Summary
Depression is the leading cause of disability around the world, but little is known about its pathology. Currently, the diagnosis of depression is made based on clinical manifestations, with little objective evidence. Magnetic resonance imaging (MRI) has been used to investigate the pathological changes in brain anatomy associated with this disorder. MRI can identify structural alterations in depressive patients in vivo, which could make considerable contributions to clinical diagnosis and treatment. Numerous studies that focused on gray and white matter have found significant brain region alterations in major depressive disorder patients, such as in the frontal lobe, hippocampus, temporal lobe, thalamus, striatum, and amygdala. The results are inconsistent and controversial because of the different demographic and clinical characteristics. However, some regions overlapped; thus, we think that there may be a “hub” in MDD and that an impairment in these regions contributes to disease severity. Brain connections contain both structural connections and functional connections, which reflect disease from a different view and support that MDD may be caused by the interaction of multiple brain regions. According to previous reports, significant circuits include the frontal‐subcortical circuit, the suicide circuit, and the reward circuit. As has been recognized, the pathophysiology of major depressive disorder is complex and changeable. The current review focuses on the significant alterations in the gray and white matter of patients with the depressive disorder to generate a better understanding of the circuits. Moreover, identifying the nuances of depressive disorder and finding a biomarker will make a significant contribution to the guidance of clinical diagnosis and treatment.
Keywords: Psychoradiology, diffusion MRI; magnetic resonance imaging; major depressive disorder; structure MRI
1. INTRODUCTION
Major depressive disorder (MDD) is characterized by persistent low mood, often accompanied by cognitive dysfunction, physical symptoms, and impaired social function.1 According to the World Health Organization's statistics, more than 300 million people worldwide suffer from depression. More severely, depression can cause suicide, and nearly 800 000 people die every day due to suicide. According to statistics from 2015,2 suicide is the second leading cause of death among people 15‐29 years of age, resulting in vast economic and social burdens. Based on epidemiological studies, more than 30% of patients with depression suffer from ineffective antidepressant treatments, and their lifetime prevalence rate is approximately 16.2%.3 Currently, the diagnosis of MDD relies on patients’ reporting and behavioral assessments. Little is known about its precise neurobiological biomarkers. Neuroimaging studies have the advantage of being noninvasive and repeatable and might provide precise evidence to clinics for more successful individualized therapies.
In recent years, neuroimaging methods, especially magnetic resonance imaging (MRI), have been used in many studies to identify disorder‐related patterns of the brain changes associated with MDD. The MRI scan sequences that are commonly used by researchers include high‐resolution structural imaging (3D‐T1), which depicts gray matter thickness in volume and brain morphology; diffusion tensor imaging (DTI), which depicts the microstructure of the white matter; and functional magnetic resonance imaging (fMRI), which depicts the neuronal activity in target brain regions. Previous studies based on MRI found that several brain regions are significantly impaired in MDD patients. Several regional gray matter changes have been identified in the frontal lobe, parietal lobe, thalamus, caudate, pallidum, putamen, and temporal lobes (eg, the hippocampus and amygdala)4, 5, 6 by anatomical MRI studies. DTI studies have shown white matter alterations, such as decreased fractional anisotropy (FA) in the cingulum,7 hippocampus, parietal regions,8 inferior temporal gyrus, and superior frontal gyrus. These findings were confirmed by a recent meta‐analysis.9 In addition, abnormal brain activity was found in the prefrontal cortex,10 occipital lobe,11 temporal gray, caudate,12 and putamen.13 Different brain regions have connections with each other and ultimately form complex brain networks. A hub is a region that is highly connected and highly central that plays a role in global information integration14 (Figure 1). The impairment of hub nodes or their connections may be a leading cause of disease, such as MDD. Structural connections between cortical and subcortical areas compose several circuits, such as the frontal‐subcortical circuit,15 basal ganglic‐thalamic‐cortical circuit,16 prefrontal hippocampus circuit,17 the limbic‐cortical‐striatal‐thalamic‐cortical circuit,18 and the limbic‐cortical‐striatal‐pallidal‐thalamic19 circuit. The most common functional connection in MDD patients is the cortical‐subcortical circuit.20 According to previous findings, the frontal lobe, parietal lobe, thalamus, putamen, and hippocampus are considered hubs in these circuits.14, 21, 22
Figure 1.
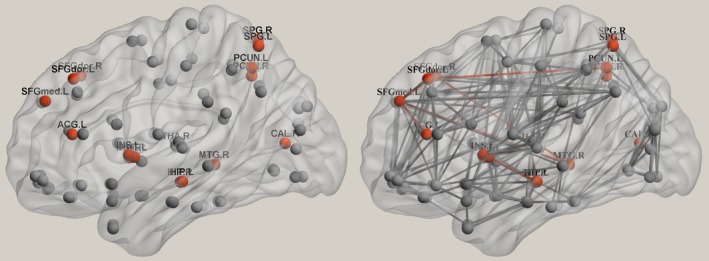
Structural network hubs of the human brain. Structural brain hubs were identified by diffusion tensor imaging, and the nodes normalized to an intramodular degree higher than 1 were defined as hubs.14, 21 ACG, anterior cingulate and paracingulate gyri; CAL, calcarine fissure and surrounding cortex; HIP, hippocampus; INS, insula; L, left; MOG, middle occipital gyrus; MTG, middle temporal gyrus; PCUN, precuneus; PUT, putamen; R, right; SOG, superior occipital gyrus; SPG, superior parietal gyrus; SFGdor, dorsolateral part of the superior frontal gyrus; SFGmed, medial part of the superior frontal gyrus; THA, thalamus
In the present review, significant structural changes in brain regions and circuits involved in MDD are reviewed, and future research directions in this field are outlined. This article focuses on the imaging findings of MDD to explore the findings of neuroimaging, which is helpful for differential diagnosis and accurate medicine.
2. SIGNIFICANT STRUCTURAL BRAIN ALTERATIONS IN MDD
2.1. Frontal lobe
According to previous reports, the change in the volume of frontal regions has been considered to be the most common region to manifest anatomic abnormalities in MDD. Important prefrontal lesions include those of Brodmann area 24 (a part of the anterior cingulate cortex), orbitofrontal cortex (OFC), middle prefrontal cortex, dorsolateral prefrontal cortex (DLPFC), and other areas of the prefrontal cortex.8, 23, 24, 25, 26 The anterior cingulate cortex (ACC) has an anatomical connection with dorsal neocortical and ventral paralimbic regions and plays a role in cognitive processes and mood regulation.27 The right anterior cingulate cortex was reported to have a decreased magnetization transfer ratio in patients with treatment‐refractory MDD relative to healthy controls,28 which increased after electroconvulsive therapy.29 Previous studies have also found that prefrontal areas undergo a significant reduction in thickness,4, 30, 31, 32 and these changes are thought to be associated with poor clinical outcomes.33 A negative correlation was observed between the Montgomery‐Asberg Depression Rating Scale score and cortical thickness in the ACC.34 It has been indicated that the thicker right caudal ACC is associated with greater symptom improvement across follow‐ups. According to an fMRI study, the ACC has increased functional correlations with the DLPFC and the amygdala35 in MDD patients, suggesting that the ACC is more similar to a bridge between the DLPFC and the amygdala and plays a critical role in attention and emotion. The OFC is involved in inhibiting background‐independent, redundant, or uncomfortable neural activity, feelings, and behaviors and also plays a crucial role in emotional/motivational management and decision making.36 It has been reported that the thickness of the right medial orbital cortex in MDD patients is thinner than that in healthy controls,37 and during treatment, increased cortical thickness was found in the OFC in MDD patients. Functional MRI results found reduced brain activity in the bilateral OFC.38 Structural and functional changes in the OFC may contribute to the reduced inhibition of negative stimuli in depressive patients. The volume of gray matter in the left middle frontal gyrus was found to be decreased in untreated depressive patients2 and to increase after drug treatment.39 These changes are associated with emotional bias, apathy, and loss of motivation.5 The fibers in the medial frontal cortex are part of the default‐mode network40 and play an essential role in the execution of long‐term mental plans from immediate environmental or internal demands.41 A study found a reduction in both the middle frontal cortex and the connection of the corpus callosum in treatment‐refractory MDD.42 Fibers extending through the bilateral medial frontal lobe to the anterior corpus callosum and passing fibers connecting the medial frontal lobe to the amygdala via the uncinate fasciculus during stimulation of the “best” contacts have been deemed to be the switch in MDD.43 The DLPFC plays an essential role in emotional, motivational, attentional, and executive functions.44 Gray matter volume reductions have also been found in the DLPFC in MDD patients compared to healthy controls.2 Brain activity in the DLPFC was also found to be decreased13 and could be increased to the average level after antidepressant treatment.2 Transcranial magnetic stimulation of the left DLPFC induces morphological increases in the left ACC and the middle frontal OFC,45 indicating that the DLPFC has connections with the ACC and the OFC.
2.2. Thalamus
The thalamus is considered a complicated sensory information node that controls emotion, memory, and arousal.46 Dysfunction and structural disruptions in the thalamus can lead to an amnestic syndrome due to impairments in recall and recognition.47 The thalamus is structured as several anatomical parts. The subthalamic nuclei accept fibers from the pallidum and motor cortex and send out fibers to the substantia nigra. The lateral dorsal thalamic nucleus sends out fibers to the parietal lobe, and the ventral lateral thalamic nucleus has connections with the cerebellum and the brainstem. Significant volume reductions and changes in shape have been observed in the left thalamus48 of patients with MDD. Based on a shape analysis of the vertex, the dorsal aspect of the left thalamus was found to be negatively correlated with the severity of depression (Hamilton Depression Rating Scale).48 A probabilistic tractography study reported that the areas with shape deformities in the bilateral putamen and left thalamus had connections with the frontal and temporal lobes.48 The gray matter volume of the right thalamus was also found to be reduced in MDD patients,42, 49 and this finding was confirmed by a recent meta‐analysis.50 As some studies have shown, drugs and acute attacks can affect the volume of the thalamus, which may suggest a state‐dependent manner of depression.5 As the anterior thalamic nuclei form a key region involved in emotional regulation,48, 51 the decreased FA in these regions in MDD might contribute to emotional deregulation and could be a target for diagnostic assessments and therapies.52, 53
2.3. Striatum
The striatum is an important part of basal ganglia.54 A large number of neuroimaging studies have reported significant changes in the striatum of MDD patients. Decreased gray matter intensity in the ventral striatum55 was also reported in MDD patients who committed suicide. Disruptions in striatal output may lead to impulsive and suicidal behavior.56 Functional magnetic resonance imaging has shown that striatal activity was reduced in reward system defects,57 and decreased reward network connections were found to be associated with depression severity.58 These findings suggest that abnormal striatal activity plays an essential role in disease progression. The striatum contains the putamen, the caudate, and the ventral striatum.47 The results of previous studies have shown that compared with a control group, the volume of the bilateral putamen decreased significantly in MDD patients.48 In addition, structural alterations in the putamen may be related to the effect of drugs59 and the age of onset in MDD. According to previous results, the putamen plays a key role in mood, cognitive processes, motivation, and regulation of movement.60 The putamen is a component of the hate circuit61 and has connections with the OFC and the ACC.62 Increased functional activity in the putamen has been reported,13 which may lead to a weakening of the ability to control emotions and a low threshold of provoking feelings of hatred toward oneself or others. The caudate nucleus is a critical component of the reward system in the brain and is often associated with the treatment of reward stimuli.63 It has been reported that the volume of the caudate is reduced in MDD patients64 and that it has a negative correlation with disease severity. Reduced activity was also reported in the caudate57 in patients with MDD. Dysfunction of the caudate nucleus may lead to a disruption in dopaminergic signaling, as this region receives inputs from the ventral tegmental dopaminergic neurons.65 Therefore, the decrease in the gray matter density in the right caudate nucleus66 of MDD patients can explain the core features of depression or even the lack of responsiveness to positive stimuli or reward constituents in patients with MDD.
2.4. Parietal lobe
The parietal lobe is involved in the organization, decision making, and predictions of rewards during conditioning that evaluates outcomes for future response choices that are uncertain.67 This region is also related to emotional processing and cognitive changes and is part of the default‐mode network. Increased cortical thickness has been noted in the left inferior parietal gyrus68 in MDD patients compared to healthy controls, and morphometric correlation analysis found a positive caudate‐cortical connection in the bilateral superior parietal lobe.68 The superior parietal lobe is part of the default‐mode network. The default‐mode network has a functional connection with the caudate via dopaminergic projections. Striatal dopaminergic circuits may regulate cognition and emotion by modulating the DMN in MDD.68 Recently, our group observed a lower magnetization transfer ratio in the left superior parietal lobule in MDD patients than that in healthy controls and increased gray matter volume in the right postcentral gyrus in MDD patients compared to healthy controls.69 Increased thickness and correlations may indicate compensatory mechanisms associated with inflammation or other aspects of the pathophysiology of depression. The regional homogeneity value was found to be increased in the right inferior parietal lobule70 and in the right frontoparietal region11 in depressive patients. The calculation of the regional homogeneity value depends on the regional cerebral blood flow. A task positron emission tomography study found that the blood flow in the parietal lobe would increase along with information complexity and decrease when the subjects adopted the information.71 This finding indicated that an increased regional homogeneity value of the parietal lobe may lead to impairments in information reception and in learning.
2.5. Hippocampus
The hippocampus is associated with memory recall and the rules of reward.72 Previous studies have shown that the hippocampus is smaller in depressed patients than in healthy controls,73 and this finding has been confirmed by meta‐analyses.74, 75 There is evidence that stress via the hypothalamic‐pituitary‐adrenal axis can result in elevated glucocorticoid levels in patients with MDD and can act on the glucocorticoid receptors in the hippocampus.76 Thus, hippocampal atrophy occurs as a result.77 Antidepressant treatment research78 and a longitudinal study of electroconvulsive therapy found an increased gray matter volume in the hippocampus in MDD patients after treatment,29, 79 suggesting that the increased hippocampal volume was associated with clinical improvement. A study reported that people with depression who are over 40 years of age, or those with severe or multiple episodes, were more likely to have a small hippocampus.80 Additionally, other studies have found that a small hippocampus may be associated with illness duration in MDD.81 According to an fMRI study, decreased brain activity in the hippocampus was reported82 in depressive patients. Reduced gray matter volume and reduced functional activity in the hippocampus would lead to negative emotion and the inability of cognitive processing in depressive patients.
3. IMPAIRED CIRCUITS IN MDD
3.1. Prefrontal‐subcortical circuit
The striatum, thalamus, and prefrontal cortex constitute the prefrontal‐subcortical circuit,47 which is involved in emotional and cognitive processing and is considered to be a potential pathophysiological target in MDD.83 The structural connectivity of the prefrontal‐subcortical circuit begins at the prefrontal cortex. The striatum receives information from the PFC and outputs the information to the globus pallidus and the substantial nigra. All information is then projected through the thalamus to the prefrontal cortex. The thalamus is the final neuronal link back to the cortex, making the circuit a closed loop. This review mainly introduces three prefrontal circuits (Figure 2), which were originally described by Alexander.84 In each circuit, two pathways have mainly been reported (Figure 3): one direct pathway goes from the striatum to the pallidum, and the other pathway projects from the striatum to the pallidum, then to the subthalamic nucleus, and back to the pallidum.85 Basic research has found that gamma‐aminobutyric acid (GABA) and glutamate participate in these two pathways.83 Directly, the prefrontal cortex, hippocampus, and thalamus elicit excitement and project to the striatum. The striatum transmits information through GABAergic neurons that project to the globus pallidus. Indirectly, the subthalamic nucleus receives information from the cortex and the globus pallidus via GABAergic neurons and then outputs through glutamatergic neurons to the globus pallidus. Both the direct and indirect pathways enter the thalamus through gamma fibers, but impairments in these two pathways could lead to differential pathology. Damage to the direct pathway results in abnormal suppression of the thalamus. In contrast, dysfunction in the indirect pathway leads to disinhibition and thalamic hyperactivity.47
Figure 2.
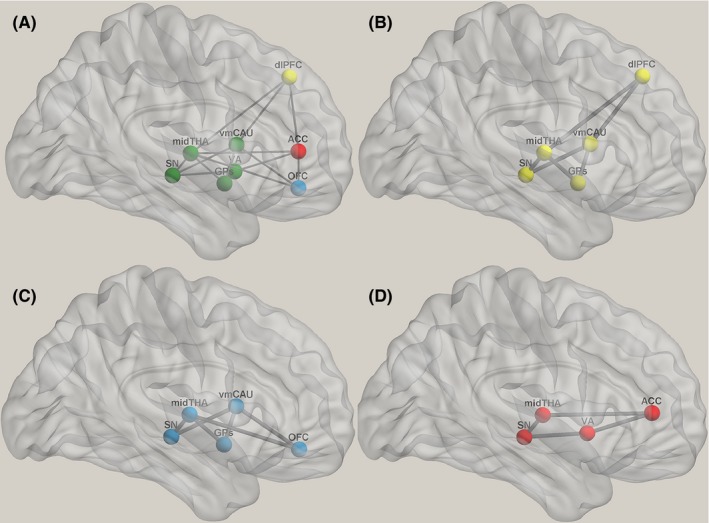
Prefrontal circuit. The striatum, thalamus, and prefrontal cortex constitute the prefrontal‐ subcortical circuit (A). The dorsolateral prefrontal circuit (B) originates in the dorsolateral prefrontal cortex, which projects to the dorsolateral caudate. Then, fibers track through the direct or indirect pathway to the lateral dorsomedial globus pallidus and the substantia nigra. The globus pallidus and the substantia nigra then project to the ventral anterior and medial dorsal thalamus.86 The thalamus collects all the information and sends it back to the dorsolateral prefrontal cortex; the orbitofrontal prefrontal circuit (C) originates in the inferolateral prefrontal cortex. The OFC fibers project to the ventromedial caudate. Then, the caudate outputs fibers through the direct and indirect pathways to the medial dorsomedial globus pallidus and the substantia nigra. The pallidum and the substantia nigra have connections with the ventral anterior and medial dorsal thalamic nuclei. All information will ultimately return to the orbitofrontal cortex; the anterior cingulate‐prefrontal circuit (D) begins in the ACC, and the fibers project to the ventral striatum and the substantia nigra. The striatum outputs to the medial dorsal thalamus. Finally, the thalamus sends all the information back to the ACC. ACC, anterior cingulate cortex; DLPFC, dorsolateral prefrontal cortex; GPs, globus pallidus; mid, middle; OFC, orbitofrontal prefrontal cortex; SN, substantia nigra; THA, thalamus
Figure 3.
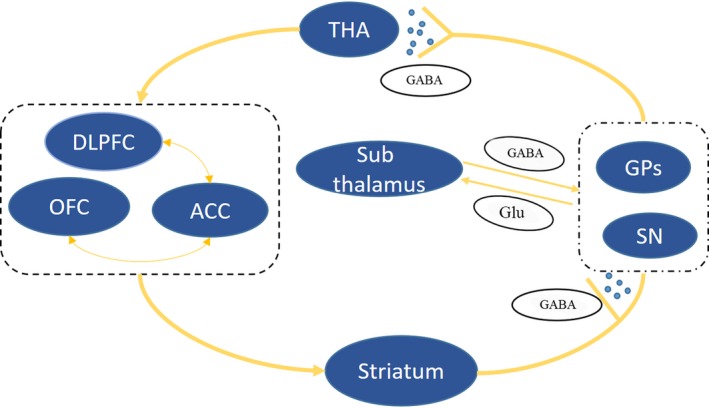
Two pathways from the caudate to the thalamus. The prefrontal‐subcortical circuit originates from the prefrontal cortex and outputs fibers projecting to the striatum. Directly, the striatum transmits information through GABAergic neurons and projects to the globus pallidus. Indirectly, the striatum projects to the globus pallidus. The subthalamic nucleus receives information from the cortex via glutamatergic neurons and from the globus pallidus via GABAergic neurons, which then output through glutamatergic neurons to the globus pallidus. All information ultimately returns to the prefrontal cortex. ACC, anterior cingulate cortex; DLPFC, dorsolateral prefrontal cortex; GABA, gamma‐aminobutyric acid; Glu, glutamatergic; GPs, globus pallidum; OFC, orbitofrontal prefrontal cortex; SN, substantia nigra; THA, thalamus
3.1.1. The dorsolateral prefrontal circuit
The dorsolateral prefrontal circuit originates from the dorsolateral prefrontal cortex (BA seed based 9 and BA 10) and connects to the dorsolateral caudate. The caudate outputs to the lateral dorsomedial globus pallidus and the substantia nigra through the direct or indirect pathway. The pallidum and substantia nigra have connections with the ventral anterior and medial dorsal thalamus.86 Finally, all messages are returned through the thalamus to the dorsolateral prefrontal cortex. A functional connectivity analysis reported coactivity in the bilateral DLPFC and the dorsal caudate.54 The dorsolateral prefrontal cortex and the caudate nucleus are unable to preserve recognition. According to a previous fMRI study, decreased activity in the caudate and increased activity in Brodmann area 10 were reported during reward anticipation57 in patients with MDD. These findings suggested that patients with depression have the intention of inducing pleasurable emotions. Thus, MDD patients with impaired dorsolateral prefrontal circuits may exhibit executive dysfunction.47
3.1.2. The orbitofrontal prefrontal circuit
The orbitofrontal prefrontal circuit starts at the inferolateral prefrontal cortex (BA 10 and BA 11).87 The OFC sends fibers to the ventromedial caudate. Then, the caudate outputs through the direct and indirect pathways to the medial dorsomedial globus pallidus and the substantia nigra. The medial dorsal thalamic nucleus receives input from the pallidum and the substantia nigra and outputs to the OFC. All the information will ultimately be sent to the orbitofrontal cortex. Functional seed‐based studies of the OFC found coactivity with these limbic regions.88 Heavy connections of the orbitofrontal gyrus with other cortical and subcortical regions, such as the parahippocampal gyrus, the ACC, and the posterior cingulate cortex,89 have been reported. A functional MRI study showed that a decrease in the OFC circuit connections was associated with unexpected reward receipt tasks90 and pleasant stimuli91 in MDD patients. The causal relationship between the OFC and the ACC is positively correlated with the severity of depression.20 In conclusion, the orbitofrontal circuit may be negatively related to the severity of depression and the source of negative thinking.92
3.1.3. The anterior cingulate‐prefrontal circuit
The anterior cingulate‐prefrontal circuit begins in the anterior cingulate cortex (BA 24). The ACC outputs messages to the ventral striatum and the substantia nigra. The striatum and the substantia nigra project to the medial dorsal thalamus.86 Finally, all the information is sent back to the anterior cingulate gray via the thalamus. It is not clear whether there is a direct or indirect connection between the ACC and the striatum. However, there is evidence that the connections between the ventral striatum and thalamic neurons are present.93 The nucleus accumbens is a part of the ventral striatum, which receives excitatory inputs from the ACC and outputs to the thalamus, and has been linked with anhedonia. The relationship between the nucleus accumbens and the thalamus is negatively correlated with the severity of depression94 and is related to emotional regulation and motivational function.95 Functional connectivity studies revealed the coordination between the ventral striatum, the bilateral middle temporal lobe (amygdala and hippocampus), and the ventral midbrain (substantia nigra).54 According to previous findings, the ACC plays an important role in anhedonic symptoms in patients with MDD.90 Therefore, damage to the anterior cingulate circuitry may be negatively correlated with the severity of depressive symptoms.
Different circuits also have correlations. As mentioned earlier, the functional connectivity of the ACC and the DLPFC is increased. This indicates an increase in the sensitivity to effective conflict.35 A correlation between the OFC and the ACC has also been mentioned. The causal interaction between the OFC and the ACC is positively related to the severity of depression.20 The ACC receives input from the PFC and outputs to other brain regions.96 A powerless DLPFC and OFC in MDD result in a failure to activate the ACC, ultimately leading to circuit impairment.
3.2. Prefrontal‐hippocampal circuit
In the early stage of MDD, a DTI study reported that the prefrontal‐hippocampal circuits,13 which originate from the fornix and output fibers to the hippocampus, project to the mammillary bodies, the anterior nuclei of the thalamus, and finally back to the prefrontal cortex. A lower FA value was observed in the fornix and the hippocampal cingulum17 of depressive patients. The fornix comprises the major fibers that track through the hippocampus, and it is related to the reduction in the hippocampal volume.97 Disruptions in the fornix would result in barriers to the transmission of information between the PFC and the hippocampus. Resting‐state functional research found decreased functional connectivity among the bilateral hippocampus, the DLPFC, and the ventral PFC17 in MDD patients, which might indicate emotional and cognitive dysfunction in MDD. There was a significant correlation between the white matter integrity of the fornix and the functional connectivity of the PFC‐hippocampal circuit in healthy controls, but this was found to be impaired in the MDD patients.17 In conclusion, prefrontal hippocampal structural damage can explain the deficits in attention, information processing, and autobiographical memory in depressive patients.17
3.3. Frontothalamic circuit
To our knowledge, Professor Jia and his team are the first ones to find and put forward the “suicide loop”6 (Figure 4). That particular DTI study found that the FA value of the left internal capsule was lower in patients who attempted suicide.1 Abnormalities in the fiber connections passing through the left anterior limb of the internal capsule that projects to the left middle frontal cortex and the OFC and finally posteriorly to the left thalamus in MDD patients who committed suicide were found to be more severe than those who did not commit suicide and controls. The middle frontal cortex and the OFC are related to decision making and problem solving and can affect modulation,49, 98 and the thalamus is involved in the mood‐related neural network.49 These three regions are considered hub nodes of brain connections.21 Changes in the frontal and thalamic circulation may lead to cognitive and emotional changes, thereby increasing the vulnerability to suicidal behavior in patients with depression. The results demonstrating these white matter changes are consistent with those of other studies.99, 100 Abnormal gray matter in the temporal and parietal lobes was also found in the patients who committed suicide. Decreased gray matter volume in the limbic cingulate gyrus and the right middle temporal gyrus and increased gray matter volume in the right parietal lobe101 were reported, and a negative correlation was observed between the limbic cingulate gyrus and dysfunctional attitude scores. The attitude score reflects the perception of oneself and the world,102 and cognitive distortions often lead to negative beliefs and behaviors, such as suicide. The reduction in the magnetization transfer ratio in the left inferior parietal lobule and the right superior parietal lobule in patients who attempted suicide was consistent with previous findings.103 Decision‐making barriers are associated with suicidal behavior.68 Thus, the alterations in the parietal lobule may lead to suicidal behaviors. In addition, the hippocampus and the angular gyrus are the biological markers for MDD, but little is known about the relationship between the volume of these regions and suicide attempters. An interesting finding reported that suicide attempters have reduced gray matter volume in the hippocampus104 and in the left angular gyrus,98 especially in cases of acute suicide. It was also found that the total volume of the hippocampal threshold of 5 cm3 had a negative predictive value of 98.2% for acute suicide attempts.104 The angular gyrus is known to be part of the default‐mode network, and impairments in this region in patients are associated with negative thoughts about the future or themselves.98 Disruptions in the angular gyrus may lead to suicidal behaviors or may increase the risk of suicide.
Figure 4.
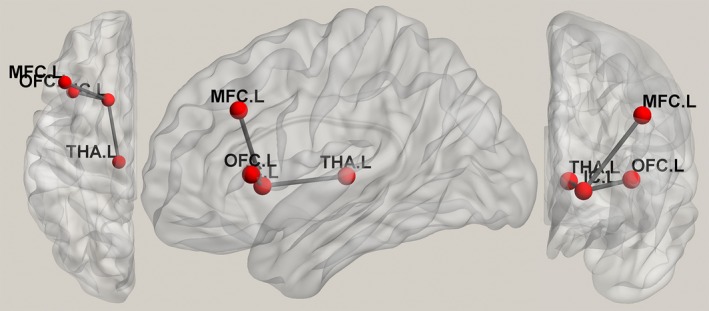
Suicide circuit. MDD patients who committed suicide were found to have abnormal connections among the left anterior limb of the internal capsule, the left middle frontal cortex, the orbital prefrontal cortex, and the left thalamus. IC, internal capsule; L, left; MDD, major depressive disorder; MFC, middle frontal cortex; OFC, orbitofrontal prefrontal cortex; THA, thalamus
4. CONCLUSION AND FUTURE DIRECTION
In vivo MRI scans have made great achievements in the study of psychiatric disorders, which have resulted in the dawn of the understanding of the pathophysiology of psychosis, especially of MDD. Many brain region alterations have been reported, and some crucial circuits have also been revealed via imaging studies. The discovery of brain network put forward new ideas in the understanding of the disease of depression, providing effective stimulation sites and efficacy evaluations for the commonly used transcranial magnetic stimulation or deep brain stimulation techniques. In addition, these findings also suggest that MDD is not only due to local lesions but is also a multiloop disorder. However, previous studies still had limitations, and more research is needed in the future. First, most of the studies mentioned small sample sizes, which could have increased the false‐positive and (or) false‐negative rates of the results. Therefore, multicenter cooperation not only would solve this problem of sample content but also could result in more in‐depth research. Second, the identification of significant lesions relies on long‐term follow‐ups and the comparison of treated and nontreated patients. Future studies need to conduct longitudinal studies with larger samples. Moreover, using animal experiments to verify the neuroimaging findings and applying the results to humans is very important and will be a big step in the application of neuroimaging to the clinical field.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation (Grant Nos. 81771812, 81621003, 81571637, and 81271532), and the Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT, Grant No. IRT16R52) of China. Q.G. received the support of Changjiang Scholar Professorship Award (Award No. T2014190) of China and the CMB Distinguished Professorship Award (Award No. F510000/G16916411) administered by the Institute of International Education, USA.
Zhang F‐F, Peng W, Sweeney JA, Jia Z‐Y, Gong Q‐Y. Brain structure alterations in depression: Psychoradiological evidence. CNS Neurosci Ther. 2018;24:994–1003. 10.1111/cns.12835
Contributor Information
Zhi‐Yun Jia, Email: zhiyunjia@hotmail.com.
Qi‐Yong Gong, Email: qiyonggong@hmrrc.org.cn.
REFERENCES
- 1. Jia ZY, Huang XQ, Wu QZ, et al. High‐field magnetic resonance imaging of suicidality in patients with major depressive disorder. Am J Psychiatry. 2010;167:1381‐1390. [DOI] [PubMed] [Google Scholar]
- 2. Kong L, Wu F, Tang Y, et al. Frontal‐subcortical volumetric deficits in single episode, medication‐naive depressed patients and the effects of 8 weeks fluoxetine treatment: a VBM‐DARTEL study. PLoS ONE. 2014;9:e79055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication. JAMA. 2003;289:3095‐3105. [DOI] [PubMed] [Google Scholar]
- 4. Eijndhoven P, Wingen GV, Katzenbauer M, et al. Paralimbic cortical thickness in first‐episode depression: evidence for trait‐related differences in mood regulation. Am J Psychiatry. 2013;170:1477‐1486. [DOI] [PubMed] [Google Scholar]
- 5. Peng W, Cheng ZQ, Yin L, Jia ZY, Gong QY. Essential brain structural alterations in major depressive disorder: a voxel‐wise meta‐analysis on first episode, medication‐naive patients. J Affect Disord. 2016;199:114‐123. [DOI] [PubMed] [Google Scholar]
- 6. Jia Z, Wang Y, Huang X, et al. Impaired frontothalamic circuitry in suicidal patients with depression revealed by diffusion tensor imaging at 3.0 T. J Psychiatry Neurosci. 2014;39:170‐177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang XH, Wang Y, Wang DF, et al. White matter microstructural abnormalities and their association with anticipatory anhedonia in depression. Psychiatry Res. 2017;264:29‐34. [DOI] [PubMed] [Google Scholar]
- 8. Srivastava S, Bhatia MS, Bhargava SK, Kumari R, Chandra S. A diffusion tensor imaging study using a voxel‐based analysis, region‐of‐interest method to analyze white matter abnormalities in first‐episode, treatment‐naive major depressive disorder. J Neuropsychiatry Clin Neurosci. 2016;28:131‐137. [DOI] [PubMed] [Google Scholar]
- 9. Chen G, Guo Y, Zhu H, et al. Intrinsic disruption of white matter microarchitecture in first‐episode, drug‐naive major depressive disorder: a voxel‐based meta‐analysis of diffusion tensor imaging. Prog Neuropsychopharmacol Biol Psychiatry. 2017;76:179‐187. [DOI] [PubMed] [Google Scholar]
- 10. Wei S, Geng H, Jiang X, et al. Amygdala‐prefrontal cortex resting‐state functional connectivity varies with first depressive or manic episode in bipolar disorder. Neurosci Lett. 2017;641:51‐55. [DOI] [PubMed] [Google Scholar]
- 11. Shen Z, Jiang L, Yang S, et al. Identify changes of brain regional homogeneity in early and later adult onset patients with first‐episode depression using resting‐state fMRI. PLoS ONE. 2017;12:e0184712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang M, Lu S, Yu L, et al. Altered fractional amplitude of low frequency fluctuation associated with cognitive dysfunction in first‐episode drug‐naive major depressive disorder patients. BMC Psychiatry. 2017;17:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhong X, Pu W, Yao S. Functional alterations of fronto‐limbic circuit and default mode network systems in first‐episode, drug‐naive patients with major depressive disorder: a meta‐analysis of resting‐state fMRI data. J Affect Disord. 2016;206:280‐286. [DOI] [PubMed] [Google Scholar]
- 14. van den Heuvel MP, Sporns O. Rich‐club organization of the human connectome. J Neurosci. 2011;31:15775‐15786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bonelli RM, Cummings JL. Frontal‐subcortical circuitry and behavior. Dialogues Clin Neurosci. 2007;9:141‐151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang W, Zhao Y, Hu X, et al. Conjoint and dissociated structural and functional abnormalities in first‐episode drug‐naive patients with major depressive disorder: a multimodal meta‐analysis. Sci Rep. 2017;7:10401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Geng H, Wu F, Kong L, et al. Disrupted structural and functional connectivity in prefrontal‐hippocampus circuitry in first‐episode medication‐naive adolescent depression. PLoS ONE. 2016;11:e0148345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yeh PH, Zhu H, Nicoletti MA, Hatch JP, Brambilla P, Soares JC. Structural equation modeling and principal component analysis of gray matter volumes in major depressive and bipolar disorders: differences in latent volumetric structure. Psychiatry Res. 2010;184:177‐185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yi S. 3D MRI studies of neuroanatomic changes in unipolar major depression: the role of stress and medical comorbidity. Biol Psychiatry. 2000;48:791‐800. [DOI] [PubMed] [Google Scholar]
- 20. Gao Q, Zou K, He ZL, Sun XL, Chen HF. Causal connectivity alterations of cortical‐subcortical circuit anchored on reduced hemodynamic response brain regions in first‐episode drug‐naïve major depressive disorder. Sci Rep. 2016;6:21861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Qin J, Liu H, Wei M, et al. Reconfiguration of hub‐level community structure in depressions: a follow‐up study via diffusion tensor imaging. J Affect Disord. 2017;207:305‐312. [DOI] [PubMed] [Google Scholar]
- 22. Qin J, Wei M, Liu H, et al. Abnormal hubs of white matter networks in the frontal‐parieto circuit contribute to depression discrimination via pattern classification. Magn Reson Imaging. 2014;32:1314‐1320. [DOI] [PubMed] [Google Scholar]
- 23. Bora E, Fornito A, Pantelis C, Yücel M. Gray matter abnormalities in major depressive disorder: a meta‐analysis of voxel based morphometry studies. J Affect Disord. 2012;138:9‐18. [DOI] [PubMed] [Google Scholar]
- 24. Lai CH. Hippocampal and subcortical alterations of first‐episode, medication naïve major depressive disorder with panic disorder patients. J Neuropsychiatry Clin Neurosci. 2014;26:142‐149. [DOI] [PubMed] [Google Scholar]
- 25. Serra‐Blasco M, Portella MJ, Gómez‐Ansón B, et al. Effects of illness duration and treatment resistance on grey matter abnormalities in major depression. Br J Psychiatry. 2013;202:434‐440. [DOI] [PubMed] [Google Scholar]
- 26. Ramezani M, Abolmaesumi P, Tahmaseb A, et al. Fusion analysis of first episode depression: where brain shape deformations meet local composition of tissue. NeuroImage. 2015;7:114‐121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mayberg HS, Brannan S, Mahurin RK, et al. Cingulate function in depression: a potential predictor of treatment response. NeuroReport. 1997;8:1057‐1061. [DOI] [PubMed] [Google Scholar]
- 28. Zhang TJ, Wu Q‐Z, Huang XQ, et al. Magnetization transfer imaging reveals the brain deficit in patients with treatment‐refractory depression. J Affect Disord. 2009;117:157‐161. [DOI] [PubMed] [Google Scholar]
- 29. Cano M, Martínez‐Zalacaín I, Bernabéu‐Sanz Á, et al. Brain volumetric and metabolic correlates of electroconvulsive therapy for treatment‐resistant depression: a longitudinal neuroimaging study. Transl Psychiatry. 2017;7:e1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu XD, Kakeda S, Watanabe K, et al. Relationship between the cortical thickness and serum cortisol levels in drug‐naive, first‐episode patients with major depressive disorder: a Surface‐based Morphometric Study. Depress Anxiety. 2015;32:702‐708. [DOI] [PubMed] [Google Scholar]
- 31. Ajilorea O, Narr K, Rosenthal J, et al. Regional cortical gray matter thickness differences associated with type 2 diabetes and major depression. Psychiatry Res. 2010;184:63‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Foland‐Ross LC, Sacchet MD, Prasad G, Gilbert B, Thompson PM, Gotlib IH. Cortical thickness predicts the first onset of major depression in adolescence. Int J Dev Neurosci. 2015;46:125‐131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yang XH, Wang Y, Huang J, et al. Increased prefrontal and parietal cortical thickness does not correlate with anhedonia in patients with untreated first‐episode major depressive disorders. J Neuropsychiatry Clin Neurosci. 2015;234:144‐151. [DOI] [PubMed] [Google Scholar]
- 34. Phillips JL, Batten LA, Tremblay P, Aldosary F, Blier P. A prospective, longitudinal study of the effect of remission on cortical thickness and hippocampal volume in patients with treatment‐resistant depression. Int J Neuropsychopharmacol. 2015;18:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lu Q, Li H, Luo G, et al. Impaired prefrontal‐amygdala effective connectivity is responsible for the dysfunction of emotion process in major depressive disorder: a dynamic causal modeling study on MEG. Neurosci Lett. 2012;523:125‐130. [DOI] [PubMed] [Google Scholar]
- 36. Milad MR, Rauch SL. The role of the orbitofrontal cortex in anxiety disorders. Ann N Y Acad Sci. 2007;1121:546‐561. [DOI] [PubMed] [Google Scholar]
- 37. Zhao K, Liu H, Yan R, et al. Altered patterns of association between cortical thickness and subcortical volume in patients with first episode major depressive disorder: a structural MRI study. Psychiatry Res. 2017;260:16‐22. [DOI] [PubMed] [Google Scholar]
- 38. Zhang X, Di X, Lei H, et al. Imbalanced spontaneous brain activity in orbitofrontal‐insular circuits in individuals with cognitive vulnerability to depression. J Affect Disord. 2016;198:56‐63. [DOI] [PubMed] [Google Scholar]
- 39. Shen Z, Cheng Y, Yang S, et al. Changes of grey matter volume in first‐episode drug‐naive adult major depressive disorder patients with different age‐onset. Neuroimage Clin. 2016;12:492‐498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang Y, Jia Y, Xu G, Ling X, Liu S, Huang L. Frontal white matter biochemical abnormalities in first‐episode, treatment‐naive patients with major depressive disorder: a proton magnetic resonance spectroscopy study. J Affect Disord. 2012;136:620‐626. [DOI] [PubMed] [Google Scholar]
- 41. Cole J, Costafreda SG, McGuffin P, Fu CH. Hippocampal atrophy in first episode depression: a meta‐analysis of magnetic resonance imaging studies. J Affect Disord. 2011;134:483‐487. [DOI] [PubMed] [Google Scholar]
- 42. Zhang X, Yao S, Zhu X, Wang X, Zhu X, Zhong M. Gray matter volume abnormalities in individuals with cognitive vulnerability to depression: a voxel‐based morphometry study. J Affect Disord. 2012;136:443‐452. [DOI] [PubMed] [Google Scholar]
- 43. Choi KS, Riva‐Posse P, Gross RE, Mayberg HS. Mapping the ‘Depression Switch’ during Intraoperative testing of subcallosal cingulate deep brain stimulation. JAMA Neurol. 2015;72:1252‐1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fettes P, Schulze L, Downar J. Cortico‐striatal‐thalamic loop circuits of the orbitofrontal cortex: promising therapeutic targets in psychiatric illness. Front Syst Neurosci. 2017;11:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lan MJ, Chhetry BT, Liston C, Mann JJ, Dubin M. Transcranial magnetic stimulation of left dorsolateral prefrontal cortex induces brain morphological changes in regions associated with a treatment resistant major depressive episode: an exploratory analysis. Brain Stimul. 2016;9:577‐583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Taber KH, Wen C, Khan A, Hurley RA. The limbic thalamus. J Neuropsychiatry Clin Neurosci. 2004;16:127‐132. [DOI] [PubMed] [Google Scholar]
- 47. Tekin S, Cummings JL. Frontal‐subcortical neuronal circuits and clinical neuropsychiatry an update. J Psychosom Res. 2002;52:647‐654. [DOI] [PubMed] [Google Scholar]
- 48. Lu Y, Liang H, Han D, et al. The volumetric and shape changes of the putamen and thalamus in first episode, untreated major depressive disorder. Neuroimage Clin. 2016;11:658‐666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhao YJ, Du MY, Huang XQ, et al. Brain grey matter abnormalities in medication‐free patients with major depressive disorder: a meta‐analysis. Psychol Med. 2014;44:2927‐2937. [DOI] [PubMed] [Google Scholar]
- 50. Zhang H, Li L, Wu M, et al. Brain gray matter alterations in first episodes of depression: a meta‐analysis of whole‐brain studies. Neurosci Biobehav Rev. 2016;60:43‐50. [DOI] [PubMed] [Google Scholar]
- 51. Zhao K, Liang H, Yan R, et al. Altered patterns of association between cortical thickness and subcortical volume in patients with first episode major depressive disorder: a structural MRI study. Psychiatry Res. 2017;260:16‐22. [DOI] [PubMed] [Google Scholar]
- 52. Kinoshita M, Nakada M, Okita H, Hamada J, Hayashi Y. Predictive value of fractional anisotropy of the arcuate fasciculus for the functional recovery of language after brain tumor resection: a preliminary study. Clin Neurol Neurosurg. 2014;117:45‐50. [DOI] [PubMed] [Google Scholar]
- 53. Chapman CH, Nazem‐Zadeh M, Lee OE, et al. Regional variation in brain white matter diffusion index changes following chemoradiotherapy: a prospective study using tract‐based spatial statistics. PLoS ONE. 2013;8:e57768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Postuma RB, Dagher A. Basal ganglia functional connectivity based on a meta‐analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cereb Cortex. 2006;16:1508‐1521. [DOI] [PubMed] [Google Scholar]
- 55. Jacobs RH, Barba A, Gowins JR, et al. Decoupling of the amygdala to other salience network regions in adolescent‐onset recurrent major depressive disorder. Psychol Med. 2016;46:1055‐1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dombrovski AY, Siegle GJ, Szanto K, Clark L, Reynolds CF, Aizenstein H. The temptation of suicide: striatal gray matter, discounting of delayed rewards, and suicide attempts in late‐life depression. Psychol Med. 2012;42:1203‐1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Forbes EE, Hariri AR, Martin SL, et al. Altered striatal activation predicting real‐world positive affect in adolescent major depressive disorder. Am J Psychiatry. 2009;166:64‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Satterthwaite TD, Kable JW, Vandekar L, et al. Common and dissociable dysfunction of the reward system in bipolar and unipolar depression. Neuropsychopharmacology. 2015;40:2258‐2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Koolschijn PCMP, van Haren NEM, Lensvelt‐Mulders GJLM, Hulshoff Pol HE, Kahn RS. Reduced volume of limbic system–affiliated basal ganglia in mood disorders: a meta‐analysis of magnetic resonance imaging studies. Hum Brain Mapp. 1999;30:3719‐3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Koolschijn PC, van Haren NE, Lensvelt‐Mulders GJ, Hulshoff Pol HE, Kahn RS. Brain volume abnormalities in major depressive disorder: a meta‐analysis of magnetic resonance imaging studies. Hum Brain Mapp. 2009;30:3719‐3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zeki S, Romaya JP. Neural correlates of hate. PLoS ONE. 2008;3:e3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Schmaal L, Veltman DJ, van Erp TG, et al. Subcortical brain alterations in major depressive disorder: findings from the ENIGMA Major Depressive Disorder working group. Mol Psychiatry. 2016;21:806‐812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Stratmann M, Konrad C, Kugel H, et al. Insular and hippocampal gray matter volume reductions in patients with major depressive disorder. PLoS ONE. 2014;9:e102692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Johnston BA, Steele JD, Tolomeo S, Christmas D, Matthews K. Structural MRI‐based predictions in patients with treatment‐refractory depression (TRD). PLoS ONE. 2015;10:e0132958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Nestler EJ, Carlezon WA. The mesolimbic dopamine reward circuit in depression. Biol Psychiat. 2006;59:1151‐1159. [DOI] [PubMed] [Google Scholar]
- 66. Wagner G, Koch K, Schachtzabel C, Schultz CC, Sauer H, Schlösser RG. Structural brain alterations in patients with major depressive disorder and high risk for suicide: evidence for a distinct neurobiological entity? NeuroImage. 2011;54:1607‐1614. [DOI] [PubMed] [Google Scholar]
- 67. Liu X, Kakeda S, Watanabe K, et al. Relationship between the cortical thickness and serum cortisol levels in drug‐naive, first‐episode patients with major depressive disorder: a surface‐based morphometric study. Depress Anxiety. 2015;32:702‐708. [DOI] [PubMed] [Google Scholar]
- 68. Yang XH, Wang Y, Huang J, et al. Increased prefrontal and parietal cortical thickness does not correlate with anhedonia in patients with untreated first‐episode major depressive disorders. Psychiatry Res. 2015;234:144‐151. [DOI] [PubMed] [Google Scholar]
- 69. Chen Z, Peng W, Sun H, et al. High‐field magnetic resonance imaging of structural alterations in first‐episode, drug‐naive patients with major depressive disorder. Transl Psychiatry. 2016;6:e942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Liang MJ, Zhou Q, Yang KR, et al. Identify changes of brain regional homogeneity in bipolar disorder and unipolar depression using resting‐state FMRI. PLoS ONE. 2013;8:e79999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Grafton ST, Mazziotta J, Presty S, Friston KJ, Frackowiak RS, Phelps ME. Functional anatomy of human procedural learning determined with regional cerebral blood flow and PET. J Neurosci. 1992;12:2542‐2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Campbell S, Macqueen G. The role of the hippocampus in the pathophysiology of major depression. J Psychiatry Neurosci. 2004;29:417‐426. [PMC free article] [PubMed] [Google Scholar]
- 73. Ballmaier M, Narr KL, Toga AW, et al. Hippocampal morphology and distinguishing late‐onset from early‐onset elderly depression. Am J Psychiatry. 2008;165:229‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Geerlings MI, Gerritsen L. Late‐life depression, hippocampal volumes, and hypothalamic‐pituitary‐adrenal axis regulation: a systematic review and meta‐analysis. Biol Psychiatry. 2017;82:339‐350. [DOI] [PubMed] [Google Scholar]
- 75. Campbell S, Marriott M, Nahmias C, MacQueen GM. Lower hippocampal volume in patients suffering from depression: a meta‐analysis. Am J Psychiatry. 2004;161:598‐607. [DOI] [PubMed] [Google Scholar]
- 76. Frodl T, O'Keane V. How does the brain deal with cumulative stress? A review with focus on developmental stress, HPA axis function and hippocampal structure in humans. Neurobiol Dis. 2013;52:24‐37. [DOI] [PubMed] [Google Scholar]
- 77. Yi JH, Brown C, Whitehead G, et al. Glucocorticoids activate a synapse weakening pathway culminating in tau phosphorylation in the hippocampus. Pharmacol Res. 2017;121:42‐51. [DOI] [PubMed] [Google Scholar]
- 78. Fu CH, Williams S, Cleare AJ, et al. Attenuation of the neural response to sad faces in major depression by antidepressant treatment: a prospective, event‐related functional magnetic resonance imaging study. Arch Gen Psychiatry. 2004;61:877‐889. [DOI] [PubMed] [Google Scholar]
- 79. Tendolkar I, Mv B, Oostrom I, et al. Electroconvulsive therapy increases hippocampal and amygdala volume in therapy refractory depression: a longitudinal pilot study. Psychiatry Res. 2013;214:197‐203. [DOI] [PubMed] [Google Scholar]
- 80. Eker C, Gonul AS. Volumetric MRI studies of the hippocampus in major depressive disorder: meanings of inconsistency and directions for future research. World J Biol Psychiatry. 2010;11:19‐35. [DOI] [PubMed] [Google Scholar]
- 81. Colla M, Kronenberg G, Deuschle M, et al. Hippocampal volume reduction and HPA‐system activity in major depression. J Psychiatr Res. 2007;41:553‐560. [DOI] [PubMed] [Google Scholar]
- 82. Shena T, Qiu M, Li C, et al. Altered spontaneous neural activity in first‐episode, unmedicated patients with major depressive disorder. NeuroReport. 2014;25:1302‐1307. [DOI] [PubMed] [Google Scholar]
- 83. Marchand WR. Cortico‐basal ganglia circuitry: a review of key research and implications for functional connectivity studies of mood and anxiety disorders. Brain Struct Funct. 2010;215:73‐96. [DOI] [PubMed] [Google Scholar]
- 84. Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357‐381. [DOI] [PubMed] [Google Scholar]
- 85. Cummings JL. Frontal‐subcortical circuits and human behavior. Arch Neurol. 1993;50:873‐880. [DOI] [PubMed] [Google Scholar]
- 86. Elliott R, Dolan RJ, Frith CD. Dissociable functions in the medial and lateral orbitofrontal cortex: evidence from human neuroimaging studies. Cereb Cortex. 2000;10:308‐317. [DOI] [PubMed] [Google Scholar]
- 87. Jarbo K, Verstynen TD. Converging structural and functional connectivity of orbitofrontal, dorsolateral prefrontal, and posterior parietal cortex in the human striatum. J Neurosci. 2015;35:3865‐3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Zald DH, McHugo M, Ray KL, Glahn DC, Eickhoff SB, Laird AR. Meta‐analytic connectivity modeling reveals differential functional connectivity of the medial and lateral orbitofrontal cortex. Cereb Cortex. 2014;24:232‐248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Cavada C, Compañy T, Tejedor J, Cruz‐Rizzolo RJ, Reinoso‐Suárez F. The anatomical connections of the macaque monkey orbitofrontal cortex. A review. Cereb Cortex. 2000;10:220‐242. [DOI] [PubMed] [Google Scholar]
- 90. Segarra N, Metastasio A, Ziauddeen H, et al. Abnormal frontostriatal activity during unexpected reward receipt in depression and schizophrenia: relationship to anhedonia. Neuropsychopharmacology. 2016;41:2001‐2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. McCabe C, Cowen PJ, Harmer CJ. Neural representation of reward in recovered depressed patients. Psychopharmacology. 2009;205:667‐677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Wegener I, Geiser F, Alfter S, et al. Changes of explicitly and implicitly measured self‐esteem in the treatment of major depression: evidence for implicit self‐esteem compensation. Compr Psychiatry. 2015;58:57‐67. [DOI] [PubMed] [Google Scholar]
- 93. Alexander GE, Crutcher MD, DeLong MR. Basal ganglia‐thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res. 1990;85:119‐146. [PubMed] [Google Scholar]
- 94. Zhu X, Helpman L, Papini S, et al. Altered resting state functional connectivity of fear and reward circuitry in comorbid PTSD and major depression. Depress Anxiety. 2017;34:641‐650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Haber SN, Calzavara R. The cortico‐basal ganglia integrative network: the role of the thalamus. Brain Res Bull. 2009;78:69‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K. Depression: perspectives from affective neuroscience. Annu Rev Psychol. 2002;53:545‐574. [DOI] [PubMed] [Google Scholar]
- 97. Davidson CA, Kuroki N, Alvarado JL, Niznikiewicz MA, McCarley RW, Levitt JJ. An MRI study of septi pellucidi in relation to hippocampus volume and fornix integrity in schizophrenia. Schizophr Res. 2012;134:165‐170. [DOI] [PubMed] [Google Scholar]
- 98. Lee YJ, Kim S, Gwak AR, et al. Decreased regional gray matter volume in suicide attempters compared to suicide non‐attempters with major depressive disorders. Compr Psychiatry. 2016;67:59‐65. [DOI] [PubMed] [Google Scholar]
- 99. Taylor WD, Boyd B, McQuoid DR, Kudra K, Saleh A, MacFall JR. Widespread white matter but focal gray matter alterations in depressed individuals with thoughts of death. Prog Neuropsychopharmacol Biol Psychiatry. 2015;62:22‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Sachs‐Ericsson N, Hames JL, Joiner TE, et al. Differences between suicide attempters and non‐attempters in depressed older patients: depression severity, white matter lesions, and cognitive functioning. Am J Geriatr Psychiatry. 2014;22:75‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Peng HJ, Wu K, Li J, et al. Increased suicide attempts in young depressed patients with abnormal temporal–parietal–limbic gray matter volume. J Affect Disord. 2014;165:69‐73. [DOI] [PubMed] [Google Scholar]
- 102. Lai CH, Wu YT. The gray matter alterations in major depressive disorder and panic disorder: putative differences in the pathogenesis. J Affect Disord. 2015;186:1‐6. [DOI] [PubMed] [Google Scholar]
- 103. Chen Z, Zhang H, Jia Z, et al. Magnetization transfer imaging of suicidal patients with major depressive disorder. Sci Rep. 2015;5:9670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Colle R, Chuoin M, Cur C, et al. Depressed suicide attempters have smaller hippocampus than depressed patients without suicide attempts. J Psychiatr Res. 2015;61:13‐18. [DOI] [PubMed] [Google Scholar]


