC, Zhang X, Eckard A, et al. Outcomes associated with apixaban use in end‐stage kidney disease patients with atrial fibrillation in the United States. Circulation. 2018;CIRCULATIONAHA.118.035418 10.1161/CIRCULATIONAHA.118.035418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Xue X, Jiang L, Duenninger E, et al. Impact of chronic kidney disease on Watchman implantation: experience with 300 consecutive left atrial appendage closures at a single center. Heart Vessels. 2018;33(9):1068‐1075. 10.1007/s00380-018-1157-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


