Abstract
Background
Transcatheter aortic valve replacement (TAVR) has become an alternative treatment to surgery in patients with severe aortic stenosis. However, patients with bicuspid aortic stenosis (BAV) are usually excluded from major TAVR studies. The aim of this study is to reexamine current evidence of TAVR in patients with severe aortic stenosis and BAV compared with tricuspid aortic valve (TAV).
Hypothesis
There might be differences in outcomes post TAVR between patients with BAV comparing to TAV.
Method
Databases were systematically searched for relevant articles featuring cohort studies that included patients with BAV and TAV who underwent TAVR studies, of which reported outcomes of interest included mortality and complications in both groups. Pooled effect size was calculated with a random‐effect model and weighted for the inverse of variance, to compare outcomes post‐TAVR between BAV and TAV.
Results
Nine studies were included in the meta‐analysis. There was no difference in 30‐day mortality rate in patients with BAV compared with TAV (OR: 1.27, 95% CI: 0.84–1.93, I 2 = 0). Patients with BAV were more likely to have a moderate to severe paravalvular leak (9 studies; OR: 1.42, 95% CI: 1.08–1.87, I 2 = 0) and conversion to surgery (5 studies; OR: 5.48, 95% CI: 1.74–17.27, I 2 = 0), and less likely to have device success compared with patients with TAV (5 studies; OR: 0.57, 95% CI: 0.40–0.81, I 2 = 0%).
Conclusions
There was no difference in mortality post‐TAVR in patients with BAV compared with TAV. Further randomized studies should be done in newer‐generation prostheses to assess this association.
Keywords: Bicuspid Aortic Valve, Transcatheter Aortic Valve Replacement
1. INTRODUCTION
Bicuspid aortic valve (BAV) is one of the most common valvular abnormalities in adults, with an estimated prevalence of 0.5% to 2%.1, 2 Individuals with BAV are at risk for rapidly progressing to aortic stenosis (AS) and ultimately requiring aortic valve surgery.3 Transcatheter aortic valve replacement (TAVR) has become an alternative treatment to surgery in patients with severe AS who are at intermediate to high risk for surgical intervention.4 However, BAV is a relative contraindication to TAVR, and patients with BAV are usually excluded from major TAVR studies. Patients with BAV undergoing TAVR are at risk of developing valve malposition, causing severe paravalvular leak, due to the asymmetrical structures of this valve.5, 6 Although studies7, 8 have shown that using newer‐generation devices in TAVR9 may be safe and feasible in some patients with BAV, there is a lack of strong evidence to suggest routine use in clinical practice.
A recent meta‐analysis of 7 observational studies10 has shown that there was no difference in midterm mortality in patients with tricuspid aortic valve (TAV) and BAV undergoing TAVR. However, more studies11, 12, 13, 14 have emerged since this previous meta‐analysis, which now suggest that these patients might have lower success rate and increased paravalvular leak after TAVR. The aim of this study is to reexamine the current evidence of TAVR in patients with severe AS and BAV compared with TAV.
2. METHODS
Studies were systematically acquired from the MEDLINE and Embase electronic databases. The search terms were “Transcatheter aortic valve implantation” or “transcatheter aortic valve replacement” or “TAVR” or “TAVI” and “Bicuspid” or “Bicuspid aortic valve.” The search was performed from the day of inception through December 20, 2017. The search was limited to studies that were in English. References sections of published studies were also manually scanned for potential relevant articles. Full‐text articles of all relevant studies were reviewed. Any discrepancies were resolved through consensus. Studies that met these eligibility criteria were included in the meta‐analysis: (1) cohort studies that included patients with BAV who underwent TAVR; (2) studies that reported outcomes of interest including mortality, paravalvular leak, pacemaker implantation, or other TAVR complications; and (3) studies in which outcomes were compared in patients with TAV.
Two independent investigators performed data extraction using a standardized data‐collection form. Primary author's last name, year of publication, demographics, and crude outcome data were extracted from all studies.
Quality of the studies was evaluated by the Newcastle‐Ottawa scale (NOS). The criteria are sample selection (S), comparability (C), and outcome assessment (O). Each domain could be scored from 0 to 9; higher scores represent higher study quality. A total score of ≥7 was considered good quality.
BAV was defined according to the classification by Sievers and Schmidtke15 by the number of cusps, presence of raphe, and spatial position and symmetry of raphe and cusps. Outcomes including mortality, device success, complications including paravalvular leak (PVL), and major bleeding were defined according to the Valve Academic Research Consortium (VARC 2) or as defined in each study.16 Due to differences in follow‐up time among the studies, we defined mid‐term mortality as mortality at 1 to 3 years postprocedure.
Random‐effect meta‐analyses were performed for each outcome of interest from all studies using the generic inverse variance method of DerSimonian and Laird.17, 18, 19 Crude outcome events were used to estimate odds ratio (OR) and 95% confidence interval (CI) for each outcome. The heterogeneity of effect estimates across the studies was assessed using I 2. I 2 values were defined as I 2 < 25%, low heterogeneity; I 2 = 25% to 50%, moderate heterogeneity; and I 2 > 50%, substantial heterogeneity.20 Publication bias was assessed with funnel plots.21 Funnel‐plot asymmetry was further confirmed with the Egger test if there were > 10 available studies.22 Statistical analyses were performed using Review Manager, version 5.3 (Nordic Cochrane Centre, the Cochrane Collaboration, Copenhagen, Denmark) and Stata software, version 13 (StataCorp LP, College Station, TX).
3. RESULTS
Our search strategy yielded 472 potentially relevant studies (203 from MEDLINE, 264 from Embase, and 5 from reference search). Duplicates and irrelevant studies were excluded by abstract review. Nineteen studies underwent full‐text review. Finally, a total of 9 studies with 854 BAV and 3615 TAV patients were included in this meta‐analysis.11–14,23–27 An outline of our search strategy is shown in Figure 1.
Figure 1.
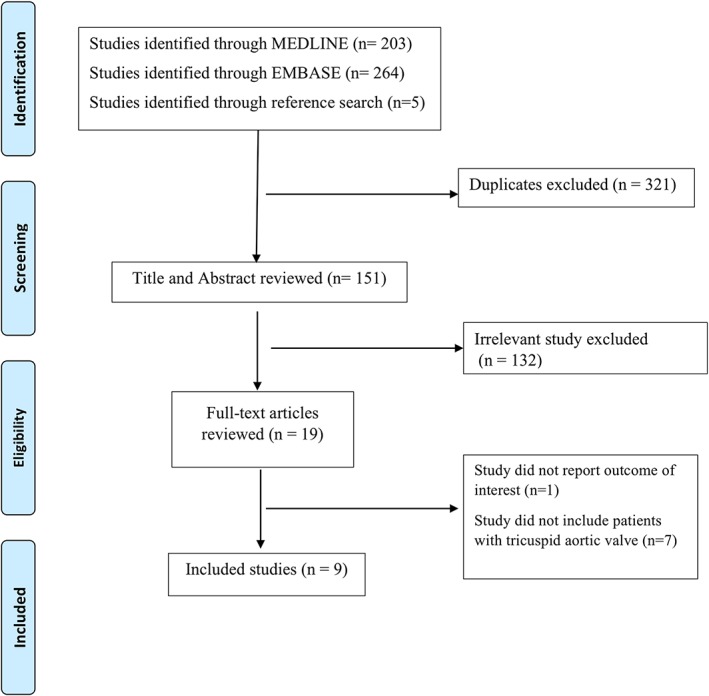
Outline of the search strategy
The NOS was used to evaluate the quality of the 9 included studies in selection (S), comparability (C), and outcomes (O). Six out of the 9 studies received a score of 7 to 9, which reflected high quality. Three studies received a score of 6, which indicated moderate quality.
Nine studies were included in the final analysis. Baseline characteristics of patients in each study are reported in the Table 1. All 9 studies reported 30‐day mortality. There was no difference in 30‐day mortality rate in patients with BAV compared with patients with TAV (OR: 1.27, 95% CI: 0.84–1.93). The analysis showed minimal heterogeneity (I 2 = 0%). Only 5 studies reported mid‐term mortality. Four studies13, 24, 25, 26 reported a 1‐year follow‐up time, and 1 study14 had a 3‐year follow‐up time. There was no difference in mid‐term mortality between patients with BAV compared with patients with TAV (OR: 1.12, 95% CI: 0.76–1.64). There was moderate heterogeneity in this analysis (I 2 = 27%). Forest plots are shown in Figure 2. Funnel plots (Figure 3) and the Egger test did not suggest publication bias for 30 days (P = 0.84) and mid‐term mortality (P = 0.97). Funnel plots are shown in the Supporting Information, Figures A and B, in the online version of this article.
Table 1.
Baseline characteristics of the included studies
| Yoon 2017 | Sannino 2017 | Liao 2017 | Arai 2017 | Liu 2015 | Kochman 2014 | Costopoulos 2014 | Bauer 2014 | Hayashida 2013 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BAV | TAV | BAV | TAV | BAV | TAV | BAV | TAV | BAV | TAV | BAV | TAV | BAV | TAV | BAV | TAV | BAV | TAV | |
| N | 546 | 546 | 88 | 735 | 87 | 70 | 10 | 143 | 15 | 25 | 28 | 84 | 21 | 447 | 38 | 1357 | 21 | 208 |
| Baseline characteristics | ||||||||||||||||||
| Male sex | 63 | 61 | 60 | 53 | 58 | 64 | 43 | 7 | 60 | 68 | 46 | 48 | 57 | 47 | 45 | 42 | 57 | 53 |
| Mean age, y | 77 ± 8 | 77 ± 9 | 80 ± 8 | 82 ± 8 | 73 ± 6 | 74 ± 7 | 81 ± 5 | 83 ± 6 | 75 ± 6 | 76 ± 6 | 78 ± 6 | 79 ± 7 | 77 ± 7 | 80 ± 7 | 81 ± 7 | 82 ± 6 | 82 ± 7 | 83 ± 7 |
| Logistic euroSCORE, % | 16 ± 12 | 17 ± 14 | N/A | N/A | N/A | N/A | N/A | N/A | 16 ± 11 | 22 ± 15 | 19 ± 9 | 19 ± 9 | 24 ± 12 | 24 ± 17 | 18 ± 10 | 20 ± 13 | 20 ± 12 | 20 ± 11 |
| STS score, % | 4.6 ± 4.6 | 4.3 ± 3 | 7.4 ± 4 | 7.6 ± 4 | 8 ± 4 | 9 ± 4 | N/A | N/A | 6 ± 4 | 8 ± 6 | N/A | N/A | 8 ± 4 | 8 ± 7 | N/A | N/A | N/A | N/A |
| DM | 23 | 23 | 38 | 40 | 16 | 19 | 20 | 26 | 0 | 12 | 39 | 35 | 29 | 30 | 37 | 34 | 5 | 24 |
| PAD | 15 | 16 | 43 | 32 | 48 | 41 | N/A | N/A | 13 | 16 | 21 | 35 | 33 | 30 | 11 | 22 | 24 | 33 |
| Prior CVA | 14 | 13 | 22 | 20 | 15 | 11 | 10 | 1 | 0 | 8 | 29 | 17 | 19 | 16 | 13 | 8 | 5 | 6 |
| AF | N/A | N/A | 18 | 20 | 22 | 17 | N/A | N/A | 7 | 8 | N/A | N/A | 19 | 16 | N/A | N/A | N/A | N/A |
| CKD | N/A | N/A | 52 | 46 | 16 | 19 | N/A | N/A | 27 | 48 | N/A | N/A | 52 | 58 | 58 | 61 | 24 | 24 |
| COPD | 18 | 15 | 20 | 22 | 58 | 64 | 0 | 3 | 27 | 16 | 21 | 20 | 33 | 31 | 21 | 24 | 57 | 60 |
| CAD | N/A | N/A | 71 | 67 | N/A | N/A | N/A | N/A | 20 | 36 | 50 | 64 | 19 | 20 | N/A | N/A | 48 | 58 |
| Prior PCI | 22 | 23 | 49 | 46 | 8 | 11 | 10 | 15 | 20 | 12 | N/A | N/A | 29 | 22 | 34 | 35 | 19 | 23 |
| Prior CABG | 11 | 12 | N/A | N/A | N/A | N/A | 10 | 6 | 0 | 0 | N/A | N/A | N/A | N/A | 13 | 18 | 10 | 14 |
| Echocardiographic findings | ||||||||||||||||||
| Mean gradient, mm Hg | 47.7 ± 17.7 | 48.5 ± 17.1 | 47 ± 17 | 44 ± 14 | 65 ± 20 | 60 ± 17 | 46 ± 20 | 48 ± 14 | 64 ± 20 | 54 ± 14 | 56 ± 18 | 53 ± 19 | 54 ± 18 | 53 ± 16 | N/A | N/A | 48 ± 19 | 54 ± 14 |
| Aortic valve area, cm2 | 0.7 ± 0.2 | 0.7 ± 0.2 | 0.7 ± 0.2 | 0.7 ± 0.2 | N/A | N/A | 0.67 ± 0.16 | 0.65 ± 0.14 | 0.47 ± 0.13 | 0.59 ± 0.14 | 0.6 ± 0.1 | 0.6 ± 0.2 | 0.7 ± 0.2 | 0.8 ± 0.5 | 0.7 ± 0.2 | 0.7 ± 0.4 | 0.67 ± 0.1 | 0.65 ± 0.1 |
| LVEF, % | 51.6 ± 15 | 51.6 ± 15.2 | 32% hasEF<50% | 27% hasEF<50% | 55 (42–68) | 62 (47–68) | 53 ± 19 | 56 ± 13 | N/A | N/A | 48 ± 13 | 50 ± 14 | N/A | N/A | 50 ± 16 | 53 ± 15 | 48 ± 15 | 54 ± 14 |
| Bicuspid morphology, % | Type 0, 13; type 1, 86; type 2, 2.0 | Type 0, 14; type 1, 85; type 2, 1.0 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | ||
| Device types | ||||||||||||||||||
| Early generation (%) | Sapien XT, 28.4; CoreValve, 30 | Sapien XT, 27.5; CoreValve, 31.3 | 75 | 75 | 100 | 100 | 0 | 0 | CoreValve, 67; Venus A, 33 | CoreValve, 68; Venus A, 32 | Edwards, 18; CoreValve, 82 | Edwards, 18; CoreValve, 82 | Edwards, 38; CoreValve, 62 | Edwards, 59; CoreValve, 41 | Edwards, 32; CoreValve, 68 | Edwards, 82; CoreValve, 18 | Edwards, 52; CoreValve, 48 | Edwards, 84; CoreValve, 16 |
| New generation (%) | Sapien 3, 29.3; Lotus, 7.9; Evolut R, 4.2 | Sapien 3 29.7; Lotus 8.6; Evolut R, 2.9 | 25 | 25 | 0 | 0 | Edwards Sapien 3, 100 | Edward Sapien 3, 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| NOS quality assessment | 7 (S‐3, C‐1, O‐3) | 7 (S‐4, C‐0, O‐3) | 7 (S‐4, C‐0, O‐3) | 6 (S‐4, C‐0, O‐2) | 6 (S‐4, C‐0, O‐2) | 7 (S‐4, C‐0, O‐3) | 6 (S‐3, C‐0, O‐3) | 7 (S‐4, C‐0, O‐3) | 7 (S‐4, C‐0, O‐3) | |||||||||
Abbreviations: AF, atrial fibrillation; BAV, bicuspid aortic valve; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; DM, diabetes mellitus; euroSCORE, Logistic European System for Cardiac Operative Risk Evaluation; IQR, interquartile range; LVEF, left ventricular ejection fraction; N/A, not available/applicable; NOS, Newcastle‐Ottawa Scale; PAD, peripheral arterial disease; PCI, percutaneous coronary intervention; SD, standard deviation; STS, Society of Thoracic Surgeons; TAV, tricuspid aortic valve.
Data are presented as %, mean ± SD, or median (IQR).
Figure 2.
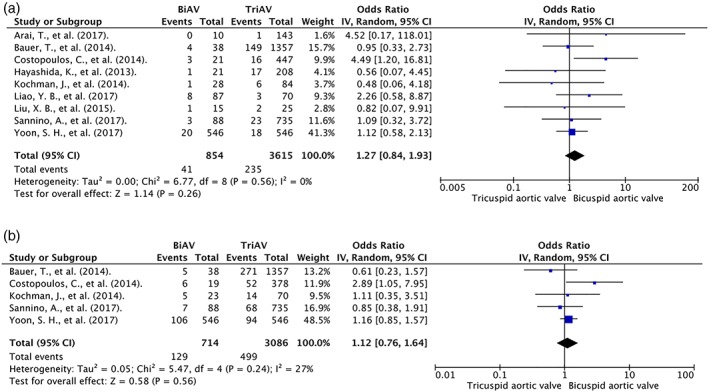
Forest plot of included studies comparing (a) 30‐day mortality and (b) mid‐term mortality in patients with BAV and TAV. Horizontal lines represent the 95% CIs, with marker size reflecting the statistical weight of the study using random‐effects model. A diamond data marker represents the overall OR and 95% CI for the outcome of interest. Abbreviations: BiAV/BAV, bicuspid aortic valve; CI, confidence interval; df, degrees of freedom; OR, odds ratio; TriAV/TAV, tricuspid aortic valve
Figure 3.
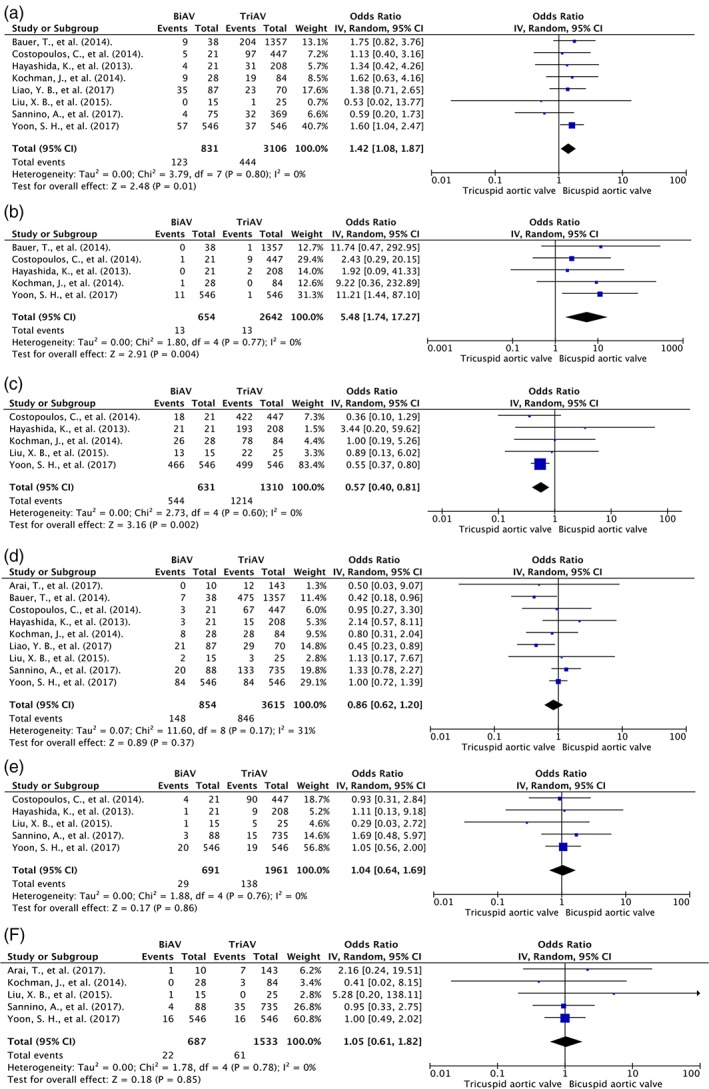
Forest plot of included studies comparing post‐TAVR complications including (a) PVL, (b) conversion to surgery, (c) device success, (d) pacemaker implantation, (e) major bleeding, and (f) major vascular complication in patients with BAV and TAV. Horizontal lines represent the 95% CIs, with marker size reflecting the statistical weight of the study using random‐effects model. A diamond data marker represents the overall OR and 95% CI for the outcome of interest. Abbreviations: BiAV/BAV, bicuspid aortic valve; CI, confidence interval; df, degrees of freedom; OR, odds ratio; PVL, paravalvular leak; TAVR, transcatheter aortic valve replacement; TriAV/TAV, tricuspid aortic valve
Eight studies reported PVL; 6 of the studies11, 12, 23, 24, 25, 26, 27 defined PVL as postoperative aortic regurgitation grade ≥ 2, and 2 studies13, 14 reported at least moderate PVL using the VARC 2 definition. PVL was more common in BAV than in TAV (OR: 1.42, 95% CI: 1.08–1.87, I 2 = 0). Subgroup analysis of 5 studies defining PVL using postoperative aortic regurgitation showed that there was no difference in PVL (OR: 1.44, 95% CI: 0.98–2.1, I 2 = 0%). Further subgroup analyses showed that the difference is mainly driven by a single large study by Yoon et al.14
Patients with BAV were more likely to have conversion to open aortic valve replacement (5 studies; OR: 5.48, 95% CI: 1.74–17.27, I 2 = 0) and less likely to have device success compared with patients with TAV (5 studies; OR: 0.57, 95% CI: 0.40–0.81, I 2 = 0%). There was a similar incidence of pacemaker placement, major bleeding, and major vascular complications in BAV and TAV. Forest plots are shown in Figure 3.
4. DISCUSSION
This study reported no significant difference in 30‐day mortality and mid‐term mortality in patients with BAV who underwent TAVR, compared with patients with TAV. Our study found that there was no difference in pacemaker implantation, major bleeding, and major vascular complication. However, we did find that patients with BAV had lower success rates, more PVL, and conversion to open aortic valve replacement post‐TAVR. To the best of our knowledge, this systematic review and meta‐analysis incorporated the largest and most updated data on patients with BAV and TAV who underwent TAVR.
After the previous meta‐analysis by Phan et al10 was published, more recent studies have also shown no difference in mortality between BAV and TAV undergoing TAVR. Thus, our findings on 30‐day mortality and mid‐term mortality are similar to this prior study. We reported lower success rates, higher rates of PVL, and higher rates of conversion to open aortic valve replacement. This finding is mainly driven by a large observational study by Yoon et al14 reporting higher incidence of these complications in BAV patients. The mechanism of PVL post‐TAVR in BAV patients is unclear. BAV was believed to be more elliptical and would not fit well with prostheses, but a recent study has found that BAV is actually not more elliptical than TAV and should be able to fit commercially available prostheses.7, 28 Other possible hypotheses include asymmetrical leaflet calcification and resistance to valve expansion.7, 28, 29 BAV is known to be associated with higher rates of pacemaker implantation in BAV cohorts compared with TAV cohorts.7 However, we found no difference in pacemaker implantation in the BAV group when directly compared with the TAV group from the same cohort.
Newer‐generation prostheses are associated with fewer postprocedural complications. Yoon et al14 reported subgroup analyses of newer‐generation devices (SAPIEN 3, Edwards Lifesciences, Irvine, CA; Lotus, Boston Scientific, Marlborough, MA; and CoreValve Evolut R, Medtronic, Minneapolis, MN) showing a similar rate of paravalvular leaks, device success rate, and conversion to open surgery between BAV and TAV patients. Higher rates of PVL and conversion to open heart surgery in our study could be driven by the fact that the majority of studies included in our meta‐analysis were done using older‐generation rather than newer‐generation devices. Further studies are needed to assess the effect of prosthesis types on outcome post‐TAVR in patients with BAV.
Currently, several randomized studies are being done to evaluate TAVR in patients with BAV. A randomized controlled trial (http://www.ClinicalTrials.gov NCT03163329) is taking place to compare TAVR and surgical aortic valve replacement in patients with BAV. Another randomized controlled trial is also being done to evaluate the appropriate valve sizing for BAV patients undergoing TAVR (http://www.ClinicalTrials.gov NCT02541877). Results from these trials will be important in the future management of patients with BAV.
4.1. Study limitations
There are several limitations to our study. Baseline characteristics of patients with BAV and TAV are different within each of the cohorts included. Multivariate analyses adjusting for potential confounders were not reported. We could not perform a pool analysis of adjusted OR; thus, we could not conclude that the associations we found are independent of potential confounders. The studies included did not report specific outcomes in newer‐generation devices; therefore, subgroup analysis comparing outcomes and complications between BAV and TAV patients undergoing TAVR with newer‐generation prostheses could not be done. Studies also did not report outcomes within subtypes of BAV. As a result, pooled analysis could not be performed to compare outcomes within each subtype of BAV.
Although studies defined outcomes based on the VARC 2 definition, there are still differences in outcome definition. PVL was defined according to VARC 2 (mild, moderate, and severe) in only 2 studies, whereas other studies still used postoperative aortic regurgitation (trace, mild, moderate, and severe). This difference resulted in increased heterogeneity among the studies.
There is a lack of studies with a longer follow‐up time to further evaluate the difference in long‐term mortality between BAV and TAV patients undergoing TAVR. Quality of life is also an important outcome post‐TAVR; however, the studies included in this meta‐analysis did not include quality of life as one of the outcomes.
5. CONCLUSION
Current evidence has shown that there was no difference in mortality post‐TAVR in patients with BAV compared with TAV. However, there might be higher rate of PVL, lower success rate, and higher conversion to surgery in patients with BAV. Further randomized studies should be done in newer‐generation prostheses to assess this association.
Conflicts of interest
The authors declare no potential conflicts of interest.
Supporting information
Supplementary figure A Funnel plot of included studies in the meta‐analysis for 30‐days mortality in patients with BiAV and TriAV undergoing TAVR. OR = Odd ratio, SE = standard error.
Supplementary figure B Funnel plot of included studies in the meta‐analysis for mid‐term mortality in patients with BiAV and TriAV undergoing TAVR. OR = Odd ratio, SE = standard error.
Kanjanahattakij N, Horn B, Vutthikraivit W, et al. Comparing outcomes after transcatheter aortic valve replacement in patients with stenotic bicuspid and tricuspid aortic valve: A systematic review and meta‐analysis. Clin Cardiol. 2018;41:896–902. 10.1002/clc.22992
REFERENCES
- 1. Tadros TM, Klein MD, Shapira OM. Ascending aortic dilatation associated with bicuspid aortic valve: pathophysiology, molecular biology, and clinical implications. Circulation. 2009;119:880–890. [DOI] [PubMed] [Google Scholar]
- 2. Siu SC, Silversides CK. Bicuspid aortic valve disease. J Am Coll Cardiol. 2010;55:2789–2800. [DOI] [PubMed] [Google Scholar]
- 3. Michelena HI, Khanna AD, Mahoney D, et al. Incidence of aortic complications in patients with bicuspid aortic valves. JAMA. 2011;306:1104–1112. [DOI] [PubMed] [Google Scholar]
- 4. Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017;70:252–289. [DOI] [PubMed] [Google Scholar]
- 5. Zegdi R, Ciobotaru V, Noghin M, et al. Is it reasonable to treat all calcified stenotic aortic valves with a valved stent? Results from a human anatomic study in adults. J Am Coll Cardiol. 2008;51:579–584. [DOI] [PubMed] [Google Scholar]
- 6. Chen M, Feng Y, Mazzitelli D, et al. Transcatheter aortic valve implantation in a patient with severe bicuspid aortic valve stenosis and ascending aortic aneurysm. JACC Cardiovasc Interv. 2014;7:e83–e84. [DOI] [PubMed] [Google Scholar]
- 7. Reddy G, Wang Z, Nishimura RA, et al. Transcatheter aortic valve replacement for stenotic bicuspid aortic valves: systematic review and meta analyses of observational studies. Catheter Cardiovasc Interv. 2017;91:975–983. [DOI] [PubMed] [Google Scholar]
- 8. Wijesinghe N, Ye J, Rodés‐Cabau J, et al. Transcatheter aortic valve implantation in patients with bicuspid aortic valve stenosis. JACC Cardiovasc Interv. 2010;3:1122–1125. [DOI] [PubMed] [Google Scholar]
- 9. Perlman GY, Blanke P, Dvir D, et al. Bicuspid aortic valve stenosis: favorable early outcomes with a next‐generation transcatheter heart valve in a multicenter study. JACC Cardiovasc Interv. 2016;9:817–824. [DOI] [PubMed] [Google Scholar]
- 10. Phan K, Wong S, Phan S, et al. Transcatheter aortic valve implantation (TAVI) in patients with bicuspid aortic valve stenosis—systematic review and meta‐analysis. Heart Lung Circ. 2015;24:649–659. [DOI] [PubMed] [Google Scholar]
- 11. Arai T, Lefèvre T, Hovasse T, et al. The feasibility of transcatheter aortic valve implantation using the Edwards SAPIEN 3 for patients with severe bicuspid aortic stenosis. J Cardiol. 2017;70:220–224. [DOI] [PubMed] [Google Scholar]
- 12. Liao YB, Li YJ, Xiong TY, et al. Comparison of procedural, clinical and valve performance results of transcatheter aortic valve replacement in patients with bicuspid versus tricuspid aortic stenosis. Int J Cardiol. 2018;254:69–74. [DOI] [PubMed] [Google Scholar]
- 13. Sannino A, Cedars A, Stoler RC, et al. Comparison of efficacy and safety of transcatheter aortic valve implantation in patients with bicuspid versus tricuspid aortic valves. Am J Cardiol. 2017;120:1601–1606. [DOI] [PubMed] [Google Scholar]
- 14. Yoon SH, Bleiziffer S, De Backer O, et al. Outcomes in transcatheter aortic valve replacement for bicuspid versus tricuspid aortic valve stenosis. J Am Coll Cardiol. 2017;69:2579–2589. [DOI] [PubMed] [Google Scholar]
- 15. Sievers HH, Schmidtke C. A classification system for the bicuspid aortic valve from 304 surgical specimens. J Thorac Cardiovasc Surg. 2007;133:1226–1233. [DOI] [PubMed] [Google Scholar]
- 16. Kappetein AP, Head SJ, Généreux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium‐2 consensus document. J Thorac Cardiovasc Surg. 2013;145:6–23. [DOI] [PubMed] [Google Scholar]
- 17. DerSimonian R. Meta‐analysis in the design and monitoring of clinical trials. Stat Med. 1996;15:1237–1252. [DOI] [PubMed] [Google Scholar]
- 18. DerSimonian R, Kacker R. Random‐effects model for meta‐analysis of clinical trials: an update. Contemp Clin Trials. 2007;28:105–114. [DOI] [PubMed] [Google Scholar]
- 19. DerSimonian R, Laird N. Meta‐analysis in clinical trials revisited. Contemp Clin Trials. 2015;45(part A):139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta‐analyses. BMJ. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Easterbrook PJ, Berlin JA, Gopalan R, et al. Publication bias in clinical research. Lancet. 1991;337:867–872. [DOI] [PubMed] [Google Scholar]
- 22. Sterne JA, Egger M. Funnel plots for detecting bias in meta‐analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046–1055. [DOI] [PubMed] [Google Scholar]
- 23. Hayashida K, Bouvier E, Lefevre T, et al. Transcatheter aortic valve implantation for patients with severe bicuspid aortic valve stenosis. Circ Cardiovasc Interv. 2013;6:284–291. [DOI] [PubMed] [Google Scholar]
- 24. Bauer T, Linke A, Sievert H, et al. Comparison of the effectiveness of transcatheter aortic valve implantation in patients with stenotic bicuspid versus tricuspid aortic valves (from the German TAVI Registry). Am J Cardiol. 2014;113:518–521. [DOI] [PubMed] [Google Scholar]
- 25. Costopoulos C, Latib A, Maisano F, et al. Comparison of results of transcatheter aortic valve implantation in patients with severely stenotic bicuspid versus tricuspid or nonbicuspid valves. Am J Cardiol. 2014;113:1390–1393. [DOI] [PubMed] [Google Scholar]
- 26. Kochman J, Huczek Z, Scisło P, et al. Comparison of one‐ and 12‐month outcomes of transcatheter aortic valve replacement in patients with severely stenotic bicuspid versus tricuspid aortic valves (results from a multicenter registry). Am J Cardiol. 2014;114:757–762. [DOI] [PubMed] [Google Scholar]
- 27. Liu XB, Jiang JB, Zhou QJ, et al. Evaluation of the safety and efficacy of transcatheter aortic valve implantation in patients with a severe stenotic bicuspid aortic valve in a Chinese population. J Zhejiang Univ Sci B. 2015;16:208–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Philip F, Faza NN, Schoenhagen P, et al. Aortic annulus and root characteristics in severe aortic stenosis due to bicuspid aortic valve and tricuspid aortic valves: implications for transcatheter aortic valve therapies. Catheter Cardiovasc Interv. 2015;86:E88–E98. [DOI] [PubMed] [Google Scholar]
- 29. Perlman GY, Blanke P, Webb JG. Transcatheter aortic valve implantation in bicuspid aortic valve stenosis. EuroIntervention. 2016;12:Y42–Y5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure A Funnel plot of included studies in the meta‐analysis for 30‐days mortality in patients with BiAV and TriAV undergoing TAVR. OR = Odd ratio, SE = standard error.
Supplementary figure B Funnel plot of included studies in the meta‐analysis for mid‐term mortality in patients with BiAV and TriAV undergoing TAVR. OR = Odd ratio, SE = standard error.


