Abstract
Airway neutrophilia is a common feature of many chronic inflammatory lung diseases and is associated with disease progression, often regardless of the initiating cause. Neutrophils and their products are thought to be key mediators of the inflammatory changes in the airways of patients with chronic obstructive pulmonary disease (COPD) and have been shown to cause many of the pathological features associated with disease, including emphysema and mucus hypersecretion. Patients with COPD also have high rates of bacterial colonisation and recurrent infective exacerbations, suggesting that neutrophil host defence mechanisms are impaired, a concept supported by studies showing alterations to neutrophil migration, degranulation and reactive oxygen species production in cells isolated from patients with COPD. Although the role of neutrophils is best described in COPD, many of the pathological features of this disease are not unique to COPD and also feature in other chronic inflammatory airway diseases, including asthma, cystic fibrosis, alpha-1 anti-trypsin deficiency, and bronchiectasis. There is increasing evidence for immune cell dysfunction contributing to inflammation in many of these diseases, focusing interest on the neutrophil as a key driver of pulmonary inflammation and a potential therapeutic target than spans diseases. This review discusses the evidence for neutrophilic involvement in COPD and also considers their roles in alpha-1 anti-trypsin deficiency, bronchiectasis, asthma, and cystic fibrosis. We provide an in-depth assessment of the role of the neutrophil in each of these conditions, exploring recent advances in understanding, and finally discussing the possibility of common mechanisms across diseases.
Keywords: Neutrophil, COPD, Asthma, Cystic Fibrosis, Bronchiectasis, Alpha-1 Anti-Trypsin, Inflammation
Introduction
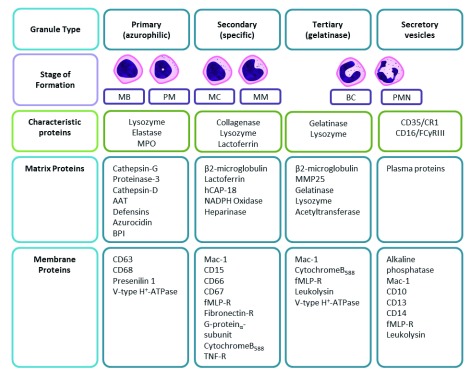
Neutrophils are the dominant circulating leucocyte, comprising around 70% of white blood cells in health and representing a key component of the innate immune system. Neutrophils are short-lived cells (with a half-life of about 8 hours), having a basal production of 1 to 2 × 10 11 neutrophils per day in health, although this can increase to 10 12 during infection and their half-life can also increase in the presence of inflammation and hypoxia 1. Neutrophils are characterised by their multi-lobed nucleus and granular cytoplasm, the latter caused by azurophillic (primary), specific (secondary) and gelatinase (tertiary) granules, as well as secretory vesicles (contents described in Figure 1). These granules and vesicles contain a complex armamentarium of products that permit cell communication, neutrophil migration, microbial killing, tissue remodelling, degradation and repair.
Figure 1. The contents of neutrophil granule subtypes split into characteristic, matrix (cytosolic), and membrane proteins.
AAT, alpha-1 anti-trypsin; BC, band cell; BPI, bacterial permeability-increasing protein; CR1, complement receptor-1; fMLP, N-formylmethionine-leucyl-phenylalanine; hCAP-18, human cathelicidin protein-18; Mac-1, macrophage-1 antigen (CD11b/CD18); MB, myeloblast; MC, myelocyte; MM, metamyelocyte; MMP, matrix metalloproteinase; MPO, myeloperoxidase; NADPH, nicotinamide adenine dinucleotide phosphate; PM, promyelocyte; PMN, polymorphonuclear neutrophil; R, receptor; TNF, tumour necrosis factor. Data were combined from 10– 12.
In response to infection, neutrophils leave the circulation and migrate to the affected sites, where they use a variety of mechanisms to contain and kill invading pathogens, preventing further dissemination. The phagocytosis of bacteria leads to intracellular pathogen killing within a contained structure (the phagolysosome) to protect the cell and surrounding tissue. The phagolysosome is formed when neutrophil granules (which contain pre-formed products such as proteinases and bactericidal proteins and newly formed reactive oxygen species [ROS]) fuse with the lysosome containing the ingested bacterium. ROS production is a convoluted process, necessary to protect the host from the free radical-associated harm. NADPH oxidase is constructed from a series of subunits and then acts as a channel for electrons from the cytosol to enter the phagolysosome, stimulating reduction of oxygen (O 2) to the superoxide anion O 2 − 2. Superoxide then can dismutate to form the highly oxidative hydrogen peroxide (H 2O 2), which can react further, forming the strongly bactericidal hypohalous acids (for example, hypochlorous acid) 3– 8. These products can also be released into the extracellular matrix by degranulation, but neutrophils require different levels of activation to release granules; secretory vesicles are released during minimal stimulation to facilitate migration and adhesion, and azurophil granules (the most cytotoxic) require the most stimulation.
Release of azurophil granules leads to areas of obligate tissue damage, as the proteinases contained therein readily digest components of the extracellular matrix until their inhibition by anti-proteinases can occur 9. Finally, in overwhelming infection or inflammation, neutrophils have been described as releasing their decondensed DNA in web-like structures outside of the cell. The extruded DNA is coated in cytotoxic products, including proteinases, termed neutrophil extracellular traps (NETs) as they can ensnare and “trap” bacteria 13. To protect the host from this immense arsenal, neutrophils are held in three states that they can fluctuate between: quiescent, primed and activated. The primed state provides both a mechanism to allow a rapid and pro-inflammatory response to infection but also a brake to prevent unwarranted degranulation 14.
There is increasing interest in neutrophil phenotypes or subtypes, and cells appear to have a broader repertoire of responses than the highly aggressive, cytotoxic response described above. The concept of cell phenotypes is well established with lymphocytes or macrophages but remains more controversial with neutrophils. However, despite previous theories, neutrophils are transcriptionally active 15, can release a wide range of context-specific products 16 and have an adaptable lifespan depending on activation status and environment 17. They also express more than 30 different receptors—including G protein–coupled receptors, Fc receptors, adhesion receptors, cytokine receptors and pattern recognition receptors—that can sense pro-inflammatory mediators and modulate neutrophil migration, function and behaviour 18, suggesting plasticity in their responses. There have been descriptions of pro-angiogenic neutrophils 19, characterised by increased matrix metalloproteinase 9 (MMP-9) release 20, 21 and anti-inflammatory neutrophils capable of suppressing other immune cells 22. Research into the exact function of these phenotypes (or indeed whether they do represent different cell types or are an adaption of the cell to environmental stimuli) remains unclear, but it might be that different subsets of cells have different functions; indeed, when neutrophils are viewed, it is clear that cells within a field behave heterogeneously 23.
What is evident is the immense potential that neutrophils have for host damage which requires constant check. Recently, it has been suggested that the lung may be involved in this process. Neutrophils are larger in diameter than some of the tortuous pulmonary vasculature that they must traverse. Initially, it was considered that the lungs hold a sequestered pool of neutrophils, slow in transit through the capillary bed and ready to respond to pulmonary infection 24– 26. However, recent research has shown that this is not the case in health, and there is no evidence of a retained neutrophil population in the lungs unless there is systemic priming of neutrophils and even this results in only a transitory retention of neutrophils 27. It was then demonstrated that the process of manoeuvring through tight spaces promoted neutrophil de-priming 28, leading to the hypothesis that the sinuous pulmonary vasculature might be a site where the primed neutrophil population (thought to be up to 40% of the whole population in some studies) can be “stood down” into a quiescent state 29.
Perhaps then it is no surprise that there is evidence of neutrophil dysregulation during lung disease, that airway neutrophilia is a feature of multiple lung pathologies and that patients with airway disease often display heightened and more damaging neutrophilic inflammation. This may represent a physiological response to an infective or inflammatory trigger (such as neutrophil recruitment to the lung in response to a respiratory infection or cigarette smoke) or a physiological response to a pathological environment (the inflamed and damaged lung causing increased neutrophil recruitment and being less able to “de-prime” cells). However, there is amassing evidence that the neutrophil itself may inflict further harm to the host by intrinsic changes to key cellular functions. Understanding whether the neutrophil is a reactive responder or a creative actor in lung disease (or indeed both) is vital when considering the development of new therapeutics: would one target the environment or the neutrophil? The evidence for these processes in airway disease has been most thoroughly described in chronic obstructive pulmonary disease (COPD). This review will explore what is currently known about neutrophils in the pathogenesis of airway disease, focusing mainly on COPD. However, neutrophil function in alpha-1 anti-trypsin deficiency (AATD), bronchiectasis, cystic fibrosis (CF) and asthma will also be considered in order to assess the likelihood of common mechanisms and therefore potential therapies which could span diseases.
Chronic obstructive pulmonary disease
COPD is a leading cause of morbidity and mortality worldwide and constitutes a significant healthcare burden 30– 32. In the UK, chronic cigarette smoking remains the largest cause of COPD, but after 25 years of smoking, only about 30 to 40% of adults will have developed COPD 33, and COPD is diagnosed in never smokers (who may have other environmental exposures which lead to disease) 34, suggesting that smoking is neither necessary nor sufficient to cause COPD.
Airway inflammation is central to the pathophysiology of COPD and contributes to tissue damage and destruction and a wealth of data support a role for the neutrophil at the heart of this inflammatory process. All patients with COPD have airway neutrophilia, regardless of clinical phenotype (chronic bronchitis, emphysema, and even eosinophilic COPD), disease severity, and rate of decline or age of onset. COPD is very heterogeneous and although patients may share a cause (such as cigarette smoking), the disease presentation is variable, suggesting that COPD is more an umbrella term than a narrow clinical entity 35 ( Table 1). Neutrophil numbers (and their products) relate to airway obstruction, decline in forced expiratory volume in 1 second (FEV 1), reduction in gas transfer, and development of emphysema 36– 40. Although patients with COPD demonstrate airway neutrophilia, they also experience airway colonisation and recurrent bacterial infections 41– 44. This raises the possibility that the function of neutrophils is impaired, leading to reduced anti-microbial function and at the same time contributing to lung damage and a number of observations support this concept.
Table 1. Recognised clinical phenotypes of chronic obstructive pulmonary disease, asthma, and bronchiectasis.
| Phenotype | Basic features |
|---|---|
| Chronic obstructive pulmonary disease (COPD) | |
| Bronchitic phenotype | The presence of productive cough (at least 3 months per year in at least
2 consecutive years) |
| Emphysema phenotype | Presence of emphysema confirmed on imaging (including computed
tomography densitometry) |
| Eosinophil COPD | Presence of eosinophilia, normally defined as at least 2% eosinophils in
either blood or sputum |
| Asthma COPD overlap | Persistent airflow limitation with several features usually associated with
asthma and several features usually associated with COPD |
| Overlap COPD and bronchiectasis | Airflow obstruction consistent with COPD alongside irreversibly dilated
airways, mucus gland hyperplasia and impaired mucus clearance associated with bronchiectasis |
| Frequent exacerbation phenotype | Two or more “exacerbation” events per year; an exacerbation is defined
as an acute worsening of respiratory symptoms that result in additional therapy. |
| Asthma | |
| Atopic asthma | Atopic and eosinophilic with increased fractional exhaled nitric oxide
(FeNO) |
| Non-eosinophilic asthma associated with obesity | Decreased lung function associated with obesity |
| Non-eosinophilic asthma (neutrophilic asthma) | Lack of eosinophilic inflammation. No raised sputum eosinophil count or
FeNO. Neutrophilic inflammation common. |
| Aetiology | Examples of causes |
| Bronchiectasis | |
| Post-infectious damage | Tuberculosis, whooping cough, and so on |
| Muco-ciliary clearance defects | Primary ciliary dyskinesia, cystic fibrosis, and Young’s syndrome |
| Immunodeficiency | Primary (for example, hypogammaglobinaemia)
Secondary (for example, malignancy such as leukaemias or immune modulation with drugs, after transplant) |
| Autoimmune conditions | Rheumatoid arteritis, systemic lupus erythematosus, and inflammatory
bowel disease |
| Congenital | Tracheobronchomegaly, cartilage deficiency, and Marfan syndrome |
| Toxic exposures, obstruction or aspiration | Toxic gas (chlorine, ammonia), foreign body, and smoke exhalation |
The first and second sections provide a table of recognised COPD and asthma phenotypes. Though not exhaustive, these represent phenotypes most often discussed in recent publications (for example, 47, 48) and it is also possible for patients to have more than one phenotype; thus, there can be considerable clinical overlap. In the third section, examples of aetiologies that can lead to bronchiectasis have been given. Again, this list is not exhaustive but for all diseases (COPD, asthma and bronchiectasis) is intended to provide an overview of how disparate clinical phenotypes associated with one umbrella term can be.
Neutrophil migration and chronic obstructive pulmonary disease
Older studies of neutrophil migration in COPD yielded conflicting results as to whether there was any compromise in migratory function 45, 46; however, more recent studies have allowed the assessment of specific neutrophil migratory dynamics and have shown that neutrophils from patients with COPD migrate with increased speed but reduced directional accuracy towards a variety of chemoattractants compared with age-matched healthy control subjects 40. This does not result from reduced chemoattractant receptor expression or impaired receptor localisation 49 but could be improved by using a broad-spectrum PI3K inhibitor (LY294002), suggesting that the defective migration results from aberrant intracellular signalling processes 40.
As a neutrophil migrates, serine proteinases, including neutrophil elastase (NE), cathepsin G and proteinase 3 (PR3), are released from azurophil granules into the extracellular space, and some active enzyme is retained on the plasma membrane 9, 50– 52. Substrates for these proteinases include elastin 53, collagen 54 and fibronectin 55, which are major components of the extracellular matrix, and their degradation is linked to all clinical facets of COPD.
Initially, NE was thought to be the most important proteinase in COPD. NE can be inhibited by a number of endogenous inhibitors, including alpha-1 anti-trypsin (AAT), secretory leucocyte proteinase inhibitor (SLPI) and α2-macroglobulin (α2M). However, at the time of NE release, its concentration (5.33 mM) is 15 to 1500 times greater than that of its inhibitors 56 (with plasma concentrations of AAT of 32.8 μM 9, SLPI of 11 μm 57 and α2M of 3.5 μM 58). NE is only partly inhibited until it diffuses away from the cell and an optimal NE-inhibitor ratio is reached. As this inhibition is not immediate, an obligate area of local, proteinase-mediated tissue damage occurs, a phenomenon known as “quantum proteolysis”. Furthermore, a proportion of NE remains bound to the neutrophil cell surface, making it less accessible to inhibitors and more resistant to inhibition, further increasing the local potential damage. There is emerging evidence that PR3 may be even more implicated in the pathology of COPD, especially the emphysema process 59. PR3 is stored in higher concentrations than NE 60 and has a lower association rate constant with AAT, suggesting that it is likely to have more prolonged activity than NE before inactivation, as demonstrated by mathematical modelling 61 and in studies of airway secretions in both AAT-deficient (AATD) and non-deficient COPD 62.
Reduced migratory accuracy of neutrophils in COPD may have implications for disease pathology because of the increased area of obligate tissue damage caused by proteinase release during poorly directed migration. In keeping with this, previous work has shown increased fibronectin degradation by migrating COPD neutrophils 45; in vitro, there are increased levels of the NE footprint Aα-Val 360 63 and newly described PR3 footprint Aα-VAL 541 64 in plasma from patients with COPD.
Of note, recent studies suggest that migration of monocytes from COPD patients to COPD sputum is also impaired 65, although similar studies using single chemokines as the chemoattractant do not replicate this finding 66. As neutrophils and monocytes are derived from the same bone marrow precursor cells, this may reflect a common genetic/epigenetic or inflammatory cause. It remains unclear when neutrophil migratory dysfunction develops in COPD: during maturation in the bone marrow or following release into the circulation. If monocyte migration were found to be similarly impaired in COPD, this might suggest that the defect lies in bone marrow precursor cells.
COPD is more commonly seen in older patients and neutrophil migratory accuracy also declines with “healthy” ageing, a phenomenon also associated with constitutive PI3K activity 67, and selective class I PI3K-δ or PI3K-γ inhibitors improve neutrophil migratory accuracy. It is possible that the poor migratory accuracy seen with age is exaggerated in patients with COPD, and inhibition of specific PI3K isoforms may offer a novel strategy to improve bacterial clearance and reduce tissue damage in COPD.
Proteinases and chronic obstructive pulmonary disease
The proteinase/anti-proteinase theory of COPD suggests that damage to the lung tissue occurs when the levels of anti-proteinases in the lung are insufficient to effectively neutralise the proteinases present 62, 68, 69. Recently, the proteinase/anti-proteinase theory of COPD was revisited in a study which elegantly demonstrates a role for exosomes (cell-derived vesicles that are present in many eukaryotic fluids, including blood, urine and cerebrospinal fluid) in promoting NE activity, effectively tipping the local protease/anti-protease balance within the lung to favour tissue damage 70. The authors describe a population of exosomes released from neutrophils which bind NE when it is released during degranulation, before its diffusion into the tissues. Unlike free enzyme, NE bound to these exosomes was found to be resistant to inhibition by AAT and to be able to bind to the extracellular matrix (via mac-1) whilst maintaining NE activity against the extracellular matrix proteins. These neutrophil-derived exosomes were found in clinical specimens from subjects with COPD but not healthy controls and importantly were capable of transferring a COPD-like phenotype from humans to mice 70. Certainly, there is evidence of increased degranulation in COPD, and CD63 (a marker of primary granules) is found to be increased on the surface of unstimulated neutrophils from patients with COPD 71.
Serine proteinases are potent stimulators of mucus secretion from submucosal and goblet cells of the airways 72, which, alongside the effects of cigarette smoke on mucosal cilia, reduces mucociliary clearance 73, 74. Mucus is able to build up in the airways, contributing to further obstruction, increasing the risk for bacterial colonisation and further inflammation 72, 75.
Neutrophil extracellular traps and chronic obstructive pulmonary disease
Recently, there has been significant interest in the role of NETs in COPD, but the current evidence is conflicting. Increased quantities of NET components have been described in the sputum of both stable and exacerbating COPD patients, alongside an increased proportion of “NET producing” neutrophils 76, 77. Furthermore, the abundance of NETs within sputum has been shown to correlate with severity of airflow limitation assessed by FEV 1 76, 78 and overall severity of COPD using a composite scale including symptoms and exacerbation frequency alongside FEV 1 78. Interestingly, the most recent study of NETs in COPD shows a correlation between NET complexes in sputum and microbial diversity, in particular a dominance of haemophilus species, whereby more than 40% haemophilus species within the lung microbiome were found to be associated with significantly greater DNA-elastase complexes 78. Despite this, neutrophils isolated from the blood of patients with exacerbations of COPD have been shown to have a reduced ability to undergo NETosis compared with both stable patients and healthy controls, despite the increased presence of cell-free DNA in plasma 79. This seems counterintuitive but it is possible that the clearance of NETs by DNases 80 is impaired in COPD or that only a proportion of cells are able to produce NETs (a phenotype of cell) but this remains unknown.
Phagocytosis and chronic obstructive pulmonary disease
Reduced phagocytic function of macrophages in COPD is well described, encompassing impaired phagocytosis of disease-relevant bacteria (non-typeable Haemophilus influenzae and Streptococcus pneumoniae), fungi 81 and apoptotic neutrophils via efferocytosis 82– 84. However, little research to date has focused on the phagocytic ability of neutrophils, and data so far provide conflicting results; some demonstrate no differences in phagocytosis 85– 87 and others suggest a reduction 88. However, few studies have used phagocytic targets relevant to disease pathology, and studies in macrophages suggest that the use of non-physiological targets may provide misleading results. For example, phagocytosis of synthetic beads by macrophages was found to be unaltered in COPD, but bacterial studies showed a reduction in phagocytosis of the disease-relevant bacteria H. influenzae and S. pneumoniae 82, suggesting that the results of studies using non-physiological targets may be misleading 85, 88. This gap in knowledge clearly needs detailed exploration.
Further to this, NE has been shown to be able to cleave complement components on bacteria as well as complement receptors on neutrophils 89, 90. If NE activity is heightened, as suggested in COPD 91 and AATD 92, the resulting opsonin-receptor mismatch may impair effective phagocytosis.
Neutrophil phenotypes in chronic obstructive pulmonary disease and retention in the lung
Despite emerging interest in the concept of neutrophil phenotypes, there are few studies of this in COPD. One recent article assessed protein expression on the surface of neutrophils from 41 patients with COPD and seven healthy, age-matched controls, describing clear clustering which could differentiate patients with COPD from the control subjects 93. Furthermore, the neutrophil proteome was different between two COPD groups; but these patient groups were not clinically different, the expressions of several activation markers were not significantly different, but there were some functional changes between groups in relation to ROS release 93.
In relation to the retention of neutrophils in COPD lungs, a very recent publication 94 has built upon the previously described studies of neutrophil transit times through the lung vasculature, clearly demonstrating increased neutrophil accumulation in COPD lungs compared with healthy individuals, with little overlap.
In summary, recent studies in COPD have built upon a strong foundation implicating the neutrophil as a key driver of COPD pathology. This includes altered cell functions which favour host tissue damage with an increased burden of proteinase activity, a clear signal of neutrophil retention in the lungs which is not seen in health, and a tantalising hint of differing cell phenotypes. However, because COPD studies invariably compare health with disease, it is unclear whether these changes are unique to COPD (and thus may represent a COPD-specific therapeutic target) or whether these changes are also seen in other diseases of the airways.
Alpha-1 anti-trypsin deficiency
AAT is a 52-kDa glycoprotein produced by hepatocytes but also macrophages and neutrophils and (as stated) functions as a serine protein inhibitor, providing essential protection of the lung tissue against the proteolytic actions of enzymes such as NE and PR3. In health, there is a constant diffusion of AAT into the lung, which is increased in the presence of inflammation (such as during respiratory infections). AATD is a genetic disorder in which the gene encoding AAT is mutated. There are many subtypes of AATD but in the most common severe form of deficiency (named PiZZ) this leads to mis-folding of the protein product, retention of AAT in AAT-producing cells and the formation of protein polymers in these cells, which causes damage and low circulating levels of AAT. AATD is the only robustly established genetic risk factor for the development of COPD and emphysema, and these disease processes can occur in patients with AATD, even in the absence of cigarette smoking 62, 91, 95, 96.
Neutrophils play a central role in the pathophysiology of emphysema associated with AATD 97, and pulmonary disease is thought to develop, in part, from an imbalance of proteinases and AAT, although AAT has many non-proteolytic functions which protect against infection and inflammation, including immunomodulation and anti-microbial activity. It is well known that AAT deficiency is associated with a reduced ability to neutralise NE and PR3 adequately, leading to more tissue damage. In response to NE activity, epithelial cells and macrophages also release pro-inflammatory mediators such as CXCL8 98 and leukotriene B 4 (LTB 4) 99, respectively. This chemoattractant production is perpetuated, further increasing neutrophil influx and increased NE activity within the lung, forming a vicious cycle of damage. In keeping with this, the inflammation present in AATD (both systemic and local) is amplified when compared with non-AATD COPD with a similar burden of disease 100 and this may influence immune function by cell priming or activation.
Lung neutrophilia has been much easier to demonstrate in AATD compared with non-AATD COPD 101 but these cells do not appear to be just “reactive responders”, and a number of studies have described abnormal neutrophil behaviour in AATD. A recent study described increased apoptosis of AATD neutrophils 102. The authors proposed that this might reflect endoplasmic reticulum stress owing to the accumulation of mis-folded AAT within the neutrophil 102. However, augmentation therapy, in which deficient AAT is “replaced” with purified plasma AAT from healthy individuals, was able to normalise cell apoptosis without altering endoplasmic reticulum stress markers, and apoptosis was a direct result of low circulating AAT. Internalised AAT is known to co-localise with and inhibit staurosporine-induced caspase-3 activation 103, a potent signal for apoptosis recently described in neutrophils 104.
The same authors propose defective bacterial killing by AATD neutrophils but this appeared to result from accelerated neutrophil apoptosis rather than an intrinsic defect in neutrophil phagocytosis per se 102. AAT augmentation both in vitro and in vivo could restore the bacterial killing capacity of ZZ-AATD neutrophils to that of non-deficient neutrophils but again this might reflect reduced apoptosis 102. AAT is known to improve phagocytosis by both human alveolar macrophages (AMs) from patients with non-AATD COPD and AMs isolated from mice exposed to cigarette smoke 105. This improvement included both efferocytosis (clearance of dead neutrophils) and phagocytosis and was associated with the upregulation of efferocytosis and scavenger receptors on the AM plasma membrane 105. These receptors were also shown to be upregulated in patients with AATD following double-dose augmentation treatment with purified AAT compared with a single dose, suggesting that a similar mechanism to enhance efferocytosis may exist in vivo.
There are limited data regarding neutrophil migratory function in patients with AATD. One study has suggested that sputum from patients with AATD has greater chemotactic activity which likely relates to increased levels of neutrophil chemoattractants CXCL8 and LTB 4 in sputum rather than the ability of neutrophils to migrate per se 106. A further study demonstrated no difference in the ability of neutrophils from patients with AATD to migrate to a standard chemoattractant (CXCL8) compared with healthy controls despite finding reduced migratory accuracy of neutrophils from patients with COPD 40. There is an increased burden of ROS in AATD, and AAT modulates neutrophil O 2 − production elicited by N-formylmethionine-leucyl-phenylalanine (fMLP) and CXCL8 in a dose-dependent manner 107. However, the burden of ROS in AATD may be multi-faceted, and AAT is known to bind to a number of products with oxidative potential, including hemin 108. There are few studies of NET formation in AATD. One study using the non-physiological stimulant phorbol myristate acetate (PMA) reported that AAT did not reduce NET formation from neutrophils isolated from patients with PiZZ AATD but this study has yet to be replicated using disease-relevant stimuli 109.
To date, there are no studies of neutrophil phenotype in PiZZ AATD to determine whether distinct patterns are seen, but when the data are considered together, it appears that AATD is not merely an exaggerated form of non-AATD COPD and there appear to be differences in cellular function between the two groups. This is highlighted in clinical and imaging studies. The predominant phenotype of emphysema observed in non-AATD COPD is typically apical centrilobular but this differs in AATD, where emphysema is predominantly basal and panlobular 110, 111, reflecting differences in pathophysiology between the two conditions. 18-fluorodeoxyglucose ( 18FDG) positron emission tomography–computed tomography (PET-CT) studies generate both quantitative and spacial data regarding pulmonary glucose uptake, which has been shown to relate to neutrophil activity in animal models 112, 113. When these studies were performed in patients with COPD, 18FDG uptake was shown to be greater in emphysematous regions of the lung and correlated with physiological measures of disease severity 114. Despite this, the increased pulmonary 18FDG demonstrated in non-AATD COPD was not demonstrated in a small cohort of patients with AATD, in whom 18FDG uptake was comparable to that of healthy controls 114. This suggests important differences in the pathogenesis of emphysema in these two conditions, in particular with respect to the role of the neutrophil.
Also, lung disease is heterogeneous in AATD, even in patients with PiZZ AATD who have never smoked. A proportion of never-smoking patients with similar deficiency levels do not develop lung disease although some do, and in those who do, rates of decline are variable and currently cannot be predicted at baseline screening 115. Furthermore, patients with AATD experience clinical phenotypes similar to those of patients with non-AATD COPD 115. Also, although AAT augmentation reduces the progression of lung disease in some patients, it has little impact on others, highlighting the fact that replenishing the deficient anti-proteinase is not enough to treat disease and more studies are needed to assess the utility of targeting the neutrophil in conjunction with augmentation strategies.
Bronchiectasis
Rather than being a pathological entity (such as AATD), bronchiectasis is a chronic lung condition caused by a number of pathological insults ( Table 1) characterised visually by irreversibly dilated airways, mucus gland hyperplasia, and impaired mucus clearance resulting in recurrent severe infections and further airway damage as described by Cole’s vicious cycle hypothesis 116– 118. Bacterial colonisation with potentially pathogenic micro-organisms is extremely common, and neutrophils are thought to play a fundamental role in bronchiectasis pathogenesis, partially in response to this infection. Impaired mucus clearance and recurrent infections cause rich sputum levels of potent neutrophil chemoattractants, including interleukin 1 beta (IL-1β), tumour necrosis factor alpha (TNFα), CXCL8, and LTB 4 119. Consequently, neutrophils dominate cell populations in both the sputum and bronchoalveolar lavage fluid of patients with bronchiectasis, and neutrophil counts positively correlate with bronchiectasis disease severity 117, 120. This heightened inflammation impacts on neutrophil function. Systemic neutrophils from individuals with bronchiectasis have a higher level of baseline activation compared with healthy individuals, as indicated by increased CD62L and CD11b 120. Furthermore, blood neutrophil viability is significantly prolonged because of delayed apoptosis (a feature of inflammation) and these neutrophils release more myeloperoxidase (MPO) when unstimulated in more severe forms of the disease (suggesting constitutive priming and activation) 120. Systemic neutrophils seem to retain their phagocytic and anti-microbial ability compared with airway counterparts 120. Airway neutrophils in bronchiectasis exhibit impaired phagocytosis of pathogens, including Pseudomonas aeruginosa (PAO1), contributing to recurrent infections 120. However, this appears to improve with antibiotic therapy 120. In a study of 103 adults with bronchiectasis, the most frequent immune cell abnormality was reduced neutrophil oxidative burst 121 but there was significant heterogeneity. A comprehensive screen of immune function confirmed that 13 subjects had low levels of IgG3, six had low levels of B-cell lymphocytes and seven had low T-helper cell lymphocytes when compared with controls. All subjects had a normal neutrophil phagocytic function, but 33 of the subjects had an oxidative burst that was below that seen in health 121. In addition, airway neutrophils in bronchiectasis exhibit higher necrosis and impaired cell death as well as reduced clearance by macrophages, delaying inflammation resolution and causing persistent inflammation and further airway damage 116, 120. Furthermore, increased neutrophil degranulation causes further airway damage and correlates with worse clinical outcome 119, 122.
Although these studies highlight themes of neutrophil function and dysfunction across bronchiectasis, the diverse causes of disease may display different patterns. For example, primary ciliary dyskinesia (PCD) is a rare genetic disease caused by abnormal structure or function of motile cilia (or both) which leads to bronchiectasis 123. Recently, neutrophils from patients with PCD have been shown to display reduced migration toward CXCR2 ligands (CXCL5 and CXCL8) but not to LTB 4 and complement component 5a. The reduced response to CXCL8 was observed in all subgroups of patients with PCD and correlated with lung function, and CXCR2 expression was downregulated on the cell surface in about 65% of the patients with PCD 124. However, in non-PCD bronchiectasis, neutrophil migration appears preserved 125, and a trial of a CXCR2 antagonist given orally for 28 days resulted in about a 70% decrease in the percentage of sputum neutrophils, suggesting that CXCR2 ligands were strong drivers of neutrophil accumulation in the lung 126.
The combination of infection and inflammation suggests that both anti-microbial and anti-inflammatory agents might help with disease management. Currently, the two main treatment options for bronchiectasis involve physiotherapy for clearance of mucus and antibiotics for treatment of infections 127, a strategy that has not changed since bronchiectasis was first characterised in the 1950s. Despite advances in understanding the pathophysiology of bronchiectasis, the rates of exacerbation and mortality amongst patients with bronchiectasis have shown little improvement 128. Therefore, further research is needed to understand how to prevent disease progression and to develop therapeutic targets accordingly but this may require better stratification of the cause of bronchiectasis and a diverse treatment strategy.
Cystic fibrosis
CF is an autosomal recessive disease whereby a loss-of-function mutation in the CF transmembrane conductance regulator ( CTFR) gene affects mucociliary clearance 117. CF is the most common inherited disorder in the Caucasian population, affecting 1 in 2000 live births 129, and a common cause of bronchiectasis, which is often more severe and progressive than non-CF bronchiectasis. With CF bronchiectasis, as with non-CF bronchiectasis, neutrophil dysfunction has been described. In particular, airway neutrophils in patients with CF exhibit a functional exhaustion and a pro-survival phenotype 129, 130, potentially reflecting the high levels of inflammation and structural damage present in the lung.
Recruitment and migration of neutrophils into the lung appear to be normal in patients with CF, but the plethora of inflammatory mediators in the CF airways makes the sputum rich in neutrophils 117. Extensive research into these airway neutrophils has uncovered some functional defects. First, CF airway neutrophils exhibit impaired degranulation which is linked to the loss of CFTR function as ivacaftor treatment reverses this 129, 131. Dysregulated degranulation of NE and MPO contributes to tissue damage which can exacerbate CF 117. Furthermore, the altered microenvironment of the CF lung is thought to be a contributing factor to lower neutrophil phagocytosis levels which are coupled with a lower respiratory burst generation shown in vitro following stimulation with PMA 117, 130. This is thought to contribute to impaired bacterial killing and recurrent infections. In addition to having functional defects, CF airway neutrophils appear to have a pro-survival phenotype. CF neutrophils have apoptosis defects which delay and impair cell death, resulting in neutrophil persistence, NET production and increased necrosis 129, 130, 132, 133. Auto-antibodies to NET components have also been described in patients with CF and the presence of these auto-antibodies has been associated with diminished lung function 134, although direct evidence linking these two observations is lacking.
New CFTR modulators have revolutionised the treatment of CF for patients with specific genetic mutations and these therapies also appear to impact on neutrophil function. Ivacaftor treatment restored neutrophil apoptosis rates in patients commencing this treatment compared with their baseline functions 129, 131. The exact mechanism of action has not yet been elucidated, but similar immune modification has been seen in macrophages from CF patients taking CFTR modulators 135, providing further evidence of effect.
Asthma
Asthma is a chronic inflammatory lung disease that affects 340 million people worldwide and accounts for 180,000 deaths worldwide every year 136, 137. Again, there are many phenotypes of asthma ( Table 1), and for many years there has been interest in the concept of “neutrophilic asthma” (where neutrophils represent 40 to 76% of total sputum cells) and this classically correlates with steroid resistance, acute exacerbations, occupational asthma and more treatment-resistant forms of the disease, suggesting that the neutrophil plays a role in asthma pathophysiology 136, 138– 140. In patients with asthma, as in those with other diseases discussed, both peripheral and airway neutrophils exhibit functional defects compared with healthy individuals. In vitro chemotactic velocity to CXCL8 and fMLP has been shown to be impaired 137. This finding has led to the suggestion that neutrophil migration could be used to differentiate asthma from non-asthma patients 141, but given that neutrophil migration is dysfunctional in a number of conditions, the utility of such a device is questionable. In asthma, as in other airway diseases, there is some evidence of increased NET formation 142, ROS generation 143 and reduced neutrophil phagocytosis 144, although results are variable. In patients with neutrophilic asthma, as in those with COPD, systemic inflammation (C-reactive protein and IL-6) is increased compared with both patients with non-neutrophilic asthma and healthy controls 145. However, whether defects in neutrophil function are intrinsic or are a consequence of—and perhaps contribute to—heightened systemic and airway inflammation remains unclear.
Of note, although neutrophils are associated with tissue damage in asthma, they have also been shown to have a role in controlling inflammation, restoring tissue homeostasis and promoting tissue repair 136, highlighting the delicate balance between protective and destructive functions of neutrophils in airway disease, a common feature across all of the diseases we have considered.
Common mechanisms across diseases
The data presented highlight many similarities in neutrophilic inflammation across airway diseases. First, an airway neutrophilia is common. Second, there are often markers of neutrophil degranulation and in particular ROS and proteinase activity which are associated with disease presentation and progression. Third, aspects of neutrophil function appear altered. Although this seems to most commonly affect migration and ROS production, studies also highlight aberrant NETosis and phagocytosis, although defects are variable. The commonality of neutrophil inflammation across different diseases might suggest common underlying mechanisms of effect, and studies have suggested potential themes as to how this might occur.
First, inflammation might impact on neutrophil functions irrespective of the initial insult (be it infection, allergen or smoking). For example, TNFα—shown to be increased in airway secretions from patients with COPD, asthma, bronchiectasis and AATD—is able to increase the expression of capture receptors and adhesion molecules on the surface of blood vascular endothelial cells, enhancing neutrophil migration into the inflamed lung 146. Furthermore, TNFα can impact on cellular functions as it is a potent priming agent and able to increase ROS production by neutrophils, which will further contribute to tissue damage 147. Second (and more speculatively), the inflammation present across diseases might impair the ability of the lungs to “de-prime” cells 29, leading to a circulating population of primed cells, which might confer a more aggressive cellular phenotype. A third putative theme is that of altered cellular subtypes. This has only recently been described in COPD, but other studies suggest that neutrophils change in response to signals such as inflammation, hypoxia or physical pressure, resulting in different functional phenotypes. These changes are subtle and may relate to the immediate cellular environment, as described in mice models of cancer 147 and pro-inflammatory culture conditions 148. Figure 2 provides a summary of how these mechanisms could lead to pathology across diseases. The evidence base for these themes is tentative but, if confirmed, may provide therapeutic insight to target fundamental inflammatory processes across diseases.
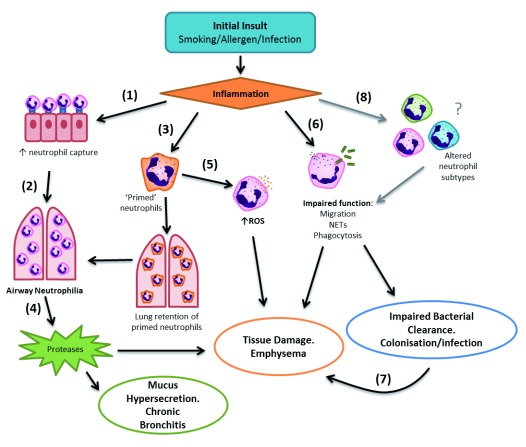
Figure 2. Inflammatory mechanisms in disease pathogenesis.
Inflammation from the initial insult (1) increases the expression of capture molecules on the bronchial epithelium and adhesion molecules on neutrophils, (2) enhancing neutrophil migration into the inflamed lung, resulting in airway neutrophilia. (3) Potentially altered neutrophil priming processes from excessive neutrophil priming, or a possible failure of the lung to “de-prime” neutrophils, further increases airway neutrophilia. (4) Release of proteases from airway neutrophils during migration, release of neutrophil extracellular traps (NETs), or frustrated phagocytosis contributes to degradation of elastin and development of emphysema. Neutrophil elastase can also cause mucus hypersecretion, contributing to development of chronic bronchitis. (5) Increased reactive oxygen species (ROS) released from primed neutrophils further contributes to tissue damage within the lung. (6) Impaired neutrophil function increases tissue-damaging potential via excessive protease release or impaired bacterial clearance, increasing susceptibility to bacterial colonisation or acute infection. (7) Bacterial colonisation further heightens pulmonary inflammation, increasing tissue damage potential. (8) Speculatively, inflammation, hypoxia or physical pressure may alter the neutrophil population, resulting in subtypes of neutrophils with different phenotypes and altered function which further contribute to local tissue damage and impaired bacterial clearance.
Targeting neutrophils in airway disease
Although modifying neutrophilic inflammation is an attractive interventional strategy, neutrophils are a challenging target and one that comes with risks associated with neutropenia or excessive inhibition of neutrophil host defence mechanisms against infection. There are two potential therapeutic avenues to explore.
The first is to target the chemoattractants responsible for neutrophil recruitment into the lung. CXCL8 and LTB 4 are the dominant chemokines thought to be responsible for this 100, 106, 149 and drugs targeting these mediators have been trialled in inflammatory airway disease. Clinical studies of a CXCR2 agonist (MK-7123) led to improvements in lung function and reduced exacerbations in active-smoking patients with COPD compared with placebo treatment 150, but a large proportion of patients experienced neutropenia, raising concerns about immunosuppression. As discussed earlier, in patients with bronchiectasis, the CXCR2 antagonist AZD5069 resulted in a 70% decrease in the percentage of sputum neutrophils but this was not associated with improved clinical outcomes 126. A small phase II trial investigated the effects of a leukotriene synthesis inhibitor, reducing LTB 4 production, on bronchial inflammation in patients with stable COPD, showing some benefit 151. However, a randomised placebo-controlled trial of an LTB 4 receptor antagonist (BIIL 284 BS) in patients with CF was terminated early because of serious adverse effects, including increased respiratory symptoms requiring intravenous antibiotics and hospitalisation, reduced pulmonary function and increased circulating neutrophil numbers 152, suggesting that LTB 4 antagonism may result in acute pulmonary exacerbations and heightened inflammation 152, although the mechanisms for this were poorly understood. An alternative strategy may be to target associated co-morbid conditions which contribute to the inflammatory load. For example, the treatment of periodontitis, a chronic inflammatory condition associated with neutrophilic inflammation and recruitment which shares many inflammatory features of COPD 153, has been shown to improve changes in both lung function and exacerbation frequency in COPD 154.
The second therapeutic option is to directly modulate neutrophil function 155. A number of in vitro studies have investigated strategies to improve the accuracy of neutrophil migration, thereby theoretically reducing the potential for migration-associated and protease-mediated tissue damage. Broad-range inhibition of PI3K signalling has been shown to restore migration of COPD neutrophils to levels similar to those of neutrophils from age-matched healthy controls 40. However, broad-spectrum inhibition of PI3K therapeutically is likely to lead to significant side effects. Selective inhibition of class I PI3K-δ and PI3K-γ, which are enriched in leucocytes 156, may offer a more acceptable therapeutic option; indeed, selective isoform inhibition of PI3K-δ and PI3K-γ has been shown to restore reduced migratory accuracy of neutrophils from healthy older adults 67. Impaired migration in COPD is hypothesised to be a further exaggeration of age-related impairment in neutrophil migration, emphasising the potential of this strategy in COPD, but whether PI3K inhibition provides benefit in vivo or in other inflammatory airway diseases requires further investigation. Simvastatin is a safe and well-tolerated drug commonly used for its cholesterol-lowering abilities. Population studies and clinical trials suggested a survival benefit for patients taking statins during infection 157, which prompted interest in the ability of these drugs to modulate immune function. In vitro simvastatin treatment has been shown to have beneficial effects on migration of neutrophils from patients with COPD 158 and during pulmonary infection in otherwise healthy older adults but not during more severe infection or sepsis 159. These beneficial effects on migration were replicated in a clinical trial of high-dose simvastatin in healthy older adults 159, 160. Similar in vivo studies are required to determine whether effects are maintained in a disease setting, but a clinical trial is currently under way 161 and outputs are expected this year. Other commonly used drugs may also provide mechanistic insight into how neutrophil could be targeted. Aspirin induces resolvin-D signalling, which has been associated with improved pneumonia outcomes in murine models 162, and aspirin is associated with improved survival in observational studies of pneumonia 163. Metformin, commonly used for glycaemic control in diabetes, has also gained interest as a potential means to target neutrophil functions, potentially modifying chemotaxis and bacterial killing through 5′ adenosine monophosphate-activated protein kinase (AMPK) activation 164.
Targeting local airway neutrophil apoptosis has also been suggested, and induction of airway neutrophil apoptosis reduced airway inflammation in mouse models 165, 166 but these models do not recapitulate all features of human disease. Effective clearance of apoptotic cells, via efferocytosis, is vital to prevent secondary necrosis and release of damaging pro-inflammatory cell contents which may heighten inflammation and contribute to further tissue damage 167. However, in human studies, clearance of apoptotic cells has been shown to be reduced in many inflammatory airway diseases, including COPD 83, 84, asthma 168, 169, CF and bronchiectasis 170. As such, induction of neutrophil apoptosis in vivo, without improvement of clearance mechanisms, needs to be approached with caution and may have the potential to cause more harm than good.
The final challenge is effective delivery of the desired drug to its target without impacting host defence against infection owing to neutropenia or excessive impairment of neutrophil function. Inhaled therapies may permit effective targeting of airway neutrophils whilst minimising systemic side effects; indeed, an inhaled PI3K-Δ inhibitor is in clinical trial for the treatment of COPD exacerbations (ClinicalTrials.gov Identifier: NCT03345407). The key would be to ensure penetration of the inhaled compounds into the smaller airways and newer devices offer the promise of these effects.
Conclusions
Neutrophilic inflammation is a common feature of many airway diseases and is associated with disease progression, often irrespective of the initiating cause or underlying diagnosis. This provides a potential therapeutic target, but the target is a challenging one. The crucial role of neutrophils in clearing bacteria means that merely inhibiting their responses in a blunt or indiscriminate fashion is likely to be detrimental to the host, as demonstrated by the manifestations of neutropenia. Targeting neutrophils requires a more subtle approach. Neutrophils appear to be susceptible to epigenetic changes 171 which are variable but impact on function and the resultant changes appear long-lived 172. This might provide a mechanism for the self-perpetuating inflammation present across many airway diseases. It might be that chronic inflammation leads to epigenetic reprogramming of neutrophils, which alters their phenotype or responses. The physical damage to the lung infrastructure and especially the pulmonary vasculature might compound this by inhibiting de-priming. Unfortunately, the current evidence base for understanding neutrophil function across diseases is limited (often small studies using different techniques across different patient groups) but this is certainly worthy of more study. To ascertain whether there are shared mechanisms of neutrophil dysfunction across disease and more importantly how these might be targetable will require collaborative research across current disease silos.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
ZhiHua Chen, Department of Respiratory and Critical Care Medicine, Second Hospital of Zhejiang University School of Medicine, Zhejiang, China
Catherine M Greene, Lung Biology Group, Department of Clinical Microbiology, Royal College of Surgeons in Ireland, Education and Research Centre, Beaumont Hospital, Dublin, Ireland
Funding Statement
This work was supported by the Alpha-1 Foundation, the Medical Research Council, the Wellcome Trust, and the National Institute for Health Research.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; peer review: 2 approved]
References
- 1. Summers C, Rankin SM, Condliffe AM, et al. : Neutrophil kinetics in health and disease. Trends Immunol. 2010;31(8):318–24. 10.1016/j.it.2010.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Segal AW: How neutrophils kill microbes. Annu Rev Immunol. 2005;23:197–223. 10.1146/annurev.immunol.23.021704.115653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Winterbourn CC, Hampton MB, Livesey JH, et al. : Modeling the reactions of superoxide and myeloperoxidase in the neutrophil phagosome: implications for microbial killing. J Biol Chem. 2006;281(52):39860–9. 10.1074/jbc.M605898200 [DOI] [PubMed] [Google Scholar]
- 4. McCord JM, Fridovich I: Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969;244(22):6049–55. [PubMed] [Google Scholar]
- 5. Klebanoff SJ: Myeloperoxidase. Proc Assoc Am Physicians. 1999;111(5):383–9. 10.1111/paa.1999.111.5.383 [DOI] [PubMed] [Google Scholar]
- 6. Levine AP, Segal AW: The NADPH Oxidase and Microbial Killing by Neutrophils, With a Particular Emphasis on the Proposed Antimicrobial Role of Myeloperoxidase within the Phagocytic Vacuole. Microbiol Spectr. 2016;4(4). 10.1128/microbiolspec.MCHD-0018-2015 [DOI] [PubMed] [Google Scholar]
- 7. Klebanoff SJ: Iodination of bacteria: a bactericidal mechanism. J Exp Med. 1967;126(6):1063–78. 10.1084/jem.126.6.1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Klebanoff SJ, Clark RA: Iodination by human polymorphonuclear leukocytes: a re-evaluation. J Lab Clin Med. 1977;89(3):675–86. [PubMed] [Google Scholar]
- 9. Liou TG, Campbell EJ: Quantum proteolysis resulting from release of single granules by human neutrophils: a novel, nonoxidative mechanism of extracellular proteolytic activity. J Immunol. 1996;157(6):2624–31. [PubMed] [Google Scholar]
- 10. Borregaard N, Cowland JB: Granules of the human neutrophilic polymorphonuclear leukocyte. Blood. 1997;89(10):3503–21. [PubMed] [Google Scholar]
- 11. Borregaard N, Sørensen OE, Theilgaard-Mönch K: Neutrophil granules: A library of innate immunity proteins. Trends Immunol. 2007;28(8):340–5. 10.1016/j.it.2007.06.002 [DOI] [PubMed] [Google Scholar]
- 12. Uriarte SM, Powell DW, Luerman GC, et al. : Comparison of proteins expressed on secretory vesicle membranes and plasma membranes of human neutrophils. J Immunol. 2008;180(8):5575–81. 10.4049/jimmunol.180.8.5575 [DOI] [PubMed] [Google Scholar]
- 13. Brinkmann V, Reichard U, Goosmann C, et al. : Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–5. 10.1126/science.1092385 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 14. Sapey E, Stockley RA: Red, amber and green: the role of the lung in de-priming active systemic neutrophils. Thorax. 2014;69(7):606–8. 10.1136/thoraxjnl-2014-205438 [DOI] [PubMed] [Google Scholar]
- 15. Yost CC, Denis MM, Lindemann S, et al. : Activated polymorphonuclear leukocytes rapidly synthesize retinoic acid receptor-alpha: a mechanism for translational control of transcriptional events. J Exp Med. 2004;200(5):671–80. 10.1084/jem.20040224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tecchio C, Micheletti A, Cassatella MA: Neutrophil-derived cytokines: facts beyond expression. Front Immunol. 2014;5:508. 10.3389/fimmu.2014.00508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Walmsley SR, Print C, Farahi N, et al. : Hypoxia-induced neutrophil survival is mediated by HIF-1alpha-dependent NF-kappaB activity. J Exp Med. 2005;201(1):105–15. 10.1084/jem.20040624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Futosi K, Fodor S, Mócsai A: Neutrophil cell surface receptors and their intracellular signal transduction pathways. Int Immunopharmacol. 2013;17(3):638–50. 10.1016/j.intimp.2013.06.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bekes EM, Schweighofer B, Kupriyanova TA, et al. : Tumor-recruited neutrophils and neutrophil TIMP-free MMP-9 regulate coordinately the levels of tumor angiogenesis and efficiency of malignant cell intravasation. Am J Pathol. 2011;179(3):1455–70. 10.1016/j.ajpath.2011.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Christoffersson G, Vågesjö E, Vandooren J, et al. : VEGF-A recruits a proangiogenic MMP-9-delivering neutrophil subset that induces angiogenesis in transplanted hypoxic tissue. Blood. 2012;120(23):4653–62. 10.1182/blood-2012-04-421040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Massena S, Christoffersson G, Vågesjö E, et al. : Identification and characterization of VEGF-A-responsive neutrophils expressing CD49d, VEGFR1, and CXCR4 in mice and humans. Blood. 2015;126(17):2016–26. 10.1182/blood-2015-03-631572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sagiv JY, Michaeli J, Assi S, et al. : Phenotypic diversity and plasticity in circulating neutrophil subpopulations in cancer. Cell Rep. 2015;10(4):562–73. 10.1016/j.celrep.2014.12.039 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 23. Rosales C: Neutrophil: A Cell with Many Roles in Inflammation or Several Cell Types? Front Physiol. 2018;9:113. 10.3389/fphys.2018.00113 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 24. Kuebler WM, Kuhnle GE, Goetz AE: Leukocyte margination in alveolar capillaries: interrelationship with functional capillary geometry and microhemodynamics. J Vasc Res. 1999;36(4):282–8. 10.1159/000025656 [DOI] [PubMed] [Google Scholar]
- 25. Lien DC, Wagner WW, Jr, Capen RL, et al. : Physiological neutrophil sequestration in the lung: visual evidence for localization in capillaries. J Appl Physiol (1985). 1987;62(3):1236–43. 10.1152/jappl.1987.62.3.1236 [DOI] [PubMed] [Google Scholar]
- 26. Hogg JC: Neutrophil kinetics and lung injury. Physiol Rev. 1987;67(4):1249–95. 10.1152/physrev.1987.67.4.1249 [DOI] [PubMed] [Google Scholar]
- 27. Summers C, Singh NR, White JF, et al. : Pulmonary retention of primed neutrophils: a novel protective host response, which is impaired in the acute respiratory distress syndrome. Thorax. 2014;69(7):623–9. 10.1136/thoraxjnl-2013-204742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ekpenyong AE, Toepfner N, Fiddler C, et al. : Mechanical deformation induces depolarization of neutrophils. Sci Adv. 2017;3(6):e1602536. 10.1126/sciadv.1602536 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 29. Vogt KL, Summers C, Chilvers ER, et al. : Priming and de-priming of neutrophil responses in vitro and in vivo. Eur J Clin Invest. 2018;48 Suppl 2:e12967. 10.1111/eci.12967 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 30. Lozano R, Naghavi M, Foreman K, et al. : Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–128. 10.1016/S0140-6736(12)61728-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Murray CJ, Lopez AD: Measuring the global burden of disease. N Engl J Med. 2013;369(5):448–57. 10.1056/NEJMra1201534 [DOI] [PubMed] [Google Scholar]
- 32. Decramer M, Janssens W, Miravitlles M: Chronic obstructive pulmonary disease. Lancet. 2012;379(9823):1341–51. 10.1016/S0140-6736(11)60968-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Løkke A, Lange P, Scharling H, et al. : Developing COPD: a 25 year follow up study of the general population. Thorax. 2006;61(11):935–9. 10.1136/thx.2006.062802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zeng G, Sun B, Zhong N: Non-smoking-related chronic obstructive pulmonary disease: a neglected entity? Respirology. 2012;17(6):908–12. 10.1111/j.1440-1843.2012.02152.x [DOI] [PubMed] [Google Scholar]
- 35. Lange P, Halpin DM, O’Donnell DE, et al. : Diagnosis, assessment, and phenotyping of COPD: beyond FEV 1. Int J Chron Obstruct Pulmon Dis. 2016;11 Spec Iss:3–12. 10.2147/COPD.S85976 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 36. Thompson AB, Daughton D, Robbins RA, et al. : Intraluminal airway inflammation in chronic bronchitis. Characterization and correlation with clinical parameters. Am Rev Respir Dis. 1989;140(6):1527–37. 10.1164/ajrccm/140.6.1527 [DOI] [PubMed] [Google Scholar]
- 37. Pilette C, Colinet B, Kiss R, et al. : Increased galectin-3 expression and intra-epithelial neutrophils in small airways in severe COPD. Eur Respir J. 2007;29(5):914–22. 10.1183/09031936.00073005 [DOI] [PubMed] [Google Scholar]
- 38. Donaldson GC, Seemungal TA, Patel IS, et al. : Airway and systemic inflammation and decline in lung function in patients with COPD. Chest. 2005;128(4):1995–2004. 10.1378/chest.128.4.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Parr DG, White AJ, Bayley DL, et al. : Inflammation in sputum relates to progression of disease in subjects with COPD: a prospective descriptive study. Respir Res. 2006;7:136. 10.1186/1465-9921-7-136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sapey E, Stockley JA, Greenwood H, et al. : Behavioral and structural differences in migrating peripheral neutrophils from patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2011;183(9):1176–86. 10.1164/rccm.201008-1285OC [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 41. Barnes PJ: Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2016;138(1):16–27. 10.1016/j.jaci.2016.05.011 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 42. Stănescu D, Sanna A, Veriter C, et al. : Airways obstruction, chronic expectoration, and rapid decline of FEV1 in smokers are associated with increased levels of sputum neutrophils. Thorax. 1996;51(3):267–71. 10.1136/thx.51.3.267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Donaldson GC, Wedzicha JA: COPD exacerbations .1: Epidemiology. Thorax. 2006;61(2):164–8. 10.1136/thx.2005.041806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fagon JY, Chastre J, Trouillet JL, et al. : Characterization of distal bronchial microflora during acute exacerbation of chronic bronchitis. Use of the protected specimen brush technique in 54 mechanically ventilated patients. Am Rev Respir Dis. 1990;142(5):1004–8. 10.1164/ajrccm/142.5.1004 [DOI] [PubMed] [Google Scholar]
- 45. Burnett D, Chamba A, Hill SL, et al. : Neutrophils from subjects with chronic obstructive lung disease show enhanced chemotaxis and extracellular proteolysis. Lancet. 1987;2(8567):1043–6. 10.1016/S0140-6736(87)91476-0 [DOI] [PubMed] [Google Scholar]
- 46. Yoshikawa T, Dent G, Ward J, et al. : Impaired neutrophil chemotaxis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175(5):473–9. 10.1164/rccm.200507-1152OC [DOI] [PubMed] [Google Scholar]
- 47. Sidhaye VK, Nishida K, Martinez FJ: Precision medicine in COPD: where are we and where do we need to go? Eur Respir Rev. 2018;27(149): pii: 180022. 10.1183/16000617.0022-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wenzel SE: Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012;18(5):716–25. 10.1038/nm.2678 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 49. Stockley J, Peat L, Walton G: The role of chemokine receptors in the aberrant migration of COPD neutrophils. Eur Respir J. 2014;44:P3859 Reference Source [Google Scholar]
- 50. Cepinskas G, Sandig M, Kvietys PR: PAF-induced elastase-dependent neutrophil transendothelial migration is associated with the mobilization of elastase to the neutrophil surface and localization to the migrating front. J Cell Sci. 1999;112(Pt 12):1937–45. [DOI] [PubMed] [Google Scholar]
- 51. Csernok E, Ernst M, Schmitt W, et al. : Activated neutrophils express proteinase 3 on their plasma membrane in vitro and in vivo. Clin Exp Immunol. 1994;95(2):244–50. 10.1111/j.1365-2249.1994.tb06518.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Owen CA, Campbell MA, Sannes PL, et al. : Cell surface-bound elastase and cathepsin G on human neutrophils: a novel, non-oxidative mechanism by which neutrophils focus and preserve catalytic activity of serine proteinases. J Cell Biol. 1995;131(3):775–89. 10.1083/jcb.131.3.775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Janoff A, Scherer J: Mediators of inflammation in leukocyte lysosomes. IX. Elastinolytic activity in granules of human polymorphonuclear leukocytes. J Exp Med. 1968;128(5):1137–55. 10.1084/jem.128.5.1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kafienah W, Buttle DJ, Burnett D, et al. : Cleavage of native type I collagen by human neutrophil elastase. Biochem J. 1998;330(Pt 2):897–902. 10.1042/bj3300897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Grinnell F, Zhu M: Identification of neutrophil elastase as the proteinase in burn wound fluid responsible for degradation of fibronectin. J Invest Dermatol. 1994;103(2):155–61. [DOI] [PubMed] [Google Scholar]
- 56. Liou TG, Campbell EJ: Nonisotropic enzyme--inhibitor interactions: a novel nonoxidative mechanism for quantum proteolysis by human neutrophils. Biochemistry. 1995;34(49):16171–7. 10.1021/bi00049a032 [DOI] [PubMed] [Google Scholar]
- 57. Vogelmeier C, Hubbard RC, Fells GA, et al. : Anti-neutrophil elastase defense of the normal human respiratory epithelial surface provided by the secretory leukoprotease inhibitor. J Clin Invest. 1991;87(2):482–8. 10.1172/JCI115021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Harpel PC: Alpha2-plasmin inhibitor and alpha2-macroglobulin-plasmin complexes in plasma. Quantitation by an enzyme-linked differential antibody immunosorbent assay. J Clin Invest. 1981;68(1):46–55. 10.1172/JCI110253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Crisford H, Sapey E, Stockley RA: Proteinase 3; a potential target in chronic obstructive pulmonary disease and other chronic inflammatory diseases. Respir Res. 2018;19(1):180. 10.1186/s12931-018-0883-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Campbell EJ, Campbell MA, Owen CA: Bioactive proteinase 3 on the cell surface of human neutrophils: quantification, catalytic activity, and susceptibility to inhibition. J Immunol. 2000;165(6):3366–74. 10.4049/jimmunol.165.6.3366 [DOI] [PubMed] [Google Scholar]
- 61. Sinden NJ, Baker MJ, Smith DJ, et al. : α-1-antitrypsin variants and the proteinase/antiproteinase imbalance in chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol. 2015;308(2):L179–90. 10.1152/ajplung.00179.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sinden NJ, Stockley RA: Proteinase 3 activity in sputum from subjects with alpha-1-antitrypsin deficiency and COPD. Eur Respir J. 2013;41(5):1042–50. 10.1183/09031936.00089712 [DOI] [PubMed] [Google Scholar]
- 63. Carter RI, Mumford RA, Treonze KM, et al. : The fibrinogen cleavage product Aα-Val360, a specific marker of neutrophil elastase activity in vivo. Thorax. 2011;66(8):686–91. 10.1136/thx.2010.154690 [DOI] [PubMed] [Google Scholar]
- 64. Crisford H, Newby PR, Sapey E, et al. : S15 Proteinase 3 activity in PiSZ alpha-1 antitrypsin deficiency and non-deficient chronic obstructive pulmonary disease. Thorax. 2018;73(Suppl 4):A10 Reference Source [Google Scholar]
- 65. Ravi AK, Plumb J, Gaskell R, et al. : COPD monocytes demonstrate impaired migratory ability. Respir Res. 2017;18(1):90. 10.1186/s12931-017-0569-y [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 66. Costa C, Traves SL, Tudhope SJ, et al. : Enhanced monocyte migration to CXCR3 and CCR5 chemokines in COPD. Eur Respir J. 2016;47(4):1093–102. 10.1183/13993003.01642-2015 [DOI] [PubMed] [Google Scholar]
- 67. Sapey E, Greenwood H, Walton G, et al. : Phosphoinositide 3-kinase inhibition restores neutrophil accuracy in the elderly: toward targeted treatments for immunosenescence. Blood. 2014;123(2):239–48. 10.1182/blood-2013-08-519520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tetley TD: New perspectives on basic mechanisms in lung disease. 6. Proteinase imbalance: its role in lung disease. Thorax. 1993;48(5):560–5. 10.1136/thx.48.5.560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Stockley RA: Neutrophils and protease/antiprotease imbalance. Am J Respir Crit Care Med. 1999;160(5 Pt 2):S49–52. 10.1164/ajrccm.160.supplement_1.13 [DOI] [PubMed] [Google Scholar]
- 70. Genschmer KR, Russell DW, Lal C, et al. : Activated PMN Exosomes: Pathogenic Entities Causing Matrix Destruction and Disease in the Lung. Cell. 2019;176(1–2):113–126.e15. 10.1016/j.cell.2018.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 71. Walton GM, Moore JS, Greenwood H, et al. : Neutrophils from Patients with Alpha 1 Anti-Trypsin Deficiency Show Evidence of Systemic Priming Which May Contribute to Tissue Damage. B101 ACUTE LUNG INJURY AND INFLAMMATION: American Thoracic Society. 2015;A3682 Reference Source [Google Scholar]
- 72. Fahy JV, Dickey BF: Airway mucus function and dysfunction. N Engl J Med. 2010;363(23):2233–47. 10.1056/NEJMra0910061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Leopold PL, O'Mahony MJ, Lian XJ, et al. : Smoking is associated with shortened airway cilia. PLoS One. 2009;4(12):e8157. 10.1371/journal.pone.0008157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Tamashiro E, Xiong G, Anselmo-Lima WT, et al. : Cigarette smoke exposure impairs respiratory epithelial ciliogenesis. Am J Rhinol Allergy. 2009;23(2):117–22. 10.2500/ajra.2009.23.3280 [DOI] [PubMed] [Google Scholar]
- 75. Sethi S, Murphy TF: Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008;359(22):2355–65. 10.1056/NEJMra0800353 [DOI] [PubMed] [Google Scholar]
- 76. Grabcanovic-Musija F, Obermayer A, Stoiber W, et al. : Neutrophil extracellular trap (NET) formation characterises stable and exacerbated COPD and correlates with airflow limitation. Respir Res. 2015;16:59. 10.1186/s12931-015-0221-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Obermayer A, Stoiber W, Krautgartner WD, et al. : New aspects on the structure of neutrophil extracellular traps from chronic obstructive pulmonary disease and in vitro generation. PLoS One. 2014;9(5):e97784. 10.1371/journal.pone.0097784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Dicker AJ, Crichton ML, Pumphrey EG, et al. : Neutrophil extracellular traps are associated with disease severity and microbiota diversity in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2018;141(1):117–27. 10.1016/j.jaci.2017.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 79. Pullan J, Greenwood H, Walton GM, et al. : Neutrophil extracellular traps (NETs) in COPD: A potential novel mechanism for host damage in acute exacerbations. Eur Respir J. 2015;46:PA5055 10.1183/13993003.congress-2015.PA5055 [DOI] [Google Scholar]
- 80. Hakkim A, Fürnrohr BG, Amann K, et al. : Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci U S A. 2010;107(21):9813–8. 10.1073/pnas.0909927107 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 81. Wrench C, Belchamber KBR, Bercusson A, et al. : Reduced Clearance of Fungal Spores by Chronic Obstructive Pulmonary Disease GM-CSF- and M-CSF-derived Macrophages. Am J Respir Cell Mol Biol. 2018;58(2):271–3. 10.1165/rcmb.2017-0351LE [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 82. Taylor AE, Finney-Hayward TK, Quint JK, et al. : Defective macrophage phagocytosis of bacteria in COPD. Eur Respir J. 2010;35(5):1039–47. 10.1183/09031936.00036709 [DOI] [PubMed] [Google Scholar]
- 83. Belchamber KB, Singh R, Wedzicha JA, et al. : Oxidative stress drives defective efferocytosis in COPD M2-like macrophages. Eur Respir J. 2014;44 Reference Source [Google Scholar]
- 84. Bewley MA, Belchamber KB, Chana KK, et al. : Differential Effects of p38, MAPK, PI3K or Rho Kinase Inhibitors on Bacterial Phagocytosis and Efferocytosis by Macrophages in COPD. PLoS One. 2016;11(9):e0163139. 10.1371/journal.pone.0163139 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 85. Venge P, Rak S, Steinholtz L, et al. : Neutrophil function in chronic bronchitis. Eur Respir J. 1991;4(5):536–43. [PubMed] [Google Scholar]
- 86. Müns G, Rubinstein I, Bergmann KC: Phagocytosis and oxidative burst of blood phagocytes in chronic obstructive airway disease. Scand J Infect Dis. 1995;27(4):369–73. 10.3109/00365549509032733 [DOI] [PubMed] [Google Scholar]
- 87. Tortorella C, Ottolenghi A, Moretti AM, et al. : Thymostimulin administration modulates polymorph metabolic pathway in patients with chronic obstructive pulmonary disease. Immunopharmacol Immunotoxicol. 1992;14(3):421–37. 10.3109/08923979209005402 [DOI] [PubMed] [Google Scholar]
- 88. Fietta A, Bersani C, De Rose V, et al. : Evaluation of systemic host defense mechanisms in chronic bronchitis. Respiration. 1988;53(1):37–43. 10.1159/000195394 [DOI] [PubMed] [Google Scholar]
- 89. Tosi MF, Zakem H, Berger M: Neutrophil elastase cleaves C3bi on opsonized pseudomonas as well as CR1 on neutrophils to create a functionally important opsonin receptor mismatch. J Clin Invest. 1990;86(1):300–8. 10.1172/JCI114699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Hartl D, Latzin P, Hordijk P, et al. : Cleavage of CXCR1 on neutrophils disables bacterial killing in cystic fibrosis lung disease. Nat Med. 2007;13(12):1423–30. 10.1038/nm1690 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 91. Carter RI, Ungurs MJ, Mumford RA, et al. : Aα-Val360: a marker of neutrophil elastase and COPD disease activity. Eur Respir J. 2013;41(1):31–8. 10.1183/09031936.00197411 [DOI] [PubMed] [Google Scholar]
- 92. Carter RI, Ungurs MJ, Pillai A, et al. : The Relationship of the Fibrinogen Cleavage Biomarker Aa-Val 360 With Disease Severity and Activity in α 1 -Antitrypsin Deficiency. Chest. 2015;148(2):382–8. 10.1378/chest.14-0520 [DOI] [PubMed] [Google Scholar]
- 93. Loi ALT, Hoonhorst S, van Aalst C, et al. : Proteomic profiling of peripheral blood neutrophils identifies two inflammatory phenotypes in stable COPD patients. Respir Res. 2017;18(1):100. 10.1186/s12931-017-0586-x [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 94. Tregay N, Begg M, Cahn A, et al. : Use of autologous 99mTechnetium-labelled neutrophils to quantify lung neutrophil clearance in COPD. Thorax. 2019; pii: thoraxjnl-2018-212509. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 95. Stoller JK, Aboussouan LS: A review of α1-antitrypsin deficiency. Am J Respir Crit Care Med. 2012;185(3):246–59. 10.1164/rccm.201108-1428CI [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 96. Stockley JA, Ismail AM, Hughes SM, et al. : Maximal mid-expiratory flow detects early lung disease in α1-antitrypsin deficiency. Eur Respir J. 2017;49(3): pii: 1602055. 10.1183/13993003.02055-2016 [DOI] [PubMed] [Google Scholar]
- 97. Stockley RA: Neutrophils and the Pathogenesis of COPD. Chest. 2002;121(5 Suppl):151S–155S. 10.1378/chest.121.5_suppl.151S [DOI] [PubMed] [Google Scholar]
- 98. Nakamura H, Yoshimura K, McElvaney NG, et al. : Neutrophil elastase in respiratory epithelial lining fluid of individuals with cystic fibrosis induces interleukin-8 gene expression in a human bronchial epithelial cell line. J Clin Invest. 1992;89(5):1478–84. 10.1172/JCI115738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Hubbard RC, Fells G, Gadek J, et al. : Neutrophil accumulation in the lung in alpha 1-antitrypsin deficiency. Spontaneous release of leukotriene B4 by alveolar macrophages. J Clin Invest. 1991;88(3):891–7. 10.1172/JCI115391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Stone H, McNab G, Wood AM, et al. : Variability of sputum inflammatory mediators in COPD and α1 -antitrypsin deficiency. Eur Respir J. 2012;40:561–9. 10.1183/09031936.00162811 [DOI] [PubMed] [Google Scholar]
- 101. Rouhani F, Paone G, Smith NK, et al. : Lung neutrophil burden correlates with increased pro-inflammatory cytokines and decreased lung function in individuals with alpha(1)-antitrypsin deficiency Chest. 2000;117(5 Suppl 1):250S–251S. 10.1378/chest.117.5_suppl_1.250S [DOI] [PubMed] [Google Scholar]
- 102. Hurley K, Lacey N, O'Dwyer CA, et al. : Alpha-1 antitrypsin augmentation therapy corrects accelerated neutrophil apoptosis in deficient individuals. J Immunol. 2014;193(8):3978–91. 10.4049/jimmunol.1400132 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 103. Petrache I, Fijalkowska I, Medler TR, et al. : alpha-1 antitrypsin inhibits caspase-3 activity, preventing lung endothelial cell apoptosis. Am J Pathol. 2006;169(4):1155–66. 10.2353/ajpath.2006.060058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Hornstein T, Lehmann S, Philipp D, et al. : Staurosporine resistance in inflammatory neutrophils is associated with the inhibition of caspase- and proteasome-mediated Mcl-1 degradation. J Leukoc Biol. 2016;99(1):163–74. 10.1189/jlb.3A1114-537RR [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 105. Serban KA, Petrusca DN, Mikosz A, et al. : Alpha-1 antitrypsin supplementation improves alveolar macrophages efferocytosis and phagocytosis following cigarette smoke exposure. PLoS One. 2017;12(4):e0176073. 10.1371/journal.pone.0176073 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 106. Woolhouse IS, Bayley DL, Stockley RA: Sputum chemotactic activity in chronic obstructive pulmonary disease: effect of α 1–antitrypsin deficiency and the role of leukotriene B 4 and interleukin 8. Thorax. 2002;57(8):709–14. 10.1136/thorax.57.8.709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Alfawaz B, Bergin DA, McElvaney NG, et al. : Alpha-1 antitrypsin regulates neutrophil reactive oxygen species production via inhibition of key players of the respiratory burst oxidase system. BMC Proc. 2013;7(Suppl 1): P7. 10.1186/1753-6561-7-S1-P7 [DOI] [Google Scholar]
- 108. Janciauskiene S, Tumpara S, Wiese M, et al. : Alpha1-antitrypsin binds hemin and prevents oxidative activation of human neutrophils: Putative pathophysiological significance. J Leukoc Biol. 2017;102(4):1127–41. 10.1189/jlb.3A0317-124R [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 109. Frenzel E, Korenbaum E, Hegermann J, et al. : Does augmentation with alpha1-antitrypsin affect neutrophil extracellular traps formation? Int J Biol Sci. 2012;8(7):1023–5. 10.7150/ijbs.4701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Parr DG, Stoel BC, Stolk J, et al. : Pattern of emphysema distribution in alpha1-antitrypsin deficiency influences lung function impairment. Am J Respir Crit Care Med. 2004;170(11):1172–8. 10.1164/rccm.200406-761OC [DOI] [PubMed] [Google Scholar]
- 111. Dowson LJ, Guest PJ, Hill SL, et al. : High-resolution computed tomography scanning in α 1-antitrypsin deficiency: relationship to lung function and health status. Eur Respir J. 2001;17(6):1097–104. 10.1183/09031936.01.00056501 [DOI] [PubMed] [Google Scholar]
- 112. Jones HA, Boobis AR, Hamacher K, et al. : PET imaging of pulmonary fibrosis. J Nucl Med. 2003;44(3):483–4; author reply 484. [PubMed] [Google Scholar]
- 113. Jones NA, Boswell-Smith V, Lever R, et al. : The effect of selective phosphodiesterase isoenzyme inhibition on neutrophil function in vitro. Pulm Pharmacol Ther. 2005;18(2):93–101. 10.1016/j.pupt.2004.10.001 [DOI] [PubMed] [Google Scholar]
- 114. Subramanian DR, Jenkins L, Edgar R, et al. : Assessment of pulmonary neutrophilic inflammation in emphysema by quantitative positron emission tomography. Am J Respir Crit Care Med. 2012;186(11):1125–32. 10.1164/rccm.201201-0051OC [DOI] [PubMed] [Google Scholar]
- 115. Stockley RA, Turner AM: α-1-Antitrypsin deficiency: clinical variability, assessment, and treatment. Trends Mol Med. 2014;20(2):105–15. 10.1016/j.molmed.2013.11.006 [DOI] [PubMed] [Google Scholar]
- 116. Watt AP, Brown V, Courtney J, et al. : Neutrophil apoptosis, proinflammatory mediators and cell counts in bronchiectasis. Thorax. 2004;59(3):231–6. 10.1136/thx.2003.008037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Russell DW, Gaggar A, Solomon GM: Neutrophil Fates in Bronchiectasis and Alpha-1 Antitrypsin Deficiency. Ann Am Thorac Soc. 2016;13 Suppl 2:S123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 118. Cole P: Host-microbe relationships in chronic respiratory infection. Respiration. 1989;55 Suppl 1:5–8. 10.1159/000195745 [DOI] [PubMed] [Google Scholar]
- 119. Gramegna A, Amati F, Terranova L, et al. : Neutrophil elastase in bronchiectasis. Respir Res. 2017;18(1):211. 10.1186/s12931-017-0691-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Bedi P, Davidson DJ, McHugh BJ, et al. : Blood Neutrophils Are Reprogrammed in Bronchiectasis. Am J Respir Crit Care Med. 2018;198(7):880–90. 10.1164/rccm.201712-2423OC [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 121. King PT, Hutchinson P, Holmes PW, et al. : Assessing immune function in adult bronchiectasis. Clin Exp Immunol. 2006;144(3):440–6. 10.1111/j.1365-2249.2006.03091.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Lloberes P, Montserrat E, Montserrat JM, et al. : Sputum sol phase proteins and elastase activity in patients with clinically stable bronchiectasis. Thorax. 1992;47(2):88–92. 10.1136/thx.47.2.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Boon M, Jorissen M, Proesmans M, et al. : Primary ciliary dyskinesia, an orphan disease. Eur J Pediatr. 2013;172(2):151–62. 10.1007/s00431-012-1785-6 [DOI] [PubMed] [Google Scholar]
- 124. Cockx M, Gouwy M, Godding V, et al. : Neutrophils from Patients with Primary Ciliary Dyskinesia Display Reduced Chemotaxis to CXCR2 Ligands. Front Immunol. 2017;8:1126. 10.3389/fimmu.2017.01126 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 125. Stockley RA, Shaw J, Hill SL, et al. : Neutrophil chemotaxis in bronchiectasis: a study of peripheral cells and lung secretions. Clin Sci (Lond). 1988;74(6):645–50. 10.1042/cs0740645 [DOI] [PubMed] [Google Scholar]
- 126. De Soyza A, Pavord I, Elborn JS, et al. : A randomised, placebo-controlled study of the CXCR2 antagonist AZD5069 in bronchiectasis. Eur Respir J. 2015;46(4):1021–32. 10.1183/13993003.00148-2015 [DOI] [PubMed] [Google Scholar]
- 127. Khoo JK, Venning V, Wong C, et al. : Bronchiectasis in the Last Five Years: New Developments. J Clin Med. 2016;5(12): pii: E115. 10.3390/jcm5120115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Snell N, Strachan D, Hubbard R, et al. : Burden of lung disease in the UK; findings from the British Lung Foundation's 'respiratory health of the nation' project. Eur Respir J. 2016;48:PA4913 10.1183/13993003.congress-2016.PA4913 [DOI] [Google Scholar]
- 129. Gray RD, Hardisty G, Regan KH, et al. : Delayed neutrophil apoptosis enhances NET formation in cystic fibrosis. Thorax. 2018;73(2):134–44. 10.1136/thoraxjnl-2017-210134 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 130. Houston N, Stewart N, Smith DS, et al. : Sputum neutrophils in cystic fibrosis patients display a reduced respiratory burst. J Cyst Fibros. 2013;12(4):352–62. 10.1016/j.jcf.2012.11.004 [DOI] [PubMed] [Google Scholar]
- 131. Pohl K, Hayes E, Keenan J, et al. : A neutrophil intrinsic impairment affecting Rab27a and degranulation in cystic fibrosis is corrected by CFTR potentiator therapy. Blood. 2014;124(7):999–1009. 10.1182/blood-2014-02-555268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Dibbert B, Weber M, Nikolaizik WH, et al. : Cytokine-mediated Bax deficiency and consequent delayed neutrophil apoptosis: a general mechanism to accumulate effector cells in inflammation. Proc Natl Acad Sci U S A. 1999;96(23):13330–5. 10.1073/pnas.96.23.13330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Moriceau S, Lenoir G, Witko-Sarsat V: In cystic fibrosis homozygotes and heterozygotes, neutrophil apoptosis is delayed and modulated by diamide or roscovitine: evidence for an innate neutrophil disturbance. J Innate Immun. 2010;2(3):260–6. 10.1159/000295791 [DOI] [PubMed] [Google Scholar]
- 134. Skopelja S, Hamilton BJ, Jones JD, et al. : The role for neutrophil extracellular traps in cystic fibrosis autoimmunity. JCI Insight. 2016;1(17):e88912. 10.1172/jci.insight.88912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Zhang S, Shrestha CL, Kopp BT: Cystic fibrosis transmembrane conductance regulator (CFTR) modulators have differential effects on cystic fibrosis macrophage function. Sci Rep. 2018;8(1): 17066. 10.1038/s41598-018-35151-7 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 136. Snelgrove RJ, Patel DF, Patel T, et al. : The enigmatic role of the neutrophil in asthma: Friend, foe or indifferent? Clin Exp Allergy. 2018;48(10):1275–85. 10.1111/cea.13191 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 137. Ciepiela O, Ostafin M, Demkow U: Neutrophils in asthma--a review. Respir Physiol Neurobiol. 2015;209:13–6. 10.1016/j.resp.2014.12.004 [DOI] [PubMed] [Google Scholar]
- 138. Gao H, Ying S, Dai Y: Pathological Roles of Neutrophil-Mediated Inflammation in Asthma and Its Potential for Therapy as a Target. J Immunol Res. 2017;2017: 3743048. 10.1155/2017/3743048 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 139. Mosca T, Menezes MC, Silva AV, et al. : Chemotactic and Phagocytic Activity of Blood Neutrophils in Allergic Asthma. Immunol Invest. 2015;44(5):509–20. 10.3109/08820139.2015.1041606 [DOI] [PubMed] [Google Scholar]
- 140. Uddin M, Nong G, Ward J, et al. : Prosurvival activity for airway neutrophils in severe asthma. Thorax. 2010;65(8):684–9. 10.1136/thx.2009.120741 [DOI] [PubMed] [Google Scholar]
- 141. Sackmann EK, Berthier E, Schwantes EA, et al. : Characterizing asthma from a drop of blood using neutrophil chemotaxis. Proc Natl Acad Sci U S A. 2014;111(16):5813–8. 10.1073/pnas.1324043111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Dworski R, Simon HU, Hoskins A, et al. : Eosinophil and neutrophil extracellular DNA traps in human allergic asthmatic airways. J Allergy Clin Immunol. 2011;127(5):1260–6. 10.1016/j.jaci.2010.12.1103 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 143. Qu J, Li Y, Zhong W, et al. : Recent developments in the role of reactive oxygen species in allergic asthma. J Thorac Dis. 2017;9(1):E32–E43. 10.21037/jtd.2017.01.05 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 144. Alexis NE, Eldridge MW, Peden DB: Effect of inhaled endotoxin on airway and circulating inflammatory cell phagocytosis and CD11b expression in atopic asthmatic subjects. J Allergy Clin Immunol. 2003;112(2):353–61. 10.1067/mai.2003.1651 [DOI] [PubMed] [Google Scholar]
- 145. Wood LG, Baines KJ, Fu J, et al. : The neutrophilic inflammatory phenotype is associated with systemic inflammation in asthma. Chest. 2012;142(1):86–93. 10.1378/chest.11-1838 [DOI] [PubMed] [Google Scholar]
- 146. Mackay F, Loetscher H, Stueber D, et al. : Tumor necrosis factor alpha (TNF-alpha)-induced cell adhesion to human endothelial cells is under dominant control of one TNF receptor type, TNF-R55. J Exp Med. 1993;177(5):1277–86. 10.1084/jem.177.5.1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Friedrichs B, Neumann U, Schüller J, et al. : Cigarette-smoke-induced priming of neutrophils from smokers and non-smokers for increased oxidative burst response is mediated by TNF-α. Toxicol In Vitro. 2014;28(7):1249–58. 10.1016/j.tiv.2014.06.007 [DOI] [PubMed] [Google Scholar]
- 148. Evrard M, Kwok IWH, Chong SZ, et al. : Developmental Analysis of Bone Marrow Neutrophils Reveals Populations Specialized in Expansion, Trafficking, and Effector Functions. Immunity. 2018;48(2):364–379.e8. 10.1016/j.immuni.2018.02.002 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 149. Beeh KM, Kornmann O, Buhl R, et al. : Neutrophil chemotactic activity of sputum from patients with COPD: role of interleukin 8 and leukotriene B 4. Chest. 2003;123(4):1240–7. 10.1378/chest.123.4.1240 [DOI] [PubMed] [Google Scholar]
- 150. Rennard SI, Dale DC, Donohue JF, et al. : CXCR2 Antagonist MK-7123. A Phase 2 Proof-of-Concept Trial for Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2015;191(9):1001–11. 10.1164/rccm.201405-0992OC [DOI] [PubMed] [Google Scholar]
- 151. Gompertz S, Stockley RA: A randomized, placebo-controlled trial of a leukotriene synthesis inhibitor in patients with COPD. Chest. 2002;122(1):289–94. 10.1378/chest.122.1.289 [DOI] [PubMed] [Google Scholar]
- 152. Konstan MW, Döring G, Heltshe SL, et al. : A randomized double blind, placebo controlled phase 2 trial of BIIL 284 BS (an LTB 4 receptor antagonist) for the treatment of lung disease in children and adults with cystic fibrosis. J Cyst Fibros. 2014;13(2):148–55. 10.1016/j.jcf.2013.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Hobbins S, Chapple IL, Sapey E, et al. : Is periodontitis a comorbidity of COPD or can associations be explained by shared risk factors/behaviors? Int J Chron Obstruct Pulmon Dis. 2017;12:1339–49. 10.2147/COPD.S127802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Zhou X, Han J, Liu Z, et al. : Effects of periodontal treatment on lung function and exacerbation frequency in patients with chronic obstructive pulmonary disease and chronic periodontitis: a 2-year pilot randomized controlled trial. J Clin Periodontol. 2014;41(6):564–72. 10.1111/jcpe.12247 [DOI] [PubMed] [Google Scholar]
- 155. Walton GM, Stockley JA, Griffiths D, et al. : Repurposing Treatments to Enhance Innate Immunity. Can Statins Improve Neutrophil Functions and Clinical Outcomes in COPD? J Clin Med. 2016;5(10): pii: E89. 10.3390/jcm5100089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Stephens L, Ellson C, Hawkins P: Roles of PI3Ks in leukocyte chemotaxis and phagocytosis. Curr Opin Cell Biol. 2002;14(2):203–13. 10.1016/S0955-0674(02)00311-3 [DOI] [PubMed] [Google Scholar]
- 157. Mansur A, Steinau M, Popov AF, et al. : Impact of statin therapy on mortality in patients with sepsis-associated acute respiratory distress syndrome (ARDS) depends on ARDS severity: a prospective observational cohort study. BMC Med. 2015;13:128. 10.1186/s12916-015-0368-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Stockley JA, Walton GM, Lord JM, et al. : Aberrant neutrophil functions in stable chronic obstructive pulmonary disease: the neutrophil as an immunotherapeutic target. Int Immunopharmacol. 2013;17(4):1211–7. 10.1016/j.intimp.2013.05.035 [DOI] [PubMed] [Google Scholar]
- 159. Sapey E, Patel JM, Greenwood HL, et al. : Pulmonary Infections in the Elderly Lead to Impaired Neutrophil Targeting, Which Is Improved by Simvastatin. Am J Respir Crit Care Med. 2017;196(10):1325–36. 10.1164/rccm.201704-0814OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Patel JM, Greenwood H, Walton G, et al. : Pre-emptive or early adjuvant simvastatin therapy in elderly patients with infection and sepsis. Lancet. 2014;383:S79 10.1016/S0140-6736(14)60342-1 [DOI] [Google Scholar]
- 161. Greenwood H, Patel J, Mahida R, et al. : Simvastatin to modify neutrophil function in older patients with septic pneumonia (SNOOPI): study protocol for a randomised placebo-controlled trial. Trials. 2014;15:332. 10.1186/1745-6215-15-332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Wang H, Anthony D, Yatmaz S, et al. : Aspirin-triggered resolvin D1 reduces pneumococcal lung infection and inflammation in a viral and bacterial coinfection pneumonia model. Clin Sci (Lond). 2017;131(18):2347–62. 10.1042/CS20171006 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 163. Falcone M, Russo A, Cangemi R, et al. : Lower mortality rate in elderly patients with community-onset pneumonia on treatment with aspirin. J Am Heart Assoc. 2015;4(1):e001595. 10.1161/JAHA.114.001595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Park DW, Jiang S, Tadie JM, et al. : Activation of AMPK enhances neutrophil chemotaxis and bacterial killing. Mol Med. 2013;19:387–98. 10.2119/molmed.2013.00065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Tian BP, Xia LX, Bao ZQ, et al. : Bcl-2 inhibitors reduce steroid-insensitive airway inflammation. J Allergy Clin Immunol. 2017;140(2):418–30. 10.1016/j.jaci.2016.11.027 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 166. Yu X, Seow HJ, Wang H, et al. : Matrine reduces cigarette smoke-induced airway neutrophilic inflammation by enhancing neutrophil apoptosis. Clin Sci (Lond). 2019;133(4):551–64. 10.1042/CS20180912 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 167. Vandivier RW, Henson PM, Douglas IS: Burying the dead: the impact of failed apoptotic cell removal (efferocytosis) on chronic inflammatory lung disease. Chest. 2006;129(6):1673–82. 10.1378/chest.129.6.1673 [DOI] [PubMed] [Google Scholar]
- 168. Simpson JL, Gibson PG, Yang IA, et al. : Impaired macrophage phagocytosis in non-eosinophilic asthma. Clin Exp Allergy. 2013;43(1):29–35. 10.1111/j.1365-2222.2012.04075.x [DOI] [PubMed] [Google Scholar]
- 169. Huynh ML, Malcolm KC, Kotaru C, et al. : Defective apoptotic cell phagocytosis attenuates prostaglandin E2 and 15-hydroxyeicosatetraenoic acid in severe asthma alveolar macrophages. Am J Respir Crit Care Med. 2005;172(8):972–9. 10.1164/rccm.200501-035OC [DOI] [PubMed] [Google Scholar]
- 170. Vandivier RW, Fadok VA, Hoffmann PR, et al. : Elastase-mediated phosphatidylserine receptor cleavage impairs apoptotic cell clearance in cystic fibrosis and bronchiectasis. J Clin Invest. 2002;109(5):661–70. 10.1172/JCI13572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Chatterjee A, Stockwell PA, Rodger EJ, et al. : Genome-wide DNA methylation map of human neutrophils reveals widespread inter-individual epigenetic variation. Sci Rep. 2015;5:17328. 10.1038/srep17328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Ecker S, Chen L, Pancaldi V, et al. : Genome-wide analysis of differential transcriptional and epigenetic variability across human immune cell types. Genome Biol. 2017;18(1):18. 10.1186/s13059-017-1156-8 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation