Summary
Aims
Autophagy has been regarded as a promising therapeutic target for spinal cord injury (SCI). Erythropoietin (EPO) has been demonstrated to exhibit neuroprotective effects in the central nervous system (CNS); however, the molecular mechanisms of its protection against SCI remain unknown. This study aims to investigate whether the neuroprotective effects of EPO on SCI are mediated by autophagy via AMP‐activated protein kinase (AMPK) signaling pathways.
Methods
Functional assessment and Nissl staining were used to investigate the effects of EPO on SCI. Expressions of proteins were detected by Western blot and immunohistochemistry.
Results
Treatment with EPO significantly reduced the loss of motor neurons and improved the functional recovery following SCI. Erythropoietin significantly enhanced the SCI‐induced autophagy through activating AMPK and inactivating mTOR signaling. The inhibitor of AMPK, compound C, could block the EPO‐induced autophagy and beneficial action on SCI, whereas the activator of AMPK, metformin, could mimic the effects of EPO. In the in vitro studies, EPO enhanced the hypoxia‐induced autophagy in an AMPK‐dependent manner.
Conclusions
The AMPK‐dependent induction of autophagy contributes to the neuroprotection of EPO on SCI.
Keywords: AMP‐activated protein kinase, autophagy, erythropoietin, spinal cord injury
1. INTRODUCTION
Spinal cord injury (SCI) is a disastrous insult of the central nervous system (CNS) with few restorative treatments1. The pathological course of SCI consists of primary and secondary injury2. In general, secondary injury which incorporates apoptosis, hypoxia, oxidative stress, inflammation, and glial scar formation is believed to evoke further damage following primary injury3, 4, 5. Although the therapeutic intervention against primary injury is inaccessible, secondary injury mechanisms could be manipulated, providing invaluable therapeutic targets for the cure of SCI2, 6, 7, 8. Autophagy is a catabolic process by which unwanted proteins and organelles are eliminated and recycled, playing an important role in intracellular homeostasis9, 10, 11. Recently, autophagy has been demonstrated to be an important therapeutic mechanism for injuries of CNS such as SCI and several CNS degeneration diseases such as Alzheimer's disease12, 13, 14. Accumulating evidence has shown that autophagy is induced after SCI, and the impairment of autophagy has been addressed to aggravate the secondary injury15, 16, 17, 18. The AMP‐activated protein kinase (AMPK) acts as an energy sensor responding to stress conditions such as oxidative stress and deprivation of nutrition, which also acts as a key player in autophagy19, 20, 21. The induction of autophagy via AMPK by several drugs such as metformin and resveratrol has recently been addressed to promote the functional recovery following SCI22, 23.
Erythropoietin has previously been reported to work in CNS in both physiological and pathological conditions24, 25, 26. Results from in vitro studies employing neurons or neuronal cell line have verified the EPO‐induced protection against a variety of insults such as neurotoxicity, serum withdrawal, and neurotrophic factor deprivation25, 27. Results from the models of cerebral ischemia, traumatic brain injury (TBI), and spinal cord ischemia suggest that EPO might exert protective action in these conditions26, 27, 28, 29. The possible mechanisms of the EPO's neuroprotection involve antiapoptosis27, 30, anti‐inflammatory28, 31, and antioxidation31, 32, yet the exact mechanisms remain unclear. The protective capacity of EPO has been previously shown to be dependent on AMPK in several conditions including neuroinflammation and oxidative stress33, 34. However, the roles of EPO in SCI, especially its action on AMPK signaling and autophagic activity, remain unknown.
This study examined the hypothesis that the neuroprotection of EPO on SCI may be mediated by autophagy via AMPK signaling pathways. We evaluated the effects of EPO on functional recovery after SCI. We found that EPO induced autophagy and activated AMPK activity in the injured spinal cords. Furthermore, we found that AMPK may be involved in the EPO‐induced autophagy and beneficial action on SCI.
2. MATERIALS AND METHODS
2.1. Animals
Adult male Sprague‐Dawley (SD) rats weighting 200‐220 g were purchased from Animal Center of the Chinese Academy of Sciences. All experiments were performed with reference to Guidelines for the Care and Use of Laboratory Animals from the National Institutes of Health and were approved by the Laboratory Animal Ethics Committee of Wenzhou Medical University (#WYDW‐2015‐0150).
2.2. Spinal cord injury
Spinal cord injury was made by the compression of a bulldog clamp35, 36. Briefly, rats were anesthetized by injecting pentobarbital sodium (50 mg/kg) intraperitoneally. A laminectomy from T7 to T10 was performed. Spinal cord was subjected to the compression of a bulldog clamp (30 g force; Oscar, China) for 1 minute. After surgery, the bladder was manually evacuated twice daily. Rats in sham group were subjected to laminectomy alone. Saline or EPO was intraperitoneally injected immediately and 24 hours after surgery. The doses of EPO (1000 U/kg, 2000 U/kg) were based on previous studies29, 37 and the instruction of manufacturers. To assess the role of the EPO‐induced modulation of AMPK in its beneficial action on SCI, the animals subjected to SCI were also treated intraperitoneally with EPO or metformin (50 mg/kg) plus compound C (20 mg/kg) immediately and 24 hours after surgery. The doses of compound C and metformin are based on previous studies22, 38, 39, 40, 41 and the instruction of manufacturers. Erythropoietin and metformin were purchased from Boyun Biotechnology (Shanghai, China). Compound C was sourced from Selleck Chemicals (Houston, TX, USA).
2.3. Locomotion recovery assessment
The Basso, Beattie, and Bresnahan (BBB) locomotor rating scale was used to evaluate the functional recovery at 1, 7, 14, and 28 days after surgery42. Three testers who were blinded to the grouping conducted these experiments, data from them averaged. The inclined plane test was utilized to objectively assess the function of hind limb43. In brief, rats were placed on an inclined plane whose angles were added at 5‐degree interval. The maximum angle where a rat could hold for 5 seconds was recorded.
2.4. Nissl staining
Spinal cord samples were collected at 28 days following surgery. The rats were transcardially perfused with 0.1 mol/L PBS followed by 4% paraformaldehyde (PFA) at 28 days after injury. The lesioned spinal cords (10 mm in length) were obtained and immersed in 4% PFA for 24 hours, transferred to 30% sucrose solution until they sank, and cut into 15‐μm‐thick transverse sections using a freezing microtome (Thermo, USA). Nissl staining was performed as previously described36. In brief, the sections were incubated with 0.1% cresyl violet for 5 minute at RT, rinsed in double distilled water followed by 95% ethanol, dehydrated in 100% ethanol and cleared in xylene, and covered by neutral resins. The sections were observed with a microscope (Nikon, Tokyo, Japan). The ventral motor neurons were counted with ImageJ software (Media Cybernetics, Bethesda, MD, USA)44.
2.5. Cell culture and hypoxia
PC12 cells were cultured in hypoxia condition as an in vitro spinal injury model. PC12 cells were purchased from Shanghai Institute of Cell Biology, Chinese Academy of Sciences. The cells were cultured in Dulbecco's modified Eagle's medium (DMEM, Gibco) containing 10% fetal bovine serum (FBS, Gibco) and 5% horse serum (Gibco). The cells were seeded at 2 × 105 cells/mL into 6‐, 24‐, or 96‐well plates and cultured under a humidified atmosphere (37°C,5% CO2) for 24 hours. The cells were then incubated for another 24 hours in an oxygen‐free incubator (Thermo Scientific, USA) with an atmosphere of 95% N2 and 5% CO2. Prior to hypoxia, experimental wells were treated with EPO (50 U/mL), or combined with compound C (5 μmol/L), or compound C (5 μmol/L) alone for 2 hours. Cell Counting Kit‐8 (CCK‐8; Beyotime, Beijing, China) was utilized to check the cell viability in accordance with the instructions of the manufacturer.
2.6. Autophagy flux monitoring
Cultures of PC 12 cells at about 80% confluence were incubated overnight with autophagy tandem sensor RFP‐GFP‐LC3B kit (Premo, Thermo Fisher, USA) according to the manufacturer's protocol. Cells were fixed with 4% PFA for 15 minute and visualized with a fluorescence microscope (Nikon, Tokyo, Japan). Representative photos were selected. The numbers of LC3 puncta per cell were counted with ImageJ44
2.7. Western blot analysis
The animals were sacrificed at 1, 3, and 7 days after surgery. PC12 cells were treated as indicated. Samples were homogenized in lysis buffer containing protease and phosphatase inhibitor cocktails. The lysate was incubated for 25 minute at 4°C, followed by 18‐min centrifuge at 15 000 g. Proteins were separated using SDS‐PAGE, electroblotted on a PVDF membrane (Millipore, MA, USA), block with 5% milk, and incubated overnight at 4°C with the following antibodies: anti‐LC3A/B (1:1000; Cell Signaling), anti‐Beclin 1 and SQSTM1/p62 (1:1000, Abcam), anti‐p‐AMPK and AMPK (1:1000; Cell Signaling), anti‐p‐mTOR and mTOR (1:1000; Cell Signaling), anti‐p‐p70S6K and p70S6K (1:1000; Cell Signaling), and anti‐β‐actin (1:1000, Sigma‐Aldrich). After being washed, incubated with appropriate secondary antibodies and the ECL kit (Thermo Scientific, USA), the protein bands were visualized with an imaging system (Bio‐Rad, CA, USA). The densities of bands were analyzed by ImageJ.
2.8. Double immunofluorescence staining
The animals were sacrificed at 3 days after surgery. After perfused transcardially with saline followed by 4% paraformaldehyde (PFA), the spinal cord samples were obtained and immersed in PFA for 24 hours, transferred to 30% sucrose in PBS until they sank. Spinal cords were cut into 20‐μm‐thick transverse sections with a freezing microtome. After treated with 0.3% Triton X‐100 for 20 minute, the sections were blocked with 5% BSA for 1 hours and incubated with anti‐LC3B (1:200, Cell Signaling) and anti‐NeuN (1:500, Abcam) for 24 hours at 4°C. After being rinses in PBS, the sections were incubated 2 hours with fluorescence‐conjugated secondary antibodies (Molecular Probes, Eugene, Oregon, USA). A fluorescence microscope (Nikon, Tokyo, Japan) was utilized to detect the fluorescence.
2.9. Statistical analysis
Data were presented as mean ± SD and were analyzed with SPSS software 17.0 (SPSS Inc., IL, USA). The results were analyzed by one‐way analysis of variance (ANOVA) followed by Bonferroni's multiple comparison test. A P value <.05 was set to be statistically significant.
3. RESULTS
3.1. Erythropoietin improves the functional recovery and decreases the loss of ventral motor neurons after SCI
To generate a traumatic spinal cord injury model, SD rats were subjected to compression injury on T9 spinal cords, and varying doses of EPO were administered to examine its therapeutic effects. Open‐field locomotion testing showed that administering EPO effectively improved the locomotion function at 14, 21, and 28 days after injury in a dose‐dependent manner, which was more remarkable for the 2000 U/kg dose compared with the 1000 U/kg dose (Figure 1B). To reconfirm the beneficial action of EPO on locomotion recovery following SCI, additional objective test was performed. As shown in Figure 1C, EPO at the 2000 U/kg dose significantly improved the angles of inclined plane at 28 days after SCI. To further examine whether the protective effects of EPO on SCI are due to decrease the loss of motor neurons, we performed the Nissl staining of spinal cord after SCI. At 28 days after SCI, the numbers of intact ventral motor neurons were significantly reduced; however, administering EPO significantly decreased the loss of neurons (Figure 1D,E). Collectively, these results suggest that treatment with EPO may improve the functional recovery and reduce the loss of ventral motor neurons following SCI in a dose‐dependent manner.
Figure 1.
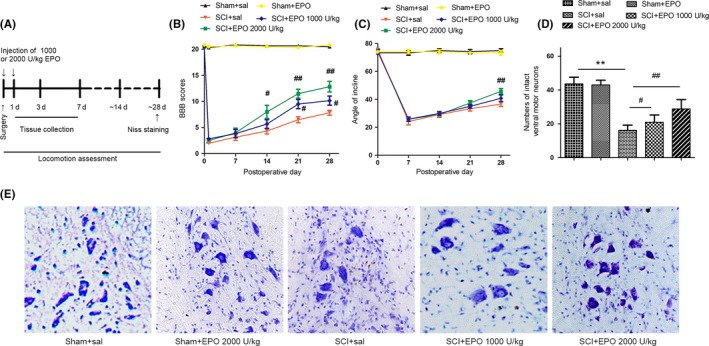
Erythropoietin (EPO) promotes the functional recovery following SCI. The animals were intraperitoneally injected with either EPO (1000 or 2000 U/kg) or saline immediately and 24 h after surgery. A, Protocol of the experiment. B, The BBB scores and (C) angles of inclined plane were evaluated in the sham + sal, sham + EPO, SCI + sal, SCI + EPO (1000 U/kg), SCI + EPO (2000 U/kg) groups. D, Representative photomicrographs of Nissl staining of ventral horn at 28 d after SCI. Magnifications: ×200. E, Histograms of the counts of intact ventral motor neurons. Data are mean ± SD, n = 5 per group. **P < .01 vs the sham group, ## P < .01, # P < .05 vs the SCI group
3.2. Erythropoietin enhances the SCI‐induced autophagy
As autophagy plays a critical role in the secondary injury mechanism of SCI15, 16, 17, 18, we examined whether the beneficial action of EPO on SCI was mediated by autophagy in our model system. Knowing that the conversion from LC3‐I to LC3‐II and expression of Beclin 1 are indispensable for the formation and expansion of autophagosomes10, we detected these autophagic markers at 1, 3, and 7 days after injury to examine the temporal pattern of autophagic activity. The levels of LC3‐II/LC3‐I ratio and Beclin 1 were significantly increased after SCI, peaked at 3 days after injury, and fell slightly at 7 days after injury (Figure S1A‐C).
Besides LC3 and Beclin 1, levels of autophagy substrates such as p62/SQSTM1 can also be used to monitor autophagic flux10, 16. Elevated levels of p62, together with higher LC3‐II/LC3‐I ratio, might indicate a decrease in autophagosomes degradation due to the impairment of autophagic flux16. We therefore checked the expression of p62 after SCI. The levels of p62 were also increased in a time‐dependent manner after SCI (Supplementary Figure 1A,D), with similar kinetics as LC3 and Beclin 1, indicating the impairments of autophagic flux. These results suggest SCI can induce autophagy in a time‐dependent manner in our model system.
To determine the effects of EPO on autophagic activity, we checked the expression pattern of autophagic markers at 3 days after SCI upon different doses of EPO. The levels of LC3‐II/LC3‐I ratio and Beclin 1 were significantly increased upon the treatment of EPO in a dose‐dependent manner (Figure 2A‐C). Conversely, the SCI‐induced accumulation of p62 was significantly decreased by EPO in a dose‐dependent manner (Figure 2A,D). To determine whether the autophagic activity happens in neurons after SCI, we have checked the immunoreactivity of LC3 and NeuN (a marker of neuron). Consistent with the results of Western blotting, at 3 days after SCI, LC3 signals were significantly enhanced in neurons, which were further enhanced upon EPO in a dose‐dependent manner (Figure 2E). Taken together, these results suggest that treatment with EPO may further enhance SCI‐induced autophagy in a dose‐dependent manner.
Figure 2.
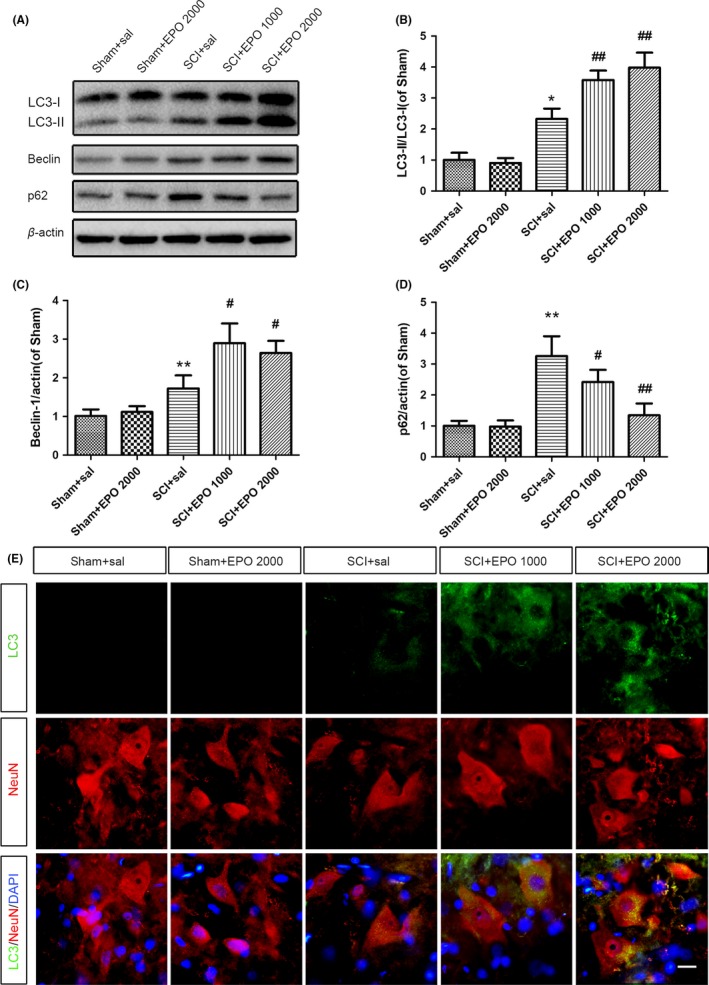
Erythropoietin (EPO) induces autophagy in the injured spinal cords. The animals were intraperitoneally injected with either EPO (1000 or 2000 U/kg) or saline immediately and 24 h after surgery. The spinal cord tissues were collected at 3 d after injury. A, The protein levels of LC3II/I, Beclin 1, and p62 were determined by Western blotting. β‐actin was used as the loading control. B‐D, Histograms of the relative expression of LC3II/I, Beclin 1, and p62. E, Representative immunofluorescent staining of LC3 (Green) and NeuN (Red). Scale bars are 5 μm. Data are mean ± SD, n = 5 per group. **P < .01, *P < .05 vs the sham + saline group, ## P < .01, # P < .05 vs the SCI + saline group
3.3. Erythropoietin activates AMPK and inactivates mTOR in the injured spinal cords
AMP‐activated protein kinase, a key modulator of energy metabolism, plays an important role in the induction of autophagy21, 45, 46, 47. The increased ratio of AMP/ATP in the condition of stress can promote the phosphorylation and activation of AMPK, but suppress the activation of mTOR and, thus, induces autophagy21, 45, 46, 47. To further examine whether EPO induces autophagy via AMPK signaling, we measured the expression of p‐AMPK and p‐mTOR after SCI. In the control groups, EPO did not significantly change the activity of AMPK and mTOR, whereas, in the SCI groups, p‐AMPK/AMPK ratio significantly rose in the spinal cords, and it was further increased under treatment with EPO (Figure 3A,C). P‐mTOR/mTOR ratio was also elevated by SCI, whereas, it tended to be decreased by EPO treatment (Figure 3A,B). To confirm mTOR activity, we have measured the level of p70S6K,the downstream signaling of mTOR. Similarly, p‐p70S6K/p70S6K ratio was significantly elevated after SCI,whereas, EPO significantly suppressed the SCI‐induced activation of p70S6K (Figure 3A,B). Taken together, these results suggest that EPO treatment may activate AMPK and inactivate mTOR in the injured spinal cords.
Figure 3.
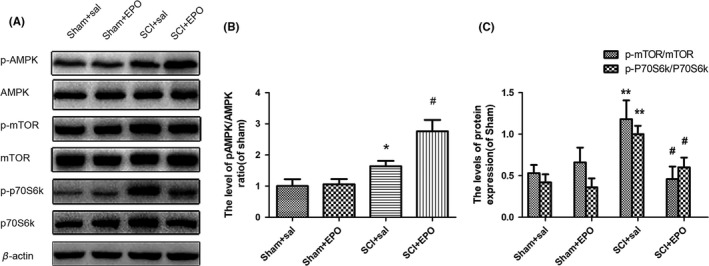
Erythropoietin (EPO) activates AMP‐activated protein kinase (AMPK) and inactivates mTOR in the injured spinal cords. The animals were intraperitoneally injected with either EPO (2000 U/kg) or saline immediately and 24 h after surgery. The spinal cord tissues were collected at 3 d after injury. A, The protein levels of p‐AMPK/AMPK, p‐mTOR/mTOR, and p‐p70S6K/p70S6K were determined by Western blotting. β‐actin was used as the loading control. (B‐D) Histograms of the relative expression of p‐AMPK/AMPK, p‐mTOR/mTOR, and p‐p70S6K/p70S6K. Data are mean ± SD, n = 5 per group. **P < .01, *P < .05 vs the sham + saline group, # P < .05 vs the SCI + saline group
3.4. Erythropoietin ‐induced autophagy is mediated by AMPK in the injured spinal cords
To assess the detailed roles of EPO‐induced activation of AMPK in the induction of autophagy after SCI, the effects of compound C (an AMPK inhibitor) and metformin (an AMPK activator) on autophagic activity were examined. Administrating compound C significantly neutralized EPO‐induced increase in p‐AMPK/AMPK and decrease in p‐mTOR/mTOR and p‐p70S6K/p70S6K ratio (Figure 4A‐C). Metformin could mimic the effects of EPO, significantly increase the p‐AMPK/AMPK ratio, and decrease the p‐mTOR/mTOR and p‐p70S6K/p70S6K ratio (Figure 4A‐C), reconfirming EPO‐induced activation of AMPK and inactivation of mTOR. More importantly, the EPO‐induced increase in LC3‐II/LC3‐I ratio and Beclin 1 and decrease in p62 were effectively blocked by compound C (Figure 4D‐F), and metformin could mimic the effects of EPO, significantly enhance the SCI‐induced increase in LC3‐II/LC3‐I ratio and Beclin 1, but decrease the level of p62, suggesting AMPK's critical role in the EPO‐induced autophagy following SCI. Taken together, these results suggest that EPO‐induced autophagy may be mediated by AMPK signaling after SCI.
Figure 4.
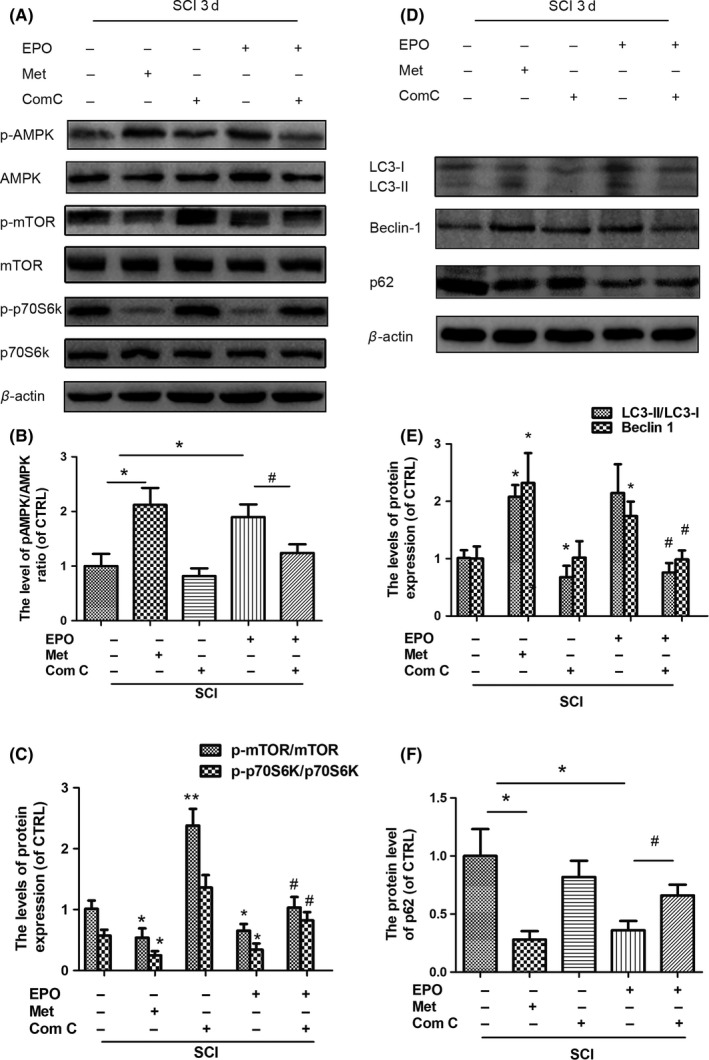
The Erythropoietin (EPO)‐induced autophagy is mediated by AMP‐activated protein kinase (AMPK) activation in the injured spinal cords. The animals subjected to SCI were intraperitoneally injected with EPO (2000 U/kg) or metformin (50 mg/kg) together with compound C (20 mg/kg) immediately and 24 h after surgery. The spinal cord tissues were collected at 3 d after injury. A‐C, The protein levels of p‐AMPK/AMPK, p‐mTOR/mTOR, and p‐p70S6K/p70S6K were determined by Western blotting. β‐actin was used as the loading control. B‐C, Histograms of the relative expression of p‐AMPK/AMPK, p‐mTOR/mTOR, and p‐p70S6K/p70S6K. D, The protein levels of LC3II/I, Beclin 1, and p62 were determined by Western blotting. β‐actin was used as the loading control. E‐F, Histograms of the relative expression of LC3‐II/I, Beclin 1, and p62. Data are mean ± SD, n = 5 per group. **P < .01, *P < .05 vs SCI + saline group, # P < .05 vs the SCI + EPO group
3.5. Inhibition of AMPK neutralizes the beneficial effects of EPO in rats after SCI
To further determine the role of the EPO‐induced activation of AMPK in its beneficial action on SCI, the effects of compound C and metformin on motor neurons and locomotion recovery were assessed. We found that treatment with compound C significantly blocked EPO‐induced improvement of BBB scores as well as angles of inclined plane (Figure 5A,B). Metformin could mimic the effects of EPO, significantly improve the BBB scores as well as angles of inclined plane (Figure 5A,B). The EPO‐induced increase in ventral motor neurons was also blocked by compound C (Figure 5C,D). Metformin could mimic the effects of EPO, decrease the loss of neurons after SCI (Figure 5C,D). Taken together, these data suggested that AMPK may play a critical role in EPO‐induced beneficial action on SCI.
Figure 5.
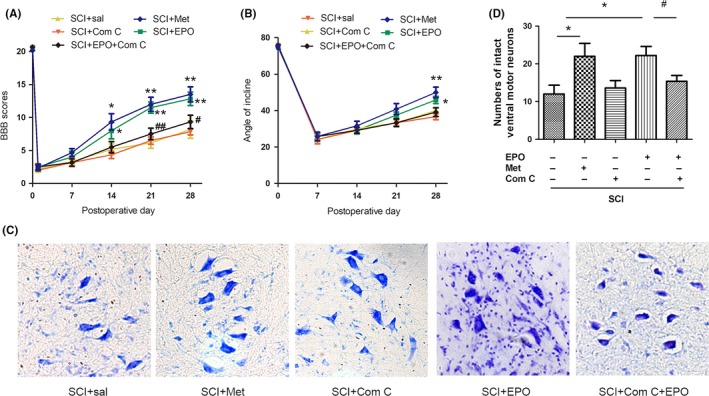
Inhibition of AMP‐activated protein kinase neutralizes the beneficial effects of Erythropoietin (EPO) in rats with SCI. The animals subjected to SCI were treated as indicated. A, The BBB scores and (B) angles of inclined plane were evaluated. C, Representative photomicrographs of Nissl staining of ventral horn at 28 d after SCI. Magnifications: ×200. D, Count of intact ventral motor neurons. Data are mean ± SD, n = 5 per group. **P < .01, *P < .05 vs SCI + saline group, ## P < .01, # P < .05 vs the SCI + EPO group
3.6. Erythropoietin enhances the hypoxia‐induced autophagy in an AMPK‐dependent manner in PC12 cells
To validate the role of AMPK‐dependent induction of autophagy in EPO's neuroprotection, we performed the in vitro study. PC12 cells (an extensively used neuronal cell line) were cultured in hypoxia condition as an in vitro neuronal injury model48. CCK8 assay showed that hypoxia significantly reduced the cell viability, whereas pretreatment with EPO for 2 hours prior to hypoxia attenuated this decrease of cell viability (Figure 6A). To further examine the protection of EPO on hypoxia‐induced cell death through promoting autophagy, the autophagic markers were detected. As shown in Figure 6B‐D, levels of LC3‐II/LC3‐I ratio, Beclin 1, and p62 were increased after hypoxia, whereas pretreatment with EPO for 2 hours prior to hypoxia further increased the LC3‐II/LC3‐I ratio and Beclin 1 but reduced the accumulation of p62. The inhibitor of AMPK, compound C, significantly blocked the effects of EPO on cell viability and autophagic activity (Figure 6A‐D), which suggests that AMPK‐dependent induction of autophagy by EPO.
Figure 6.
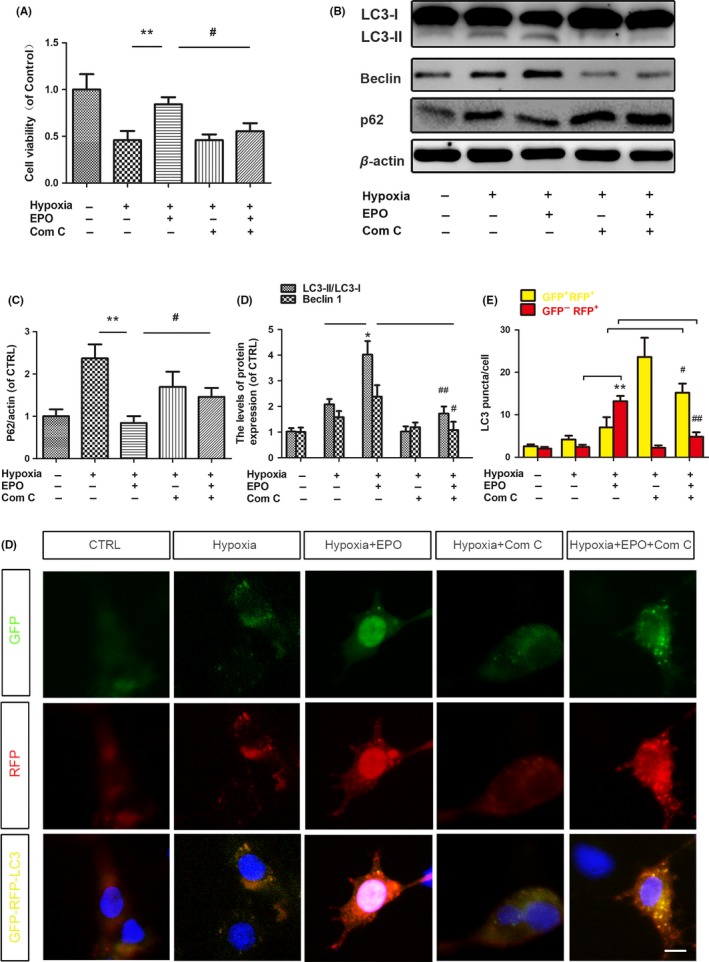
Erythropoietin (EPO) enhances the hypoxia‐induced autophagy in an AMP‐activated protein kinase‐dependent manner in PC12 cells. PC12 cells were cultured under a conventional atmosphere for 24 h followed by an oxygen‐free atmosphere of 95% N2 and 5% CO2. Prior to hypoxia, experimental wells were treated with EPO (50 U/mL), or combined with compound C (5 μmol/L), or compound C (5 μmol/L) alone for 2 h. A, The cell viability was determined by CCK8 assay. B,The protein levels of LC3II/I, Beclin 1, and p62 were determined by Western blotting. β‐actin was used as the loading control. C‐D, Histograms of the relative expression of LC3‐II/I, Beclin 1, and p62. E, Representative fluorescent images of PC12 cells incubated with tandem sensor RFP‐GFP‐LC3B kit and treated as indicated. F, The numbers of yellow puncta (autophagosomes) and red puncta (autolysosomes) in the merged images were counted. Data are mean ± SD, n = 4 per group. **P < .01, *P < .05 vs hypoxia group, # P < .05 vs the hypoxia + EPO group
To confirm autophagic flux, PC12 cells were monitored with tandem sensor RFP‐GFP‐LC3B kit and treated as indicated. As shown in Figure 6E, hypoxia induced many more autophagosomes (GFP+RFP+) in PC12 cells compared with control, although there was no statistical significance (Figure 6F). Administrating EPO dramatically increased the autolysosomes (GFP−RFP+) in PC12 cells; however, the compound C (an inhibitor of AMPK) effectively blocked the EPO‐induced formation of autolysosomes (Figure 6E‐F), suggesting that autophagic flux was induced by EPO via AMPK signaling. Taken together, these data suggested that EPO enhances the hypoxia‐induced autophagy in an AMPK‐dependent manner in PC12 cells.
4. DISCUSSION
In the present study, we validated EPO's beneficial effects on SCI and found that EPO may induce autophagy and activate AMPK in the injured spinal cords. And more importantly, we found that AMPK may be involved in the EPO‐induced autophagy and its beneficial effects on SCI. This study suggests that EPO may be a potential therapeutic drug for SCI targeting AMPK and autophagy.
Our results showed that either 1000 or 2000 U/kg EPO effectively improved the functional recovery following SCI, yet its therapeutic action was dose‐dependent. The reported effective doses of EPO varied from 500 U/kg to 5000 U/kg27, 29, 37, which were consistent with our results. In the present study, we further validated EPO's beneficial effect by Nissl staining and count of ventral motor neurons.
Increasing evidence indicates that autophagy plays critical roles in neuroprotection23, 49, 50. Previous studies employing spinal cord contusion injury model have shown that levels of LC3 and Beclin 1 were increased and peaked at 7 or 10 days after injury22, 51, while studies employing hemi‐section injury model showed that the increase in LC3 signals was detected as early as 4 hours and peaked at 3 days after hemi‐section injury52. The inconsistency of temporal pattern of autophagy following SCI could be prompted by the difference in SCI models or injury severity16, 53. Under our experimental condition, autophagic activity was significantly enhanced after SCI, peaked at 3 days, and fell slightly at 7 days after injury. A novel finding of our study is that treatment with EPO further promotes autophagic activity under SCI condition.
AMP‐activated protein kinase, which is a key regulator of energy metabolism, plays a critical role in the induction of autophagy21, 46. The increase in AMP/ATP ratio in the state of stress can promote the phosphorylation and activation of AMPK and suppress the activation the mTOR, thus induces autophagy19, 21, 45, 46. AMP‐activated protein kinase phosphorylation was increased after SCI under our experimental condition, yet mTOR activity was also increased. The identical between AMPK and mTOR activity may be caused by the distinction of antibodies or other unknown cause. A novel finding of the present study is that AMPK activity was upregulated by EPO under SCI condition, which could be mimicked by the positive control, metformin.
To determine the role of AMPK in EPO‐induced autophagy, we further examined the effect of the inhibition of AMPK. The EPO‐induced increase in LC3‐II/LC3‐I ratio and Beclin 1 and decrease in p62 were effectively diminished by compound C, suggestive of AMPK's involvement in the EPO‐induced autophagy. However, the effect of compound C on EPO‐induced autophagy appeared to be partial, suggesting other undetected mechanisms. The induction of autophagy via AMPK by several drugs including metformin22 and resveratrol23 has recently been addressed to promote the functional recovery following SCI. On the other side, EPO was reported to induce AMPK‐mediated therapeutic action in microglial cells and adipocytes33, 34. In our condition, inhibition of AMPK with compound C could neutralize the beneficial effects of EPO in rats with SCI. Our findings suggest that AMPK play at least partial role in EPO‐induced beneficial effects on SCI.
The present study had several limitations. To reconfirm the role of AMPK in EPO's action following SCI, experiments with AMPK shRNA or AMPK KO mice are needed to reconfirm our findings in the future54. Autophagy was assessed only by the ratio of LC3‐II/LC3‐I, levels of Beclin 1, and p62, added tools such as electron microscopic analysis are needed55. Also, as autophagy is a dynamic process, examining p62 only is not adequate to monitor autophagy flux because it is difficult to distinguish it happened on the process of autophagy formation or of autophagy degradation. Therefore, further methods such as tandem fluorescent‐tagged LC3 (mRFP‐EGFP‐LC3) can be used in the in vivo study55.
Overall, EPO has a therapeutic effect on SCI, at least in part, through the AMPK‐dependent induction of autophagy. These novel mechanisms of EPO's neuroprotection may provide invaluable therapeutic target for the exploitation of potential therapeutic strategies for SCI.
CONFLICT OF INTERESTS
The authors declare no conflict of interests.
Supporting information
ACKNOWLEDGMENTS
This study was supported by the Natural Science Foundation of Zhejiang Province (LR18C090001) and National Natural Science Foundation (81571190, 31671071, 81771348, 81371350, 81701202).
Wang P, Xie Z‐D, Xie C‐N, et al. AMP‐activated protein kinase‐dependent induction of autophagy by erythropoietin protects against spinal cord injury in rats. CNS Neurosci Ther. 2018;24:1185–1195. 10.1111/cns.12856
The first two authors contributed equally to this work.
Contributor Information
Zhi‐Hui Huang, Email: hzhzju021@163.com.
Hong‐Lin Teng, Email: honlinten@163.com.
REFERENCES
- 1. Silva NA, Sousa N, Reis RL, Salgado AJ. From basics to clinical: a comprehensive review on spinal cord injury. Prog Neurobiol. 2014;114:25‐57. [DOI] [PubMed] [Google Scholar]
- 2. Dumont RJ, Okonkwo DO, Verma S, et al. Acute spinal cord injury, part I: pathophysiologic mechanisms. Clin Neuropharmacol. 2001;24:254‐264. [DOI] [PubMed] [Google Scholar]
- 3. Penas C, Guzman MS, Verdu E, Fores J, Navarro X, Casas C. Spinal cord injury induces endoplasmic reticulum stress with different cell‐type dependent response. J Neurochem. 2007;102:1242‐1255. [DOI] [PubMed] [Google Scholar]
- 4. Sirbulescu RF, Zupanc GK. Inhibition of caspase‐3‐mediated apoptosis improves spinal cord repair in a regeneration‐competent vertebrate system. Neuroscience. 2010;171:599‐612. [DOI] [PubMed] [Google Scholar]
- 5. Tator CH, Fehlings MG. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg. 1991;75:15‐26. [DOI] [PubMed] [Google Scholar]
- 6. Fehlings MG, Nguyen DH. Immunoglobulin G: a potential treatment to attenuate neuroinflammation following spinal cord injury. J Clin Immunol. 2010;30(Suppl 1):S109‐S112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sekhon LH, Fehlings MG. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine (Phila Pa 1976). 2001;26:S2‐S12. [DOI] [PubMed] [Google Scholar]
- 8. Zhang HY, Zhang X, Wang ZG, et al. Exogenous basic fibroblast growth factor inhibits ER stress‐induced apoptosis and improves recovery from spinal cord injury. CNS Neurosci Ther. 2013;19:20‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kanno H, Ozawa H, Sekiguchi A, Itoi E. The role of autophagy in spinal cord injury. Autophagy. 2009;5:390‐392. [DOI] [PubMed] [Google Scholar]
- 10. Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290:1717‐1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang Y, Song H, Guo T, et al. Overexpression of annexin II receptor‐induced autophagy protects against apoptosis in uveal melanoma cells. Cancer Biother Radiopharm. 2016;31:145‐151. [DOI] [PubMed] [Google Scholar]
- 12. Huang J, Klionsky DJ. Autophagy and human disease. Cell Cycle. 2007;6:1837‐1849. [DOI] [PubMed] [Google Scholar]
- 13. Shintani T, Klionsky DJ. Autophagy in health and disease: a double‐edged sword. Science. 2004;306:990‐995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang P, Miao CY. Autophagy in the disorders of central nervous system: vital and/or fatal? CNS Neurosci Ther. 2012;18:955‐956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Park Y, Liu C, Luo T, Dietrich WD, Bramlett H, Hu B. Chaperone‐mediated autophagy after traumatic brain injury. J Neurotrauma. 2015;32:1449‐1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bisicchia E, Latini L, Cavallucci V, et al. Autophagy inhibition favors survival of rubrospinal neurons after spinal cord hemisection. Mol Neurobiol. 2017;54:4896‐4907. [DOI] [PubMed] [Google Scholar]
- 17. Kanno H, Ozawa H, Sekiguchi A, Itoi E. Spinal cord injury induces upregulation of Beclin 1 and promotes autophagic cell death. Neurobiol Dis. 2009;33:143‐148. [DOI] [PubMed] [Google Scholar]
- 18. Duan XC, Wang W, Feng DX, et al. Roles of autophagy and endoplasmic reticulum stress in intracerebral hemorrhage‐induced secondary brain injury in rats. CNS Neurosci Ther. 2017;23:554‐566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Egan DF, Shackelford DB, Mihaylova MM, et al. Phosphorylation of ULK1 (hATG1) by AMP‐activated protein kinase connects energy sensing to mitophagy. Science. 2011;331:456‐461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mao K, Klionsky DJ. AMPK activates autophagy by phosphorylating ULK1. Circ Res. 2011;108:787‐788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang D, Xuan J, Zheng BB, et al. Metformin improves functional recovery after spinal cord injury via autophagy flux stimulation. Mol Neurobiol. 2017;54:3327‐3341. [DOI] [PubMed] [Google Scholar]
- 23. Zhao H, Chen S, Gao K, et al. Resveratrol protects against spinal cord injury by activating autophagy and inhibiting apoptosis mediated by the SIRT1/AMPK signaling pathway. Neuroscience. 2017;348:241‐251. [DOI] [PubMed] [Google Scholar]
- 24. Dame C, Juul SE, Christensen RD. The biology of erythropoietin in the central nervous system and its neurotrophic and neuroprotective potential. Biol Neonate. 2001;79:228‐235. [DOI] [PubMed] [Google Scholar]
- 25. Morishita E, Masuda S, Nagao M, Yasuda Y, Sasaki R. Erythropoietin receptor is expressed in rat hippocampal and cerebral cortical neurons, and erythropoietin prevents in vitro glutamate‐induced neuronal death. Neuroscience. 1997;76:105‐116. [DOI] [PubMed] [Google Scholar]
- 26. Sakanaka M, Wen TC, Matsuda S, et al. In vivo evidence that erythropoietin protects neurons from ischemic damage. Proc Natl Acad Sci U S A. 1998;95:4635‐4640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Siren AL, Fratelli M, Brines M, et al. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc Natl Acad Sci U S A. 2001;98:4044‐4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brines ML, Ghezzi P, Keenan S, et al. Erythropoietin crosses the blood‐brain barrier to protect against experimental brain injury. Proc Natl Acad Sci U S A. 2000;97:10526‐10531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Celik M, Gokmen N, Erbayraktar S, et al. Erythropoietin prevents motor neuron apoptosis and neurologic disability in experimental spinal cord ischemic injury. Proc Natl Acad Sci U S A. 2002;99:2258‐2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Digicaylioglu M, Lipton SA. Erythropoietin‐mediated neuroprotection involves cross‐talk between Jak2 and NF‐kappaB signalling cascades. Nature. 2001;412:641‐647. [DOI] [PubMed] [Google Scholar]
- 31. Sela S, Shurtz‐Swirski R, Sharon R, et al. The polymorphonuclear leukocyte–a new target for erythropoietin. Nephron. 2001;88:205‐210. [DOI] [PubMed] [Google Scholar]
- 32. Kristal B, Shurtz‐Swirski R, Shasha SM, et al. Interaction between erythropoietin and peripheral polymorphonuclear leukocytes in hemodialysis patients. Nephron. 1999;81:406‐413. [DOI] [PubMed] [Google Scholar]
- 33. Wang L, Di L, Noguchi CT. AMPK is involved in mediation of erythropoietin influence on metabolic activity and reactive oxygen species production in white adipocytes. Int J Biochem Cell Biol. 2014;54:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tsai CF, Kuo YH, Yeh WL, et al. Regulatory effects of caffeic acid phenethyl ester on neuroinflammation in microglial cells. Int J Mol Sci. 2015;16:5572‐5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rivlin AS, Tator CH. Effect of duration of acute spinal cord compression in a new acute cord injury model in the rat. Surg Neurol. 1978;10:38‐43. [PubMed] [Google Scholar]
- 36. Wang P, Lin C, Wu S, et al. Inhibition of Autophagy is Involved in the Protective Effects of Ginsenoside Rb1 on Spinal Cord Injury. Cell Mol Neurobiol. 2017;38:679‐690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gorio A, Madaschi L, Di Stefano B, et al. Methylprednisolone neutralizes the beneficial effects of erythropoietin in experimental spinal cord injury. Proc Natl Acad Sci U S A. 2005;102:16379‐16384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kim YM, Kim MY, Kim HJ, et al. Compound C independent of AMPK inhibits ICAM‐1 and VCAM‐1 expression in inflammatory stimulants‐activated endothelial cells in vitro and in vivo. Atherosclerosis. 2011;219:57‐64. [DOI] [PubMed] [Google Scholar]
- 39. Yu PB, Hong CC, Sachidanandan C, et al. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol. 2008;4:33‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li J, Zeng Z, Viollet B, Ronnett GV, McCullough LD. Neuroprotective effects of adenosine monophosphate‐activated protein kinase inhibition and gene deletion in stroke. Stroke. 2007;38:2992‐2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhou G, Myers R, Li Y, et al. Role of AMP‐activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167‐1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1‐21. [DOI] [PubMed] [Google Scholar]
- 43. Rivlin AS, Tator CH. Objective clinical assessment of motor function after experimental spinal cord injury in the rat. J Neurosurg. 1977;47:577‐581. [DOI] [PubMed] [Google Scholar]
- 44. Jensen EC. Quantitative analysis of histological staining and fluorescence using ImageJ. Anat Rec (Hoboken). 2013;296:378‐381. [DOI] [PubMed] [Google Scholar]
- 45. Hu G, McQuiston T, Bernard A, et al. TOR‐dependent post‐transcriptional regulation of autophagy. Autophagy. 2015;11:2390‐2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Xu Z, Klionsky DJ. The AMPK‐SKP2‐CARM1 axis links nutrient sensing to transcriptional and epigenetic regulation of autophagy. Ann Transl Med. 2016;4:S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhao J, Dong JN, Wang HG, et al. Docosahexaenoic acid attenuated experimental chronic colitis in interleukin 10‐deficient mice by enhancing autophagy through inhibition of the mTOR pathway. JPEN J Parenter Enteral Nutr. 2017;41:824‐829. [DOI] [PubMed] [Google Scholar]
- 48. Macks C, Gwak SJ, Lynn M, Lee JS. Rolipram‐loaded polymeric micelle nanoparticle reduces secondary injury after rat compression spinal cord injury. J Neurotrauma. 2017;35:582‐592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fang B, Li XQ, Bao NR, et al. Role of autophagy in the bimodal stage after spinal cord ischemia reperfusion injury in rats. Neuroscience. 2016;328:107‐116. [DOI] [PubMed] [Google Scholar]
- 50. Smith CM, Chen Y, Sullivan ML, Kochanek PM, Clark RS. Autophagy in acute brain injury: feast, famine, or folly? Neurobiol Dis. 2011;43:52‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chen HC, Hsu PW, Tzaan WC, Lee AW. Effects of the combined administration of vitamins C and E on the oxidative stress status and programmed cell death pathways after experimental spinal cord injury. Spinal Cord. 2014;52:24‐28. [DOI] [PubMed] [Google Scholar]
- 52. Kanno H, Ozawa H, Sekiguchi A, Yamaya S, Itoi E. Induction of autophagy and autophagic cell death in damaged neural tissue after acute spinal cord injury in mice. Spine (Phila Pa 1976). 2011;36:E1427‐E1434. [DOI] [PubMed] [Google Scholar]
- 53. Zhou K, Sansur CA, Xu H, Jia X. The temporal pattern, flux, and function of autophagy in spinal cord injury. Int J Mol Sci. 2017;18:466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sohn M, Kim K, Uddin MJ, et al. Delayed treatment with fenofibrate protects against high‐fat diet‐induced kidney injury in mice: the possible role of AMPK autophagy. Am J Physiol Renal Physiol. 2017;312:F323‐F334. [DOI] [PubMed] [Google Scholar]
- 55. Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140:313‐326. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


