Summary
Aims
Oxidative stress and inflammation have been implicated in the pathogenesis of vascular dementia (VD). Thioredoxin‐interacting protein (TXNIP) plays a vital role in oxidative stress and NOD‐like receptor protein 3 (NLRP3) inflammasome activation. There is evidence that acupuncture has an antioxidative and neuroprotective effect in VD. In this study, we investigated whether acupuncture can attenuate cognitive impairment via inhibiting TXNIP‐associated oxidative stress and inflammation in VD rats.
Methods
Both common carotid arteries were occluded (2‐vessel occlusion [2VO]) in rats to model VD. The neuroprotective effect of acupuncture was assessed by the Morris water maze and Nissl staining. Oxidative stress was assessed by detecting levels of reactive oxygen species, DNA oxidation, and antioxidase. Western blot, real‐time PCR, and immunofluorescence were used to detect the expression of TXNIP, NLRP3, caspase‐1, and IL‐1β. A TXNIP siRNA intraventricular injection was applied to investigate whether acupuncture mimicked the effect of TXNIP inhibitor.
Results
Our findings demonstrated that VD rats treated with acupuncture had reduced hippocampal neuronal loss and oxidative stress. The upregulation of TXNIP, NLRP3, caspase‐1, and IL‐1β induced by 2VO was also reversed by acupuncture. Furthermore, TXNIP siRNA had a similar effect as acupuncture on cognition, hippocampal neurons, and ROS production in VD rats.
Conclusion
In conclusion, our study suggests that the neuroprotective effects of acupuncture in VD are mediated through reducing expression of TXNIP‐associated oxidative stress and inflammation.
Keywords: cognitive impairment, inflammation, oxidative stress, thioredoxin‐interacting protein, vascular dementia
1. INTRODUCTION
Vascular dementia (VD) is the second most cognitive disorders after Alzheimer's disease (AD), which is strongly related to aging and a range of vascular conditions. There is difficulty identifying diagnostic criteria and therapy of VD, because of uncertainties over its mechanisms.1 Chronic cerebral hypoperfusion (CCH) is closely correlated with the development and progression of degenerative cognitive impairment, including VD and AD.2, 3 In experimental studies, bilateral common carotid artery occlusion, which is also called 2‐vessel occlusion (2VO), was established to reproduce CCH‐induced cognitive impairment. As the most commonly used VD model, 2VO has been used to find out the pathological changes and molecular mechanisms involved in cognitive impairment in CCH.4
Overproduction of reactive species oxygen (ROS) and inflammation resulting from ischemia are two major contributors to the pathogenesis of VD.5, 6 During CCH, endogenous antioxidants decrease, along with an increase in oxidative damage to protein, lipid, and DNA.7 As an intracellular endogenous regulator of oxidative stress and inflammation, thioredoxin‐interacting protein (TXNIP) played a critical part in ischemic diseases in many studies.8, 9 Under oxidative stress, TXNIP would oxidize thioredoxin (Trx) via binding to the Trx active site, enhancing oxidative stress and activating the apoptosis signal‐regulating kinase 1 (ASK1)‐mediated signaling pathway.10, 11 TXNIP was also found to mediate and activate immune response in response to overproduction of ROS, via binding to NOD‐like receptor protein 3 (NLRP3) inflammasome.12 The NLRP3 inflammasome is a molecular protein complex mediating sterile inflammation during ischemia.13 After the combination of NLRP3 and TXNIP, NLRP3 inflammasome was activated, and an another component pro‐caspase‐1 transformed to caspase‐1, from which pro‐interleukin‐1β (pro‐IL‐1β) was cleaved and IL‐1β was secreted.12 Recently, TXNIP has been found to participate in the biochemical process of cerebral ischemia. Inhibiting TXNIP or TXNIP‐knockout played a neuroprotective role against cerebral ischemia.14, 15
Acupuncture is widely accepted as a complementary and alternative medicinal therapy, which has proven effective in many neurological diseases, including VD.16, 17, 18 Studies have also provided evidence that acupuncture could promote stroke rehabilitation.19 Our previous studies found that improving the cognition of 2VO rats could be achieved by acupuncture via inhibiting O2‐ production.20 However, whether and how the regulation of both oxidative stress and inflammation, TXNIP, is involved in acupuncture's effect on VD remains unclear. In this study, we evaluated whether acupuncture can reduce TXNIP and inhibit TXNIP‐related oxidative stress and inflammation to provide neuroprotective effects in VD, and explored the potential TXNIP inhibition for treating VD.
2. MATERIALS AND METHODS
2.1. Animals
Male Wistar rats (aged 10 weeks, from Vital River Laboratory Animal Technology Co. Ltd, Beijing, China) were housed at room temperature (22 ± 2°C) and standard humidity (50 ± 10%), with 12 hours dark/light cycle and free access to water and food. Rats were randomly into 6 groups: sham‐operated group (sham), 2‐vessel occlusion group (2VO), 2VO with acupuncture group (2VO+Acu), 2VO with nonacupoint acupuncture group (2VO+Non‐acu), 2VO with stereotactic injection of control siRNA (2VO+ con siRNA), and 2VO with injection of TXNIP siRNA (2VO+ TX siRNA). All experimental procedures were performed in accordance with Ethics Committee for Animal Experimentation and Use Committee of China Academy of Chinese Medical Sciences (Beijing, China).
2.2. Surgery, siRNA injection, and acupuncture treatment
Rats were anesthetized with sodium pentobarbital (40 mg/kg), and permanent bilateral common carotid artery occlusion was performed to induce a model of global cerebral hypoperfusion.21 The sham‐operated rats were treated with the same procedures except for the occlusion of common carotid arteries. TXNIP siRNA or control siRNA with a scrambled sequence (1 ug/uL; GenePharma, China; 10 μL) was injected into the ipsilateral lateral ventricle (−1 mm anteroposterior, 2 mm lateral, 4 mm deep) before 2VO or acupuncture. Mini‐osmotic pump was used to continuously deliver siRNA that was dissolved in the transfection medium (Engreen Biosystem Co., Ltd, Beijing, China). The sequence of the TXNIP siRNA was sense 5′ ‐GCU GGA UAG ACC UAA ACA UTT‐ 3′ and antisense 5′ ‐AUG UUU AGG UCU AUC CAG CTT‐ 3′. On the third day after operation, acupuncture treatment was performed by the acupuncturist at specific acupoints bilateral “Zusanli” (ST36, 5 mm distal to the head of the fibula beneath the stifle and 2 mm lateral to the tibial tuberosity) and “Baihui” (DU20, on the midline of the head and the line connecting the apices of both auricles), with needles (0.3 mm×40 mm, Hwato, China). Detailed method and procedure of acupuncture treatment were as previously described.22 Other groups were given the same catching‐grasping stimulus as the acupuncture group.
2.3. Morris water maze
After 14 days of acupuncture treatment, rats were trained and tested in a Morris water maze to evaluate the spatial learning and memory as described before.23 All data were recorded by a camera above the pool and calculated by the computer with tracking system (TopScan Lite Animal Behavior Analysis System, USA) that was connected to the camera.
2.4. Nissl staining
Brain‐frozen section (10 um) was stained with cresyl violet solution (Beyotime, Shanghai, China) at 50°C for 45 minutes. Then they were dehydrated in serially diluted ethanol and cleared in xylene. Nissl staining images were captured using microscope (ECLIPSE 90i, Nikon, Japan), and quantitative analysis was also performed using NIH Image J.
2.5. Dihydroethidium (DHE) staining
For visualization of superoxide, sections were incubated with the DHE reagent (GENMED, Plymouth, MN, USA) away from light for 30 minutes at 37°C. Hippocampal ROS production was examined by fluorescence microscopy under an excitation wavelength of 540 nm and an emission wavelength of 590 nm.
2.6. Measurement of superoxide dismutase (SOD) activity
The procedure of examining SOD was performed with SOD assay kit from Nanjing Jiancheng Bioengineering Institute. Hippocampal samples were weighed and homogenized in ice‐cold saline. After centrifugation, the obtained supernatants were determined with the Pierce BCA Protein Assay kit (Thermo Fisher Scientific, Inc., Waltham, MA, USA) to get the protein concentrations. The activity of SOD was treated by assay kit and measured by spectrophotometer. The result of measurements was expressed as nmol/mg total protein.
2.7. Western blot
The total protein was extracted from isolated hippocampal tissues. Protein concentration was quantified by the Pierce BCA Protein Assay kit (Thermo Fisher Scientific, Inc.). The protein samples were separated by 7.5%‐12% SDS‐PAGE gels (Applygen, Beijing, China) and electrically transferred to polyvinylidene fluoride membranes. The membranes were blocked with 5% nonfat milk in TBST for 1 hour at room temperature and then incubated with primary antibodies including anti‐TXNIP (1:1000; Abcam, Cambridge, UK), anti‐NLRP3 (1:500; Abcam), anti‐caspase‐1 (1:200; ENZO, New York, NY, USA), anti‐IL‐1β (1:200; Abcam), and anti‐β‐actin (1:10000; Bioss, Beijing, China) overnight at 4 °C. After being washed 4 times (5 minutes each time) with TBST, membranes were incubated with secondary antibody (1:10000; KPL, Gaithersburg, MD, USA) away from light for 1 hour at room temperature. Staining was scanned and analyzed by Odyssey Infrared Imaging System (LI‐COR Biosciences, Nebraska, NE, USA).
2.8. Immunofluorescence and immunohistochemistry
After being perfused with normal saline and 4% paraformaldehyde in PBS, the fixed brains were removed and the water was removed in sucrose. Frozen brain tissues were cut into serial coronal sections (10um) in optimal cutting temperature compound (OCT, Miles Inc., Elkhart, IN, USA). For immunofluorescence, brain sections were incubated at 37°C with a blocking solution (5% donkey serum and 0.1% Triton X‐100 in PBS) for 30 minutes. Then they were incubated with primary antibodies anti‐TXNIP (1:1000; Abcam) and anti‐NeuN (1:2000; Abcam) at 4 °C overnight and with secondary antibodies anti‐mouse (1:10 000; KPL) and anti‐rabbit (1:10 000; KPL) at room temperature for 1 hour. For immunohistochemistry, brain sections were treated with heat‐induced epitope retrieval and then with 3% hydrogen peroxide for 10 minutes. After being incubated with primary antibodies anti‐8‐OhdG (1:500; Abcam) at 4°C overnight, sections were incubated with secondary antibodies at room temperature for 1 hour. 3, 3′‐Diaminobenzidine (DAB) was used for visualization. All Images were captured using microscope (ECLIPSE 90i, Nikon, Japan), and quantitative analysis was also performed using NIH Image J.
2.9. Real‐time polymerase chain reaction (real‐time PCR)
Total RNA was extracted from hippocampus tissues using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA). The primers were designed for rat as follows: TXNIP, 5′ ‐AAG CGT TGA GTA GTA CAG ATG AG ‐3′ (forward primer), 5′ ‐GGT ATG GCG TGG CAA GAG TC‐ 3′ (reverse primer), NLRP3, 5′ ‐AGC CTC AGG GCA CCA AA‐ 3′ (forward primer), 5′ ‐GGT ATG GCG TGG CAA GAG TC‐ 3′ (reverse primer), caspase‐1, 5′ ‐ATG GAT TGC TGG ATG AAC T‐ 3′ (forward primer), 5′ ‐GAT AAC CTT GGG CTT GTC TT‐ 3′ (reverse primer), IL‐1β, 5′ ‐AGC TTC AGG AAG GCA GTG TC‐ 3′ (forward primer), 5′ ‐TCA GAC AGC ACG AGG CAT TT‐ 3′ (reverse primer), and GAPDH, 5′ ‐TCC ATG ACA ACT TTG GCA TC‐ 3′ (forward primer), 5′ ‐CAT GTC AGA TCC ACC ACG GA‐ 3′ (reverse primer). The cDNA was obtained from 2 μg of total RNA using AMV reverse transcriptase (Promega, Madison, WI). Quantitative PCR was used to analyze corresponding mRNA levels by BIO‐RAD iCycler iQ5 (Bio‐Rad, Hercules, CA, USA).
2.10. Data analysis and statistics
Parameters for spatial learning and memory were analyzed by a two‐way repeated measures ANOVA. One‐way ANOVA was used to compare the results of quantitative the data of the Nissl staining, DHE staining, Western blot analysis, real‐time PCR, immunofluorescence, and immunohistochemistry (mean ± SE). Significant differences were determined if the P‐values were under 0.05. Data analyses were performed with the SPSS software version 17.0.
3. RESULTS
3.1. Acupuncture reduced neuronal death and oxidative stress in the hippocampus of 2VO‐operated rats
To determine the effect of acupuncture on hippocampal neuronal death in 2VO‐operated rats, we used Nissl staining of frozen brain sections. After the 2VO operation, the number of Nissl‐positive cells significantly decreased in the hippocampal CA1 region of 2VO‐operated rats, compared to the sham group. Acupuncture markedly reversed 2VO‐induced neuronal loss in the CA1 region (P < 0.05), and nonacupuncture point treatment showed no effect on 2VO‐induced neuronal loss (Figure 1A,B; P > 0.05).
Figure 1.

Acupuncture inhibited hippocampal oxidative stress and neuronal loss in 2VO‐operated rats. Representative images and quantitative graph of Nissl staining (A,B) and DHE staining (C,D) in hippocampal CA1 region in sham, 2VO, 2VO + Acu, and 2VO + Non‐acu groups. Immunohistochemical staining was used to show the 8‐OHdG expression in CA1 region in sham, 2VO, 2VO + Acu, and 2VO + Non‐acu groups (200 × , scale bar= 100 μm) (E,F). Quantitative analysis of hippocampal SOD activity in these four groups (G). Values are means ± SEM (n = 10 for each group). *P < 0.05, 2VO vs Sham. # P < 0.05, 2VO+Acu vs 2VO. & P < 0.05, 2VO+Acu vs 2VO+Non‐acu
ROS, 8‐OHdG, and SOD were used to determine the effect of acupuncture on 2VO‐induced hippocampal oxidative stress. ROS generation was evaluated by DHE staining. As depicted in Figure 1C,D, an increase in ethidium fluorescence (red) was shown in the hippocampal CA1 region of 2VO‐operated rats, compared to the sham‐operated rats (P < 0.05). Acupuncture significantly inhibited 2VO‐induced ROS production in the hippocampal CA1 subfield (P < 0.05). Nonacupuncture point treatment did not change the ROS level after 2VO operation. 8‐OHdG, a well‐established biomarker of oxidative DNA damage, was significantly elevated in the hippocampal CA1 subfield after 2VO operation (P < 0.05). However, in acupuncture‐treated rats, hippocampal 8‐OHdG decreased compared with either 2VO group or non‐acu group (P < 0.05), which indicates that acupuncture attenuated CCH‐induced oxidative DNA damage (Figure 1E,F). The activity of SOD, which reflects the antioxidant resistance, decreased in the hippocampus after the 2VO operation (P < 0.05). Two weeks of acupuncture treatment improved hippocampal SOD activity, in comparison with 2VO group and non‐acu group (Figure 1G; P < 0.05).
3.2. Acupuncture inhibited the expression of TXNIP in the hippocampus of 2VO‐operated rats
As a negative regulator of the Trx antioxidant system, TXNIP is not only upregulated in response to oxidative stress, but also oxidizes and inhibits Trx and enhances oxidative damage and activating NLRP3 inflammasome. Both mRNA and protein expression of TXNIP were detected after occlusion in our study. As depicted in Figure 2A, TXNIP immunostaining (red) was mainly colocalized with NeuN (green), indicating that hippocampal TXNIP was mostly expressed in neurons. Neuronal TXNIP increased in hippocampal CA1 region after the 2VO operation. Acupuncture treatment inhibited its expression, while there was no difference in neuronal TXNIP between the 2VO group and non‐acu group. mRNA was identified using real‐time PCR, and TXNIP protein in the hippocampus was detected by Western blot analysis. We found that both the protein and mRNA expression of TXNIP increased in the hippocampus of 2VO‐operated rats compared to the sham‐operated rats. Acupuncture inhibited the 2VO‐induced upregulation of TXNIP in the hippocampus, while nonacupuncture could not (Figure 2B,C) (P < 0.05). These results suggested that acupuncture reversed the upregulation of TXNIP expressed in hippocampal neurons caused by 2VO operation.
Figure 2.
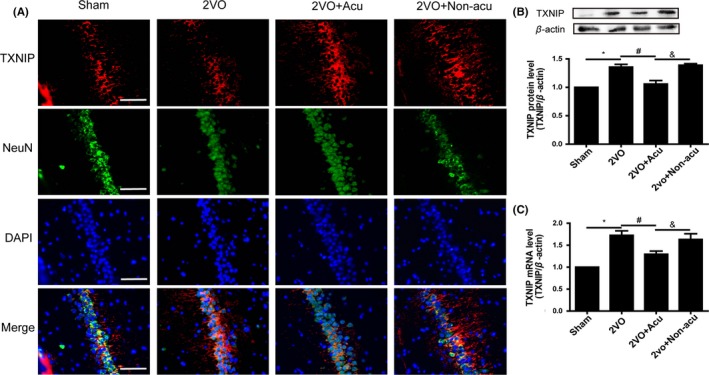
Acupuncture decreased hippocampal TXNIP expression in 2VO‐operated rats. Double immunofluorescence of TXNIP (green), NeuN (red), and nuclei (blue) of hippocampal CA1 region in sham, 2VO, 2VO + Acu, and 2VO + Non‐acu rats (200 × , scale bar = 100 μm) (A). The protein level of TXNIP and corresponding β‐actin bands was determined by Western blot analysis, and the corresponding quantitative densitometry of the blots was shown in following histogram (B) (n = 6 for each group). Graphic presentation shows the expression of TXNIP mRNA level at day 14 after acupuncture treatment analyzed by real‐time PCR (C) (n = 6 for each group).Values are means ± SEM. *P < 0.05, 2VO vs Sham. # P < 0.05, 2VO+Acu vs 2VO. & P < 0.05, 2VO+Acu vs 2VO+Non‐acu
3.3. Acupuncture decreased NLRP3 inflammasome and IL‐1β expression in the hippocampus of 2VO‐operated rats
Studies have found that TXNIP was involved in regulating inflammation via binding to NLRP3 inflammasome, inducing the generation and secretion of IL‐1β. To verify the effect of acupuncture on the expression of NLRP3 inflammasome (NLRP3 and caspase‐1) and IL‐1β in the hippocampus of 2VO‐operated rats, Western blot and real‐time PCR were respectively used to detect the protein and mRNA level of NLRP3, caspase‐1, and IL‐1β. As shown in Figure 3A‐D, protein expressions of NLRP3, caspase‐1, and IL‐1β were upregulated after 2VO. The 2VO rats with acupuncture treatment showed less expression of NLRP3, caspase‐1, and IL‐1β compared with the 2VO rats (P > 0.05), while 2VO rats with nonacupoint acupuncture treatment showed no difference with the 2VO rats (P < 0.05). The result was similar in Figure 3E‐G, which showed decreased hippocampal NLRP3, caspase‐1, and IL‐1β mRNA after 2VO was reversed by acupuncture treatment (P > 0.05), while nonacupoint acupuncture treatment did not have an effect (P < 0.05).
Figure 3.
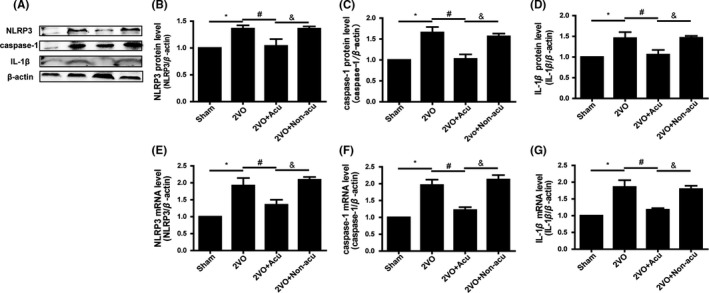
Acupuncture inhibited expression of hippocampal NLRP3 inflammasome and IL‐1β in 2VO‐operated rats. The protein level of NLRP3, caspase‐1, IL‐1β, and corresponding β‐actin bands was determined by Western blot analysis (A). The corresponding quantitative densitometry of the blots was shown in following histogram (n = 6 for each group) (B‐D). Graphic presentation shows the expression of NLRP3, caspase‐1, IL‐1β, and corresponding β‐actin mRNA at day 14 after acupuncture treatment analyzed by quantitative real‐time PCR (E‐G) (n = 6 for each group).Values are means ± SEM. *P < 0.05, 2VO vs Sham. # P < 0.05, 2VO+Acu vs 2VO. & P < 0.05, 2VO+Acu vs 2VO+Non‐acu
3.4. Inhibition of TXNIP mimicked the neuroprotective potential of acupuncture on 2VO rats
Escape latency, total distance, and the time spent in the target quadrant were calculated in the Morris water maze. As shown in Figure 4E‐G, both acupuncture treatment and TXNIP siRNA injection decreased escape latency, total distance, and the time spent in the target quadrant in 2VO rats in comparison with 2VO rats with control siRNA injection, which failed to ameliorate 2VO‐induced cognitive impairment (P < 0.05). In addition, Nissl staining was performed to determine the effect of TXNIP inhibition on hippocampal neuronal damage. The amelioration of hippocampal neuronal death in 2VO rats which were injected with TXNIP siRNA was similar to 2VO rats with acupuncture treatment (Figure 4A,B; P > 0.05). However, control siRNA injection did not reduce hippocampal neuronal damage in 2VO rats (P > 0.05). DHE staining was used to detect ROS generation in hippocampus, assessing the effect of TXNIP siRNA on oxidative stress. In 2VO rats, the increase in hippocampal ROS was inhibited by TXNIP siRNA injection (P < 0.05), as well as by acupuncture treatment (P > 0.05). By contrast, scrambled siRNA injection failed to decrease ROS level (P > 0.05; Figure 4C,D).
Figure 4.
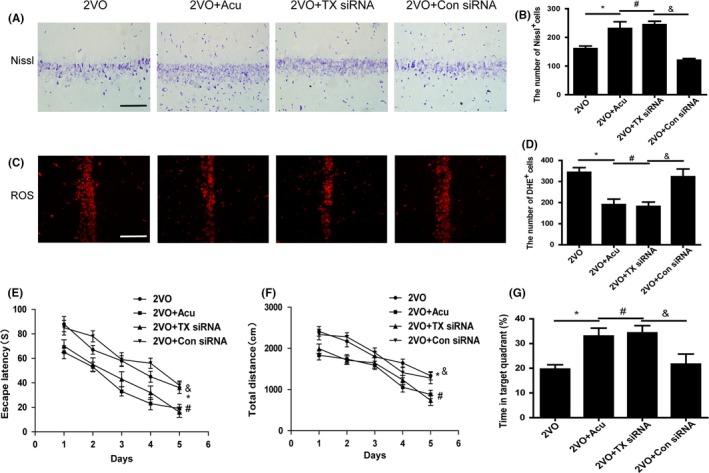
Acupuncture mimicked the antioxidant and neuroprotective potential of TXNIP siRNA injection. Representative images and quantitative graph of Nissl staining (A,B) and DHE staining (C,D) in hippocampal CA1 region of 2VO, 2VO + Acu, 2VO + TX siRNA, and 2VO + Con siRNA rats (200 × , scale bar = 100 μm). Quantification of escape latency (E), total distance (F) for reaching the hidden platform during 5 days in hidden platform trial, and time spent in the target quadrant (G) were measured in 2VO, 2VO + Acu, 2VO + TX siRNA, and 2VO + Con siRNA rats using Morris water maze task. Values are expressed as means ± SEM (n = 10 for each group). *P < 0.05, 2VO vs Sham. # P < 0.05, 2VO+Acu vs 2VO. & P < 0.05, 2VO+Acu vs 2VO+Non‐acu
4. DISCUSSION
In this study, we investigated the neuroprotective effect of acupuncture on VD rats. We found that acupuncture treatment alleviated cognitive decline and hippocampal neuronal death by inhibiting cerebral oxidative stress and inflammation, which was probably through downregulating TXNIP expression. In addition, we also demonstrated that the injection of siRNA targeting TXNIP had the similar effects to acupuncture on cognition, hippocampal neuronal loss, and ROS production, indicating the potential role of acupuncture as TXNIP inhibitor.
VD is a frequent, encountered type of dementia caused by reduced cerebral blood flow, whose main symptom is progressive cognitive dysfunction including impaired learning and memory.24 Acupuncture treatment tends to be a popular and effective complementary replacement therapy. Previous studies have shown that acupuncture treatment can apparently ease main symptoms of the impairments of VD patients.25, 26, 27 Our results demonstrated that acupuncture reversed spatial learning and memory deficits caused by 2VO via reducing hippocampal neuronal death. Besides VD, studies have suggested that acupuncture was helpful for the inhibition of neuronal damage and cognitive deficits, eliciting neuroprotective function in many other CNS diseases.28, 29, 30, 31
The molecular mechanisms of acupuncture treatment for cerebral ischemia include the regulation of multiple molecules and signaling pathways, especially those related to oxidative stress and inflammation.18 Oxidative stress is the result of a disequilibrium of pro‐oxidant and antioxidant systems, which have been well documented in the pathophysiology of CCH‐induced cognitive dysfunction.32 Acupuncture has proven to have the ability to suppress oxidative stress in many diseases including VD.16, 33, 34 Our previous study demonstrated that the major ROS‐producing enzyme, NADPH oxidase, was suppressed by acupuncture in VD rats.20 In this study, we found that overproduction of ROS and 8‐OHdG and SOD inhibitory activity induced by 2VO were reversed by acupuncture treatment. A mechanism was demonstrated as to how acupuncture could improve cognition and impede neuronal loss of VD rats partly through suppressing oxidative stress.
After establishing the benefit of acupuncture on CCH‐induced dementia, we investigated the mechanisms underlying the antioxidative and neuroprotective effects of acupuncture. TXNIP was the focus of many studies as a significant regulator of redox homeostasis and inflammation, and has become a therapeutic target in the neuroprotection of cerebral ischemia.14, 15 Thioredoxin (Trx) is a key antioxidant protein producing electrons to thiol‐dependent peroxidases to protect tissues and cells against oxidative stress.35 Under high concentrations of ROS, TXNIP would oxidize and inactivate Trx via binding to Trx, enhancing oxidative stress.10, 11 Studies have shown that TXNIP deletion in mice improved antioxidant capacity as compared to controls.36 Additionally, overexpression of TXNIP promoted inflammation and neurotoxicity in rat retinal injury.37 In our study, we confirmed for the first time that TXNIP was expressed in neurons in the hippocampus of VD rats. We also found that CCH increased hippocampal TXNIP and that acupuncture inhibited TXNIP expression, protecting the brain against neurodegeneration. In addition, TXNIP inhibition by siRNA intraventricular injection ameliorated cognitive impairment, neuronal death, and ROS production, just like acupuncture did. Acupuncture mimicked the neuroprotective effect of TXNIP inhibition on VD. Besides oxidative stress, TXNIP is also a pro‐apoptotic protein via interacting with ASK‐1, activating the p38‐MAPK pathway and leading to cell death.38
Activation of inflammatory pathways, inflammatory factors, and mediators, important mechanisms might contribute to neuronal loss during CCH. As a part of the innate immune system, NLRP3 inflammasome contributes to the pathogenesis of many CNS diseases via regulating inflammation.39 Under oxidative stress, TXNIP is stimulated and binds to NLRP3, inducing NLRP3 inflammasome activation, which activates caspase‐1 and promotes IL‐1β secretion. Mice with NLRP3 deficiency showed reduced infarct volumes and reduced neuronal death after cerebral ischemia. This indicates that NLRP3 inflammasome‐dependent microglial neurotoxicity could mediate neuronal apoptosis.40 Our findings provide the evidence that acupuncture suppressed TXNIP‐mediated NLRP3 and IL‐1β upregulation in VD rats.
Our study has some limitations. First, we did not use an agonist or knockout mice to confirm the specific role of TXNIP relating to the mechanisms of acupuncture‐related neuroprotection in VD. Additionally, besides TXNIP and NLRP3 inflammasome, there are many other mediators of oxidative stress and inflammatory response in the pathophysiological process of VD, of which we could not exclude the possible effects.
In conclusion, our data suggests that acupuncture attenuates cognitive impairment and neuronal death in VD rats. The mechanisms of this beneficial effect may involve the downregulation of hippocampal TXNIP to inhibit oxidative stress and inflammation. Our findings further suggest that inhibiting TXNIP has similar neuroprotective effect on VD as acupuncture, indicating the possible role of acupuncture as a TXNIP inhibitor.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
We would like to thank the Beijing Hospital of Traditional Chinese Medicine affiliated to Capital Medical University, Beijing Institute of Traditional Chinese Medicine, and Chinese Academy of Traditional Chinese Medicine for scientific, technical, and logistical support.
Du S‐Q, Wang X‐R, Zhu W, et al. Acupuncture inhibits TXNIP‐associated oxidative stress and inflammation to attenuate cognitive impairment in vascular dementia rats. CNS Neurosci Ther. 2018;24:39–46. 10.1111/cns.12773
Funding information
This work was supported by the National Natural Science Foundation of China—Outstanding Youth Foundation (no.81222050).
REFERENCES
- 1. O'Brien JT, Thomas A. Vascular dementia. Lancet. 2015;386:1698‐1706. [DOI] [PubMed] [Google Scholar]
- 2. Lin MS, Chiu MJ, Wu YW, et al. Neurocognitive improvement after carotid artery stenting in patients with chronic internal carotid artery occlusion and cerebral ischemia. Stroke. 2011;42:2850‐2854. [DOI] [PubMed] [Google Scholar]
- 3. Pimentel‐Coelho PM, Michaud JP, Rivest S. Effects of mild chronic cerebral hypoperfusion and early amyloid pathology on spatial learning and the cellular innate immune response in mice. Neurobiol Aging. 2013;34:679‐693. [DOI] [PubMed] [Google Scholar]
- 4. Farkas E, Luiten PG, Bari F. Permanent, bilateral common carotid artery occlusion in the rat: a model for chronic cerebral hypoperfusion‐related neurodegenerative diseases. Brain Res Rev. 2007;54:162‐180. [DOI] [PubMed] [Google Scholar]
- 5. Choi DH, Lee KH, Kim JH, et al. NADPH oxidase 1, a novel molecular source of ROS in hippocampal neuronal death in vascular dementia. Antioxid Redox Signal. 2014;21:533‐550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rosenberg GA, Bjerke M, Wallin A. Multimodal markers of inflammation in the subcortical ischemic vascular disease type of vascular cognitive impairment. Stroke. 2014;45:1531‐1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Du SQ, Wang XR, Xiao LY, et al. Molecular mechanisms of vascular dementia: what can be learned from animal models of chronic cerebral hypoperfusion? Mol Neurobiol. 2017;54:3670‐3682. [DOI] [PubMed] [Google Scholar]
- 8. Yoshioka J, Chutkow WA, Lee S, et al. Deletion of thioredoxin‐interacting protein in mice impairs mitochondrial function but protects the myocardium from ischemia‐reperfusion injury. J Clin Invest. 2012;122:267‐279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chutkow WA, Lee RT. Thioredoxin regulates adipogenesis through thioredoxin‐interacting protein (Txnip) protein stability. J Biol Chem. 2011;286:29139‐29145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lu J, Holmgren A. Thioredoxin system in cell death progression. Antioxid Redox Signal. 2012;17:1738‐1747. [DOI] [PubMed] [Google Scholar]
- 11. Nishiyama A, Matsui M, Iwata S, et al. Identification of thioredoxin‐binding protein‐2/vitamin D(3) up‐regulated protein 1 as a negative regulator of thioredoxin function and expression. J Biol Chem. 1999;274:21645‐21650. [DOI] [PubMed] [Google Scholar]
- 12. Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin‐interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11:136‐140. [DOI] [PubMed] [Google Scholar]
- 13. Abderrazak A, Syrovets T, Couchie D, et al. NLRP3 inflammasome: from a danger signal sensor to a regulatory node of oxidative stress and inflammatory diseases. Redox Biol. 2015;4:296‐307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ishrat T, Mohamed IN, Pillai B, et al. Thioredoxin‐interacting protein: a novel target for neuroprotection in experimental thromboembolic stroke in mice. Mol Neurobiol. 2015;51:766‐778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guo ZN, Xu L, Hu Q, et al. Hyperbaric oxygen preconditioning attenuates hemorrhagic transformation through reactive oxygen species/thioredoxin‐interacting protein/nod‐like receptor protein 3 pathway in hyperglycemic middle cerebral artery occlusion rats. Crit Care Med. 2016;44:e403‐e411. [DOI] [PubMed] [Google Scholar]
- 16. Wang XR, Shi GX, Yang JW, et al. Acupuncture ameliorates cognitive impairment and hippocampus neuronal loss in experimental vascular dementia through Nrf2‐mediated antioxidant response. Free Radic Biol Med. 2015;89:1077‐1084. [DOI] [PubMed] [Google Scholar]
- 17. Ashford JW, Mahoney L, Burkett T. A role for complementary and integrative medicine in alzheimer's disease prevention. J Alzheimers Dis. 2015;48:13‐14. [DOI] [PubMed] [Google Scholar]
- 18. Zhu W, Ye Y, Liu Y, et al. Mechanisms of acupuncture therapy for cerebral ischemia: an evidence‐based review of clinical and animal studies on cerebral ischemia. J Neuroimmune Pharmacol. 2017; [Epub ahead of print]. 10.1007/s11481-017-9747-4. [DOI] [PubMed] [Google Scholar]
- 19. Wu P, Mills E, Moher D, Seely D. Acupuncture in poststroke rehabilitation: a systematic review and meta‐analysis of randomized trials. Stroke. 2010;41:e171‐e179. [DOI] [PubMed] [Google Scholar]
- 20. Shi GX, Wang XR, Yan CQ, et al. Acupuncture elicits neuroprotective effect by inhibiting NAPDH oxidase‐mediated reactive oxygen species production in cerebral ischaemia. Sci Rep. 2015;10:17981. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21. Choi BR, Kim DH, Back DB, et al. Characterization of white matter injury in a rat model of chronic cerebral hypoperfusion. Stroke. 2016;47:542‐547. [DOI] [PubMed] [Google Scholar]
- 22. Li H, Liu Y, Lin LT, et al. Acupuncture reversed hippocampal mitochondrial dysfunction in vascular dementia rats. Neurochem Int. 2016;92:35‐42. [DOI] [PubMed] [Google Scholar]
- 23. Ye Y, Li H, Yang JW, et al. Acupuncture attenuated vascular dementia‐induced hippocampal long‐term potentiation impairments via activation of D1/D5 receptors. Stroke. 2017;48:1044‐1051. [DOI] [PubMed] [Google Scholar]
- 24. Tsivgoulis G, Katsanos AH, Papageorgiou SG, et al. The role of neurosonology in the diagnosis of vascular dementia. J Alzheimers Dis. 2014;42:S251‐S257. [DOI] [PubMed] [Google Scholar]
- 25. Zhao L, Zhang H, Zheng Z, Huang J. Electroacupuncture on the head points for improving gnosia in patients with vascular dementia. J Tradit Chin Med. 2009;29:29‐34. [DOI] [PubMed] [Google Scholar]
- 26. Shi GX, Liu CZ, Guan W, et al. Effects of acupuncture on Chinese medicine syndromes of vascular dementia. Chin J Integr Med. 2010;20:661‐666. [DOI] [PubMed] [Google Scholar]
- 27. Shi GX, Li QQ, Yang BF, et al. Acupuncture for vascular dementia: a pragmatic randomized clinical trial. Sci World J. 2010;2015:161439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee B, Sur B, Shim J, Hahm DH, Lee H. Acupuncture stimulation improves scopolamine‐induced cognitive impairment via activation of cholinergic system and regulation of BDNF and CREB expressions in rats. BMC Complement Altern Med. 2010;17:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu H, Sun X, Zou W, et al. Scalp acupuncture attenuates neurological deficits in a rat model of hemorrhagic stroke. Complement Ther Med. 2017;32:85‐90. [DOI] [PubMed] [Google Scholar]
- 30. Jeon S, Kim YJ, Kim ST, et al. Proteomic analysis of the neuroprotective mechanisms of acupuncture treatment in a Parkinson's disease mouse model. Proteomics. 2008;8:4822‐4832. [DOI] [PubMed] [Google Scholar]
- 31. Kang KA, Shin ES, Hur J. Acupuncture attenuates neuronal cell death in middle cerebral artery occlusion model of focal ischemia. Neurol Res. 2010;32:84‐87. [DOI] [PubMed] [Google Scholar]
- 32. Bennett S, Grant MM, Aldred S. Oxidative stress in vascular dementia and Alzheimer's disease: a common pathology. J Alzheimers Dis. 2009;17:245‐257. [DOI] [PubMed] [Google Scholar]
- 33. Santos EL, Dias BH, Andrade AC, et al. Effects of acupuncture and electroacupuncture on estradiol‐induced inflammation and oxidative stress in health rodents. Acta Cir Bras. 2013;28:582‐588. [DOI] [PubMed] [Google Scholar]
- 34. Yu JB, Dong SA, Luo XQ, et al. Role of HO‐1 in protective effect of electro‐acupuncture against endotoxin shock‐induced acute lung injury in rabbits. Exp Biol Med (Maywood). 2010;238:705‐712. [DOI] [PubMed] [Google Scholar]
- 35. Mahmood DF, Abderrazak A, El Hadri K, Simmet T, Rouis M. The thioredoxin system as a therapeutic target in human health and disease. Antioxid Redox Signal. 2013;19:1266‐1303. [DOI] [PubMed] [Google Scholar]
- 36. Abdelsaid MA, Matragoon S, El‐Remessy AB. Thioredoxin‐interacting protein expression is required for VEGF‐mediated angiogenic signal in endothelial cells. Antioxid Redox Signal. 2013;19:2199‐2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Al‐Gayyar MM, Abdelsaid MA, Matragoon S, Pillai BA, El‐Remessy AB. Thioredoxin interacting protein is a novel mediator of retinal inflammation and neurotoxicity. Br J Pharmacol. 2011;164:170‐180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Martinon F. Signaling by ROS drives inflammasome activation. Eur J Immunol. 2010;40:616‐619. [DOI] [PubMed] [Google Scholar]
- 39. Trendelenburg G. Molecular regulation of cell fate in cerebral ischemia: role of the inflammasome and connected pathways. J Cereb Blood Flow Metab. 2014;34:1857‐1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yang F, Wang Z, Wei X, et al. NLRP3 deficiency ameliorates neurovascular damage in experimental ischemic stroke. J Cereb Blood Flow Metab. 2014;34:660‐667. [DOI] [PMC free article] [PubMed] [Google Scholar]


