Abstract
Background
Electrical cardioversion (CV) is essential in rhythm management of atrial fibrillation (AF). However, optimal timing of CV remains unknown.
Hypothesis
Timing of CV in AF is associated with risk of adverse events.
Methods
We analyzed the effect of AF episode duration on safety and efficacy of electrical CV in a multicenter, multicohort study exploring 4356 CVs in 2530 patients on oral anticoagulation. The composite adverse outcome included unsuccessful CV, acute arrhythmic complications, thromboembolic events, mortality, and AF recurrence within 30‐day follow‐up.
Results
Study groups were stratified according to duration of index AF episode (<24 h, 24–48 h, 48 h–30d, and > 30d), consisting of 1767, 516, 632, and 1441 CVs, respectively. CVs were unsuccessful in 8.5% (<24 h), 5.4% (24–48 h), 11.1% (48 h–30d), and 13.9% (>30d), respectively (P < 0.01). Occurrence of thromboembolic events (0.1%), mortality (0.1%), and asystole >5 seconds (0.7%) within 30‐day follow‐up was infrequent and comparable in the study groups. AF recurrence within 30 days after initially successful CVs was 29.8% (<24 h), 26.5% (24–48 h), 37.3% (48 h–30d), and 30.3% (>30d), respectively (P < 0.01). Composite adverse outcome occurred in 1669 (38.4%) CVs, and index AF episode >48 hours was an independent predictor for the composite endpoint (OR: 1.49, 95% CI: 1.28–1.74, P < 0.01) in multivariate analysis.
Conclusions
Optimal timing of CV for AF showed a J‐shaped curve, with fewest adverse outcomes in patients with CV performed 24 to 48 hours after onset of AF. In patients with rhythm‐control strategy, delaying CV >48 hours is associated with increased risk for adverse outcomes.
Keywords: Arrhythmic Complications, Atrial Fibrillation, Cardioversion, Recurrence, Success Rate, Thromboembolism
1. INTRODUCTION
Atrial fibrillation (AF) is the most prevalent cardiac arrhythmia, and it is estimated that every fourth middle‐aged adult will ultimately develop AF during their lifespan.1 Electrical cardioversion (CV) has remained the cornerstone of rhythm‐control management of AF for decades and is widely used in symptomatic patients despite the lack of evidence of any survival benefit.2, 3, 4 In previous studies, longer duration of AF episode increased the possibility of unsuccessful CV and stroke.5, 6, 7 However, little is known regarding efficacy and safety of CV with different AF episode lengths in patients on oral anticoagulation (OAC). Therefore, we sought to investigate the optimal timing of CV for AF in this multicenter study.
2. METHODS
The Safety of Cardioversion of Acute Atrial Fibrillation (Finnish CardioVersion [FinCV]) studies constitute an observational study program exploring thrombotic and bleeding complications of AF after electrical CV.6, 7, 8, 9
The present study cohort was gathered from datasets of the FinCV (http://www.ClinicalTrials.gov NCT01380574), the FinCV2 (http://www.ClinicalTrials.gov NCT02850679), and the FinCV3 (http://www.ClinicalTrials.gov NCT02911545) study populations. Data were collected from patient registries at 3 university hospitals, 2 central hospitals, and 3 regional hospitals from the time periods of 2003–2010, 2003–2015, and 2011–2016 in the FinCV, FinCV2, and FinCV3 studies, respectively. The details of the catchment areas of each study have been described in detail previously.7, 8, 10 Initially, patient registries were screened in each hospital for International Classification of Diseases, Tenth Revision (ICD‐10) code I48 (AF) and for Nordic Classification of Surgical Procedures (NCSP) code TFP20 (cardioversion). Patients were then included in each study according to specified inclusion criteria: all patients admitted to the emergency department because of an acute (<48 h) AF in whom electrical or pharmacological CV was attempted (FinCV); all patients with persistent (>48 h) AF in whom elective electrical CV was attempted (FinCV2); and all patients with AF using non–vitamin K oral anticoagulants (NOAC) in whom electrical or pharmacological CV was attempted (FinCV3). Altogether, these studies comprised 5441 patients and 10 852 CVs.
Only patients on OAC, either vitamin K antagonist or NOAC, prior to electrical CV for AF were included in the present analysis. Patients undergoing pharmacological CV and those with unavailable data on the duration of the index AF episode were excluded. The final study cohort included a total of 2530 patients and 4356 CVs (Figure 1). Patients were divided into 4 groups according to the duration of index episode of AF: <24 hours, 24 to 48 hours, 48 hours to 30 days, and > 30 days.
Figure 1.
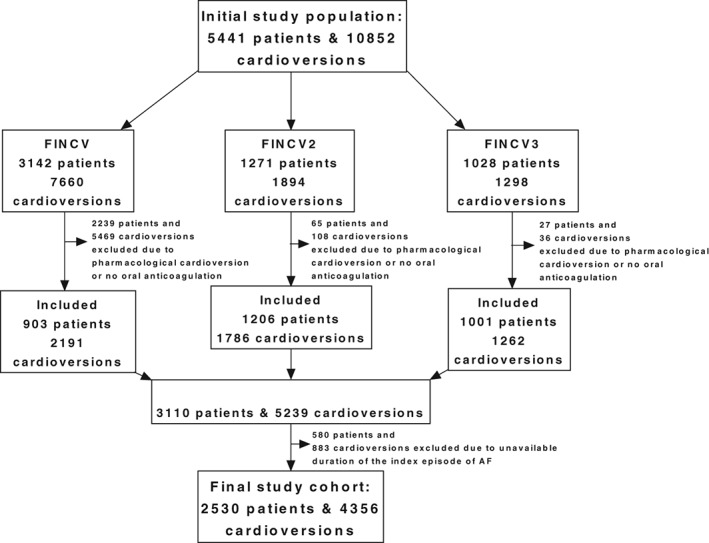
Flow chart showing patient cohort selection
All patient data were collected from patient records using a structured electronic case‐report form consisting of general disease and AF history and medications.
The primary endpoint of this study was a composite adverse outcome defined as the occurrence of any of the following events: death, thromboembolic (TE) event, unsuccessful CV, and acute arrhythmic complication and/or AF recurrence within the 30‐day follow‐up. The inability to maintain sinus rhythm until discharge despite initially successful CV was considered an unsuccessful CV. Asystole lasting >5 seconds, ventricular tachycardia, or ventricular fibrillation immediately after the CV were considered acute arrhythmic complications. A TE event was classified as an ischemic nonhemorrhagic stroke adjudicated by the treating neurologist and imaging. Occurrence of AF within 30 days after a successful index CV confirmed by electrocardiogram or pacemaker log was defined as an AF recurrence.
All AFs were confirmed by the clinician performing the procedure based on electrocardiographic findings. AF and atrial flutter were not segregated. CVs were performed according to the contemporary guidelines in general anesthesia. The positioning of the paddles or pads (anteroposterior or anterolateral configuration) was at the discretion of the clinician. The energy ranged from 70 J to 360 J with monophasic and 70 J to 200 J with biphasic defibrillator devices. Biphasic defibrillators were used after 2004.
The study received approval of the Medical Ethics Committee of the Hospital District of Southwest Finland and the ethics committee of the National Institute for Health and Welfare. The study conforms to the Declaration of Helsinki. Informed consent was not required because of the retrospective nature of the study.
2.1. Statistical analysis
Normally distributed continuous variables were reported as mean ±SD, whereas skewed continuous variables were denoted as median (interquartile range). Normality in continuous covariates was tested with Kolmogorov–Smirnov and Shapiro–Wilk tests. Categorical variables were reported as counts and percentages. The unpaired t test or Mann–Whitney test was used to compare continuous variables and the Pearson χ2 or Fisher exact test to compare categorical variables in the study subgroups, as appropriate. Logistic regression with backward selection was used to identify the independent predictors of adverse events. Baseline variables with P < 0.10 level in univariate analysis were entered into the logistic regression models. The Hosmer‐Lemeshow test was used to estimate the calibration of the regression models. All tests were 2‐sided, and P < 0.05 was considered statistically significant. SPSS Statistics software, version 22.0 (IBM Corp., Armonk, NY), was used to perform all analyses.
3. RESULTS
Altogether, 1767, 516, 632, and 1441 CVs were performed for index AF episodes lasting <24 hours, 24 to 48 hours, 48 hours to 30 days, and > 30 days, respectively. Baseline characteristics of each patient group are depicted in Table 1. Patients with shorter index AF episode duration (<24 h or 24–48 h) were more likely to be female and have a history of hypertension, coronary artery disease, peripheral arterial disease, prior stroke or myocardial infarction, or antiarrhythmic medication. Patients with longer AF episodes (48 h–30 d or > 30 d) had lower CHA2DS2‐VASc scores and ventricular rate at the time of CV, but they were more likely to have a history of congestive heart failure or chronic kidney disease.
Table 1.
Baseline characteristics of CVs according to duration of index episode of AF
| AF <24 h, n = 1767 | AF 24–48 h, n = 516 | AF 48 h–30 d, n = 632 | AF >30 d, n = 1441 | P Value | |
|---|---|---|---|---|---|
| Age, y | |||||
| Mean | 64 ± 12 | 64 ±10 | 63 ±11 | 64 ±10 | <0.01 |
| Median (IQR) | 66 (60–73) | 64 (59–71) | 63 (56–70) | 65 (58–72) | <0.01 |
| > 75 | 294 (16.7) | 62 (12.1) | 73 (12.7) | 223 (16.7) | <0.01 |
| Female sex | 796 (45.0) | 197 (38.2) | 159 (25.2) | 401 (28.2) | <0.01 |
| Prior cardioversiona | 1301 (75.8) | 330 (69.6) | 246 (81.5) | 347 (49.2) | <0.01 |
| CHA2DS2‐VASc‐score ≥ 2 | 1190 (67.3) | 327 (63.4) | 305 (48.3) | 766 (53.2) | <0.01 |
| Ventricular rate < 60 bpm | 23 (1.4) | 12 (2.5) | 16 (3.0) | 46 (3.5) | <0.01 |
| Median (IQR) | 109 (92–125) | 102 (88–120) | 91 (77–110) | 86 (75–100) | <0.01 |
| History of HF | 147 (8.3) | 66 (12.8) | 110 (17.7) | 193 (13.8) | <0.01 |
| HTN | 989 (56.0) | 271 (52.5) | 295 (47.0) | 775 (54.3) | <0.01 |
| CKD | 34 (1.9) | 13 (2.5) | 29 (4.7) | 70 (5.1) | <0.01 |
| DM | 226 (12.8) | 81 (15.7) | 87 (13.9) | 213 (15.2) | 0.18 |
| Cirrhosis | 0 (0.0) | 2 (0.4) | 0 (0.0) | 4 (0.3) | 0.49 |
| Prior stroke | 244 (13.8) | 58 (11.3) | 31 (5.0) | 39 (2.8) | <0.01 |
| CAD | 632 (35.8) | 178 (34.5) | 78 (12.6) | 164 (11.7) | <0.01 |
| Prior MI | 201 (11.4) | 73 (14.1) | 43 (6.9) | 88 (6.3) | <0.01 |
| PAD | 475 (26.9) | 131 (25.5) | 19 (3.1) | 26 (1.9) | <0.01 |
| Pacemaker | 157 (8.9) | 58 (11.3) | 67 (11.0) | 69 (5.0) | <0.01 |
| Medication at CV | |||||
| β‐Blocker | 1542 (87.3) | 430 (83.5) | 543 (86.2) | 1262 (88.0) | 0.06 |
| Digoxin | 175 (9.9) | 58 (11.5) | 88 (15.3) | 246 (19.4) | <0.01 |
| Verapamil | 37 (2.1) | 17 (3.4) | 4 (0.7) | 13 (1.0) | <0.01 |
| Any antiarrhythmic agentb | 466 (26.4) | 132 (26.1) | 132 (22.9) | 122 (9.7) | <0.01 |
Abbreviations: AF, atrial fibrillation; CAD, coronary artery disease; CHA2DS2‐VASc, congestive HF, HTN, age ≥ 75 years (doubled), DM, prior stroke/TIA/TE (doubled), vascular disease, age 65–74 years, and sex category (female, unless age < 65 years and no other risk factors); CKD, chronic kidney disease; CV, cardioversion; DM, diabetes mellitus; HF, heart failure; HTN, hypertension; IQR, interquartile range; MI, myocardial infarction; PAD, peripheral arterial disease; SD, standard deviation; TE, thromboembolism; TIA, transient ischemic attack.
Data are presented as n (%), mean ± SD, or median (IQR).
Data are missing in 1158 (26.6) cases.
Antiarrhythmic agents comprised flecainide, amiodarone, propafenone, quinidine, or disopyramide, and dronedarone.
The associations between index AF episode duration and adverse outcomes are summarized in Table 2. A total of 448 (10.3%) CVs were unsuccessful. The relationship between AF episode duration and unsuccessful CV showed a J‐shaped curve (Figure 2). Patients with AF episodes lasting 24 to 48 hours had the lowest rate of unsuccessful CVs. In multivariate analysis, >48‐hour duration of index episode of AF independently predicted unsuccessful CV (odds ratio [OR]: 1.79, 95% CI: 1.41–2.26, P < 0.01). Asystole lasting >5 seconds was detected after 32 (0.7%) CVs, and there were no differences in the occurrence of asystole >5 seconds between the groups.
Table 2.
Adverse outcomes of CVs according to duration of index episode of AF
| Outcomes | AF <24 h, n = 1767 | AF 24–48 h, n = 516 | AF 48 h–30 d, n = 632 | AF >30 d, n = 1441 | P Value, H‐L Test |
|---|---|---|---|---|---|
| Composite adverse outcome | 645 (36.5) | 160 (31.1) | 283 (45.0) | 581 (40.4) | <0.01 |
| Adjusted OR (95% CI) | 1.33 (1.07–1.66) | Ref | 2.00 (1.52–2.60) | 1.89 (1.49–2.40) | 0.37 |
| Unsuccessful CV | 150 (8.5) | 28 (5.4) | 70 (11.1) | 200 (13.9) | <0.01 |
| Adjusted OR (95% CI) | 1.81 (1.16–2.84) | Ref | 2.10 (1.17–3.60) | 3.60 (2.27–5.90) | 0.74 |
| Asystole >5 s | 15 (0.8) | 4 (0.8) | 6 (0.9) | 7 (0.5) | 0.58 |
| Adjusted OR (95% CI) | 1.00 (0.33–3.03) | Ref | 1.24 (0.33–4.65) | 0.54 (0.15–1.92) | 0.84 |
| TE event | 2 (0.1) | 0 (0.0) | 0 (0.0) | 3 (0.2) | 0.29 |
| Adjusted OR (95% CI) | — | Ref | — | — | — |
| Mortality | 0 (0.0) | 1 (0.2) | 0 (0.0) | 3 (0.2) | 0.18 |
| Adjusted OR (95% CI) | — | Ref | — | — | — |
| AF recurrence | 481 (29.8) | 129 (26.5) | 209 (37.3) | 375 (30.3) | <0.01 |
| Adjusted OR (95% CI) | 1.23 (0.97–1.56) | Ref | 1.83 (1.36–2.46) | 1.64 (1.25–2.14) | 0.26 |
Abbreviations: AF, atrial fibrillation; CI, confidence interval; CV, cardioversion; H‐L, Hosmer‐Lemeshow; OR, odds ratio; Ref, reference; TE, thromboembolic.
Data are presented as n (%).
Figure 2.
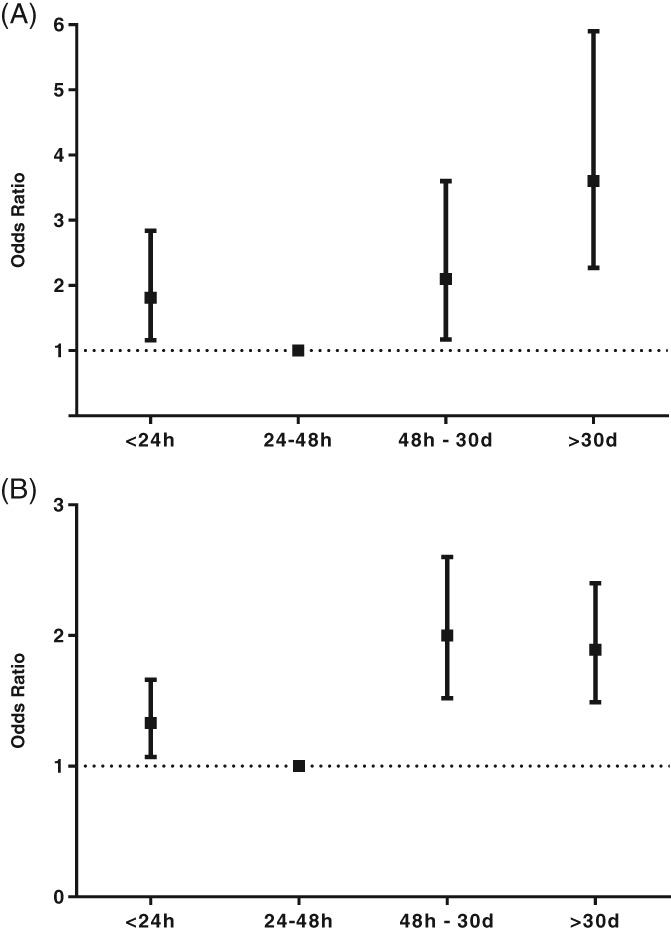
Main outcomes of the study. Adjusted ORs with 95% CIs of (A) unsuccessful CV and (B) composite adverse outcome according to duration of the index episode of AF. Abbreviations: AF, atrial fibrillation; CI, confidence interval; CV, cardioversion; FINCV, Finnish CardioVersion Study (Safety of Cardioversion of Acute Atrial Fibrillation); OR, odds ratio
Within 30 days of follow‐up, 4 (0.1%) patients died and 5 (0.1%) definite TE events were observed. None of the studied covariates were associated with an increased risk of TE events. An early recurrence of AF was detected after 1194 (30.6%) initially successful CVs within the 30‐day follow‐up. Patients with shorter AF episode duration (<24 h and 24–48 h) had lower risk for recurrence, whereas an index AF episode lasting >48 hours was an independent predictor of AF recurrence (OR: 1.38, 95% CI: 1.16–1.64, P < 0.01).
Altogether, composite adverse outcomes occurred after 1669 (38.4%) CVs within 30‐day follow‐up, and the risk of adverse events was lowest in patients with an index AF episode lasting 24 to 48 hours (Figure 2). An index episode of AF lasting >48 hours (OR: 1.49, 95% CI: 1.28–1.74, P < 0.01), congestive heart failure (OR: 1.52, 95% CI: 1.22–1.89, P < 0.01), chronic kidney disease (OR: 1.56, 95% CI: 1.07–2.27, P = 0.02), peripheral arterial disease (OR: 1.23, 95% CI: 1.01–1.49, P = 0.04), β‐blocker medication (OR: 1.43, 95% CI: 1.16–1.76, P < 0.01), and antiarrhythmic medication at discharge (OR: 1.73, 95% CI: 1.46–2.05, P < 0.01) independently predicted the development of composite adverse outcome in multivariate analysis.
In an additional analysis, the study patient cohort with known duration of the index AF episode was compared with the excluded patient cohort with uncertain duration of the index AF episode (see Supporting Information, Table S1, in the online version of this article). The patients with AF episodes of uncertain duration were generally healthier, more often female, and less often had prior history of AF or medications. The rate of unsuccessful CVs was higher (17.5% vs 10.3%; P < 0.01), but fewer (0.1% vs 0.7%; P = 0.03) episodes of asystole lasting >5 seconds occurred in the excluded patient cohort compared with the study patient cohort, respectively. There were no other differences between the groups in the other efficacy or safety endpoints of the composite adverse outcome.
4. DISCUSSION
The present large multicenter study demonstrated that the risk for adverse outcomes after CV was doubled in patients with an index AF episode >48 hours when compared with patients with a shorter duration of AF before CV, irrespective of other predictors of CV failure and complications. Concordantly, rates of unsuccessful CVs and recurrences of AF within 30‐day follow‐up after CV were lowest in the patient group with AF episode lasting 24 to 48 hours. The occurrence of safety endpoints (eg, TE events, mortality, and episodes of asystole [>5 s] after CV) was low and spread evenly among patient groups. These observations support the view that performing CV early is better than delaying it when aiming at rhythm control.
It was not a surprise that there were fewer unsuccessful CVs among patients with acute AF episodes. The overall rates of unsuccessful CVs and recurrences of AF within the 30‐day follow‐up were in line with previous studies.5, 11, 12, 13, 14 The relationship between AF episode duration and rate of unsuccessful CV is also in agreement with previous studies,5, 7, 15 whereas the predictors for AF recurrence after CV have been inconsistent11, 13, 14, 15, 16 and no association between the episode duration and recurrence rate of AF has been observed previously. However, prior studies were based on small patient populations, and they were less balanced with respect to AF episode duration. Importantly, in our study only 5% of CVs performed for AF lasting 24 to 48 hours were unsuccessful, whereas the patient group with the shortest AF episodes had slightly more unsuccessful CVs. Similarly, the recurrence rate of AF after CV was slightly lower in patients with AF lasting 24 to 48 hours when compared with patients with AF lasting <24 hours.
The number of TE events, deaths, and acute arrhythmic complications was scant and in line with prior studies,14, 17, 18 and these adverse events were not associated with AF episode duration. Prior studies have, however, suggested that elective CV of AF lasting >48 hours introduces patients to 5‐fold risk (~0.5% for the post‐CV month) for TE events despite anticoagulation, compared with overall low risk in patients on OAC (0.1%/mo). Although recent data suggest that this extra risk associated with elective CV of AF lasting >48 hours could be countered with more intensive anticoagulation,19 non‐anticoagulated AF patients should be referred for prompt (preferably <12 h) rhythm conversion, because shorter delay to CV has been tied to a lower risk of stroke.6
Although there appears to be relatively few safety issues with the CV procedure itself in appropriately anticoagulated patients, there is substantially more room for improvement with efficacy outcomes after CV for AF. The composite adverse outcome was largely driven by the efficacy endpoints (ie, the rates of unsuccessful CV and recurrence of AF after CV). Modifiable independent risk factors for the composite adverse outcome were scarce, and this further underscores the importance of minimizing the delay between AF diagnosis and CV in rhythm management of AF. This view is supported by the data analysis revealing lower efficacy outcomes in patients with AF episodes of unknown duration (see Supporting Information, Table S1, in the online version of this article).
4.1. Study limitations
The major limitations of our study are related to the retrospective setup. First, the inclusion of study population from 3 different cohorts may cause imbalance in the study cohort. Nevertheless, patients were from the same hospitals and catchment areas, and CV procedures were performed using a standardized protocol. Second, the definition of index episode duration is subjective; this is why we excluded patients with unknown AF index episode duration from the main analysis but provided the data in the Supporting Information. Despite these limitations, we believe that these data can guide future research and treatment decisions in patients undergoing CV.
5. CONCLUSION
Our findings provide evidence that the longer delay from AF onset to CV is associated with reduced procedural success rate together with increased AF recurrence after successful CV. Sooner seems better in scheduling CV for patients in rhythm management of AF.
Conflicts of interest
The authors declare no potential conflicts of interest.
Supporting information
Table S1. Baseline characteristics and outcome of patients with known vs unknown duration of index AF episode.
Hellman T, Kiviniemi T, Nuotio I, et al. Optimal timing for cardioversion in patients with atrial fibrillation. Clin Cardiol. 2018;41:966–971. 10.1002/clc.22986
Funding information This work was supported by the Finnish Foundation for Cardiovascular Research, Turku University Hospital (TYKS) Foundation, the Finnish Medical Foundation and the Finnish Medical Society Duodecim, the Finnish Cultural Foundation, and the Finnish‐Norwegian Medical Foundation.
REFERENCES
- 1. Heeringa J, van der Kuip DA, Hofman A, et al. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J. 2006;27:949–953. [DOI] [PubMed] [Google Scholar]
- 2. Lown B. Electrical reversion of cardiac arrhythmias. Br Heart J. 1967;29:469–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wyse DG, Waldo AL, Di Marco J, et al; AFFIRM Investigators . A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825–1833. [DOI] [PubMed] [Google Scholar]
- 4. Kirchhof P, Benussi S, Kotecha D, et al; ESC Scientific Document Group . 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. [DOI] [PubMed] [Google Scholar]
- 5. Elhendy A, Gentile F, Khandheria BK, et al. Predictors of unsuccessful electrical cardioversion in atrial fibrillation. Am J Cardiol. 2002;89:83–86. [DOI] [PubMed] [Google Scholar]
- 6. Nuotio I, Hartikainen JE, Grönberg T, et al. Time to cardioversion for acute atrial fibrillation and thromboembolic complications. JAMA. 2014;312:647–649. [DOI] [PubMed] [Google Scholar]
- 7. Hellman T, Kiviniemi T, Vasankari T, et al. Prediction of ineffective elective cardioversion of atrial fibrillation: a retrospective multi‐center patient cohort study. BMC Cardiovasc Disord. 2017;17:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grönberg T, Nuotio I, Nikkinen M, et al. Arrhythmic complications after electrical cardioversion of acute atrial fibrillation: the FinCV study. Europace. 2013;15:1432–1435. [DOI] [PubMed] [Google Scholar]
- 9. Jaakkola S, Lip GY, Biancari F, et al. Predicting unsuccessful electrical cardioversion for acute atrial fibrillation (from the AF‐CVS Score). Am J Cardiol. 2017;119:749–752. [DOI] [PubMed] [Google Scholar]
- 10. Itäinen S, Lehto M, Vasankari T, et al. Non–vitamin K antagonist oral anticoagulants in atrial fibrillation patients undergoing elective cardioversion. Europace. 2017. 10.1093/europace/eux116. [DOI] [PubMed] [Google Scholar]
- 11. Raitt MH, Volgman AS, Zoble RG, et al; AFFIRM Investigators . Prediction of the recurrence of atrial fibrillation after cardioversion in the Atrial Fibrillation Follow‐up Investigation of Rhythm Management (AFFIRM) study. Am Heart J. 2006;151:390–396. [DOI] [PubMed] [Google Scholar]
- 12. Alegret JM, Viñolas X, Sagristá J, et al; REVERSE Study Investigators . Predictors of success and effect of biphasic energy on electrical cardioversion in patients with persistent atrial fibrillation. Europace. 2007;9:942–946. [DOI] [PubMed] [Google Scholar]
- 13. Melduni RM, Lee HC, Bailey KR, et al. Real‐time physiologic biomarker for prediction of atrial fibrillation recurrence, stroke, and mortality after electrical cardioversion: a prospective observational study. Am Heart J. 2015;170:914–922. [DOI] [PubMed] [Google Scholar]
- 14. Pisters R, Nieuwlaat R, Prins MH, et al; Euro Heart Survey Investigators . Clinical correlates of immediate success and outcome at 1‐year follow‐up of real‐world cardioversion of atrial fibrillation: the Euro Heart Survey. Europace. 2012;14:666–674. [DOI] [PubMed] [Google Scholar]
- 15. Kuppahally SS, Foster E, Shoor S, et al. Short‐term and long‐term success of electrical cardioversion in atrial fibrillation in managed care system. Int Arch Med. 2009;2:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fumagalli S, Boncinelli L, Bondi E, et al. Does advanced age affect the immediate and long‐term results of direct‐current external cardioversion of atrial fibrillation? J Am Geriatr Soc. 2002;50:1192–1197. [DOI] [PubMed] [Google Scholar]
- 17. Goette A, Merino JL, Ezekowitz MD, et al; ENSURE‐AF Investigators . Edoxaban versus enoxaparin‐warfarin in patients undergoing cardioversion of atrial fibrillation (ENSURE‐AF): a randomised, open‐label, phase 3b trial [published correction appears in Lancet 2016;388:1984]. Lancet. 2016;388:1995–2003. [DOI] [PubMed] [Google Scholar]
- 18. Cappato R, Ezekowitz MD, Klein AL, et al; X‐VeRT Investigators . Rivaroxaban vs. vitamin K antagonists for cardioversion in atrial fibrillation. Eur Heart J. 2014;35:3346–3355. [DOI] [PubMed] [Google Scholar]
- 19. Hellman T, Kiviniemi T, Nuotio I, et al. Intensity of anticoagulation and risk of thromboembolism after elective cardioversion of atrial fibrillation. Thromb Res. 2017;156:163–167. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline characteristics and outcome of patients with known vs unknown duration of index AF episode.


