Abstract
Background
Decision making regarding a patient who has experienced a first clinical episode of atrial fibrillation (AF) is challenging, and the AF recurrences should be a significant consideration. Continuous long‐term rhythm monitoring via implantable loop recorders (ILRs) has enabled us to evaluate the AF recurrence profile after the first clinical episode and to investigate clinical parameters associated with the course of the arrhythmia.
Hypothesis
Continuous rhythm monitoring via ILRs in AF patients after the first clinical episode is of clinical significance and precisely evaluate the AF recurrence profile.
Methods
Thirty consecutive patients with paroxysmal AF received an ILR after their first symptomatic episode. We evaluated the maximum duration of episodes and the recurrence rate of the arrhythmia during a follow‐up period of 3 years.
Results
Three patients (10%) had no AF recurrence, whereas 4 patients (13.3%) presented only 1 episode. Almost half of the patients (46.7%) had a low recurrence rate (<5 episodes/year), whereas the majority of patients (19/30) suffered from episodes with maximum duration ≤24 hours. Eleven patients (36.7%) presented either no episode or a low recurrence rate with episodes lasting ≤24 hours. The use of statins was greater in patients with a low recurrence rate (P = 0.025).
Conclusions
A significant percentage of patients either suffer no AF recurrence after their first symptomatic episode or show a low recurrence rate. Most patients present episodes of short duration. If these findings are confirmed in larger studies, they could have clinical implications ensuring individualized management of the arrhythmia in the future.
Keywords: Anticoagulation, Arrhythmia, Atrial Fibrillation, Continuous Monitoring, Implantable Loop Recorder
1. INTRODUCTION
A common clinical scenario faced by physicians is therapeutic decision making concerning a patient who has experienced a first electrocardiogram (ECG)‐documented episode of atrial fibrillation (AF). Should they recommend antiarrhythmic drugs or pulmonary vein ablation as an early rhythm control strategy? More importantly, should they recommend oral anticoagulants, if the CHA2DS2‐VASc (Congestive heart failure, Hypertension, Age ≥ 75 years [doubled risk weight], Diabetes mellitus, previous Stroke/transient ischemic attack [doubled risk weight], Vascular disease, Age 65 to 74 years, Sex) score is appropriate, to prevent thromboembolic stroke and/or systemic embolism? The first step in this direction is to search for and treat any reversible factor that may have precipitated the arrhythmia, whereupon the patient can be reevaluated. However, when a thorough evaluation fails to reveal any reversible cause, the patient's AF recurrence profile and AF burden may play a key role in long‐term management. A high percentage of patients may not suffer any other AF episode after their first,1, 2 or may present a low recurrence rate of the arrhythmia in the future. In a previous study by Pappone et al.,1 which is the only investigation to evaluate AF progression prospectively after the first clinical episode, it was found that almost half of the patients had no further AF recurrence after a 5‐year follow‐up period. Clearly, these patients should be treated differently than those with frequent recurrences of the arrhythmia. The weak point of that study was that the recurrence rate was mainly evaluated in terms of symptomatic episodes, whereas it is known that asymptomatic AF is common in patients who have exhibited symptomatic episodes2, 3 and is associated with a similarly increased thromboembolic risk.4, 5
Nowadays, continuous long‐term rhythm monitoring and remote rhythm monitoring have been made feasible by the use of implantable loop recorders (ILRs). ILRs are small, subcutaneously implanted devices that are able to detect and store AF episodes lasting longer than 2 minutes with high sensitivity (96.1%–100%) and good specificity (67%–85.4%).6, 7 In this study, using such devices, we evaluated the recurrence profile of AF, including symptomatic and asymptomatic episodes, after the first ECG‐documented symptomatic episode, and we searched for clinical, demographic, and echocardiographic factors associated with the recurrence profile of the arrhythmia.
2. METHODS
The methods of this study have been partly described in a previous publication.2 Briefly, consecutive patients older than 18 years who had a first ECG‐documented episode of paroxysmal AF were included. Once sinus rhythm had been restored, an ILR (Reveal XT 9529; Medtronic, Minneapolis, MN) was inserted subcutaneously. All the patients received anticoagulation therapy according to the CHA2DS2‐VASc score.8 The European Heart Rhythm Association (EHRA) score8 was used for the classification of AF‐related symptoms. We analyzed the maximum duration of AF episodes and the recurrence rate of the arrhythmia (Figure 1). A low recurrence rate of the arrhythmia was defined as less than 5 paroxysmal AF episodes per year. The data from 2 representative patients, 1 with a low recurrence rate and another with a high recurrence rate, are shown in Figure 2. Follow‐up visits were scheduled to be performed after 1 month, 3 months, and every 3 months thereafter for a period of 3 years.
Figure 1.
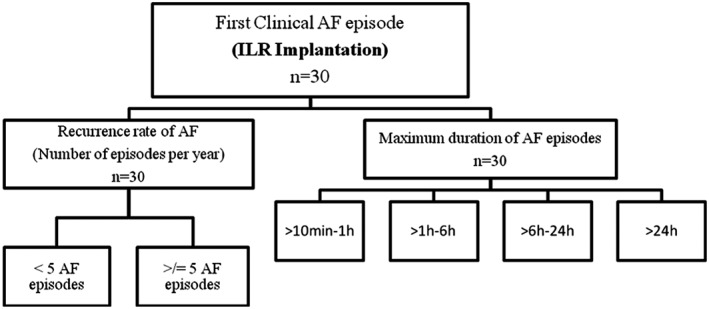
Categorization of AF patients according to the recurrence rate and maximum duration of the arrhythmia. The patients were divided according to the number of AF episodes per year (<5 AF episodes or ≥5 AF episodes) and the maximum duration of AF episodes occurring during the follow‐up period. Abbreviations: AF, atrial fibrillation; ILR, implantable loop recorder
Figure 2.
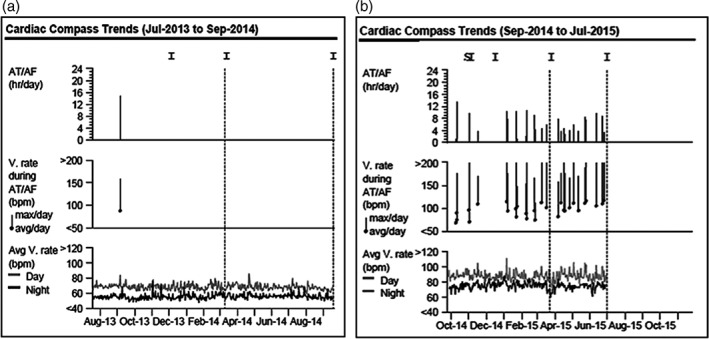
Representative data from the ILRs of 2 patients. (a) Data from a 63‐year‐old male patient with a low recurrence rate of AF. The histograms show an episode of paroxysmal AF that lasted 14 hour 54 minutes, with a median ventricular rate of 87 bpm and a maximum ventricular rate of 158 bpm. (b) Data from a 58‐year‐old patient with a high recurrence rate of AF. The histograms show several episodes of paroxysmal AF that were cardioverted spontaneously. Abbreviations: AF, atrial fibrillation; AT, atrial tachyarrhythmia; ILRs, implantable loop recorders; V, ventricular
All patients gave written informed consent, the study was organized according to ethical considerations as described in the Declaration of Helsinki for human medical studies, and the protocol was approved by the institutional medical ethics committee. Types of AF were defined according to the 2016 European Society of Cardiology (ESC) guidelines.8 ILR‐recorded AF episodes with duration longer than 10 minutes were included in our analysis. We excluded patients with persistent or permanent AF on admission and patients with an episode of AF attributed to reversible or transient causes.
2.1. Implantable loop recorder
The Reveal XT detects the occurrence of AF episodes using an automatic algorithm based on the pattern of R‐wave interval variability within 2‐minute periods. It stores up to 30 AF episodes in an episode log. When the log is full, data from the most recent episode will overwrite the oldest stored episode data. For each automatically detected AF episode the Reveal XT stores an ECG of the first 2 minutes of the episode, whereas for each patient‐activated episode it stores the ECG 6.5 minutes before the patient's activation and 1 minute after. The device's memory was reset after each patient's visit. The AF episodes were classified as either asymptomatic (the patient was unaware of the arrhythmia throughout the episode) or symptomatic (the patient suffered from symptoms indicative of the arrhythmia). All the recorded episodes were reviewed by 2 independent physicians from our department who had full access to the data and were responsible for their interpretation.
2.2. Statistics
Summary descriptive statistics are presented as mean ± standard deviation or frequencies (%), as appropriate. Comparisons of continuous variables between the 2 AF groups were performed using the independent samples t test. Categorical variables were compared between the 2 groups using the χ2 or Fisher exact test. All statistical tests were carried out using IBM‐SPSS version 21 software (IBM, Armonk, NY) at the 2‐sided 5% level of significance.
3. RESULTS
The patients' baseline demographic and clinical characteristics are shown in Table 1. Thirty consecutive patients (mean age = 66.9 ± 10 years, 14 men) were included in our study after the restoration of sinus rhythm (Table 2). The mean follow‐up period was 34.63 ± 2.96 months after the implantation of the ILR. The mean CHA2DS2‐VASc score was 2.83 ± 1.66; 6 patients (20%) had a score of 0 to 1, 10 patients (33.3%) a score of 2, and 14 patients (46.7%) a score of >2. The mean EHRA score was 1.7 ± 0.99; 23 patients (76.7%) had EHRA score class I or II, and 7 patients (23.3%) had EHRA score class III or IV. Seven patients (23.3%) received antiarrhythmic drug therapy for heart rhythm control; 3 patients (10%) received propafenone, 2 patients (6.6%) received flecainide, and 2 patients (6.6%) received amiodarone. Twenty‐eight patients (93.3%) received oral anticoagulants. No thromboembolic episode, death, or major bleeding was observed during the follow‐up period.
Table 1.
Demographic and clinical characteristics of the enrolled patients and AF recurrence rate during the follow‐up period
| All, N = 30, 100% | Low AF Recurrence Rate, n = 14, 100% | High AF Recurrence Rate, n = 16, 100% | P Value | |
|---|---|---|---|---|
| Age, y | 66.9 ± 10 | 65.43 ± 10.72 | 68.25 ± 9.53 | 0.45 |
| Men | 14 (46.7) | 5 (35.7) | 9 (56.3) | 0.3 |
| Height, m | 1.67 ± 0.09 | 1.65 ± 0.09 | 1.70 ± 0.09 | 0.14 |
| Weight, kg | 84.4 ± 13.8 | 82.93 ± 13.48 | 85.92 ± 15.06 | 0.59 |
| BMI, kg/m2 | 30.2 ± 4.6 | 30.6 ± 5.33 | 29.74 ± 4.06 | 0.64 |
| Hypertension | 25 (83.3) | 12 (85.7) | 13 (81.3) | 0.99 |
| Smoking | 14 (46.7) | 7 (50) | 7 (43.8) | 0.99 |
| Dyslipidemia | 21 (70) | 11 (78.6) | 10 (62.5) | 0.44 |
| Diabetes mellitus | 7 (23.3) | 3 (21.4) | 4 (25) | 0.99 |
| Stable coronary artery disease | 3 (10) | 3 (21.4) | 0 (0) | 0.09 |
| Heart failure | 6 (20) | 3 (21.4) | 3 (18.8) | 0.99 |
| History of ΤΙΑ/stroke | 2 (6.6) | 0 (0) | 2 (12.5) | 0.485 |
| CHADS2 | 1.67 ± 1.3 | 1.5 ± 1.09 | 1.81 ± 1.47 | 0.52 |
| CHA2DS2‐VASc | 2.87 ± 1.74 | 2.93 ± 1.54 | 2.81 ± 1.94 | 0.86 |
| Antiarrhythmic drug therapy | 7 (23.3) | 3 (21.4) | 4 (25) | 0.99 |
| Other medications | ||||
| β‐blockers | 9 (30) | 4 (28.6) | 5 (31.3) | 0.99 |
| ACEI | 8 (26.6) | 5 (35.7) | 3 (18.8) | 0.43 |
| ARB | 15 (50) | 6 (42.9) | 9 (56.3) | 0.47 |
| Statins | 16 (53.3) | 11 (78.6) | 5 (31.3) | 0.025 |
| Echocardiographic parameters | ||||
| Diameter of the left atrium, mm | 41.14 ± 5.77 | 40.28 ± 5.22 | 42 ± 6.37 | 0.46 |
| Ejection fraction, % | 59.03 ± 6.32 | 58.07 ± 7.2 | 60 ± 5.4 | 0.45 |
| End‐diastolic diameter of the left ventricle, mm | 48.78 ± 5.03 | 47.9 ± 6.37 | 49.57 ± 3.48 | 0.42 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; BMI, body mass index; CHADS2, Congestive heart failure, Hypertension, Age ≥ 75 years, Diabetes mellitus, Stroke (double risk weight);
CHA2DS2‐VASc, Congestive heart failure, Hypertension, Age ≥ 75 years (doubled risk weight), Diabetes mellitus, previous Stroke/transient ischemic attack (doubled risk weight), Vascular disease, Age 65 to 74 years, Sex; TIA, transient ischemic attack.
The patients were separated into 2 groups according to the recurrence rate of the arrhythmia: low (<5 AF episodes per year) or high (≥5 AF episodes per year).
Data are presented as mean ± standard deviation or number (%).
Table 2.
Duration, recurrence rate, and clinical profile of AF episodes
| No. of Patients (%), N = 30, 100% | |
|---|---|
| Maximum duration of AF episodes | |
| >24 hours | 8 (26.7%) |
| >6–24 hours | 14 (46.7%) |
| >1–6 hours | 4 (13.3%) |
| >10 minutes–1 hour | 1 (3.3%) |
| No AF episode | 3 (10%) |
| Recurrence rate of AF, per year | |
| ≥5 AF episodes | 16 (53.3%) |
| <5 AF episodes | 14 (46.7%) |
| Clinical profile of AF episodes | |
| Only symptomatic AF episodes, clinical AF episodes | 7 (23.3%) |
| Only asymptomatic AF episodes, subclinical AF episodes | 9 (30%) |
| Both symptomatic and asymptomatic AF episodes | 11 (36.7%) |
| No AF episode | 3 (10%) |
| Restoration of the sinus rhythm after the first AF episode | |
| Spontaneously | 10 (33.3%) |
| Pharmacologic (chemical cardioversion) with amiodarone | 17 (56.7%) |
| Electrical cardioversion | 3 (10%) |
Abbreviations: AF, atrial fibrillation.
3.1. AF episodes
A total of 543 AF episodes were stored in the ILR memory and reviewed by 2 cardiologists in our department. After the physicians' review, 479 of them (88.2%) were finally included in our analysis. The remaining episodes (64 episodes [11.8%]) were incorrectly classified as AF due to signal noise, irregular atrioventricular conduction, and premature atrial or ventricular contractions. The agreement in the classification of episodes between the 2 reviewers was 98.5%. In case of disagreement between the 2 reviewers, a third cardiologist, who is an expert in cardiac arrhythmias, reviewed the episodes and made the final decision.
3.2. AF recurrence
Twenty‐seven patients presented at least 1 AF recurrence. Seven patients (23.3%) suffered only from symptomatic episodes, whereas in 9 patients (30%) only asymptomatic episodes were recorded. Eleven patients (36.7%) had both types of episodes (symptomatic and asymptomatic). Fourteen patients (46.7%) had a low recurrence rate of the arrhythmia during the follow‐up period (Table 2). In 3 patients (10%), no episode was recorded, whereas in 4 patients (13.3%) only 1 episode was recorded. Four patients with a high recurrence rate also presented episodes of persistent AF. The use of statins was significantly greater in the patients with a low recurrence rate (P = 0.025) (Table 1). No significant difference was observed between the method of restoration of the sinus rhythm at inclusion (spontaneous, chemical or electrical cardioversion) and the recurrence rate of AF.
3.3. Maximum duration of AF episodes (≤24 hours vs >24 hours)
Nineteen patients (63.3%) presented only AF episodes with a duration ≤24 hours, whereas in 5 patients (16.7%) the maximum duration of AF episodes did not exceed 6 hours (Table 2). The use of antiarrhythmic drugs (P = 0.033) and angiotensin receptor blockers (P = 0.036) was higher in the group of patients who suffered episodes with maximum duration >24 hours (Table 3). No significant difference was observed between the method of restoration of the sinus rhythm at inclusion and the maximum duration of the episodes.
Table 3.
Maximum duration of AF episodes during the follow‐up period
| AF Maximum Duration ≤24 hours, n = 19, 100% | AF Maximum Duration >24 Hours, n = 8, 100% | P Value | |
|---|---|---|---|
| Age, y | 70.26 ± 6.28 | 65 ± 11.65 | 0.14 |
| Men | 6 (31.6) | 6 (75) | 0.087 |
| Height, m | 1.66 ± 0.09 | 1.70 ± 0.1 | 0.35 |
| Weight, kg | 81.65 ± 16.1 | 87.85 ± 9.3 | 0.35 |
| BMI, kg/m2 | 29.75 ± 5.1 | 30.77 ± 4.39 | 0.64 |
| Hypertension | 14 (73.7) | 8 (100) | 0.28 |
| Smoking | 7 (36.8) | 5 (62.5) | 0.4 |
| Dyslipidemia | 13 (68.4) | 6 (75) | 0.99 |
| Diabetes mellitus | 4 (21.1) | 2 (25) | 0.99 |
| Stable coronary artery disease | 1 (5.3) | 2 (25) | 0.2 |
| Heart failure | 4 (21.1) | 2 (25) | 0.99 |
| History of ΤΙΑ/stroke | 2 (10.5) | 0 (0) | 0.99 |
| CHADS2 | 1.68 ± 1.45 | 1.75 ± 1.16 | 0.9 |
| CHA2DS2‐VASc | 3.1 ± 1.76 | 2.75 ± 1.9 | 0.6 |
| Antiarrhythmic drug therapy | 2 (10.5) | 4 (50) | 0.044 |
| Amiodarone | 0 (0) | 2 (25) | |
| Propafenone | 1 (5.3) | 1 (12.5) | |
| Flecainide | 1 (5.3) | 1 (12.5) | |
| Other medications | |||
| β‐blockers | 5 (26.3) | 3 (37.5) | 0.67 |
| ACEI | 6 (31.6) | 0 (0) | 0.132 |
| ARB | 7 (36.8) | 7 (87.5) | 0.036 |
| Statins | 9 (47.4) | 5 (62.5) | 0.68 |
| Echocardiographic parameters | |||
| Diameter of the left atrium, mm | 40.33 ± 5.2 | 42.75 ± 7.2 | 0.36 |
| Ejection fraction, % | 58.66 ± 5.8 | 59.37 ± 8.2 | 0.8 |
| End‐diastolic diameter of the left ventricle, mm | 49 ± 5.2 | 49.1 ± 4.46 | 0.96 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; BMI, body mass index; CHADS2, Congestive heart failure, Hypertension, Age ≥ 75 years, Diabetes mellitus, Stroke (double risk weight);
CHA2DS2‐VASc, Congestive heart failure, Hypertension, Age ≥ 75 years (doubled risk weight), Diabetes mellitus, previous Stroke/transient ischemic attack (doubled risk weight), Vascular disease, Age 65 to 74 years, Sex; TIA, transient ischemic attack.
The patients were separated into 2 groups: maximum AF duration ≤24 hours and maximum AF duration >24 hours.
Data are presented as mean ± standard deviation or number (%).
3.4. Patients with no AF episode or low recurrence rate and AF episodes lasting ≤24 hours vs other AF patients
Eleven patients (36.7%) had no recurrent AF episode or a low recurrence rate with episodes lasting ≤24 hours. Patients with no AF or a low recurrence rate with episodes lasting ≤24 hours had a lower mean CHADS2 (Congestive heart failure, Hypertension, Age ≥ 75 years, Diabetes mellitus, Stroke [double risk weight]) (1.18 ± 0.75 vs 1.95 ± 1.47, P = 0.12) and CHA2DS2‐VASc scores (2.45 ± 1.21 vs 3.11 ± 1.97, P = 0.33), though the differences did not reach statistical significance. Although the use of statins was greater in patients with no AF or a low recurrence rate with AF ≤24 hours, the difference was only of borderline statistical significance (P = 0.052). As far as the method of restoration of the sinus rhythm at inclusion is concerned, no significant difference was observed between the 2 groups.
4. DISCUSSION
In this prospective study, we found for the first time that after their first ECG‐documented symptomatic AF episode, a high percentage of patients either do not suffer any other episode or present a low recurrence rate of the arrhythmia, with most of the episodes being of short duration. To the best of our knowledge, this is the first study to prospectively evaluate the natural history of paroxysmal AF after the first ECG‐documented clinical AF episode via long‐term rhythm monitoring with ILRs. In the literature, there is only 1 prospective study by Pappone et al1 that evaluated the progression of AF over a 5‐year follow‐up period after the first‐detected symptomatic paroxysmal AF episode. In contrast to our study, Pappone et al1 followed up 106 patients using intermittent rhythm monitoring. They reported that 50 patients (47%) did not suffer any other AF episode during the 5‐year period. This proportion of AF‐free patients is likely to have been overestimated, given the sporadic nature of the episodes and the potential absence of provoked symptoms. Large prospective studies using continuous ECG monitoring via implantable devices showed a high prevalence of subclinical AF in selected populations,5, 9 including patients with no previous history of AF.5, 9 In our study, 3 patients (10%) had no AF episode, whereas 9 patients (30%) did not experience any other symptomatic episode. These findings provide strong evidence that only continuous monitoring of the arrhythmia can exclude AF recurrences and identify AF‐free patients.7
The AF recurrence rate during the natural course of the arrhythmia should be viewed as very important, as it may have serious clinical implications that affect the management of the patient after the first appearance of the arrhythmia. Should the physician recommend antiarrhythmics and/or pulmonary vein ablation? What about anticoagulants? Is the strategy watch and decide, based on the total burden (symptomatic and asymptomatic) of the arrhythmia, better than the management of patients based only on symptoms? Should some AF patients, who may constitute a high percentage, avoid the long‐term use of oral anticoagulants or use these drugs intermittently?
The first ECG‐documented AF episode could be considered an alert that leads to the early detection and modification of any potentially reversible factors that predispose to arrhythmia. Subsequently, continuous monitoring with ILRs could be used as the most reliable way to bridge the existing gap between the management of asymptomatic episodes and the clinical outcome. Interrogation of ILRs could guide, in selected cases, a strategy of early rhythm control, with antiarrhythmic drugs and/or catheter ablation. This strategy might prevent the detrimental self‐perpetuating effects that AF exerts through complex mechanisms, leading to electrophysiological and structural remodeling of the atria, a main reason for the long‐term failure to maintain sinus rhythm.
In the absence of strong evidence about the exact natural history of subclinical AF,10 current ESC guidelines8 recommend that long‐term oral anticoagulation therapy should be considered in patients with a CHA2DS2‐VASc score equal to 1, whereas it is indicated in all AF patients with a CHA2DS2‐VASc score ≥ 2. Nevertheless, the use of non–vitamin K antagonist oral anticoagulants (NOACs) in combination with devices that provide a continuous remote rhythm monitoring capability has encouraged anticoagulation strategies based on the AF burden.11 By monitoring atrial tachyarrhythmias in patients with implanted defibrillators and cardiac resynchronization devices, Martin et al12 evaluated the strategy of early anticoagulation for incident AF and withdrawal after arrhythmia‐free periods to reduce the risk of stroke and bleeding. In their study (IMPACT [Combined Use of BIOTRONIK Home Monitoring and Predefined Anticoagulation to Reduce Stroke Risk]),12 the introduction and termination of anticoagulation based upon atrial tachyarrhythmia monitoring did not improve outcomes (stroke and bleeding) compared with conventional long‐term continuous anticoagulation management.
A recent study evaluating the same hypothesis (REACT.COM [Rhythm Evaluation for Anticoagulation With Continuous Monitoring]13) analyzed data from 59 patients who suffered from paroxysmal or persistent AF. The patients were treated with NOACs, whereas anticoagulation management was based upon the recurrence profile of the arrhythmia via rhythm monitoring with ILRs. The anticoagulants were administered for every AF episode lasting at least 60 minutes and for a duration of 30 days after each episode. The study showed that this strategy yielded a 94% reduction in time on anticoagulation compared to conservative management, whereas no stroke or death was observed. TACTIC‐AF (Tailored Anticoagulation for non‐continuous Atrial Fibrillation)11 is another ongoing study that aims to test this tailored anticoagulation strategy in patients with a CHADS2 score < 3 and a low AF burden, and its results are expected soon. Our results also support the concept of on–off anticoagulation management and the organization of large trials to evaluate this strategy.
Another intriguing issue, the association between AF duration and stroke risk, is still controversial.5, 14, 15, 16 Patients who present less common episodes of short duration may be less prone to develop a left atrial thrombus.11 A recently published report by Van Gelder et al15 analyzing the duration of subclinical AF (SCAF) and the occurrence of ischemic stroke in the ASSERT (Asymptomatic Atrial Fibrillation and Stroke Evaluation in Pacemaker Patients and the Atrial Fibrillation Reduction Atrial Pacing Trial)5 study's population, and showed that only patients who suffered from SCAF with duration >24 ours had a significantly increased thromboembolic risk. However, in the MOST (MOde Selection Trial)14 study, the risk of stroke or death was increased by a factor of 2.5 in patients who had at least 1 atrial high‐rate episode with a duration >5 minutes, whereas the TRENDS (A Prospective Study of the Clinical Significance of Atrial Arrhythmias Detected by Implanted Device Diagnostics)16 study showed that the thromboembolic risk is a quantitative function of AF burden. Cappucci et al.....,17 in accordance with Van Gelder et al,15 showed that AF episodes with duration >24 hours were independently associated with increased thromboembolic risk.17 In a well‐conducted study by Botto et al,18 the authors suggested that in patients with recurrent AF episodes, the prediction of thromboembolic events can be improved by combining AF duration with CHADS2 score. In our study, most of the patients (63.3%) presented episodes of short duration (≤24 hours), and it is unknown whether these AF episodes increase thromboembolic risk to a level requiring anticoagulants for the prevention of stroke or systemic embolism. Nevertheless, the precise cutoff value for the AF duration and its relation to other thromboembolic risk factors is a matter for further research.
Regarding the correlation of the recurrence rate or the duration of AF episodes with demographic, clinical, and echocardiographic parameters, the use of statins was significantly associated with a low recurrence rate of the arrhythmia (P = 0.025). In the literature, the impact of statins on the prevention of AF is controversial.19, 20 Furthermore, the use of antiarrhythmic drugs was higher in patients who suffered from prolonged episodes. This was at the borderline of statistical significance (P = 0.044) and it may be attributed to the preference of the treating physicians for the administration of antiarrhythmic drugs in patients with long‐duration episodes. The use of angiotensin receptor blockers was also greater in patients with longer AF episodes (P = 0.036)—perhaps because all the patients with AF lasting >24 hours had hypertension, and these drugs are a major class of antihypertensive agents. However, given the small number of patients enrolled, the above results should be interpreted with caution.
4.1. Limitations
In our study, we arbitrarily used the cutoff of 10 minutes to increase the specificity in the detection of AF episodes. The Reveal XT does not have the ability to provide the available ECG for all the AF recorded episodes, but only for the last 10 stored episodes. Episodes of short duration (eg, AF episodes of 2 minutes) usually were incorrectly classified as AF as there was signal noise, irregular atrioventricular conduction, and premature atrial or ventricular contractions.
The specificity of the ILR6 in the automatic detection of AF episodes (85.4%) could have slightly influenced our results. To avoid losing data through overwriting of the older episodes, we scheduled the follow‐up visits for every 3 months. However, for patients who had a high recurrence rate, some data may have been lost. A low recurrence rate was arbitrarily defined as less than 5 AF episodes per year. We believe that patients who have this recurrence rate with episodes of short duration (≤24 hours) could be a target group of clinical interest as far as their long‐term anticoagulation management is concerned. Nevertheless, our sample was small and heterogeneous, influencing the ability to reliably evaluate determinants of AF recurrences.
We also included patients who had experienced their first symptomatic, ECG‐documented paroxysmal AF episode. It is not known whether these patients suffered from subclinical AF episodes in the past before their first symptomatic episode, but that is of questionable clinical significance. These episodes may accelerate the progression of the arrhythmia to persistent or permanent; nevertheless, the first clinical episode is the time point for decision‐making regarding the management of AF. The number of patients included in this study is small, and the results should be evaluated with caution. However, due to the lack of evidence in the natural course of the arrhythmia and to the long‐term follow‐up period after the first AF episode (3 years for each patient), we believe that our results are of interest and deserve some merit as they may trigger further larger studies in this field.
5. CONCLUSION
We observed in our study that after their first clinical AF episode, a significant percentage of patients do not suffer any other AF episode or have a low recurrence rate of the arrhythmia. Furthermore, most of the patients presented episodes with short duration. If these findings are confirmed in larger studies, they could have clinical implications ensuring individualized evaluation and long‐term management of the arrhythmia in the future. Further large randomized studies are needed, not only to evaluate such strategies, but also to elucidate the role of ILRs in the long‐term management of the arrhythmia.
Author contributions
Emmanuel N. Simantirakis and Panteleimon E. Papakonstantinou wrote the manuscript, collected the data, performed the data analysis and the literature research, and took part in the care of the patients. Emmanuel Kanoupakis took part in the care of the patients, collected the data, performed literature research, and reviewed the manuscript. Gregory I. Chlouverakis performed the statistical analysis of the data. Stylianos Tzeis performed literature research and reviewed the manuscript. Panos E. Vardas took part in the coordination and final editing of the manuscript. All authors read and approved the final manuscript. Emmanuel N. Simantirakis and Panteleimon E. Papakonstantinou contributed equally to this work.
Conflicts of interest
Emmanuel N. Simantirakis received a research grant from Medtronic Inc. (A 1065096 and SQ‐1679).
Simantirakis EN, Papakonstantinou PE, Kanoupakis E, Chlouverakis GI, Tzeis S, Vardas PE. Recurrence rate of atrial fibrillation after the first clinical episode: A prospective evaluation using continuous cardiac rhythm monitoring. Clin Cardiol. 2018;41:594–600. 10.1002/clc.22904
Funding information Medtronic, Grant/Award number: A 1065096 and SQ‐1679
REFERENCES
- 1. Pappone C, Radinovic A, Manguso F, et al. Atrial fibrillation progression and management: a 5‐year prospective follow‐up study. Heart Rhythm. 2008;5:1501–1507. [DOI] [PubMed] [Google Scholar]
- 2. Simantirakis EN, Papakonstantinou PE, Chlouverakis GI, et al. Asymptomatic versus symptomatic episodes in patients with paroxysmal atrial fibrillation via long‐term monitoring with implantable loop recorders. Int J Cardiol. 2017;231:125–130. [DOI] [PubMed] [Google Scholar]
- 3. Hindricks G, Piorkowski C, Tanner H, et al. Perception of atrial fibrillation before and after radiofrequency catheter ablation: relevance of asymptomatic arrhythmia recurrence. Circulation. 2005;112:307–313. [DOI] [PubMed] [Google Scholar]
- 4. Flaker GC, Belew K, Beckman K, et al. Asymptomatic atrial fibrillation: demographic features and prognostic information from the Atrial Fibrillation Follow‐up Investigation of Rhythm Management (AFFIRM) study. Am Heart J. 2005;149:657–663. [DOI] [PubMed] [Google Scholar]
- 5. Healey JS, Connolly SJ, Gold MR, et al. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med. 2012;366:120–129. [DOI] [PubMed] [Google Scholar]
- 6. Hindricks G, Pokushalov E, Urban L, et al. Performance of a new leadless implantable cardiac monitor in detecting and quantifying atrial fibrillation: results of the XPECT trial. Circ Arrhythm Electrophysiol. 2010;3:141–147. [DOI] [PubMed] [Google Scholar]
- 7. Ciconte G, Saviano M, Giannelli L, et al. Atrial fibrillation detection using a novel three‐vector cardiac implantable monitor: the atrial fibrillation detect study. Europace. 2017;19:1101–1108. [DOI] [PubMed] [Google Scholar]
- 8. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace. 2016;18:1609–1678. [DOI] [PubMed] [Google Scholar]
- 9. Healey JS, Alings M, Ha AC, et al. Subclinical atrial fibrillation in older patients. Circulation. 2017;136:1276–1283. [DOI] [PubMed] [Google Scholar]
- 10. Rahimi K. Subclinical atrial fibrillation in need of more assertive evidence. Eur Heart J. 2017;38:1345–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zimetbaum P, Ellis ER, Waks JW, et al. The importance of atrial fibrillation burden and the origin of device‐tailored anticoagulation. Pacing Clin Electrophysiol. 2013;36:1319–1324. [DOI] [PubMed] [Google Scholar]
- 12. Martin DT, Bersohn MM, Waldo AL, et al. Randomized trial of atrial arrhythmia monitoring to guide anticoagulation in patients with implanted defibrillator and cardiac resynchronization devices. Eur Heart J. 2015;36:1660–1668. [DOI] [PubMed] [Google Scholar]
- 13. Passman R, Leong‐Sit P, Andrei AC, et al. Targeted anticoagulation for atrial fibrillation guided by continuous rhythm assessment with an insertable cardiac monitor: the Rhythm Evaluation for Anticoagulation With Continuous Monitoring (REACT.COM) pilot study. J Cardiovasc Electrophysiol. 2016;27:264–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Glotzer TV, Hellkamp AS, Zimmerman J, et al. Atrial high rate episodes detected by pacemaker diagnostics predict death and stroke: report of the Atrial Diagnostics Ancillary Study of the MOde Selection Trial (MOST). Circulation. 2003;107:1614–1619. [DOI] [PubMed] [Google Scholar]
- 15. Van Gelder IC, Healey JS, Crijns HJ, et al. Duration of device‐detected subclinical atrial fibrillation and occurrence of stroke in ASSERT. Eur Heart J. 2017;38:1339–1344. [DOI] [PubMed] [Google Scholar]
- 16. Glotzer TV, Daoud EG, Wyse DG, et al. The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: the TRENDS study. Circ Arrhythm Electrophysiol. 2009;2:474–480. [DOI] [PubMed] [Google Scholar]
- 17. Capucci A, Santini M, Padeletti L, et al. Monitored atrial fibrillation duration predicts arterial embolic events in patients suffering from bradycardia and atrial fibrillation implanted with antitachycardia pacemakers. J Am Coll Cardiol. 2005;46:1913–1920. [DOI] [PubMed] [Google Scholar]
- 18. Botto GL, Padeletti L, Santini M, et al. Presence and duration of atrial fibrillation detected by continuous monitoring: crucial implications for the risk of thromboembolic events. J Cardiovasc Electrophysiol. 2009;20:241–248. [DOI] [PubMed] [Google Scholar]
- 19. Fauchier L, Clementy N, Babuty D. Statin therapy and atrial fibrillation: systematic review and updated meta‐analysis of published randomized controlled trials. Curr Opin Cardiol. 2013;28:7–18. [DOI] [PubMed] [Google Scholar]
- 20. Rahimi K, Emberson J, McGale P, et al. Effect of statins on atrial fibrillation: collaborative meta‐analysis of published and unpublished evidence from randomised controlled trials. BMJ. 2011;342:d1250. [DOI] [PubMed] [Google Scholar]


