Abstract
Cardiovascular disease (CVD) remains the leading cause of death in the United States. Healthcare expenditures have been principally allocated toward treatment of CVD at the end of the health/disease continuum, rather than toward health promotion and disease prevention. A focused effort on both primordial and primary prevention can promote cardiovascular health and reduce the burden of CVD. Risk‐factor assessment for predicting atherosclerotic CVD events serves as the foundation of preventive cardiology and has been driven by population‐based scoring algorithms based on traditional risk factors. Incorporating individual nontraditional risk factors, biomarkers, and selective use of noninvasive measures may help identify more at‐risk patients as well as truly low‐risk individuals, allowing for better targeting of treatment intensity. Using a combination of validated population‐based atherosclerotic CVD risk‐assessment tools, nontraditional risk factors, social health determinants, and novel markers of atherosclerotic disease, we should be able to improve our ability to assess CVD risk. Through scientific evidence, clinical judgment, and discussion between the patient and clinician, we can implement an effective evidence‐based strategy to assess and reduce CVD risk.
Keywords: General Clinical Cardiology/Adult, Ischemic Heart Disease, Preventive Cardiology
1. INTRODUCTION
As the leading cause of mortality in the United States, CVD accounts for >787 000 deaths annually.1 To define CV health, the American Heart Association (AHA) Life's Simple 7 (LS7) uses 7 metrics for clinicians: smoking, body mass index, physical activity level, healthy diet, total cholesterol, blood pressure, and fasting glucose. CV health in the United States is relatively poor, with only 3.3% of the population in ideal CV health among a survey of 356 411 adults.2 Patients with poor LS7 metrics have traditional risk factors, contributing to an increase in lifetime CVD risk. Favorably impacting the development of these risk factors is the cornerstone for CVD primordial prevention. By focusing on the cumulative effects of small population‐based interventions, healthcare systems can effectively practice primordial prevention (Figure 1).
Figure 1.
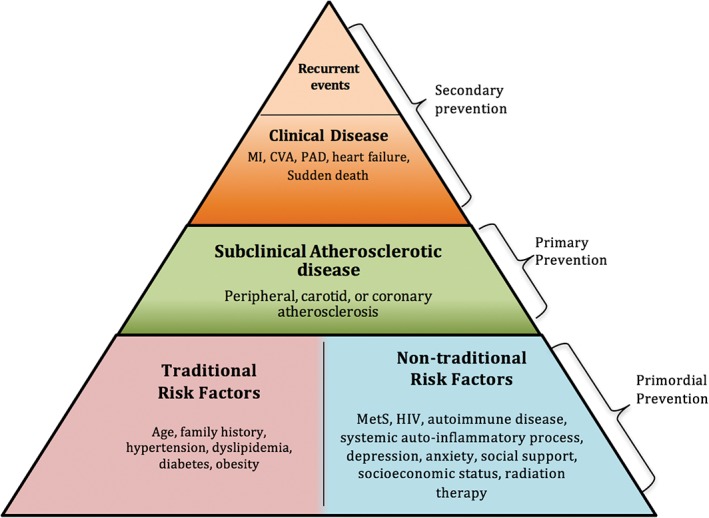
The pyramid to address CVD prevention by population level. Adapted and modified from Sandesara PB et al.61 Abbreviations: CV, cardiovascular; CVA, cerebrovascular accident; CVD, cardiovascular disease; HIV, human immunodeficiency virus; MetS, metabolic syndrome; MI, myocardial infarction; PAD, peripheral arterial disease
To prevent CVD‐related mortality, it is essential both to understand factors encompassing CV health and to systematically assess CV risk. Population‐based CVD risk models that focus on traditional risk factors have been incorporated into guidelines to target primary prevention, attempting to shift the population toward better CV health. Despite these models, a sizeable gap in identifying asymptomatic individuals who develop CVD remains, as many have few, if any, traditional risk factors.3 More than one‐third of individuals with hypertension in the United States are undetected, and the Adult Treatment Panel III (ATP‐III) approximates nearly 36 million patients needing treatment for elevated low‐density lipoprotein cholesterol (LDL‐C), whereas only 10 to 15 million are on lipid‐lowering therapy.3 Concomitantly, novel risk markers that underlie the pathogenesis of CVD are being investigated to potentially bridge this gap.
2. GENERAL APPROACH TO RISK ASSESSMENT
Risk assessment begins with a detailed history and physical examination for the evaluation of traditional and nontraditional risk factors. The use of a population‐based risk calculator, such as the American College of Cardiology (ACC)/AHA pooled cohort equations for ASCVD risk, should be employed to determine both absolute and lifetime CVD risk.4 Based on this estimation, evidence‐based guidelines can be utilized to determine individuals who would most likely benefit from statin and aspirin therapy and possibly more aggressive antihypertensive therapy. After determination of risk, a clinician‐patient risk discussion should take place in which risk data are reviewed, evidence‐based clinical guidelines are considered, and potential side effects to therapy are discussed with the patient as part of a shared–decision‐making approach to care.5 For patients in whom risk remains intermediate or uncertain, selective utilization of biomarkers, nontraditional risk factors, social determinants of health, and noninvasive measures of subclinical atherosclerosis can be applied to further inform treatment decisions (Figure 2).6
Figure 2.

Approach to CV risk assessment. “ABCDE: Assess, Base Risk Estimation, Consider, Develop, Engage” is the recommended approach to initiate risk assessment from a population perspective and, subsequently, individualize CV risk. Abbreviations: CV, cardiovascular
2.1. Traditional risk factors
In 1961, the coining of the term “risk factor” resulted from the identification of an initial set of traditional risk factors for coronary heart disease (CHD) in the Framingham Heart Study.7 The important risk factors identified by the Framingham study were age (males ≥45 years or females ≥55 years), male sex, hypertension (HTN), dyslipidemia, smoking, and diabetes mellitus (DM). Over the past several decades, family history of premature ASCVD (age < 55 years for males and < 65 years for females) has been added.
2.2. Population‐based risk assessment
2.2.1. CVD risk‐prediction tools
Periodic assessment of risk provides a starting point for office‐based discussion and initiation of primary‐prevention strategies. Several multivariate risk models have evolved through many iterations. The original Framingham Risk Score (FRS) was developed in 1998 as a means to assess CHD risk. This model was refined by the third ATP in 2002 with a focus on hard CHD endpoints, death, and nonfatal MI.8 The 2008 Framingham General CVD Risk Score incorporated the additional CV endpoints of stroke, heart failure, and peripheral arterial disease (PAD).9 More recent recommendations apply the 2013 ACC/AHA Pooled Cohort ASCVD Risk Score, derived from several patient cohorts: the Framingham original and offspring cohorts, the Atherosclerosis Risk in Communities (ARIC) study, the Coronary Artery Risk Development in Young Adults (CARDIA) study, and the Cardiovascular Health Study (CHS).4, 7, 10, 11, 12, 13
This algorithm consists of age, sex, ethnicity, total cholesterol, high‐density lipoprotein cholesterol (HDL‐C), systolic blood pressure, treatment for HTN, DM, and smoking.4 Endpoints are limited to hard ASCVD outcomes including CHD death, nonfatal myocardial infarction (MI), fatal stroke, and nonfatal stroke.4 Both a 10‐year risk for adults age 40 to 79 years and a lifetime risk for these adults can be calculated.4 The ACC/AHA Pooled Cohort Risk Calculator does not include prediction of angina pectoris or PAD, nor arterial revascularization or risk of heart failure from HTN or ischemic heart disease, thus underestimating total CVD risk. The ASCVD risk estimator also has been shown to overestimate hard ASCVD endpoints in the modern era when attempts have been made to validate it in more contemporary cohorts; this is likely due in part to the fact that the derivation cohort was predominantly from the 1970s and ‘80s.14
Other multivariate risk models have been developed; the European Systematic Coronary Risk Evaluation algorithm (SCORE), the QRISK Calculator, the Prospective Cardiovascular Münster Model (PROCAM), and the Reynolds Risk Score (RRS) are among the risk‐stratification tools incorporated into international guidelines (Table 1).15, 16, 17, 18, 19 These differ in the endpoints evaluated (eg, the SCORE algorithm predicts only CV mortality) and predictor variables (eg, the SCORE algorithm does not have DM nor HDL‐C as factors). Thus, the risk estimates using these other algorithms are often quite different from the FRS or ACC/AHA Pooled Cohort Risk Calculator.
Table 1.
An overview of CV risk scores and algorithms and their components
| Risk Algorithm | Components |
|---|---|
| ACC/AHA ASCVD Pooled Cohort Risk Calculator | Assesses risk of an adverse CV event (CHD death, nonfatal MI, fatal stroke, and nonfatal stroke) over 10 years and over lifetime |
| Comprised of age, sex, race, TC, HDL‐C, SBP, DBP, DM status, smoking status, treatment for HTN | |
| European Systematic Coronary Risk Evaluation (SCORE) algorithm | Separated into low and high risk based on European country |
| Assesses fatal CVD risk over 10 years | |
| Comprised of age, sex, TC, SBP, smoking status | |
| QRISK Calculator (2–2017) | Assesses 10‐year adverse events (MI or stroke) |
| Comprised of age, sex, ethnicity, smoking status, DM status, family history of premature MI, CKD (stage 4/5), AF, treatment for HTN, RA, cholesterol/HDL‐C ratio, SBP, BMI | |
| Prospective Cardiovascular Münster (PROCAM) model | Assesses 10‐year risk of acute MI or SCD |
| Comprised of age, LDL‐C, HDL‐C, TG, smoking status, DM status, family history of premature MI, SBP | |
| Reynolds Risk Score (RRS) | Assesses 10‐year risk of MI, stroke, CABG, angioplasty, or CVD death |
| Comprised of age, sex, SBP, TC, HDL‐C, family history of premature MI, hsCRP |
Abbreviations: ACC, American College of Cardiology; AF, atrial fibrillation; AHA, American Heart Association; ASCVD, atherosclerotic cardiovascular disease; BMI, body mass index; CABG, coronary artery bypass grafting; CHD, coronary heart disease; CKD, chronic kidney disease; CV, cardiovascular; CVD, cardiovascular disease; DBP, diastolic blood pressure; DM, diabetes mellitus; HDL‐C, high‐density lipoprotein cholesterol; hsCRP, high‐sensitivity C‐reactive protein; HTN, hypertension; LDL‐C, low‐density lipoprotein cholesterol; MI, myocardial infarction; RA, rheumatoid arthritis; SBP, systolic blood pressure; SCD, sudden coronary death; TC, total cholesterol; TG, triglycerides.
2.3. Nontraditional risk factors for ASCVD
Nontraditional risk factors related to ASCVD risk include the metabolic syndrome (MetS), inflammatory factors, autoimmune disease (eg, systemic lupus erythematosus [SLE], rheumatoid arthritis [RA], systemic sclerosis), human immunodeficiency virus status, gestational syndromes, extensive comorbidities, psychosocial stressors, and social determinants.
MetS is a complex, pathophysiologic state defined by abdominal obesity (males, >40 inches; females, >35 inches, but with different cutpoints recommended for non‐US Caucasians), hypertriglyceridemia (≥150 mg/dL), low HDL‐C (males, <40 mg/dL; females, <50 mg/dL), increased blood pressure (≥130/85 mm Hg or on therapy for HTN), and elevated fasting glucose suggestive of insulin resistance (≥100 mg/dL or on hypoglycemic medication). MetS comprises a constellation of risk factors. The presence of MetS, even in the absence of DM, along with its individual components not found in traditional risk algorithms, confers an increased ASCVD risk that is often underrecognized.20, 21
Rheumatologic disease and systemic autoinflammatory processes are associated with elevated CV risk and are more likely in young to middle‐age females who are classically considered low risk. These diseases contribute to chronic inflammation, endothelial dysfunction, and accelerated atherosclerosis. Women ages 35 to 44 years with diagnosed SLE were 50× more likely (risk ratio: 52.43, 95% confidence interval [CI]: 21.6–98.5) to suffer a MI than were their age‐matched counterparts without SLE.22 Women diagnosed with RA are twice as likely to suffer from a MI. Furthermore, an estimated 40% to 50% of the mortality in patients with RA has been attributed to CVD,23 and medications used for this disorder can be associated with worsening traditional risk factors, including dyslipidemia.24
Human immunodeficiency virus is associated with several factors that contribute to increased CV risk. Accelerated atherosclerosis secondary to viral‐mediated damage to the vascular endothelium is seen in both highly active antiretroviral therapy (HAART)‐naïve and HAART‐treated populations.25 Increased expression of adhesion molecules (E‐selectin and intracellular adhesion molecule 1) and inflammatory cytokines (tumor necrosis factor‐α, interleukin‐6) contribute to the pathogenesis of CVD.
Gestational syndromes, including gestational HTN and gestational DM, may have an impact on future CV risk. In 3909 patients in the Kentucky Women's Health Registry, the prevalence of CVD was significantly greater in patients with gestational DM compared with those without the syndrome (43.8% vs 22.4%; 95% CI: 13.2–29.6).26 Patients who experience gestational HTN, preeclampsia, or other placental‐mediated adverse events are often at risk of developing adverse CV risk factors after pregnancy.27 The retrospective Cardiovascular Health After Maternal Placental Syndromes (CHAMPS) study cohort comprised 1.03 million women without CVD prior to pregnancy.28 Adverse CV events were twice as common (hazard ratio: 2.0, 95% CI: 1.7–2.2) in women with preeclampsia, placental abruption, or placental infarction, and even higher when these maternal placental syndromes were associated with poor fetal growth or intrauterine fetal death.28
Extensive burden of comorbidities, particularly affecting older populations, can affect CV risk. In patients with chronic obstructive pulmonary disease, underlying low‐grade inflammation and oxidative stress have led to increased frequency of coronary artery disease when adjusting for other factors.29 Patients with chronic liver disease associated with hepatic steatosis, including alcoholic liver disease, chronic hepatitis C infection, and nonalcoholic fatty liver disease, have increased CV risk.30 Chronic kidney disease is a well‐known contributor to CV morbidity and mortality.31 This risk is inversely related to glomerular filtration rate and persists after adjusting for the traditional ASCVD risk factors.32 In end‐stage renal disease patients on dialysis, the rate of CV mortality is 10× to 20× higher than in the general population.33
Other determinants of health, including social support, social networks, socioeconomic status, and mental health disorders, affect CVD risk. Individuals of lower socioeconomic status are at higher risk of traditional risk factors and participating in at‐risk behaviors, including tobacco use. Rates of CVD significantly increase in countries with the greatest income inequality.34 Individuals with major depression following an MI are at an elevated risk of death and future CV events.35 Additionally, mental health disorders may serve as a barrier to adherence with cardiac medications. Hence, it is sometimes useful to screen for anxiety and depression using the Patient Health Questionnaire (PHQ‐9) and the Generalized Anxiety Disorder scale (GAD‐7).36
2.4. Risk reclassification
Current guidelines stratify patients into low or high risk, with a group falling between the 2 categories.4 However, patients stratified between the low‐ and high‐risk groups, defined by an ASCVD pooled risk score of 5% to 7.5%, can have a highly variable comprehensive CVD risk.37 This lack of clarity may cause difficulty for clinicians deciding between a conservative or more aggressive therapeutic approach.37 Certain risk markers can augment the predictive value of population‐based risk calculators and potentially reclassify individuals into a new risk category.
The C‐statistic, a commonly reported standard for CVD risk‐prediction models, is a statistical tool to discriminate future adverse events from nonevents.15 It ranges from 0.5 (the score applied is no better than random chance) to 1.0 (perfect discrimination) and can discern and rank the accuracy of one risk model over another.15 A clinically significant increase in C‐statistic for the coronary artery calcification (CAC) score typically seems to be around a threshold of ≥0.10. The C‐statistic cannot say how much more likely an adverse event is to occur, and it cannot estimate the similarity between observed and estimated risk.15 For instance, when examining multiple risk indices including the RRS and the ATP‐III score in the Women's Health Initiative observational cohort, statistically significant differences in the C‐statistic were seen. A C‐statistic of 0.765 from the RRS vs a C‐statistic of 0.757 from the ATP‐III score (P = 0.04) suggested that the RRS is better discriminated against future adverse events compared with the ATP‐III score.38
Two important metrics to assess reclassification of risk are the net reclassification improvement (NRI) index and the integrative discrimination index (IDI).39 The NRI indicates the quantity of reclassification occurring that is statistically significant, whereas the IDI determines how far subjects move on a continuum of predicted risk.39 When applying the NRI to the ASCVD risk score through the investigation of novel markers of subclinical atherosclerosis, the potential enhancement in risk assessment can be demonstrated. For example, some investigators have examined several markers to improve risk stratification in the Multi‐Ethnic Study of Atherosclerosis (MESA) cohort, composed of nondiabetic, intermediate‐risk patients.40 These included traditional risk factors such as family history, biomarkers such as high‐sensitivity C‐reactive protein (hsCRP), and other indicators of subclinical atherosclerosis such as CAC, ankle‐brachial index (ABI), carotid intima‐media thickness, and brachial flow‐mediated dilation.40 Added to the FRS, the CAC had the highest improvement in accurately predicting risk compared with several other markers. The NRI effectively reclassified a net 25.5% of events to a higher risk category while reclassifying a net 40.4% of nonevents to a lower risk category.40 Multiple risk indices within a certain population can be compared with the NRI and the IDI. For example, the FRS, ATP‐III score and the RRS were applied to the multiethnic Women's Health Initiative observational cohort.38 The RRS compared with the ATP‐III score showed an improvement in predicting risk, with a 4% (P = 0.02) improvement in prediction of events based on the NRI (4.9%; 95% CI: 1.2–8.7, P = 0.010) and with improvement in discrimination of risk based on the IDI (4.1%; 95% CI: 2.7–5.7, P < 0.0001).38
3. ADDITIONAL POTENTIAL TOOLS FOR CV RISK ASSESSMENT
3.1. Biomarkers
3.1.1. High‐sensitivity CRP
An acute‐phase reactant, CRP is a surrogate measure of subclinical, systemic inflammation. In the Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) trial, hsCRP was shown to independently predict events in asymptomatic individuals.41 Asymptomatic patients with elevated hsCRP and low LDL‐C randomized to statin therapy had a 20% risk reduction in all‐cause mortality.41 However, the cost‐effectiveness of screening an asymptomatic population is unclear; moreover, there was no low‐hsCRP group studied in JUPITER, and the Heart Outcomes Prevention Evaluation (HOPE‐3) trial did not find that hsCRP values predicted benefit from statin use. High‐sensitivity CRP has been incorporated into the RRS, a population‐based risk calculator, which also incorporates family history of premature CVD. The 2013 ACC/AHA risk assessment guidelines recommend consideration of hsCRP measurement when the treatment decision is uncertain, with a level of ≥2 mg/L considered in support for upward risk stratification.42 This cutpoint is challenging because African Americans tend to have significantly higher hsCRP levels than do Caucasians, and females tend to have higher baseline levels than do men.43, 44 In the Canakinumab Anti‐inflammatory Thrombosis Outcome Study (CANTOS) trial, canakinumab use led to a statistically significant reduction in hsCRP despite no lipid effects and a reduction in recurrent CV events, proving the inflammatory hypothesis of atherothrombosis.45
3.1.2. Lipoprotein(a)
Lipoprotein(a), or Lp(a), composed of apolipoprotein B100 and a heterogeneous glycoprotein apolipoprotein(a), is a pro‐inflammatory, proatherogenic marker conferring a genetic risk of CVD. Adding Lp(a) to the FRS and RRS enhanced the predictive capability of patients stratified as intermediate risk over a 15‐year follow‐up period.46 Currently, antisense oligonucleotides are being developed to lower Lp(a); however, in the absence of clinical trials, the impact on CVD risk reduction remains unknown.47 At this time, current European Atherosclerosis Society (EAS) and US National Lipid Association (NLA) guidelines—but not the ACC/AHA 2013 Cardiovascular Risk Assessment guidelines—recommend a single measurement in patients with premature CVD, familial hypercholesterolemia, recurrent CVD despite treatment, and markedly elevated risk of fatal or nonfatal CVD.48
3.1.3. Apolipoprotein B
Apolipoprotein B (apoB), a structural protein found in atherogenic lipoproteins, can be directly measured to calculate a collective atherogenic burden.3 A meta‐analysis involving 91 307 statin‐naïve, primary‐prevention patients demonstrated that apoB was one of the best predictors of future CVD compared with other atherogenic measures.49 The Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS), composed of 6605 primary‐prevention patients on statin therapy, showed apoB outperforming LDL‐C in predicting future CV events.50 In the CARDIA study comprising 2794 young adults without CVD, elevated apoB levels—despite a low non–HDL‐C—predicted a nonzero CAC score at age 25.51 The NLA specified apoB‐containing lipoproteins as “a root cause of atherosclerosis” contributing to clinical ASCVD events and recommends non–HDL‐C as a primary target of therapy along with LDL‐C.48 Although simple, nonfasting non–HDL‐C measurement is more predictive than LDL‐C, the NRI for apoB has been marginal. Its identity as a separate laboratory test with higher cost and slower turnaround time has contributed to a lack of recommendation for routine use by the ACC/AHA.4
3.2. HDL‐C and HDL‐C functionality
The use of HDL‐C in large, prospective, observational studies suggest an inverse relationship between HDL‐C levels and adverse CV events.52 HDL‐C is a central component of the ASCVD risk equation and other risk calculators.4 In post hoc analyses of the Treating to New Targets (TNT) study and JUPITER trial, there was no statistically significant association between HDL‐C levels and adverse CV events in patients on statin therapy.41 Moreover, increasing HDL‐C, using therapy such as niacin on a background of statin use, does not appear to favorably affect CV outcomes.53
HDL‐C function at the molecular level may be a more robust predictor of adverse CV events than HDL‐C levels.52 Cholesterol efflux capacity, the most studied aspect of HDL‐C functionality, involves the ability to transport cholesterol molecules from cells, like arterial macrophages, to the extracellular environment in a process termed reverse cholesterol transport. Within an atherosclerotic plaque, there is impaired cholesterol efflux due to dysfunctional apolipoprotein‐A1.54 A prospective cohort study involving 2924 individuals from the Dallas Heart Study evaluated the role of cholesterol efflux capacity in predicting adverse CV events.54 Adjusting for traditional risk factors including HDL‐C levels, there was a 67% risk reduction in adverse CV events in the highest quartile of efflux capacity, compared with the lowest.54 However, measures of HDL‐C function remain investigational and are not currently recommended by guidelines.
4. NONINVASIVE MEASURES OF SUBCLINICAL ATHEROSCLEROSIS
4.1. Ankle‐brachial index
The ABI is utilized for the detection of PAD. Additionally, an abnormal ABI <0.9 is diagnostic of PAD and is associated with increased CV risk as well as atherosclerosis in other vascular territories. A meta‐analysis involving 24 375 men and 20 377 females free of CVD showed that adding ABI to the FRS better predicted future coronary events, with a significant NRI for both men and women (4.3% and 9.6%, respectively).55 The highest NRI values for both men and women were seen in those with 10‐year CVD risk scores of 10% to 19%. ABI levels of <0.9 were associated with a > 2‐fold increase in mortality, and borderline levels of 0.9 to 1.0 were still associated with nearly 2‐fold increased risk.56 Although the data suggest that ABI is effective for CV risk prediction independent of conventional assessment, intention to treat and as‐treated analysis of quality‐adjusted life‐years, lifetime costs, and incremental cost‐effectiveness ratios have demonstrated that ABI is not as cost‐effective compared with other modalities.56 Recent ACC/AHA guidelines designated ABI as a class IIb recommendation if management decisions remain unclear.4 Moreover, in many states this test is not reimbursable if it is not done in an accredited vascular laboratory.
4.2. Coronary CT calcium score
CAC, as detected by computed tomography, involves noninvasive measurement of coronary calcification burden. A score of 0 suggests no identifiable calcified disease and low risk of an ASCVD event over 10 to 15 years, being the best predictor of total survival out to 15 years.57 Increasing CAC scores indicate the presence of and increasing severity of disease. The MESA study and a meta‐analysis comprising 4 studies demonstrated increased risk of CHD events with increasing levels of CAC in patients without CVD.57
A subgroup of the MESA cohort looked at 6814 men and women without CVD who were statin‐eligible per the ASCVD risk score58; 44% of the subgroup had a CAC score of 0, with a very low 10‐year ASCVD rate of 4.2 per 1000 person‐years.58 These findings were corroborated in a prospective study of 687 treatment‐naïve patients free of CVD.59 However, if the expected 22% relative risk reduction per 1‐mmol/L decrease in LDL‐C from statin meta‐analyses were applied, then clinical utility appeared severely limited in this population.58 Among those deemed reasonable to treat based on ASCVD guidelines (10‐year risk, 5%–7%), 40% had a CAC score of 0.59 Collectively, this indicates a high incidence (40%–50%) of CAC scores of 0 in patients in whom statin therapy is being considered based on the current ACC/AHA guidelines, but who are unlikely to benefit based on their low risk of ASCVD and correspondingly high number needed to treat.
In the appropriate population, CAC detection is consistently superior to other novel markers of CVD risk. In an intermediate‐risk population, CAC score outperformed other risk markers such as carotid intima‐media thickness, ABI, and hsCRP for predicting CVD based on C‐statistic and NRI.40 Drawbacks to widespread use of CAC measurement include cost ($75–$100) and exposure to ionizing radiation (equivalent to 1–2 mammograms). The 2013 ACC/AHA guidelines noted that CAC was likely the most useful of the current approaches to improve risk assessment among those with indeterminate risk, with a class IIb‐B recommendation, but the level of evidence is likely to be elevated in future ACC/AHA guidelines of ASCVD risk assessment.4 However, the Society of Cardiovascular Computed Tomography (SCCT) does not recommend the use of a CAC score in asymptomatic, low‐risk patients with an ASCVD risk of <10%.60
5. INDIVIDUALIZED RISK ASSESSMENT
Using traditional risk factors, nontraditional risk factors, novel biomarkers, and noninvasive techniques to more authoritatively classify patients may improve a clinician's prediction of cardiac risk, bridging the detection gap in asymptomatic patients. Further use of precision medicine, where clinicians incorporate individual genetic, environmental, and experiential variability and actively overcome socioeconomic barriers, may improve CVD outcomes.3 Although many novel risk markers may predict CVD, their tangible impact on the NRI varies greatly. In further studies of markers, incorporation of risks such as radiation exposure and cost‐effectiveness should be taken into consideration. Based on the NRI, a patient who fits between the low‐ and high‐risk groups, as defined by guidelines, can potentially be further stratified into a higher‐risk category if the CAC score is ≥300 or 75th percentile, there is a family history of premature ASCVD, the CRP is ≥2 mg/L, and/or the ABI is <0.9.4
6. CONCLUSION
A focus on prevention is an essential, cost‐effective way to reduce CVD burden globally. Population‐based approaches, such as the ASCVD Pooled Cohort Risk Calculator, provide an initial efficient method to identify at‐risk patients. Patients should be further assessed for nontraditional risk factors, and if CV risk remains unclear, consideration should be given to use novel risk markers to improve detection of subclinical atherosclerosis.
CONFLICTS OF INTEREST
The authors declare no potential conflicts of interest.
Khambhati J, Allard‐Ratick M, Dhindsa D, et al. The art of cardiovascular risk assessment. Clin Cardiol. 2018;41:677–684. 10.1002/clc.22930
REFERENCES
- 1. Mozaffarian D, Benjamin EJ, Go AS, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2015 update: a report from the American Heart Association [published corrections appear in Circulation 2015;131:e535 and Circulation 2016;133:e417]. Circulation. 2015;131:e29–e322. [DOI] [PubMed] [Google Scholar]
- 2. Fang J, Yang Q, Hong Y, et al. Status of cardiovascular health among adult Americans in the 50 States and the District of Columbia, 2009. J Am Heart Assoc. 2012;1:e005371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pasternak RC, Abrams J, Greenland P, et al. 34th Bethesda Conference: Task force #1—Identification of coronary heart disease risk: is there a detection gap? J Am Coll Cardiol. 2003;41:1863–1874. [DOI] [PubMed] [Google Scholar]
- 4. Goff DC Jr, Lloyd‐Jones DM, Bennett G, et al. 2013. ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published correction appears in Circulation. 2014;129(25 suppl 2):S74–S75]. Circulation. 2014;129(25 suppl 2):S49–S73. [DOI] [PubMed] [Google Scholar]
- 5. Martin SS, Sperling LS, Blaha MJ, et al. Clinician‐patient risk discussion for atherosclerotic cardiovascular disease prevention: importance to implementation of the 2013 ACC/AHA guidelines. J Am Coll Cardiol. 2015;65:1361–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gaziano JM, Wilson PW. Cardiovascular risk assessment in the 21st century. JAMA. 2012;308:816–817. [DOI] [PubMed] [Google Scholar]
- 7. Kannel WB, Dawber TR, Kagan A, et al. Factors of risk in the development of coronary heart disease—six year follow‐up experience: the Framingham Study. Ann Intern Med. 1961;55:33–50. [DOI] [PubMed] [Google Scholar]
- 8. National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report . Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 9. D'Agostino RB Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. [DOI] [PubMed] [Google Scholar]
- 10. Kannel WB, Feinleib M, McNamara PM, et al. An investigation of coronary heart disease in families: the Framingham offspring study. Am J Epidemiol. 1979;110:281–290. [DOI] [PubMed] [Google Scholar]
- 11. ARIC investigators . The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 12. Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–1116. [DOI] [PubMed] [Google Scholar]
- 13. Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. [DOI] [PubMed] [Google Scholar]
- 14. DeFilippis AP, Young R, McEvoy JW, et al. Risk score overestimation: the impact of individual cardiovascular risk factors and preventive therapies on the performance of the American Heart Association–American College of Cardiology–Atherosclerotic Cardiovascular Disease risk score in a modern multi‐ethnic cohort. Eur Heart J. 2017;38:598–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lloyd‐Jones DM. Cardiovascular risk prediction: basic concepts, current status, and future directions. Circulation. 2010;121:1768–1777. [DOI] [PubMed] [Google Scholar]
- 16. Conroy RM, Pyörälä K, Fitzgerald AP, et al; SCORE Project Group . Estimation of ten‐year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24:987–1003. [DOI] [PubMed] [Google Scholar]
- 17. Hippisley‐Cox J, Coupland C, Vinogradova Y, et al. Derivation and validation of QRISK, a new cardiovascular disease risk score for the United Kingdom: prospective open cohort study. BMJ. 2007;335:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ridker PM, Buring JE, Rifai N, et al. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score [published correction appears in JAMA. 2007;297:1433]. JAMA. 2007;297:611–619. [DOI] [PubMed] [Google Scholar]
- 19. Ridker PM, Paynter NP, Rifai N, et al. C‐reactive protein and parental history improve global cardiovascular risk prediction: the Reynolds Risk Score for men. Circulation. 2008;118:2243–2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mottillo S, Filion KB, Genest J, et al. The metabolic syndrome and cardiovascular risk: a systematic review and meta‐analysis. J Am Coll Cardiol. 2010;56:1113–1132. [DOI] [PubMed] [Google Scholar]
- 21. Sperling LS, Mechanick JI, Neeland IJ, et al. The CardioMetabolic Health Alliance: working toward a new care model for the metabolic syndrome. J Am Coll Cardiol. 2015;66:1050–1067. [DOI] [PubMed] [Google Scholar]
- 22. Manzi S, Meilahn EN, Rairie JE, et al. Age‐specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham Study. Am J Epidemiol. 1997;145:408–415. [DOI] [PubMed] [Google Scholar]
- 23. Bacon PA, Stevens RJ, Carruthers DM, et al. Accelerated atherogenesis in autoimmune rheumatic diseases. Autoimmun Rev. 2002;1:338–347. [DOI] [PubMed] [Google Scholar]
- 24. Miller LW. Cardiovascular toxicities of immunosuppressive agents. Am J Transplant. 2002;2:807–818. [DOI] [PubMed] [Google Scholar]
- 25. Crane HM, Paramsothy P, Drozd DR, et al; Centers for AIDS ResearchNetwork of Integrated Clinical Systems (CNICS) . Types of myocardial infarction among human immunodeficiency virus‐infected individuals in the United States. JAMA Cardiol. 2017;2:260–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Archambault C, Arel R, Filion KB. Gestational diabetes and risk of cardiovascular disease: a scoping review. Open Med. 2014;8:e1–e9. [PMC free article] [PubMed] [Google Scholar]
- 27. Ahmed R, Dunford J, Mehran R, et al. Pre‐eclampsia and future cardiovascular risk among women: a review. J Am Coll Cardiol. 2014;63:1815–1822. [DOI] [PubMed] [Google Scholar]
- 28. Ray JG, Vermeulen MJ, Schull MJ, et al. Cardiovascular health after maternal placental syndromes (CHAMPS): population‐based retrospective cohort study. Lancet. 2005;366:1797–1803. [DOI] [PubMed] [Google Scholar]
- 29. Bhatt SP, Dransfield MT. Chronic obstructive pulmonary disease and cardiovascular disease. Transl Res. 2013;162:237–251. [DOI] [PubMed] [Google Scholar]
- 30. Loria P, Marchesini G, Nascimbeni F, et al. Cardiovascular risk, lipidemic phenotype and steatosis: a comparative analysis of cirrhotic and non‐cirrhotic liver disease due to varying etiology. Atherosclerosis. 2014;232:99–109. [DOI] [PubMed] [Google Scholar]
- 31. Sarnak MJ , Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension. 2003;42:1050–1065. [DOI] [PubMed] [Google Scholar]
- 32. Manjunath G, Tighiouart H, Ibrahim H, et al. Level of kidney function as a risk factor for atherosclerotic cardiovascular outcomes in the community. J Am Coll Cardiol. 2003;41:47–55. [DOI] [PubMed] [Google Scholar]
- 33. Foley RN, Parfrey PS, Sarnak MJ. Epidemiology of cardiovascular disease in chronic renal disease. J Am Soc Nephrol. 1998;9(12 suppl):S16–S23. [PubMed] [Google Scholar]
- 34. Marmot M, Bobak M. International comparators and poverty and health in Europe. BMJ. 2000;321:1124–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bush DE, Ziegelstein RC, Tayback M, et al. Even minimal symptoms of depression increase mortality risk after acute myocardial infarction. Am J Cardiol. 2001;88:337–341. [DOI] [PubMed] [Google Scholar]
- 36. Coulter SA, Campos K. Identify and treat depression for reduced cardiac risk and improved outcomes. Tex Heart Inst J. 2012;39:231–234. [PMC free article] [PubMed] [Google Scholar]
- 37. Martin SS, Blumenthal RS. Concepts and controversies: the 2013 American College of Cardiology/American Heart Association risk assessment and cholesterol treatment guidelines. Ann Intern Med. 2014;160:356–358. [DOI] [PubMed] [Google Scholar]
- 38. Cook NR, Paynter NP, Eaton CB, et al. Comparison of the Framingham and Reynolds Risk scores for global cardiovascular risk prediction in the multiethnic Women's Health Initiative. Circulation. 2012;125:1748–1756, S1–S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pencina MJ, D'Agostino RB Sr, D'Agostino RB Jr, et al. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172, 207–212. [DOI] [PubMed] [Google Scholar]
- 40. Yeboah J, McClelland RL, Polonsky TS, et al. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate‐risk individuals. JAMA. 2012;308:788–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ridker PM, Genest J, Boekholdt SM, et al. HDL cholesterol and residual risk of first cardiovascular events after treatment with potent statin therapy: an analysis from the JUPITER trial. Lancet. 2010;376:333–339. [DOI] [PubMed] [Google Scholar]
- 42. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013. ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published corrections appear in J Am Coll Cardiol 2014;63(25 part B):3024–3025, J Am Coll Cardiol 2015;66:2812]. J Am Coll Cardiol 2014;63(25 part B):2889–2934. [DOI] [PubMed]
- 43. Khera A, McGuire DK, Murphy SA, et al. Race and gender differences in C‐reactive protein levels. J Am Coll Cardiol. 2005;46:464–469. [DOI] [PubMed] [Google Scholar]
- 44. Lakoski SG, Cushman M, Criqui M, et al. Gender and C‐reactive protein: data from the Multiethnic Study of Atherosclerosis (MESA) cohort. Am Heart J. 2006;152:593–598. [DOI] [PubMed] [Google Scholar]
- 45. Ridker PM, Everett BM, Thuren T, et al; CANTOS TrialGroup. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 46. Willeit P, Kiechl S, Kronenberg F, et al. Discrimination and net reclassification of cardiovascular risk with lipoprotein(a): prospective 15‐year outcomes in the Bruneck Study [published correction appears in J Am Coll Cardiol. 2016;67:737]. J Am Coll Cardiol. 2014;64:851–860. [DOI] [PubMed] [Google Scholar]
- 47. Tsimikas S. Lipoprotein(a): novel target and emergence of novel therapies to lower cardiovascular disease risk. Curr Opin Endocrinol Diabetes Obes. 2016;23:157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jacobson TA, Ito MK, Maki KC, et al. National Lipid Association recommendations for patient‐centered management of dyslipidemia: part 1—full report. J Clin Lipidol. 2015;9:129–169. [DOI] [PubMed] [Google Scholar]
- 49.Di Angelantonio E, Sarwar N, Perry P, et al; Emerging Risk Factors Collaboration . Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302:1993–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gotto AM Jr, Whitney E, Stein EA, et al. Relation between baseline and on‐treatment lipid parameters and first acute major coronary events in the Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS). Circulation. 2000;101:477–484. [DOI] [PubMed] [Google Scholar]
- 51. Wilkins JT, Li RC, Sniderman A, et al. Discordance between apolipoprotein B and LDL‐cholesterol in young adults predicts coronary artery calcification: the CARDIA study. J Am Coll Cardiol. 2016;67:193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rader DJ, Hovingh GK. HDL and cardiovascular disease. Lancet. 2014;384:618–625. [DOI] [PubMed] [Google Scholar]
- 53. Boden WE, Probstfield JL, Anderson T, et al; AIM‐HIGH Investigators . Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–2267. [DOI] [PubMed] [Google Scholar]
- 54. Rohatgi A, Khera A, Berry JD, et al. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med. 2014;371:2383–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fowkes FG, Murray GD, Butcher I, et al; Ankle Brachial Index Collaboration . Development and validation of an ankle brachial index risk model for the prediction of cardiovascular events. Eur J Prev Cardiol. 2014;21:310–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Van Kempen BJ, Ferket BS, Steyerberg EW, et al. Comparing the cost‐effectiveness of four novel risk markers for screening asymptomatic individuals to prevent cardiovascular disease (CVD) in the US population. Int J Cardiol. 2016;203:422–431. [DOI] [PubMed] [Google Scholar]
- 57. Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. [DOI] [PubMed] [Google Scholar]
- 58. Nasir K, Bittencourt MS, Blaha MJ, et al. Implications of coronary artery calcium testing among statin candidates according to American College of Cardiology/American Heart Association Cholesterol Management Guidelines: MESA (Multi‐Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2015;66:1657–1668. [DOI] [PubMed] [Google Scholar]
- 59. Isma'eel H, Min D, Al‐Shaar L, et al. Assessing level of agreement for atherosclerotic cardiovascular disease risk categorization between coronary artery calcium score and the American College of Cardiology/American Heart Association cardiovascular prevention guidelines and the potential impact on treatment recommendations. Am J Cardiol. 2016;118:1480–1485. [DOI] [PubMed] [Google Scholar]
- 60. Greenland P, Bonow RO, Brundage BH, et al. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) developed in collaboration with the Society of Atherosclerosis Imaging and Prevention and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol. 2007;49:378–402. [DOI] [PubMed] [Google Scholar]
- 61. Sandesara PB, Lambert CT, Gordon NF, et al. Cardiac rehabilitation and risk reduction: time to “rebrand and reinvigorate.” J Am Coll Cardiol. 2015;65:389–395. [DOI] [PubMed] [Google Scholar]


