Abstract
Type 2 diabetes mellitus (T2DM) is a major independent risk factor for cardiovascular disease, and diabetic dyslipidemia is a major contributor to cardiovascular risk in these patients. Here we report the rationale and design of a phase 3, double‐blind study specifically designed to evaluate the lipid‐lowering efficacy of the proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor evolocumab in patients with T2DM and hyperlipidemia or mixed dyslipidemia who are on background statin therapy. In the BERSON (evolocumaB Efficacy for LDL‐C Reduction in subjectS with T2DM On background statiN) trial, patients with T2DM, a screening low‐density lipoprotein cholesterol (LDL‐C) level of ≥ 2.6 mmol/L (≥100 mg/dL) or ≥ 3.4 mmol/L (≥130 mg/dL), and with or without statin treatment at screening, respectively, were enrolled and started on atorvastatin 20 mg/day for a lipid stabilization period of at least 4 weeks. Then, patients were randomly assigned in a 2:2:1:1 ratio to receive atorvastatin 20 mg once daily plus either evolocumab 140 mg every 2 weeks (Q2W), evolocumab 420 mg every month (QM), placebo Q2W, or placebo QM. The co‐primary outcome measures were the percentage change from baseline in LDL‐C at week 12 and the percentage change from baseline in LDL‐C at the mean of weeks 10 and 12. The BERSON trial has completed enrollment. The study completed in the first half of 2018, and will provide information on the efficacy and safety of evolocumab in patients with T2DM and dyslipidemia.
Keywords: diabetes, diabetic dyslipidemia, dyslipidemia, hypercholesterolemia, monoclonal antibody, PCSK9, PCSK9 inhibitor
1. INTRODUCTION
When compared with healthy individuals, patients with diabetes mellitus (T2DM) have a two or three times higher rate of comorbid cardiovascular disease (CVD),1, 2 the latter of which is a major cause of mortality. While the risk of cardiovascular (CV) events is high in patients with atherosclerotic cardiovascular disease (ASCVD) and comorbid T2DM,3 CV event risk in patients with T2DM remains elevated even in those without established CVD.4 The global burden of ASCVD associated with diabetes is also of concern in China, where the overall prevalence of diabetes is 11%5 and the magnitude of increased risk of major CVD is similar to that observed in Western populations.6
In patients with T2DM, mortality due to coronary heart disease increases exponentially as a function of LDL cholesterol (LDL‐C) level.4 Because elevated levels of LDL‐C are associated with an increased risk of ASCVD, and LDL‐C is known to be a strong independent predictor of heart disease, an even modest increase in LDL‐C of 0.26 mmol/L (10 mg/dL) in patients with T2DM can increase a patient's CV risk by 12%.7
Lowering LDL‐C with statin therapy significantly reduces CV events in patients with diabetes8, 9, 10, 11 and the addition of ezetimibe provides additional, modest reductions in the incidences of myocardial infarction and stroke in patients with T2DM and previous acute coronary syndrome.9 For this reason, various guidelines recommend aggressive LDL‐C lowering through a moderate‐ or high‐intensity statin regimen.12 Despite the substantive lipid lowering achieved with statin therapy, many patients with diabetes continue to have high residual lipid risk as well as profound lipid profile abnormalities.13
Proprotein convertase subtilisin/kexin type 9 (PCSK9) reduces the recycling of LDL receptors by binding to the receptor along with LDL and targeting the receptor for lysosomal degradation.14 Evolocumab is a human immunoglobulin G subclass 2 monoclonal antibody to PCSK9 that blocks circulating PCSK9 from binding to the LDL receptor. This, in turn, increases the number of LDL receptors available in the liver cell surface, which facilitates the clearance of LDL‐C from plasma and results in lower levels of LDL‐C. In phase 2 and 3 studies, evolocumab consistently reduced LDL‐C levels across diverse patient populations, including patients with familial hypercholesterolemia, those with a range of CV risk, those with statin intolerance, and those on various background therapies including diet (monotherapy), a range of statins at various doses, and statins with other lipid‐lowering therapies.15, 16, 17, 18, 19, 20 Evolocumab also reduced ApoB, non‐HDL cholesterol (non‐HDL‐C), VLDL cholesterol (VLDL‐C), triglycerides, and lipoprotein (a) [Lp(a)], and increased HDL‐C and apolipoprotein A1. Improvements in these lipid parameters in 12‐week studies were consistent with those observed following longer‐term use of evolocumab.15, 19
Evolocumab is indicated as an adjunct to diet and maximally tolerated statin in adults with primary hypercholesterolemia or mixed dyslipidemia who require additional lowering of LDL‐C. Evolocumab is also indicated in adults and adolescents aged 12 years and over for the treatment of homozygous familial hypercholesterolemia. Recently, evolocumab has also been approved for the prevention of CV events (myocardial infarction, stroke, and coronary revascularization) in adults with established CVD.21 In a prespecified subanalysis from a large CV outcomes study,22 as well as post‐hoc analyses of data from phase 3 studies,23, 24 evolocumab significantly reduced LDL‐C levels by approximately 60% in patients with diabetes. Reductions in LDL‐C were consistent in patients with or without diabetes, were sustained over longer‐term use, and were not associated with adverse effects on glycemic control.15, 22, 23, 24 Additionally, there was no observed increase in the risk of new‐onset diabetes in patients without T2DM at baseline.22, 24, 25 These data are consistent with those reported for patients treated with another PCSK9 inhibitor, alirocumab, strongly suggesting that monoclonal antibodies against PCSK9 improve lipid profiles and likely have no adverse effects on measures of glycemic control and do not cause new‐onset diabetes in patients without diabetes.26, 27, 28 Here, we present the study design and rationale for the BERSON study, which is one of the two dedicated clinical trials specifically designed to evaluate the efficacy and safety of 12 weeks of evolocumab in patients with T2DM. BERSON was recently conducted in a global population that included half of its patients from China, due to both the global burden of diabetes and the high prevalence of diabetes in China.
2. METHODS
2.1. Study design
BERSON was a 12‐week, phase 3, randomized, double‐blind, placebo‐controlled, multicenter study in patients with T2DM and hyperlipidemia or mixed dyslipidemia, designed to evaluate if evolocumab plus background atorvastatin therapy is effective, safe, and well tolerated (ClinicalTrials.gov identifier: NCT02662569). The study was conducted in 10 countries (Argentina, Brazil, Canada, China, Colombia, France, South Korea, Russia, Turkey, and the United States; Supplemental Appendix).
2.2. Study hypothesis and objectives
The primary hypothesis of the study is that both dosing regimens of evolocumab (140 mg Q2W and 420 mg QM) in combination with atorvastatin will be well tolerated and will result in greater reduction of LDL‐C, defined as mean percent change from baseline at weeks 10 and 12 and percent change from baseline at week 12, compared with placebo (Q2W and QM), in combination with atorvastatin in diabetic patients with hyperlipidemia or mixed dyslipidemia. The primary objective of the study was to evaluate the efficacy (vs placebo) of 12 weeks of subcutaneous (SC) evolocumab administered every 2 weeks (Q2W) or every month (QM) when used in combination with 20 mg/day of atorvastatin therapy on the percentage change from baseline in LDL‐C level.
2.3. Study endpoints
Endpoints are listed in Table 1. The co‐primary endpoints of the study were the percentage change from baseline in LDL‐C at week 12 and the percentage change from baseline in LDL‐C at the mean of weeks 10 and 12. Secondary endpoints included but were not limited to the change from baseline in LDL‐C and the percentage change from baseline in non‐HDL‐C, ApoB, total cholesterol, triglycerides, HDL‐C, and VLDL‐C. Safety endpoints included patient incidence of treatment‐emergent adverse events, laboratory values, and the incidence of antidrug antibodies (binding and neutralizing). Measures of glycemic control—fasting plasma glucose and hemoglobin A1c—were also assessed at baseline and week 12 (Supplemental Appendix).
Table 1.
Key endpoints
Co‐primary
Secondary
Safety
|
Abbreviations: ApoB, apolipoprotein B; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; VLDL‐C, very low‐density lipoprotein cholesterol.
2.4. Study ethics
All patients were required to provide written informed consent prior to screening and administration of investigational product (IP). An independent review board or independent ethics committee at each study site reviewed the study and approved the protocol and the subsequent amendments to the study protocol. An external, independent data monitoring committee (DMC) periodically reviewed study data, and analyses for the DMC were provided by an independent biostatistical group.
2.5. Study population
Eligible patients were aged 18 to 80 years, had a diagnosis of T2DM for at least 6 months prior to screening, were on stable diabetes therapy prior to randomization, and had a diagnosis of hyperlipidemia or mixed dyslipidemia. Patients receiving statin therapy at screening were required to have an LDL‐C of ≥2.6 mmol/L (≥ 100 mg/dL), and patients not on background statin therapy at screening were required to have an LDL‐C of ≥3.4 mmol/L (≥130 mg/dL). Type 2 diabetes was defined as a patient receiving pharmacologic treatment for type 2 diabetes for ≥6 months prior to screening, with stable diabetes therapy prior to randomization to IP and not expected to change during the duration of study participation. Stable diabetes therapy was defined as no new agents added, no dose change of any oral antihyperglycemic drug within 2 months, and daily insulin dose not changed by >25% and > 25 units within 1 month prior to randomization. Patients were also required to have fasting triglycerides ≤4.5 mmol/L (≤ 400 mg/dL). Patients were excluded from the study if they had medical contraindications to receiving 20 mg atorvastatin, if they had type 1 diabetes or poorly controlled T2DM, a history of myocardial infarction, unstable angina, percutaneous coronary intervention, coronary artery bypass graft, or stroke in the past 6 months. Full exclusion criteria are provided in Table 2.
Table 2.
Major exclusion criteria
|
Abbreviations: PCSK9, proprotein convertase subtilisin/kexin type 9; QD, once daily; ULN, upper limit of normal.
Poorly controlled T2DM defined as: HbA1c > 10.0% at screening and at lipid stabilization or not on stable pharmacologic therapy for type 2 diabetes. Stable therapy was defined as no new agents added, no dose change of any oral antihyperglycemic drug within 2 months, and daily insulin dose not changed by >25% and >25 units within 1 month prior to randomization.
2.6. Study procedures
This study was designed with a duration of 12 weeks because previous phase 2 and 3 studies of evolocumab demonstrated that 12 weeks is sufficient to measure primary and secondary endpoints at peak pharmacodynamic effect. In addition, most pivotal LDL‐C‐lowering studies fall within an 8‐ to 16‐week study length; thus, there is regulatory precedent for a 12‐week study duration.16, 17, 18, 19, 29, 30
Patients first underwent screening procedures, including laboratory tests and the administration of a single dose of placebo using an auto‐injector device (AI/Pen). Eligible patients were then enrolled and underwent a lipid stabilization period of up to 8 weeks with atorvastatin 20 mg/day orally, followed by a 2:2:1:1 randomization to IP (evolocumab 140 mg SC Q2W or 420 mg QM, or placebo SC Q2W or QM). Atorvastatin was selected as the background statin therapy for this study in part because clinical outcomes data are available for this drug in patients with diabetes.8 Half of the patients in this study were recruited from study sites in China. A dose of 20 mg/day atorvastatin was selected based on standard medical practice in China, the 2007 Chinese dyslipidemia guidelines,31 and the expected LDL‐C response with atorvastatin 20 mg/day in the Chinese population.32 This dose was used at all study sites to maintain consistency among study participants in all regions. After completion of the lipid stabilization period, patients were then randomized to IP (Figure 1). Each study arm continued receiving atorvastatin until the end of the study. Randomization to 1 of 4 treatment arms was based on a computer‐generated randomization schedule. For the duration of the study, investigators, site staff, and patients remained blinded to treatment assignments. The study had a planned enrollment of 900 patients. Of these, approximately 300 were to be randomized to each evolocumab plus atorvastatin dosing regimen, and approximately 150 to each placebo plus atorvastatin dosing regimen.
Figure 1.
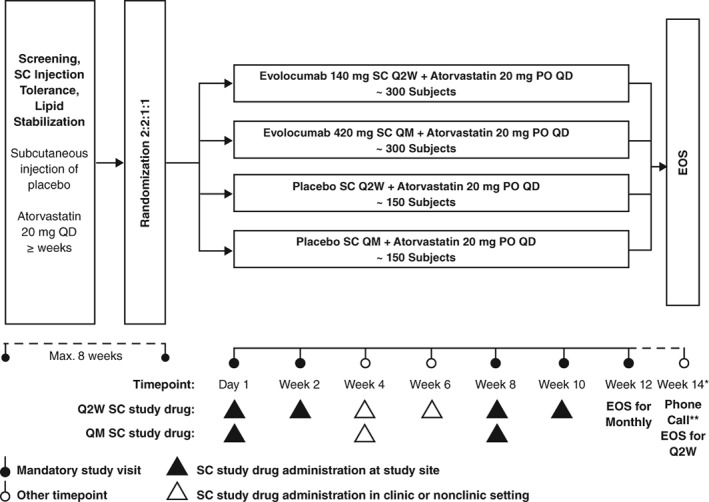
Treatment schema. Patients who met the inclusion criteria and completed the lipid stabilization period were assigned to one of four treatment arms. Abbreviations: EOS, end of study; PO, orally; Q2W, every 2 weeks; QD, once daily; QM, every month; SC, subcutaneous
2.7. Data collection
Fasting lipid levels (total cholesterol, LDL‐C, triglycerides, VLDL‐C, non‐HDL‐C, and HDL‐C) were measured at screening, at the end of lipid stabilization, and at study visits on day 1, week 2, week 8, week 10, and week 12. ApoB was measured at day 1, week 10, and week 12.
Central laboratory results of the lipid panel and lipoproteins remained blinded until unblinding of the clinical database occurred, and were not reported to the investigator post‐screening. Investigators were not permitted to perform nonprotocol testing of study analytes during a patient's study participation and until at least 12 weeks after last IP administration or the patient's end of study, whichever was later. Adverse device events, adverse events, and serious adverse events were collected at every study visit. Final administration of IP was at week 10 for Q2W treatment and week 8 for QM treatment. End of study for Q2W patients was at week 14 and for QM patients was at week 12.
2.8. Statistical design and analysis
A sample size of 900 was planned to provide ≥96% power to detect at least a 30% reduction in the LDL‐C level in both evolocumab groups compared with placebo, assuming a common SD of 30%, after accounting for an estimated 15% treatment attenuation (patients not completing study), and assuming that 2% of the patients would not receive any study drug.
Efficacy and safety analyses will include all patients randomized to treatment and receiving at least one dose of study drug. Repeated‐measures linear mixed‐effects models will be used to assess the co‐primary endpoint of percentage change from baseline in LDL‐C at week 12 and the mean of week 10 and week 12, as well as the secondary efficacy endpoints. Models will include terms for treatment group, stratification factors (statin therapy at study entry [none vs non‐intensive vs intensive], geographic region [China, Korea, other countries]), scheduled visit, and the interaction of treatment with the scheduled visit. Analyses for the secondary endpoints will be adjusted for multiplicity to preserve the family‐wise error rate at 0.05. Baseline covariates for this study include, but are not limited to, statin therapy at study entry, age, sex, race, and the site's geographic region.
All lipids were assayed in serum samples by Medpace (Cincinnati, Ohio; and Leuven, Belgium). Serum glucose and hemoglobin A1c were assessed as part of chemistry by Q2 Solutions (Valencia, California; Livingston, UK).
A Mixed Meal Tolerance Test (MMTT) substudy is planned for this study. The MMTT was performed after an overnight fast at the day 1 and the week 12 study visits. Fasting venous blood samples were collected at each of these 2 visits as close as possible before consuming a standardized mixed meal and before the subject received any food or drink (other than water) or study drug. Postprandial blood samples were then collected at time 0, and 120 ±10 minutes after consumption of the standardized mixed meal for assessment of plasma glucose, insulin, proinsulin, C‐peptide, free fatty acids, glucagon, lipids, chylomicrons, ApoB48, IL‐6, adiponectin, vitamin E.
Adverse events, collected at each study visit, are coded using the Medical Dictionary for Regulatory Activities (MedDRA) and patient incidences will be summarized by system organ class and preferred term by treatment group. Other safety measurements (eg, safety laboratory parameters, vital signs, electrocardiogram, anti‐evolocumab antibodies) will be summarized by treatment group using descriptive statistics.
3. RESULTS
Randomization was completed in September 2017, and the clinical trial completed in the first half of 2018. In total, 986 patients were randomized from 10 countries (Argentina, Brazil, Canada, China, Colombia, France, South Korea, Russia, Turkey, and the United States). A total of 453 patients were recruited from China. The mean age [SD] of patients was 61 (9) years, 57% were women, 42% were white, and the mean (SD) baseline LDL‐C was 93 (33) mg/dL.
4. DISCUSSION
The BERSON study is one of the two dedicated evolocumab clinical trials in patients with T2DM. The other study, BANTING, is different in that it requires enrolled patients to be on maximally tolerated statin of at least moderate intensity at screening. In addition, in BANTING, lipid eligibility criteria includes both LDL‐C and non‐HDL‐C. Additional differences between the studies include dosing regimens, device use, participating countries, and sample size. Thus, while both studies include patients with type 2 diabetes and with hyperlipidemia or mixed dyslipidemia, there are notable differences in eligibility requirements, study design, and regions between the two, which make them complementary.
Results from the BERSON study will supplement the data already available from a prespecified subanalysis of a large CV outcomes trial of evolocumab in high‐risk patients, as well as other post‐hoc analyses of patients with diabetes from phase 3 evolocumab trials.22, 23, 24, 25, 33 In the prespecified subgroup analysis of the FOURIER outcomes trial, LDL‐C lowering was comparable in the 11 031 patients with diabetes (57% reduction) and 16 533 patients without diabetes (60% reduction), with no safety signals observed in evolocumab‐treated patients. In patients with diabetes, the incidence of adverse events was similar between patients receiving evolocumab (78.5%) and placebo (78.3%). There were also no adverse effects on measures of glycemic control.22 At a median follow‐up of 2.2 years, glycated hemoglobin and fasting plasma glucose levels were similar between patients receiving evolocumab or placebo, regardless of their baseline glycemic status (diabetes, prediabetes, or normoglycemia). In addition, despite Mendelian randomization studies suggesting that PCSK9 deficiency may be linked to new‐onset diabetes,34 the FOURIER analysis demonstrated that there was also no signal of new‐onset diabetes following treatment with evolocumab.22, 24 These data confirm what was observed in prior, post‐hoc analyses of evolocumab clinical trials,23, 24, 25 as well as data from another PCSK9 inhibitor, alirocumab.22, 27, 35, 36 The BERSON study we describe here differs from the FOURIER subgroup analysis in that it is specifically designed to test the effects of evolocumab in patients with diabetes and hyperlipidemia or mixed dyslipidemia on background statin therapy. In addition, the BERSON trial enrolled half of its patients from China, allowing for the examination of the effect of evolocumab in this population.
5. CONCLUSION
This study was designed to specifically assess the efficacy and safety of evolocumab in reducing atherogenic lipids such as LDL‐C in patients with T2DM. While prior subanalyses show consistent efficacy and safety of evolocumab in patients with and without T2DM, this randomized, double‐blind study will provide additional, rigorous evidence of the effects of evolocumab in this patient population. The study completed in the first half of 2018.
Supporting information
Appendix S1.
ACKNOWLEDGMENTS
We acknowledge Annalise M. Nawrocki, PhD, of Amgen Inc., for writing and editorial assistance. This study was funded by Amgen Inc.
Conflict of interest
The authors take responsibility for all aspects of the reliability and freedom from bias of the data presented here and their discussed interpretation. Maria Laura Monsalvo, Ransi Somaratne, and Hui‐Chun Hsu report that they are employees of Amgen Inc. (or were at the time of the study) and own Amgen stock/stock options. Ransi Somaratne is also an inventor on at least one pending patent application owned by Amgen Inc. relating to evolocumab. Alberto J. Lorenzatti has received research funding and personal fees from Amgen during the study. Freddy G. Eliaschewitz has served as a speaker and has received grants for research from Amgen, Sanofi, Boehringer, Eli Lilly, Novo Nordisk, and AstraZeneca. Yundai Chen, Juming Lu, and Jun‐bo Ge have no conflicts of interest to report. Jonathan Fialkow has served on speakers' bureau for Amgen Inc. and Amarin. Alexis Baass is a consultant or scientific advisor for Amgen and Regeneron, receives research grants from Amgen, Regeneron, Merck, and AstraZeneca and participates in clinical trials for Amgen, Pfizer, Ionis, Regeneron, and Sanofi.
Lorenzatti AJ, Eliaschewitz FG, Chen Y, et al. Rationale and design of a randomized study to assess the efficacy and safety of evolocumab in patients with diabetes and dyslipidemia: The BERSON clinical trial. Clin Cardiol. 2018;41:1117–1122. 10.1002/clc.23018
Funding information Amgen Inc.
REFERENCES
- 1. Sarwar N, Gao P, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta‐analysis of 102 prospective studies. Lancet. 2010;375:2215‐2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Turner RC, Millns H, Neil HA, et al. Risk factors for coronary artery disease in non‐insulin dependent diabetes mellitus: United Kingdom prospective diabetes study (UKPDS: 23). BMJ. 1998;316:823‐828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Catapano AL, Graham I, et al. 2016 ESC/EAS guidelines for the Management of Dyslipidaemias: the task force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and European atherosclerosis society (EAS) developed with the special contribution of the European Assocciation for Cardiovascular Prevention & Rehabilitation (EACPR). Atherosclerosis. 2016;253:281‐344. [DOI] [PubMed] [Google Scholar]
- 4. Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229‐234. [DOI] [PubMed] [Google Scholar]
- 5. Wang L, Gao P, Zhang M, et al. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA. 2017;317:2515‐2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bragg F, Li L, Yang L, et al. Risks and population burden of cardiovascular diseases associated with diabetes in China: a prospective study of 5 million adults. PLoS Med. 2016;13:e1002026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Howard BV, Robbins DC, Sievers ML, et al. LDL cholesterol as a strong predictor of coronary heart disease in diabetic individuals with insulin resistance and low LDL: the strong heart study. Arterioscler Thromb Vasc Biol. 2000;20:830‐835. [DOI] [PubMed] [Google Scholar]
- 8. Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the collaborative atorvastatin diabetes study (CARDS): multicentre randomised placebo‐controlled trial. Lancet. 2004;364:685‐696. [DOI] [PubMed] [Google Scholar]
- 9. Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387‐2397. [DOI] [PubMed] [Google Scholar]
- 10. Sever PS, Poulter NR, Dahlof B, et al. Reduction in cardiovascular events with atorvastatin in 2,532 patients with type 2 diabetes: Anglo‐Scandinavian cardiac outcomes trial‐‐lipid‐lowering arm (ASCOT‐LLA). Diabetes Care. 2005;28:1151‐1157. [DOI] [PubMed] [Google Scholar]
- 11. Jellinger PS, Handelsman Y, Rosenblit PD, et al. American Association of Clinical Endocrinologists and American College of endocrinology guidelines for Management of Dyslipidemia and Prevention of cardiovascular disease. Endocr Pract. 2017;23:1‐87. [DOI] [PubMed] [Google Scholar]
- 12. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1‐S45. [DOI] [PubMed] [Google Scholar]
- 13. Mora S, Wenger NK, Demicco DA, et al. Determinants of residual risk in secondary prevention patients treated with high‐ versus low‐dose statin therapy: the treating to new targets (TNT) study. Circulation. 2012;125:1979‐1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Awan Z, Baass A, Genest J. Proprotein convertase subtilisin/kexin type 9 (PCSK9): lessons learned from patients with hypercholesterolemia. Clin Chem. 2014;60:1380‐1389. [DOI] [PubMed] [Google Scholar]
- 15. Blom DJ, Hala T, Bolognese M, et al. A 52‐week placebo‐controlled trial of evolocumab in hyperlipidemia. N Engl J Med. 2014;370:1809‐1819. [DOI] [PubMed] [Google Scholar]
- 16. Koren MJ, Giugliano RP, Raal FJ, et al. Efficacy and safety of longer‐term administration of evolocumab (AMG 145) in patients with hypercholesterolemia: 52‐week results from the open‐label study of long‐term evaluation against LDL‐C (OSLER) randomized trial. Circulation. 2014;129:234‐243. [DOI] [PubMed] [Google Scholar]
- 17. Koren MJ, Lundqvist P, Bolognese M, et al. Anti‐PCSK9 monotherapy for hypercholesterolemia: the MENDEL‐2 randomized, controlled phase III clinical trial of evolocumab. J Am Coll Cardiol. 2014;63:2531‐2540. [DOI] [PubMed] [Google Scholar]
- 18. Raal FJ, Stein EA, Dufour R, et al. PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD‐2): a randomised, double‐blind, placebo‐controlled trial. Lancet. 2015;385:331‐340. [DOI] [PubMed] [Google Scholar]
- 19. Robinson JG, Nedergaard BS, Rogers WJ, et al. Effect of evolocumab or ezetimibe added to moderate‐ or high‐intensity statin therapy on LDL‐C lowering in patients with hypercholesterolemia: the LAPLACE‐2 randomized clinical trial. JAMA. 2014;311:1870‐1882. [DOI] [PubMed] [Google Scholar]
- 20. Stroes E, Colquhoun D, Sullivan D, et al. Anti‐PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS‐2 randomized, placebo‐controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol. 2014;63:2541‐2548. [DOI] [PubMed] [Google Scholar]
- 21. Repatha® : Full prescribing information. Evolocumab: Amgen Inc., 2017.
- 22. Sabatine MS, Leiter LA, Wiviott SD, et al. Cardiovascular safety and efficacy of the PCSK9 inhibitor evolocumab in patients with and without diabetes and the effect of evolocumab on glycaemia and risk of new‐onset diabetes: a prespecified analysis of the FOURIER randomised controlled trial. N Engl J Med. 2017;376:1713‐1722. [DOI] [PubMed] [Google Scholar]
- 23. Sattar N, Preiss D, Robinson JG, et al. Lipid‐lowering efficacy of the PCSK9 inhibitor evolocumab (AMG 145) in patients with type 2 diabetes: a meta‐analysis of individual patient data. Lancet Diabetes Endocrinol. 2016;4:403‐410. [DOI] [PubMed] [Google Scholar]
- 24. Sattar N, Toth PP, Blom DJ, et al. Effect of the proprotein convertase subtilisin/kexin type 9 inhibitor Evolocumab on Glycemia, body weight, and new‐onset diabetes mellitus. Am J Cardiol. 2017;120:1521‐1527. [DOI] [PubMed] [Google Scholar]
- 25. Blom DJ, Koren MJ, Roth E, et al. Evaluation of the efficacy, safety and glycaemic effects of evolocumab (AMG 145) in hypercholesterolaemic patients stratified by glycaemic status and metabolic syndrome. Diabetes Obes Metab. 2017;19:98‐107. [DOI] [PubMed] [Google Scholar]
- 26. Colhoun HM, Ginsberg HN, Robinson JG, et al. No effect of PCSK9 inhibitor alirocumab on the incidence of diabetes in a pooled analysis from 10 ODYSSEY phase 3 studies. Eur Heart J. 2016;37:2981‐2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Leiter LA, Cariou B, Muller‐Wieland D, et al. Efficacy and safety of alirocumab in insulin‐treated individuals with type 1 or type 2 diabetes and high cardiovascular risk: the ODYSSEY DM‐INSULIN randomized trial. Diabetes Obes Metab. 2017;19:1781‐1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ray KK, Leiter LA, Muller‐Wieland D, et al. Alirocumab vs usual lipid‐lowering care as add‐on to statin therapy in individuals with type 2 diabetes and mixed dyslipidaemia: the ODYSSEY DM‐DYSLIPIDEMIA randomized trial. Diabetes Obes Metab. 2018;20:1479‐1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Raal FJ, Honarpour N, Blom DJ, et al. Inhibition of PCSK9 with evolocumab in homozygous familial hypercholesterolaemia (TESLA part B): a randomised, double‐blind, placebo‐controlled trial. Lancet. 2015;385:341‐350. [DOI] [PubMed] [Google Scholar]
- 30. Stein EA, Giugliano RP, Koren MJ, et al. Efficacy and safety of evolocumab (AMG 145), a fully human monoclonal antibody to PCSK9, in hyperlipidaemic patients on various background lipid therapies: pooled analysis of 1359 patients in four phase 2 trials. Eur Heart J. 2014;35:2249‐2259. [DOI] [PubMed] [Google Scholar]
- 31. Joint Committee for Developing Chinese guidelines on Prevention and Treatment of Dyslipidemia in Adults . Chinese guidelines on prevention and treatment of dyslipidemia in adults. Zhonghua Xin Xue Guan Bing Za Zhi. 2007;35:390‐419. [PubMed] [Google Scholar]
- 32. Lipitor® . Atorvastatin. Dublin: Pfizer Ireland; 2009. [Google Scholar]
- 33. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713‐1722. [DOI] [PubMed] [Google Scholar]
- 34. Ference BA, Robinson JG, Brook RD, et al. Variation in PCSK9 and HMGCR and risk of cardiovascular disease and diabetes. N Engl J Med. 2016;375:2144‐2153. [DOI] [PubMed] [Google Scholar]
- 35. Leiter LA, Muller‐Wieland D, Baccara‐Dinet MT, Letierce A, Samuel R, Cariou B. Efficacy and safety of alirocumab in people with prediabetes vs those with normoglycaemia at baseline: a pooled analysis of 10 phase III ODYSSEY clinical trials. Diabet Med. 2018;35:121‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Leiter LA, Zamorano JL, Bujas‐Bobanovic M, et al. Lipid‐lowering efficacy and safety of alirocumab in patients with or without diabetes: a sub‐analysis of ODYSSEY COMBO II. Diabetes Obes Metab. 2017;19:989‐996. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1.


