Abstract
Background
Evolocumab significantly lowers low‐density lipoprotein cholesterol (LDL‐C) when dosed 140 mg every 2 weeks (Q2W) or 420 mg monthly (QM) subcutaneously.
Hypothesis
LDL‐C changes are comparable among different patient subgroups in a pooled analysis of data from phase 3 trials.
Methods
A total of 3146 patients received ≥1 dose of evolocumab or control in four 12‐week phase 3 studies. Percent change from baseline in LDL‐C for evolocumab 140 mg Q2W or 420 mg QM vs control was reported as the average of week 10 and 12 values. Quantitative and qualitative interactions between treatment group and subgroup by dose regimen were tested.
Results
In the pooled analysis, treatment differences vs placebo or ezetimibe were similar for both 140 mg Q2W and 420 mg QM doses across ages (<65 years, ≥65 years); gender; race (Asian, black, white, other); ethnicity (Hispanic, non‐Hispanic); region (Europe, North America, Asia Pacific); glucose tolerance status (type 2 diabetes mellitus, metabolic syndrome, neither); National Cholesterol Education Program risk categories (high, moderately high, moderate, low); and European Society of Cardiology/European Atherosclerosis Society risk categories (very high, high, moderate, or low). Certain low‐magnitude variations in LDL‐C lowering among subgroups led to significant quantitative interaction P values that, when tested by qualitative interaction, were not significant. The incidences of adverse events were similar across groups treated with each evolocumab dosing regimen or control.
Conclusions
Consistent reductions in LDL‐C were observed in the evolocumab group regardless of demographic and disease characteristics.
Keywords: age, cardiovascular disease, diabetes, dose, gender, race
1. INTRODUCTION
The importance of reducing low‐density lipoprotein cholesterol (LDL‐C) to lower morbidity and mortality associated with cardiovascular disease is well established. Current guidelines recommend statins as first‐line treatment for hypercholesterolemia in patients at high risk for cardiovascular mortality.1, 2, 3, 4, 5 Despite the cholesterol‐lowering effect of statins, a subset of patients may require additional LDL‐C‐lowering to reach risk‐stratified LDL‐C levels or to further reduce cardiovascular risk.4, 6, 7, 8, 9 The development of monoclonal antibodies that bind proprotein convertase subtilisin/kexin type 9 (PCSK9) has allowed for additional highly effective treatment options for hypercholesterolemia.
Evolocumab is a human monoclonal antibody against PCSK9. The Program to Reduce LDL‐C and Cardiovascular Outcomes Following Inhibition of PCSK9 In Different Populations (PROFICIO) is a comprehensive clinical trial program that established evolocumab efficacy and safety in diverse patient populations with hypercholesterolemia, including those with familial hypercholesterolemia or statin intolerance.10, 11, 12, 13, 14, 15, 16, 17, 18, 19 Within each of these studies, approved evolocumab dosing regimens have substantially and consistently reduced LDL‐C.
To further elucidate the LDL‐C lowering associated with each evolocumab dosing regimen for patient subsets defined by demographic and disease characteristics, we performed a pooled analysis to assess evolocumab efficacy compared to placebo or control from patients enrolled in four randomized placebo‐ or ezetimibe‐controlled phase 3 trials.
2. METHODS
Data were analyzed from patients enrolled in four randomized 12‐week phase 3 evolocumab clinical trials (Table S1, Supporting information).13, 16, 17, 18 Background lipid therapies included statin alone or with ezetimibe. The evolocumab dosing regimens were 140 mg subcutaneously every 2 weeks (Q2W) and 420 mg monthly (QM) (Table S1). An ezetimibe treatment arm was included in three trials.13, 17, 18 All patients provided written informed consent. The individual protocols were approved by each institutional review board and the investigations were in accordance with the Declaration of Helsinki. Additional methods for each trial have been reported elsewhere.13, 16, 17, 18
Patient subgroups for the current analysis were defined according to baseline demographic and disease characteristics (Table 1) and were prespecified in the statistical analysis plans.
Table 1.
LDL‐C reductions by evolocumab dosing regimen and patient subgroup
| Subgroup | Evolocumab Q2W vs placebo | Evolocumab QM vs placebo | Evolocumab Q2W vs ezetimibe | Evolocumab QM vs ezetimibe |
|---|---|---|---|---|
| Treatment differencea in percent change from baseline in LDL‐C between evolocumab and control (95% CI) at the mean of 10 and 12 weeks | ||||
| Age, years | ||||
| <65 | −65.4 (−68.2, −62.6) | −65.3 (−68.3, −62.3) | −39.5 (−43.0, −35.9) | −44.0 (−47.2, −40.8) |
| ≥65 | −65.9 (−69.7, −62.0) | −64.4 (−68.8, −60.1) | −40.1 (−44.8, −35.4) | −35.6 (−40.1, −31.0) |
| Interaction P value (quantitative) | 0.86 | 0.74 | 0.83 | 0.003 |
| Interaction P value (qualitative) | NA | NA | NA | 0.5 |
| Gender | ||||
| Male | −68.5 (−71.8, −65.2) | −67.2 (−70.3, −64.1) | −43.0 (−47.3, −38.7) | −43.8 (−47.5, −40.1) |
| Female | −62.6 (−65.7, −59.4) | −62.9 (−66.8, −59.0) | −36.6 (−40.2, −33.0) | −38.8 (−42.5, −35.1) |
| Interaction P value (quantitative) | 0.01 | 0.09 | 0.025 | 0.06 |
| Interaction P value (qualitative) | 0.5 | NA | 0.5 | NA |
| Race | ||||
| Asian | −61.1 (−70.4, −51.8) | −66.3 (−75.6, −56.9) | −41.5 (−55.4, −27.7) | −52.9 (−62.2, −43.7) |
| Black | −72.6 (−82.5, −62.6) | −58.4 (−70.5, −46.3) | −44.3 (−58.9, −29.8) | −47.3 (−61.0, −33.6) |
| White | −65.7 (−68.1, −63.3) | −65.2 (−67.8, −62.5) | −39.2 (−42.2, −36.3) | −40.6 (−43.4, −37.7) |
| Other | −70.5 (−89.1, −52.0) | NA | NA | NA |
| Interaction P value (quantitative) | 0.37 | 0.52 | 0.75 | 0.034 |
| Interaction P value (qualitative) | NA | NA | NA | 0.875 |
| Ethnicity | ||||
| Hispanic | −77.4 (−92.6, −62.3) | −63.5 (−79.0, −48.1) | −36.6 (−46.8, −26.3) | −38.1 (−52.1, −24.2) |
| Non‐Hispanic | −64.9 (−67.2, −62.7) | −65.2 (−67.7, −62.7) | −39.8 (−42.7, −36.9) | −41.6 (−44.2, −38.9) |
| Interaction P value (quantitative) | 0.11 | 0.83 | 0.54 | 0.63 |
| Region | ||||
| Europe | −66.7 (−69.9, −63.6) | −63.2 (−66.8, −59.7) | −38.5 (−43.2, −33.7) | −40.6 (−44.7, −36.4) |
| North America | −65.6 (−69.4, −61.9) | −67.2 (−71.1, −63.2) | −41.4 (−45.0, −37.9) | −42.1 (−45.7, −38.5) |
| Asia Pacific | −57.8 (−64.3, −51.3) | −66.2 (−72.2, −60.2) | −36.5 (−45.0, −28.1) | −48.0 (−55.9, −40.0) |
| Interaction P value (quantitative) | 0.051 | 0.32 | 0.43 | 0.26 |
| Glucose tolerance | ||||
| Diabetic | −66.4 (−74.9, −57.9) | −62.0 (−72.6, −51.3) | −36.5 (−46.3, −26.6) | −42.5 (−52.2, −32.9) |
| Metabolic syndromeb | −70.0 (−74.1, −65.9) | −63.8 (−67.7, −59.8) | −40.9 (−44.8, −37.0) | −44.8 (−49.2, −40.4) |
| No diabetes or metabolic syndrome | −63.5 (−66.5, −60.5) | −66.7 (−69.7, −63.6) | −39.7 (−43.7, −35.6) | −39.1 (−42.5, −35.7) |
| Interaction P value (quantitative) | 0.04 | 0.42 | 0.70 | 0.12 |
| Interaction P value (qualitative) | 0.75 | NA | NA | NA |
| NCEP risk | ||||
| High | −65.0 (−69.1, −61.0) | −64.6 (−69.6, −59.6) | −40.4 (−46.6, −34.2) | −42.0 (−47.9, −36.1) |
| Moderately high | −72.6 (−80.5, −64.6) | −62.0 (−67.9, −56.1) | −48.0 (−57.8, −38.3) | −39.7 (−46.0, −33.4) |
| Moderate | −67.9 (−72.5, −63.4) | −64.9 (−69.5, −60.3) | −39.4 (−43.9, −34.9) | −42.1 (−47.0, −37.2) |
| Low | −61.8 (−65.7, −57.9) | −65.6 (−69.7, −61.6) | −36.7 (−40.9, −32.4) | −41.0 (−44.8, −37.2) |
| Interaction P value (quantitative) | 0.016 | 0.47 | 0.007 | 0.89 |
| Interaction P value (qualitative) | 0.875 | NA | 0.875 | NA |
| ESC/EAS risk | ||||
| Very high | −66.5 (−70.4, −62.5) | −62.7 (−67.2, −58.1) | −41.1 (−46.8, −35.3) | −40.4 (−45.7, −35.1) |
| High | −65.7 (−72.1, −59.3) | −68.9 (−74.9, −62.9) | −44.2 (−54.9, −33.4) | −45.5 (−57.8, −33.2) |
| Moderate | −66.0 (−69.7, −62.3) | −65.0 (−68.9, −61.1) | −37.9 (−41.8, −34.1) | −38.8 (−42.4, −35.3) |
| Low | −60.5 (−67.3, −53.8) | −67.8 (−73.7, −62.0) | −41.5 (−47.4, −35.7) | −48.5 (−54.1, −43.0) |
| Interaction P value (quantitative) | 0.53 | 0.19 | 0.65 | 0.009 |
| Interaction P value (qualitative) | NA | NA | NA | 0.875 |
Abbreviations: CI, confidence interval; EAS, European Atherosclerosis Society; ESC, European Society of Cardiology; LDL‐C, low‐density lipoprotein cholesterol; NA, not applicable; NCEP, National Cholesterol Education Program; Q2W, every 2 weeks; QM, monthly.
All treatment differences between evolocumab and control were statistically significant with a P value of <0.001.
Defined as no type 2 diabetes mellitus and three or more of the following conditions: fasting glucose ≥100 mg/dL, triglycerides ≥150 mg/dL, high blood pressure based on systolic blood pressure ≥ 130 mmHg or diastolic blood pressure ≥ 85 mmHg or answer of “Yes” to the hypertension question on case report form (CRF), elevated waist circumference, or answer of “Yes” to the question “Low HDL” on CRF.
2.1. Efficacy and safety endpoints
For this analysis, the primary outcome was the difference in percent change from baseline in LDL‐C between each evolocumab dosing regimen and control using the mean of week 10 and 12 LDL‐C values. Key safety endpoints were treatment‐emergent and serious adverse events (AEs), laboratory parameters, and anti‐evolocumab antibodies.
2.2. Statistical analysis
Data from 3146 patients who were randomized and received at least one dose of evolocumab or control were evaluated for efficacy and safety. Mean treatment effect differences and 95% confidence intervals (CIs) within each subgroup were estimated on the average of week 10 and week 12 LDL‐C percent reduction using a repeated measures linear effect model. The model included treatment group, study, baseline value, visit, and treatment by visit interaction. Comparisons between treatment groups were tested separately for the Q2W and QM dosing regimens. Quantitative interactions between treatment group and subgroups were tested on the average of week 10 and 12 LDL‐C percent reductions through an analysis of covariance (ancova) model, which included the treatment group, study, baseline LDL‐C, each subgroup variable, and the interaction of treatment with subgroup as covariates. For cases in which quantitative interaction testing showed that treatment efficacy varied in magnitude among subgroups, qualitative interaction was performed via Gail‐Simon's method20 to test if the treatment efficacy varied in direction among subgroups. Waterfall plots illustrated individual‐patient percent change from baseline in LDL‐C at a mean of weeks 10 and 12. Response was defined as a ≥ 15% LDL‐C reduction at the mean of weeks 10 and 12; patients evaluable were those with an LDL‐C value at that timepoint. No missing data imputation or multiplicity adjustments were performed. Baseline demographics, baseline lipid parameters, and safety data were assessed using descriptive statistics. All analyses were conducted with SAS/STAT, version 9.2 (SAS Institute, Cary, North Carolina). The studies were not powered for safety endpoints; therefore, no inferential statistical analyses with associated P values were conducted for adverse events.
3. RESULTS
3.1. Baseline characteristics
The patient populations of the evolocumab trials included in this analysis are summarized in Table S1.13, 16, 17, 18 Included in these trials were patients with primary hypercholesterolemia and cardiovascular risk of various levels, familial hypercholesterolemia, and prior intolerance to ≥2 statins. Baseline characteristics of the pooled population from the trials are summarized in Table S2. In the pooled population, the mean age of participants was 57.8 years, 49.4% of patients were women, 91.5% were white, and 54.1% were receiving statins. The mean (SD) baseline calculated LDL‐C was 3.3 (1.3) mmol/L, and 33.8% of patients were at high risk according to National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATPIII) cardiovascular risk categories.
3.2. LDL‐C reduction in overall population
In the individual studies, the mean percent change from baseline in LDL‐C ranged from −74.9% (95% CI: −84.5, −65.3) to −56.5% (95% CI: −59.9, −53.0) in patients receiving evolocumab 140 mg Q2W vs placebo; from −74.8% (95% CI: −83.0, −66.6) to −57.4% (95% CI: −60.7, −54.1) in patients receiving evolocumab 420 mg QM vs placebo; from −44.9% (95% CI: −54.3, −35.6) to −36.9% (95% CI: −42.3, −31.5) in patients receiving evolocumab 140 mg Q2W vs ezetimibe; and from −43.8% (95% CI: −52.1, −35.5) to −38.7% (95% CI: −43.1, −34.3) in patients receiving evolocumab 420 mg QM vs ezetimibe (Table S3).
Among all patients in this integrated population from all trials, mean percent changes from baseline in LDL‐C were −65.7% (95% CI: −70.9, −60.6; evolocumab 140 mg Q2W) and −65.0% (95% CI: −69.5, −60.4; evolocumab 420 mg QM) vs placebo, and −38.9% (95% CI: −41.3, −36.4; evolocumab 140 mg Q2W) and −40.3% (95% CI: −42.6, −38.0; evolocumab 420 mg QM) vs ezetimibe (Table S4).
At the week 2, 8, 10, and 12 scheduled post‐baseline assessments, mean percent change from baseline in LDL‐C ranged from −53.7% to −60.5% and from −55.5% to −67.8% for the Q2W and QM regimens, respectively, and consistent reductions in LDL‐C were observed in evolocumab‐treated groups compared to placebo‐ or ezetimibe‐treated groups (Figure 1). Waterfall plots demonstrate consistent patient‐level LDL‐C reductions with evolocumab plus statins compared to statin plus placebo (Figure 2A) or with evolocumab monotherapy compared to placebo alone (Figure 2B) among patients who were not statin intolerant. In the statin combination studies, the proportion of responders (LDL‐C reduction of ≥15%) was 98.3% among evaluable patients receiving evolocumab 140 mg Q2W plus statins, 96.9% among patients receiving evolocumab 420 mg QM plus statins, and 12.5% among patients receiving placebo plus statins. In the monotherapy study, the proportion of responders was 100% among evaluable patients receiving evolocumab 140 mg Q2W, 100% among patients receiving evolocumab 420 mg QM, and 8.8% among patients receiving placebo.
Figure 1.
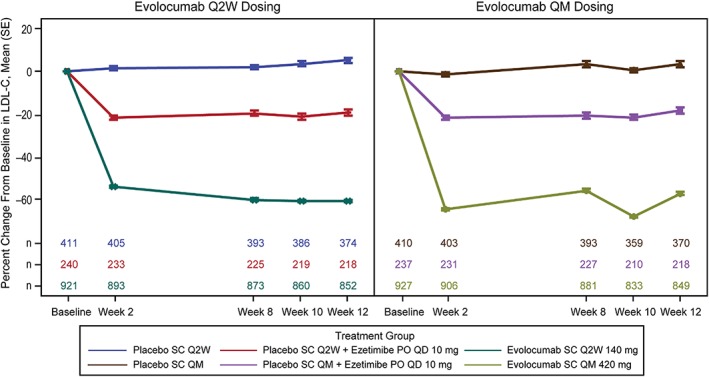
Percent change from baseline in LDL‐C by scheduled visit and treatment group. Abbreviations: LDL‐C, low‐density lipoprotein cholesterol; PO, orally; QD, daily; QM, monthly; Q2W, every 2 weeks; SC, subcutaneously; SE, standard error
Figure 2.
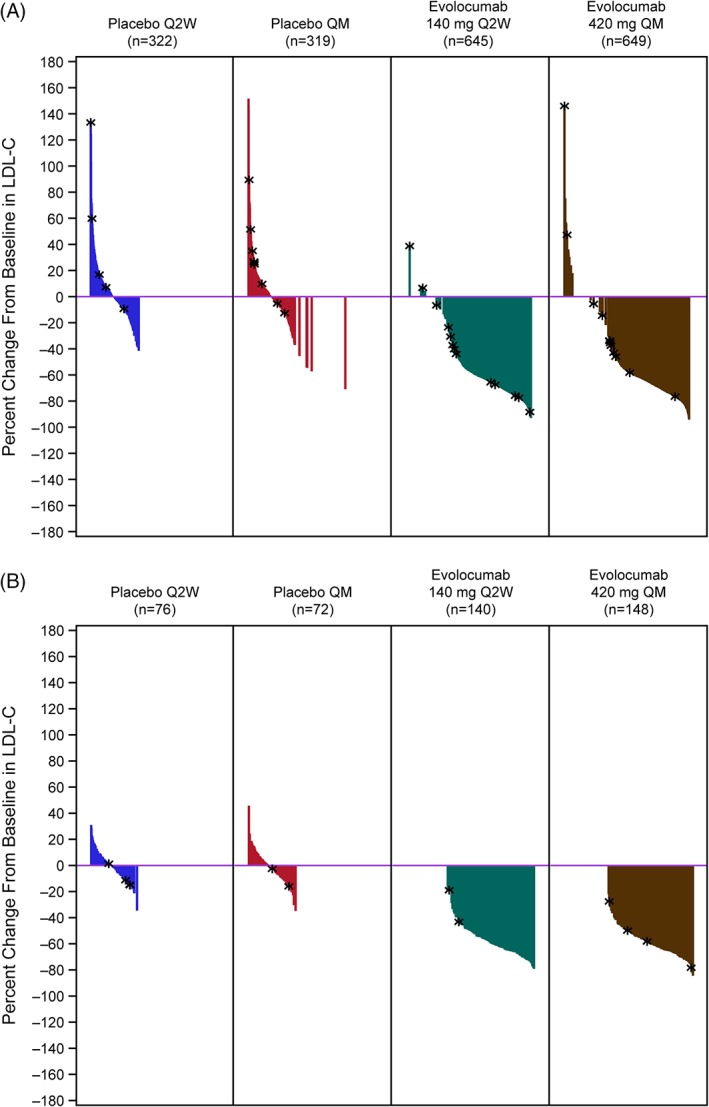
Waterfall plots showing percent change from baseline in LDL‐C at the mean of weeks 10 and 12 in patients who did (A) and did not (B) receive combination statin therapy. Plot is based on observed data; no imputation is used for missing values. *patients with early termination. Abbreviations: LDL‐C, low‐density lipoprotein cholesterol; n, number of patients randomized, dosed and who were evaluable for LDL‐C at the timepoint; QM, monthly; Q2W, every 2 weeks
3.3. LDL‐C reduction by subgroup
Treatment differences in mean percent change from baseline in LDL‐C between evolocumab and placebo and between evolocumab and ezetimibe were similar for both 140 mg Q2W and 420 mg QM doses across studies and subgroups (Table 1 and Figure 3). Within each subgroup, evolocumab 140 mg Q2W and 420 QM demonstrated statistically significant mean reductions in LDL‐C from baseline as compared to placebo or ezetimibe (P < 0.001).
Figure 3.
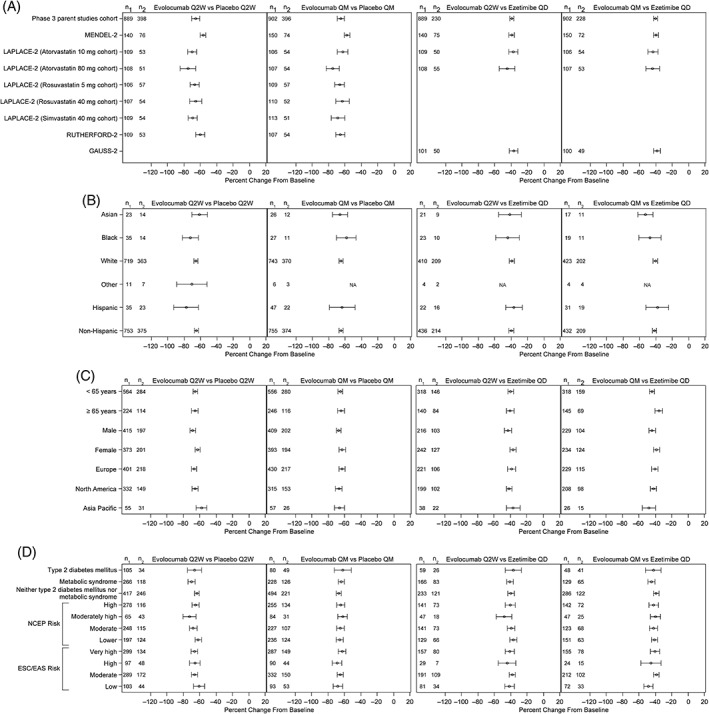
Percent change from baseline in LDL‐C at mean of weeks 10 and 12 for evolocumab vs placebo or ezetimibe according to individual study (A), race (B), patient demographic characteristics (C), and disease status (D). Abbreviations: EAS, European atherosclerosis society; ESC, European Society of Cardiology; LDL‐C, low‐density lipoprotein cholesterol; NA, not applicable; NCEP, National Cholesterol Education Program; n1, number of patients in the subgroup of interest included in the repeated measures model receiving evolocumab; n2, number of patients in the subgroup of interest included in the repeated measures model receiving placebo; QD, daily; QM, monthly; Q2W, every 2 weeks
Due to the large sample size from the four integrated studies, a small magnitude of variation in LDL‐C reduction among subgroups led to quantitative interaction P values of <0.05 for certain subgroups. In patients treated with evolocumab Q2W vs ezetimibe, greater LDL‐C reductions were observed in men (interaction P = 0.025) and patients with NCEP risk category of moderately high vs other risk categories (interaction P = 0.007). In patients treated with evolocumab Q2W vs placebo, greater LDL‐C reductions were observed in men vs women (interaction P = 0.01), in patients with metabolic syndrome vs those with diabetes or without diabetes/metabolic syndrome (interaction P = 0.04), and in patients with NCEP risk category of moderately high vs other risk categories (interaction P = 0.016). In patients treated with evolocumab QM vs ezetimibe, greater LDL‐C reductions were observed in patients younger than 65 years old (interaction P = 0.003), Asian patients (interaction P = 0.034), and patients with ESC/EAS risk category of low (interaction P = 0.009). For these subgroups, qualitative interaction testing demonstrated common directionality of LDL‐C lowering effect among subgroups and non‐significant P values. No significant quantitative interactions were observed in the evolocumab QM vs placebo group.
The relationship between gender and LDL‐C reduction with evolocumab Q2W vs placebo or ezetimibe was further evaluated. Results from the individual phase 3 studies did not show a consistent pattern. Exploratory analyses adjusting for the covariates of age, body mass index, baseline LDL‐C, baseline PCSK9, and baseline statin in both univariate and multivariate settings did not result in notable changes in treatment effect and interaction P value of treatment by gender, indicating no evidence of confounding factors.
3.4. Safety
In the integrated population, the incidences of AEs and laboratory parameter elevations were similar across groups treated with each evolocumab dosing regimen or control. The rates of overall AEs were 43.8% (evolocumab 140 mg Q2W), 43.4% (evolocumab 420 mg QM), 48.8% (ezetimibe), and 41.8% (placebo) (Table 2). Serious AEs occurred in 2.6%, 1.7%, 1.5%, and 2.3% of patients across the same groups, respectively. Muscle‐related AEs were highest in the ezetimibe‐treated group (7.8%) as compared to evolocumab 140 mg Q2W (3.5%), evolocumab 420 mg QM (3.8%), or placebo (2.9%). A creatine kinase elevation of >5 times the upper limit of normal (xULN) occurred in <1% of patients in any arm. Injection site reactions occurred with similar incidence between the evolocumab‐treated arms (2.5%, 140 mg Q2W; 3.0%, 420 mg QM) and the control‐treated arms (3.6%, ezetimibe; 2.4%, placebo). Neurocognitive events were infrequent, occurring in 0.1% of evolocumab‐treated patients, 0.6% of ezetimibe‐treated patients, and in no patients receiving placebo. Liver enzyme elevations of >3 xULN occurred in 0.5% (evolocumab 140 mg Q2W), 0.2% (evolocumab 420 mg QM), 0.8% (ezetimibe), and 1.3% (placebo) of patients. One of the four studies evaluated evolocumab in patients not receiving statins. Among this cohort, new‐onset diabetes was observed in 1 (0.6%) evolocumab‐treated patient. Binding anti‐evolocumab antibodies were observed in three patients after evolocumab dosing; of these patients, one had binding antibodies at baseline. No neutralizing anti‐evolocumab antibodies were detected. The low numbers of individual AEs precluded analysis of safety by subgroup.
Table 2.
Summary of AEs and laboratory parameters
| % | Evolocumab; 140 mg Q2W (N = 921) | Evolocumab; 420 mg QM (N = 927) | Ezetimibe (N = 477) | Placebo (N = 821) |
|---|---|---|---|---|
| Any AE | 43.8 | 43.4 | 48.8 | 41.8 |
| Serious AEs | 2.6 | 1.7 | 1.5 | 2.3 |
| Muscle‐related AEs | 3.5 | 3.8 | 7.8 | 2.9 |
| Injection site reactions | 2.5 | 3.0 | 3.6 | 2.4 |
| Neurocognitive AEs | 0.1 | 0.1 | 0.6 | 0 |
| Creatine kinase >5× ULN | 0.1 | 0.4 | 0.6 | 0.7 |
| ALT or AST >3× ULN | 0.5 | 0.2 | 0.8 | 1.3 |
| Neutralizing anti‐evolocumab antibodies | 0 | 0 | Not tested | Not tested |
Abbreviations: AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase; Q2W, every 2 weeks; QM, monthly; ULN, upper limit of normal.
4. DISCUSSION
This pooled analysis demonstrated substantial reductions in LDL‐C across various patient subgroups treated with evolocumab. A very low nonresponder rate was seen compared to placebo or placebo plus statins with or without other lipid‐lowering therapy (such as ezetimibe), as illustrated in the waterfall plots. These plots indicate patient‐level data for all patients enrolled with the exception of statin‐intolerant patients (those enrolled in GAUSS‐2), who were not eligible for efficacy analysis according to statin use. No substantial differences in responsiveness across dosing regimens and subgroups defined by demographic and disease characteristics were observed. Certain quantitative interactions between subgroup and LDL‐C reduction by evolocumab reached statistical significance despite their small magnitude. For example, in patients treated with evolocumab Q2W vs placebo or vs ezetimibe, greater LDL‐C reductions were observed in men vs women, and in patients with NCEP risk category of moderately high vs other risk categories. These observations are not surprising, given that the sample size for each subgroup was large and evolocumab has a substantial treatment effect. The quantitative interaction test detected small differences in the magnitude of the treatment effect across the subgroups that are not clinically meaningful. Furthermore, qualitative interaction analysis demonstrated consistent directionality of effect and no statistically significant differences.
Potentially explanatory investigations into the cause of the observed numerical treatment effect in gender did not reveal alternative factors that could explain the results. In addition, P values were not adjusted for multiplicity; therefore, it is reasonable to suggest that the observed difference of treatment effect in gender was obtained by chance.
Results of the current study are consistent with those of a pooled analysis of phase 2 evolocumab studies.21 That pooled analysis, which included 1359 patients from 4 studies, demonstrated similar reductions in LDL‐C with evolocumab dosed at 140 mg Q2W or 420 mg QM among subgroups defined by age, gender, statin use, baseline LDL‐C level, and baseline triglyceride level. In this analysis, the interaction between evolocumab Q2W dosing and gender was also statistically significant (interaction P = 0.03), with women showing less LDL‐C response than men. In the literature, various impacts of gender or gender‐specific conditions on cholesterol and PCSK9 have been identified, including hormone therapy and menopause.22, 23, 24, 25, 26, 27, 28, 29 However, data regarding hormone therapy or menopause were not collected in PROFICIO, and the potential role of any of the identified factors on the results of our current dataset is unknown. While this study was not designed to explore the biological and physiological pathways that underlie the gender differences in LDL‐C reduction, it is hypothesis‐generating for additional mechanistic studies.
The safety profile revealed no new concerns. Together with efficacy data, these results support a favorable benefit–risk profile for evolocumab across diverse patient populations.
A strength of this analysis is that it includes a very diverse population with patients who had participated in monotherapy, statin combination therapy, statin intolerance, and heterozygous familial hypercholesterolemia evolocumab trials. The analysis also includes data from two dosing options, Q2W and QM, and from both placebo‐ and ezetimibe‐controlled trials. A limitation of our analysis is that this analysis was post hoc, with pooled data from four randomized studies.
5. CONCLUSIONS
In this pooled analysis of data from patients enrolled in four phase 3 trials, evolocumab 140 mg Q2W and 420 mg QM demonstrated significantly greater reductions in LDL‐C vs placebo or ezetimibe for all demographic and disease status subgroups. Substantial reductions in LDL‐C were observed in the evolocumab group, regardless of age, race, background statin dose, or cardiovascular risk. Although several subgroup quantitative interaction comparisons were significant at the P < 0.05 level, the differences were of small magnitude. Very few nonresponders were observed in comparison to patients on statins and other commonly employed compounds, including ezetimibe. Adverse events for the evolocumab 140 mg Q2W and 420 mg QM dosing regimens were overall similar to those observed with control.
Supporting information
Table S1. Phase 3 trials with evolocumab
Table S2. Baseline characteristics of patients in the overall population
Table S3. LDL‐C reductions by evolocumab dosing regimen and individual clinical trial
Table S4. LDL‐C reduction by dosing regimens in the overall population
ACKNOWLEDGMENTS
The authors thank Mahta Nili, PhD (formerly of Amgen Inc.) and Laura Evans, PharmD (on behalf of Amgen Inc.) for preliminary drafting and editorial support.
This study was funded by Amgen Inc.
Conflict of interest
Dr. Stroes reports that his institution has received lecturing fees/grants from Amgen Inc., Merck, Novartis, Regeneron Pharmaceuticals, and Sanofi.
Dr. Robinson reports consulting fees from Amgen Inc., Eli Lilly, Merck, Pfizer, Regeneron Pharmaceuticals, and Sanofi, and reports that her institution has received grants from Acasti Pharma, Amarin Corporation, Amgen Inc., AstraZeneca, Eisai, Esperion, Merck, Pfizer, Regeneron Pharmaceuticals, Sanofi, and Takeda Pharmaceutical Company Ltd.
Dr. Raal reports consulting fees from Amgen Inc. and Sanofi related to PCSK9 inhibitors, and institutional research funding related to PCSK9 inhibitor clinical trials from Amgen Inc. and Sanofi.
Dr. Dufour reports consulting fees from Amgen Inc., Regeneron Pharmaceuticals/Sanofi, Aegerion Pharmaceuticals, and Janssen Pharmaceuticals, research funding from Amgen Inc. and lecturing fees form Amgen Inc., Valeant Pharmaceuticals International and Sanofi.
Dr. Sullivan reports advisory committee/lecture fees from Abbott, Amgen Inc., AstraZeneca, Mylan, and Pfizer; and that he has received grants from Abbott, Amgen Inc., Mylan, Sanofi, and Novartis.
Drs. Kassahun and Wasserman are employees of and stockholders in Amgen Inc. Dr. Wasserman appears on a number of pending patents owned by Amgen Inc. relating to evolocumab and PCSK9 inhibition. Dr. Ma is employed on behalf of Amgen Inc.
Dr. Koren is employed by a company that has received research grants and consulting fees from Amgen Inc. and other companies developing therapies for hyperlipidemia.
Stroes E, Robinson JG, Raal FJ, et al. Consistent LDL‐C response with evolocumab among patient subgroups in PROFICIO: A pooled analysis of 3146 patients from phase 3 studies. Clin Cardiol. 2018;41:1328–1335. 10.1002/clc.23049
Funding information Amgen Inc.
[The copyright line for this article was changed on 30‐July 2019 after original online publication]
REFERENCES
- 1. Anderson TJ, Grégoire J, Pearson GJ, et al. 2016 Canadian cardiovascular society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol. 2016;32:1263‐1282. [DOI] [PubMed] [Google Scholar]
- 2. Expert Dyslipidemia Panel , Grundy SM. An International Atherosclerosis Society position paper: global recommendations for the management of dyslipidemia. J Clin Lipidol. 2013;7:561‐565. [DOI] [PubMed] [Google Scholar]
- 3. Perk J, De Backer G, Gohlke H, et al. European guidelines on cardiovascular disease prevention in clinical practice (version 2012). The fifth joint task force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts). Eur Heart J. 2012;33:1635‐1701. [DOI] [PubMed] [Google Scholar]
- 4. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation. 2014;129:S1‐S45. [DOI] [PubMed] [Google Scholar]
- 5. European Association for Cardiovascular Prevention, Rehabilitation , Reiner Z, et al. ESC/EAS Guidelines for the management of dyslipidaemias: the task force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J. 2011;32:1769‐1818. [DOI] [PubMed] [Google Scholar]
- 6. Boekholdt SM, Hovingh GK, Mora S, et al. Very low levels of atherogenic lipoproteins and the risk for cardiovascular events: a meta‐analysis of statin trials. J Am Coll Cardiol. 2014;64:485‐494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bohula EA, Giugliano RP, Cannon CP, et al. Achievement of dual low‐density lipoprotein cholesterol and high‐sensitivity C‐reactive protein targets more frequent with the addition of ezetimibe to simvastatin and associated with better outcomes in IMPROVE‐IT. Circulation. 2015;132:1224‐1233. [DOI] [PubMed] [Google Scholar]
- 8. Waters DD, Brotons C, Chiang CW, et al. Lipid treatment assessment project 2: a multinational survey to evaluate the proportion of patients achieving low‐density lipoprotein cholesterol goals. Circulation. 2009;120:28‐34. [DOI] [PubMed] [Google Scholar]
- 9. Lloyd‐Jones DM, Morris PB, Ballantyne CM, et al. 2017 focused update of the 2016 ACC expert consensus decision pathway on the role of non‐statin therapies for LDL‐cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American College of Cardiology task force on expert consensus decision pathways. J Am Coll Cardiol. 2017;70:1785‐1822. [DOI] [PubMed] [Google Scholar]
- 10. Blom DJ, Hala T, Bolognese M, et al. A 52‐week placebo‐controlled trial of evolocumab in hyperlipidemia. N Engl J Med. 2014;370:1809‐1819. [DOI] [PubMed] [Google Scholar]
- 11. Giugliano RP, Desai NR, Kohli P, et al. Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 in combination with a statin in patients with hypercholesterolaemia (LAPLACE‐TIMI 57): a randomised, placebo‐controlled, dose‐ranging, phase 2 study. Lancet. 2012;380:2007‐2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hirayama A, Honarpour N, Yoshida M, et al. Effects of evolocumab (AMG 145), a monoclonal antibody to PCSK9, in hypercholesterolemic, statin‐treated Japanese patients at high cardiovascular risk‐‐primary results from the phase 2 YUKAWA study. Circ J. 2014;78:1073‐1082. [DOI] [PubMed] [Google Scholar]
- 13. Koren MJ, Lundqvist P, Bolognese M, et al. Anti‐PCSK9 monotherapy for hypercholesterolemia: the MENDEL‐2 randomized, controlled phase III clinical trial of evolocumab. J Am Coll Cardiol. 2014;63:2531‐2540. [DOI] [PubMed] [Google Scholar]
- 14. Koren MJ, Scott R, Kim JB, et al. Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 as monotherapy in patients with hypercholesterolaemia (MENDEL): a randomised, double‐blind, placebo‐controlled, phase 2 study. Lancet. 2012;380:1995‐2006. [DOI] [PubMed] [Google Scholar]
- 15. Raal F, Scott R, Somaratne R, et al. Low‐density lipoprotein cholesterol‐lowering effects of AMG 145, a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease in patients with heterozygous familial hypercholesterolemia: the reduction of LDL‐C with PCSK9 inhibition in heterozygous familial hypercholesterolemia disorder (RUTHERFORD) randomized trial. Circulation. 2012;126:2408‐2417. [DOI] [PubMed] [Google Scholar]
- 16. Raal FJ, Stein EA, Dufour R, et al. PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD‐2): a randomised, double‐blind, placebo‐controlled trial. Lancet. 2015;385:331‐340. [DOI] [PubMed] [Google Scholar]
- 17. Robinson JG, Nedergaard BS, Rogers WJ, et al. Effect of evolocumab or ezetimibe added to moderate‐ or high‐intensity statin therapy on LDL‐C lowering in patients with hypercholesterolemia: the LAPLACE‐2 randomized clinical trial. JAMA. 2014;311:1870‐1882. [DOI] [PubMed] [Google Scholar]
- 18. Stroes E, Colquhoun D, Sullivan D, et al. Anti‐PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS‐2 randomized, placebo‐controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol. 2014;63:2541‐2548. [DOI] [PubMed] [Google Scholar]
- 19. Sullivan D, Olsson AG, Scott R, et al. Effect of a monoclonal antibody to PCSK9 on low‐density lipoprotein cholesterol levels in statin‐intolerant patients: the GAUSS randomized trial. JAMA. 2012;308:2497‐2506. [DOI] [PubMed] [Google Scholar]
- 20. Gail M, Simon R. Testing for qualitative interactions between treatment effects and patient subsets. Biometrics. 1985;41:361‐372. [PubMed] [Google Scholar]
- 21. Stein EA, Giugliano RP, Koren MJ, et al. Efficacy and safety of evolocumab (AMG 145), a fully human monoclonal antibody to PCSK9, in hyperlipidaemic patients on various background lipid therapies: pooled analysis of 1359 patients in four phase 2 trials. Eur Heart J. 2014;35:2249‐2259. [DOI] [PubMed] [Google Scholar]
- 22. Mayne J, Raymond A, Chaplin A, et al. Plasma PCSK9 levels correlate with cholesterol in men but not in women. Biochem Biophys Res Commun. 2007;361:451‐456. [DOI] [PubMed] [Google Scholar]
- 23. Smith PM, Cowan A, White BA. The low‐density lipoprotein receptor is regulated by estrogen and forms a functional complex with the estrogen‐regulated protein ezrin in pituitary GH3 somatolactotropes. Endocrinology. 2004;145:3075‐3083. [DOI] [PubMed] [Google Scholar]
- 24. Croston GE, Milan LB, Marschke KB, Reichman M, Briggs MR. Androgen receptor‐mediated antagonism of estrogen‐dependent low density lipoprotein receptor transcription in cultured hepatocytes. Endocrinology. 1997;138:3779‐3786. [DOI] [PubMed] [Google Scholar]
- 25. Paganini‐Hill A, Dworsky R, Krauss RM. Hormone replacement therapy, hormone levels, and lipoprotein cholesterol concentrations in elderly women. Am J Obstet Gynecol. 1996;174:897‐902. [DOI] [PubMed] [Google Scholar]
- 26. Lakoski SG, Lagace TA, Cohen JC, Horton JD, Hobbs HH. Genetic and metabolic determinants of plasma PCSK9 levels. J Clin Endocrinol Metab. 2009;94:2537‐2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mora S, Buring JE, Ridker PM. Discordance of low‐density lipoprotein (LDL) cholesterol with alternative LDL‐related measures and future coronary events. Circulation. 2014;129:553‐561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Furuhashi M, Omori A, Matsumoto M, et al. Independent link between levels of proprotein convertase subtilisin/kexin type 9 and FABP4 in a general population without medication. Am J Cardiol. 2016;118:198‐203. [DOI] [PubMed] [Google Scholar]
- 29. Matthews KA, Crawford SL, Chae CU, et al. Are changes in cardiovascular disease risk factors in midlife women due to chronological aging or to the menopausal transition? J Am Coll Cardiol. 2009;54:2366‐2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Phase 3 trials with evolocumab
Table S2. Baseline characteristics of patients in the overall population
Table S3. LDL‐C reductions by evolocumab dosing regimen and individual clinical trial
Table S4. LDL‐C reduction by dosing regimens in the overall population


