Abstract
Background
Ventricular sensing in transvenous cardiac implantable electronic devices (CIEDs) occurs conventionally from the right ventricular (RV) channel, though it evolved from epicardial sensing both in pacemakers and implantable cardioverter‐defibrillators (ICDs).
Hypothesis
The objective of this study was to observe the reliability of left ventricular (LV) sensing by transvenous leads placed in coronary veins.
Methods
LV leads were used for sensing and arrhythmia detection in clinical situations where placement of an RV lead across the tricuspid valve was either not preferred or not feasible, or RV signal was unsuitable for arrhythmia detection, or in the event of sensing failure of an RV lead under advisory in cardiac resynchronization therapy defibrillator (CRTD) recipients.
Results
Thirty‐seven patients had an IS‐1 LV lead connected to the RV port of CIEDs (17 pacemakers, 5 cardiac resynchronization therapy pacemaker [CRTP], 2 ICDs, and 13 CRTDs). Along a median 41 (25‐67) months follow‐up, lead performance remained stable; there were neither undersensing nor oversensing of non‐cardiac signals. VT/VF were correctly detected and terminated by ATP and shocks (one and three patients, respectively); no inappropriate arrhythmia detection. Device reprogramming occurred in four CRTD recipients because of transient counting the QRS (short intervals) when paced in LV‐only, and in two with T‐wave oversensing.
Conclusions
Ventricular sensing by an LV lead is feasible in transvenous devices. Sensing programmability is an unmet need: to fix RV lead sensing issues in cardiac resynchronization therapy (CRT) recipients at no risk of infection (no pocket opening); to avoid interaction with the tricuspid valve; to avoid lead redundancy in the vasculature. Moreover, it will be mandatory owing to the loss of lead interchangeability due to the adoption of DF‐4 and quadripolar leads.
Keywords: Arrhythmia detection, Cardiac stimulation, Left ventricular lead, Sensing, Tricuspid regurgitation
1. INTRODUCTION
Cardiac stimulation is conventionally based on endocardial right ventricular sensing owing to the easy accessibility to the right cardiac chambers via the transvenous route. However, it has historically evolved from epicardial stimulation in the pioneering phase of cardiac surgery1; this approach has maintained its applicability with comparable results to endocardial pacing in congenital heart disease patients, owing to the improvement in suture‐lead technology.2 Epicardial sensing was the source firstly used for the detection of ventricular arrhythmias3 also in the early stage of implantable cardioverter‐defibrillator (ICD) therapy. Epicardial sensing and pacing for bradyarrhythmias by leads placed into coronary veins via the customary transvenous route was reported to be safe and effective some years ago,4 and has been adopted also for arrhythmia detection in patients with failure of an ICD lead5 or for the management of functional ICD leads under advisory notice.6 We investigated the efficacy of sensing and arrhythmia detection by a coronary sinus lead over the long term in a sizeable cohort of patients.
2. METHODS
This is a prospective observational study gathering patients implanted along 15 years with a left ventricular (LV) lead working the sensing and pacing functionality of pacemakers and ICD/cardiac resynchronization therapy defibrillator (CRTDs). We investigated the reliability of ventricular sensing and arrhythmia detection by an LV lead placed in a coronary vein in a cohort of cardiac implantable electronic device (CIED) recipients (pacemaker, ICD, cardiac resynchronization therapy pacemaker [CRTP/D]) who had at least one of the following clinical conditions:
tricuspid mechanical valve; tricuspid regurgitation ≥ grade 2 at the time of pacemaker/CRTP implantation (Figure 1);
malfunction of an right ventricular (RV) lead in place >3 years with ≥ grade 2 tricuspid regurgitation;
RV lead instability due to severe tricuspid regurgitation with or without pulmonary hypertension;
RV sensing issues due to low signal amplitude (<2.5 mV) in ICD recipients with arrhythmogenic RV cardiomyopathy;
univentricular heart with ventricular septal defect;
sensing issues of an ICD lead or ICD lead under advisory notice at device end of life, with stable shock impedance since implantation;
left bundle branch block (LBBB) or EF between 35% and 49% in patients with a class 1 indication to pacing.
Figure 1.
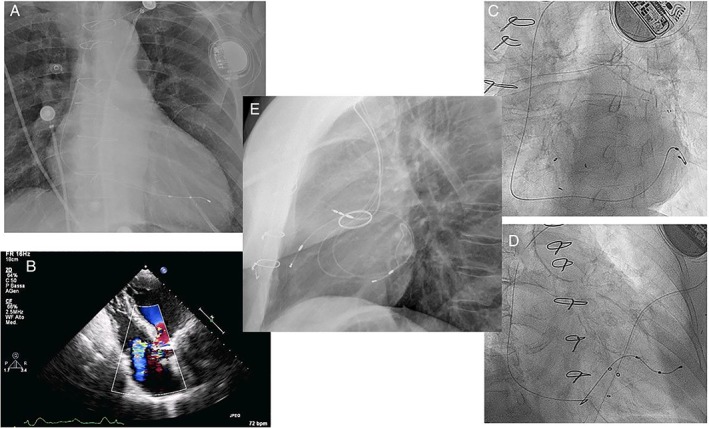
LV lead use in patients with ≥ grade 2 tricuspid regurgitation (panel B) and pacemaker (panels A,C,D) or CRTP (panel E) indication. Note the presence of a tricuspid biological valve (C,D) and of a mechanical aortic valve (E). The LV lead is placed in posterior or lateral coronary veins (A, C, D), whereas for CRTP an anterior vein is also targeted instead of the customary RV lead (E)
All the patients had an IS‐1 LV lead placed in a coronary vein, that was connected to the RV port of pacemakers, CRTP/Ds, ICDs. The opportunity to avoid a new lead insertion in CRTD recipients, the balance of worsening tricuspid regurgitation vs the risk of LV displacement, and the option of surgical placement when instability of the RV lead was proven, were discussed with each patient based on the individual situation, and an off‐label solution to minimize complications and infection risk was offered, according to the principles of our Hospital Ethic Committee. The study was approved by the Hospital Ethic Committee; informed consent was signed by each patient.
2.1. Follow‐up
RV sensing and pacing parameters were set to work with an LV lead, where phrenic nerve stimulation (PNS), double counting of the QRS in extended‐bipolar configurations (tip‐to‐coil), and T‐wave oversensing might be the major challenges. RV sensing was programmed by the shipment setting of automatically adjusting algorithms in ICD/CRTDs recipient first, and was later individualized according to sensing performance at follow‐up visits. The sensing configuration was tip‐to‐coil in two CRTD recipients with a unipolar LV lead, otherwise it was standard bipolar. In pacemaker recipients, sensing amplitude was set as 1/3 average sensing amplitude. RV pacing output was programmed with auto‐adaptive algorithms when the starting voltage of automated threshold measurement was at least 1 V lower than PNS threshold, otherwise it was programmed as threshold+1.5 V. Integrity and functionality of the LV lead were periodically checked during follow‐up (twice in the first year after surgery, than yearly), and via remote device monitoring where available. Battery performance, arrhythmia occurrence, appropriateness of arrhythmia detection and of tachyarrhythmia therapy delivery were collected. Phrenic stimulation was verified manually during follow‐up visits.
Adverse events such as lead dislodgments, infections, hospitalizations, and clinical events (cardiac, infectious, non‐cardiac, and non‐infectious) were collected to evaluate a possible relationship with the implanted device. The effect of pacing via the LV lead on ventricular volume and contractility, and on tricuspid valve function, was evaluated by echocardiography at baseline (first implant) or before lead switch (CRTD patients) and at last follow up visit. The inter and intraobserver measurements reproducibility of our Echocardiography Unit is reported.7
2.2. Statistical analysis
Continuous variables with non normal distribution were expressed as median value and first and third quartile. Categorical variables are reported as percentages. Diagnostic parameters were compared between groups by means of the Student's t test or the Wilcoxon‐Mann‐Whitney test as appropriate. All statistical tests were two sided, and deemed to be statistically significant if P < 0.05. Analyses were carried out using SAS 9.4 version software (SAS Institute Inc., Cary, NC, USA).
3. RESULTS
The population is described in Table 1. Pacemaker recipients had a class 1 indication due to symptomatic bradyarrhythmias; 8/17 had an LV lead placed because of a coexistent LBBB or LV systolic dysfunction, whereas 8 out of 17 received an LV lead to avoid interaction with the tricuspid valve (Figure 1A‐D, two with tricuspid bioprosthesis); a single patient had a univentricular heart with Eisenmenger syndrome and a VSD. Five CRTP recipients had two ventricular leads placed in coronary veins to avoid worsening of tricuspid regurgitation (one also had complete atrio‐ventricular block with a poorly functioning pre‐existent RV lead): one lead was placed anteriorly in the great cardiac vein and one posterolateral to ensure cardiac resynchronization therapy (CRT) (Figure 1E).
Table 1.
Patients characteristics and clinical follow‐up
| Pacemaker/CRTP; N = 22 (59%) | ICD/CRTD; N = 15 (41%) | All patients (N = 37) | |
|---|---|---|---|
| Male sex | 14 (64%) | 11 (73%) | 25 (68%) |
| AGE (years) | 66 [58‐81] | 72 [64‐78] | 72 [64‐79] |
| BMI (kg/m 2 ) | 25 [21.8‐26.6] | 25.2 [23.2‐26.6] | 25.3 [22.5‐26.8] |
| GFR (ml/min/1.73m 2 ) | 68.5 [45.5‐80.8] | 70 [54.5‐78.5] | 66 [46‐77] |
| Severe CKD (GFR < 30 mL/min) | 2 (9%) | 2 (12%) | 4 (11%) |
| Underlying heart disease | |||
| Ischemic | 4 (18%) | 2 (13%) | 6 (16%) |
| Dilatative | 3 (14%) | 11 (73%) | 14 (38%) |
| Hypertensive | 3 (14%) | 0 (0%) | 4 (11%) |
| Valvular | 2 (8%) | 1 (6%) | 3 (8%) |
| Other | 4 (18%) | 1 (6%) | 4 (11%) |
| None | 6 (27%) | 0 (0%) | 6 (16%) |
| Hypertension | 11 (50%) | 10 (67%) | 21 (57%) |
| Diabetes | 1 (4%) | 0 (0%) | 1 (3%) |
| Smoke | 2 (9%) | 1 (6%) | 3 (8%) |
| CRT indication | 5 (13%) | 13 (35%) | 18 (48%) |
| AVB in permanent AF | 3 (8%) | — | 3 (8%) |
| AVB second‐3rd in sinus rhythm | 8 (21%) | — | 8 (21%) |
| SSS + (AVB first or LBBB or CSH) | 7 (19%) (3/2/1) |
— | 7 (19%) |
| Clinical events at follow‐up | |||
| VT/VF | 1 (4%) | 3 (20%) | 4 (11%) |
| NSVT | 7 (32%) | 8 (53%) | 15 (40%) |
| Appropriate ATP and/or shock | NA | 3 (20%) | 3 (8%) |
| Inappropriate therapy delivery | NA | 0 (0%) | 0 (0%) |
| Infections | 0 (0%) | 1 (7%) | 1 (3%) |
| Hospitalization for HF | 4 (18%) | 1 (7%) | 5 (13%) |
| Hospitalization for VT recurrence | 0 (0%) | 1 (7%) | 1 (3%) |
| Hospitalization for non‐cardiac reasons | 7 (32%) | 2 (14%) | 9 (24%) |
| Death from cardiac causes | 1 (4%) | 0 (0%) | 1 (3%) |
Abbreviations: AF, atrial fibrillation; ATP, antitachycardia pacing; AVB, atrio‐ventricular block; BMI, body mass index; CKD, chronic kidney disease; CRT, cardiac resynchronization therapy; CSH, carotid sinus hypersensitivity; GFR, glomerular filtration rate; HF, heart failure; LBBB, left bundle branch block; NA, not applicable; NSVT, non‐sustained ventricular tachycardia (four beats up to 30 seconds or to detection cutoff time); SSS, sick sinus syndrome; VF, ventricular fibrillation; VT, ventricular tachycardia.
3.1. ICD/CRTDs
Fourteen of 15 CRTD/ICDs recipients had a primary prevention indication, one had monomorphic VT. Two patients with a single chamber ICD received an LV lead for sensing, respectively to overcome a tricuspid mechanical valve,8 and because of a very low (1.5 to 2 mV at nine different RV sites) signal amplitude at implantation in ARVC. Twelve of 13 CRTD patients had a previously implanted RV lead under advisory notice (11 Sprint Fidelis 6949, Medtronic Inc., Minneapolis, Minnesota; 2 Riata 1580, St Jude Medical, St Paul, Minnesota) with at least one inappropriate shock due to ring electrode failure (10 patients, Figure 2 panel A‐B), or had a prophylactic lead switch at the second replacement procedure (three patients, one with short V‐V intervals detected). Thus, LV lead switch occurred as the third CIED surgery in patients' life at a 93 months (62‐117) median time from the first implant. Three of 13 CRTDs were upgraded from an ICD and received an active fixation LV lead (Attain Stability 20 066, Medtronic Inc.) that was connected to the RV port. These 13 CRTD recipients had the old RV lead connected to the LV port of the new device (Figure 2 panel A), where the tip electrode could be used for biventricular stimulation in tip‐to‐coil or in tip‐to‐can/RV ring (this being the LV ring electrode) configuration (Figure 2 Panel B). The median follow‐up of CRTD recipients after the switch procedure was 43 months (27‐56).
Figure 2.
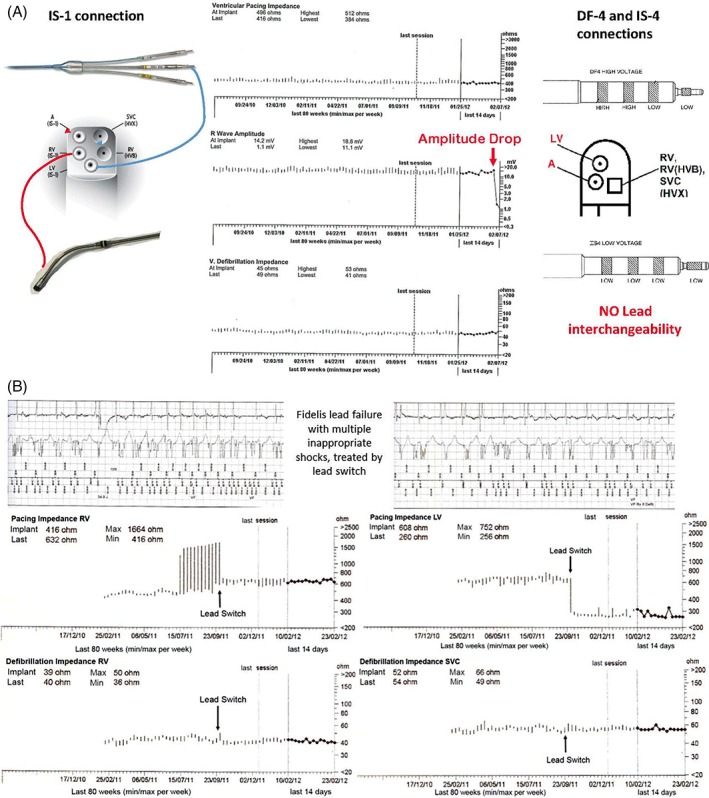
A, Prophylactic LV and fidelis RV lead switch in a CRTD (left side) due to drop of signal amplitude (central panel). Loss of lead interchangeability with DF‐4 and quadripolar LV lead connections would prevent such action (right side). B, Lead switch to treat failure of an RV lead under advisory notice in a CRTD patient. From top to bottom: Inappropriate shocks due to ring electrode fracture; trends of RV and LV lead impedance before and after lead switch, and of shocking coils impedance. Note the return to normal RV impedance after the LV lead was connected (the LV maintains a similar impedance regardless of the port whom it is connected to), and the drop of LV impedance once the RV lead was connected in tip to coil configuration (from out of range to the typical extend‐bipolar impedance). Shock impedance is up to now unchanged after 6 more years
3.2. Follow‐up with LV sensing in the overall population
During a median 41 months (25‐67) follow‐up, a single LV lead dislodgement occurred (2.7%) in a pacemaker recipient within 3 months from implantation, and was managed by lead repositioning. No other reoperation occurred because of LV lead‐related sensing issues or loss of capture. Clinical events along follow‐up were comparable to a general CIED population (Table 1), cardiac mortality being higher in pacemaker recipients and non‐cardiac events being the leading cause of hospitalization. The functionality of the LV lead connected to the RV port was stable, with only minor changes of pacing threshold and impedance, as observed with the aging of electrode/tissue interface (Table 2). A significant decrease of LV impedance working as an RV lead occurred due to reprogramming of pacing configuration from bipolar to unipolar in seven patients to lower the voltage output and avoid voltage multipliers (Table 2). There were no failures of the coil/s function in Fidelis/Riata lead patients until now (Tables 1 and 2, Figure 2B): three patients (8%) received appropriate and effective shock therapy (two with a malfunctioning Fidelis lead). In one pacemaker patient an asymptomatic self‐terminating VT <1 min was recorded. Reprogramming of pacing and sensing parameters were deemed necessary: 0.5 V increase of RV output (increased LV threshold between 1.5 and 2.5 V not compromising capture in five patients); prolongation of post‐paced ventricular blanking due transient QRS double‐counting in four CRTD recipients, two of which were RV‐paced only (ie, LV) by a unipolar LV lead (double counting occurring in extended‐bipolar sensing configuration); and use of T‐wave suppression algorithm in two who had transitory sensing of the T‐wave. There were no episodes of oversensing leading to arrhythmias misdetection, while ventricular arrhythmias were correctly detected in all patients (Table 1). Far‐field P‐wave oversensing could occur with a truly basal LV lead placement, but was not observed in three such patients in our series owing to a tip‐to‐ring sensing configuration.
Table 2.
Performance of LV leads connected to the RV port of CIEDS, and of RV leads connected to the LV port of CRTDs
| LV leads connected to the RV port | PM/CRTP (n = 22) | ICD/CRTD (n = 15) |
|---|---|---|
| LV threshold (V@0.4 ms) | ||
| Baseline | 0.75 [0.4‐0.8] | 1 [0.7‐1.5] |
| FU | 1.15 [0.8‐1.7] | 1.38 [1‐1.5] |
| LV signal amplitude (mV) | ||
| Baseline | 14.6 [10.7‐19.2] | 11.3 [7.1‐7.8] |
| FU | 10 [9.2‐15.7] | 15.25 [9.6‐18.1] |
| LV impedance (ohm) | ||
| Baseline | 815 [613‐966] | 627 [504‐815] |
| FU | 580 [468‐741] * | 665 [585‐798] |
| RV leads connected to the LV port (n = 13) | Not applicable | CRTD |
|---|---|---|
|
RV impedance (ohm)
Tip‐coil | ||
| At lead switch | 380 [350‐465] | |
| FU | 397 [365‐446] | |
| SVC coil impendance (ohm) | ||
| At lead switch | 59 [55‐62] | |
| FU | 60 [55‐61] | |
| RV coil impedance (ohm) | ||
| At lead switch | 46 [45‐47] | |
| FU | 46 [43‐48] | |
|
RV threshold (V@0.4 ms)
Tip‐coil | ||
| At lead switch | 1 [1‐1.3] | |
| FU | 1 [1‐1.3] | |
Abbreviations: FU, follow‐up; LV, left ventricular; RV, right ventricular; SVC, superior vena cava
P < 0.01.
Nine patients (24%) underwent replacement for battery end‐of‐life after 80 months (49‐105) median service life. Pocket infection with lead endocarditis occurred in a lady with a Riata lead after the fourth device surgery, 12 years from the first implant (6 years after lead switch). The effect of LV pacing in CRT patients and in pacemaker candidates are reported in Table 3: 10 CRTD patients had been treated for longer than 5 years on average, being super‐responders, that explains LV volume and EF; pacemaker recipients had unchanged ventricular volume compared to baseline, and no worsening of tricuspid and mitral function at follow‐up.
Table 3.
Echocardiographic follow‐up
| CRTP/D patients | Patients with ≥ 90% LVp | Patients with < 90% LVp | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N = 18 (49%) | N = 11 (30%) | N = 8 (21%) | |||||||
| Baseline | FU | P | Baseline | FU | P | Baseline | FU | p | |
| LV EDV (mL) | 118 [106‐211] | 115 [106‐135] | NS | 146 [125‐163] | 110[99‐130] | NS | 98 [78‐151] | 90 [71‐161] | NS |
| LV ESV (mL) | 58 [45‐131] | 56 [50‐64] | NS | 74 [70‐99] | 61 [46‐75] | NS | 35 [24‐89] | 32 [30‐95] | NS |
| LV EF (%) | 46 [31‐59] | 50 [43‐59] | NS | 47 [37‐49] | 53 [37‐56] | NS | 53 [38‐61] | 53 [32‐65] | NS |
| LA (mm) | 4.5 [3.9‐4.9] | 4.5 [4.1‐4.8] | NS | 4.6 [4.2‐5.2] | 4.1 [3.9‐5.5] | NS | 4.5 [4.1‐5] | 4.1 [3.8‐4.6] | NS |
| Patients with grade 3 or 4 MR | 3 | 1 | NS | 2 | 2 | NS | 3 | 1 | NS |
| Patients with grade 1 or 2 MR | 15 | 17 | NS | 9 | 9 | NS | 5 | 7 | NS |
| Patients with grade 3 or 4 TR | 2 | 1 | NS | 2 | 2 | NS | 2 | 1 | NS |
| Patients with grade 1 or 2 TR | 16 | 17 | NS | 9 | 9 | NS | 6 | 7 | NS |
| RV pressure (mmHg) | 25 [25‐35] | 25 [25‐30] | NS | 30 [25‐35] | 30 [25‐30] | NS | 25 [25‐30] | 30 [25‐30] | NS |
Abbreviations: EDV, end‐diastolic volume; EF, ejection fraction; ESV, end‐systolic volume; LA, left atrium; LV, left ventricular; MP, mitral regurgitation; NS, XXX; RV, right ventricular; TR, tricuspid regurgitation.
4. DISCUSSION
Ventricular sensing and arrhythmia detection by an LV lead placed in a coronary vein are reliable at long term in a mixed population of pacemaker and ICD recipients, where either RV lead failure was observed or anticipated (advisory notice), or lead placement across the tricuspid valve was not preferred because of tricuspid regurgitation/lead instability. The reliability of this setting has minor implications at this stage of medical practice, possibly limited only to the management of an RV lead failure, or of tricuspid function in a pacemaker candidate, but paves the way to new perspectives in device development for the future.
4.1. LV lead in the scenario of lead malfunction
RV lead functionality is the weakest point of CIED therapy at long term, with an estimated rate of serious issues around 40% over 10 years in ICD recipients.9 Lead advisory involved both ICD and pacemaker leads in the past 20 years, dictating re‐interventions for new lead addition and/or extraction of the malfunctioning lead in patients at risk of serious adverse events10, 11, 12; however, the risks associated to prophylactic lead extraction recommend a careful evaluation of the individual risk/benefit ratio,13 as it does not seem the safer approach in patients with leads in place for several years.14 We considered a risk‐minimization approach for 13 patients who had a CRTD in place or were going to be upgraded, where the LV lead could fix the sensing issue of the lead under advisory or malfunctioning (11/13). Indeed, this proved effective during follow‐up, the shock functionality still working as expected (Figure 2, Table 2). The priority to minimize infections recommends against multiple lead additions, that are associated to worse outcome,15 hence the lead switch procedure should be the first step of sensing issues management/advisory notice in CRTD recipients, as previously reported.5, 6 These latter case series did not report ICD therapy delivery after switching the leads,5, 6 while all patients in our study had reliable arrhythmia detection, and three had shock termination of life‐threatening VT/VF following lead switching. There is little evidence of the risk of progressive Fidelis failure leading to shock malfunction (Table 2), thus the best management strategy is uncertain; as highlighted by Burri, early lead extraction (< 2 years) can be the optimal strategy to minimize both procedure risks and hardware burden in the vasculature.16, 17 Indeed, worsening of the sensing functionality is the prominent aspect of ICD lead failure, rather than shocking coil malfunction, independently of an advisory notice status.9 In this perspective, our approach in CRTD recipients provides the best risk/benefit ratio, minimizing lead redundancy and procedure risk. Regretfully, LV lead switch demands pocket opening, that is linked to bacterial contamination and infection.18, 19 Multiple pocket openings dramatically increase infection risk20: this awareness demands that electronic re‐programming of the sensing channel and configuration in ICD/CRT devices becomes available in the next future to fix RV lead sensing issues while minimizing infections (for instance, use of any bipolar vector in RV leads with DF4 connections). This is especially true nowadays, where first‐choice are DF‐4 connection leads and multipolar LV leads, that blunt the flexibility to adapt at the changes of signal amplitude (related to disease progression or to a worsening lead‐tissue interface) by lead interchangeability (Figure 2A). This limitation was anticipated at DF‐4 introduction into the market.21
4.2. Hardware minimization and tricuspid valve function
Lead interaction with the tricuspid valve may seriously compromise its functionality, as progressively emerged with the expansion of CIED therapy.22 Though predictive factors are not precisely defined, pre‐existing valve abnormalities, burden of lead/s across the tricuspid valve, heart failure history seem associated to the development of clinically significant tricuspid regurgitation.23 In our pacemaker patients we did not observe significant changes of tricuspid function along follow‐up, owing to the absence of redundant hardware across the valve (Figure 1, Table 3). Beyond the physical interaction with valve leaflets, tricuspid function was unchanged owing to preservation of the LV volume and function irrespective of the extent of LV stimulation (Table 3). The effect of LV pacing on ventricular mechanics (Table 3) is indeed consistent with the concept that pacing the systemic ventricle helps to minimize the deleterious effect of a non‐physiologic ventricular activation, as learnt both in pediatric and adult patients with either normal hearts24 or systolic ventricular dysfunction.25
4.3. Challenges of LV lead use for sensing and arrhythmia detection
LV lead stability is key to enable programmability of any channel for sensing. Though a major concern in the past in the first months after implantation,26, 27 it is now comparable to transvenous RV leads owing to the quadripolar lead technology28, 29 and to the novel active fixation leads.30, 31 A practical approach to maximize patient safety in non‐active fixation lead recipients is to make the LV channel accessible for sensing programmability only after LV lead stabilization has been proven (for instance: coded unlock of LV sensing).
5. CONCLUSION
This is an observational study reporting the reliability of ventricular sensing via an LV lead placed in a coronary vein. At the stage of actual technology28, 29, 30, 31 LV leads performance is similar to transvenous RV leads, thus representing a trustful alternative to our customary practice. We believe that programmability of the sensing channel and configuration in CRTP/D should become available in the next future to minimize hardware redundancy in the vasculature,32 tricuspid valve dysfunction,33 and infections. This is especially required because of the loss of lead interchangeability due to the adoption of DF‐4 leads and quadripolar LV leads. While single chamber ICD candidates are already taking advantage of non‐transvenous devices32 and leadless technology,34 leadless CRT is still at an exploratory stage,35 thus CRT recipients can significantly benefit of this feature to reduce unnecessary and potentially dangerous lead‐related procedures. Future technologic developments should target the clinical needs of CIED recipients while helping to minimize complications, as for the case of the subcutaneous ICD.36
ACKNOWLEDGMENTS
No funding was received to support this study.
Conflicts of interest
No other potential conflict of interest exists.
Author contributions
Mauro Biffi has held educational activity and received speaker's bureau on behalf of Boston Scientific, Biotronik, and Medtronic. M. Ziacchi has held educational activity and received speaker's bureau on behalf of Medtronic.
Biffi M, de Zan G, Massaro G, et al. Is ventricular sensing always right, when it is left? Clin Cardiol. 2018;41:1238–1245. 10.1002/clc.23033
REFERENCES
- 1. Lillehei CW, Gott VL, Hodges PC Jr, Long DM, Bakken EE. Transistor pacemaker for treatment of complete atrioventricular dissociation. JAMA. 1960;172:2006‐2010. [DOI] [PubMed] [Google Scholar]
- 2. Kubus P, Materna O, Gebauer RA, et al. Permanent epicardial pacing in children: long‐term results and factors modifying outcome. Europace. 2012;14:509‐514. [DOI] [PubMed] [Google Scholar]
- 3. Mirowski M, Reid PR, Mower MM, et al. Termination of malignant ventricular arrhythmias with an implanted automatic defibrillator in human beings. N Engl J Med. 1980;303:322‐324. [DOI] [PubMed] [Google Scholar]
- 4. Biffi M, Bertini M, Ziacchi M, Boriani G. Left ventricular pacing by automatic capture verification. Europace. 2007;9:1177‐1181. [DOI] [PubMed] [Google Scholar]
- 5. Venkataraman G, Moore H, Karasik P, Singh S, Fletcher R. Biventricular implantable cardioverter defibrillator right ventricle pace‐sense ring electrode failure: lead switch fix. Pacing Clin Electrophysiol. 2008;31:899‐903. [DOI] [PubMed] [Google Scholar]
- 6. Zhu DWX, Chu MM, House CM. Management of functional Sprint fidelis leads at cardiac resynchronization therapy‐defibrillator generator replacement: a novel option for preventing inappropriate shocks from lead failure in fragile patients with high risk of sudden death. Europace. 2017;19:2007‐2014. [DOI] [PubMed] [Google Scholar]
- 7. Rocchi G, Bertini M, Biffi M, et al. Exercise stress echocardiography is superior to rest echocardiography in predicting left ventricular reverse remodelling and functional improvement after cardiac resynchronization therapy. Eur Heart J. 2009;30:89‐97. [DOI] [PubMed] [Google Scholar]
- 8. Biffi M, Bertini M, Ziacchi M, Boriani G. Transvenous cardioverter‐defibrillator implantation in a patient with tricuspid mechanical prosthesis. J Cardiovasc Electrophysiol. 2007;18:329‐331. [DOI] [PubMed] [Google Scholar]
- 9. Kleemann T, Becker T, Doenges K, et al. Annual rate of transvenous defibrillation lead defects in implantable cardioverter‐defibrillators over a period of >10 years. Circulation. 2007;115:2474‐2480. [DOI] [PubMed] [Google Scholar]
- 10. Gould PA, Krahn AD, Canadian Heart Rhythm Society Working Group on Device Advisories . Complications associated with implantable cardioverter‐defibrillator replacement in response to device advisories. JAMA. 2006;295:1907‐1911. [DOI] [PubMed] [Google Scholar]
- 11. Maytin M, Wilkoff BL, Brunner M, et al. Multicenter experience with extraction of the riata/riata ST ICD lead. Heart Rhythm. 2014;11:1613‐1618. [DOI] [PubMed] [Google Scholar]
- 12. Priori SG, Auricchio A, Nisam S, Yong P. To replace or not to replace: a systematic approach to respond to device advisories. J Cardiovasc Electrophysiol. 2009;20:164‐170. [DOI] [PubMed] [Google Scholar]
- 13. Auricchio A, Gropp M, Ludgate S, et al. European heart rhythm association guidance document on cardiac rhythm management product performance. Europace. 2006;8:313‐322. [DOI] [PubMed] [Google Scholar]
- 14. Parsonnet V, Roelke M, Bernstein AD, Stern M. Reduced frequency of retention wire fractures suggests that elective explantation of affected atrial leads is no longer indicated. Pacing Clin Electrophysiol. 2000;23:380‐383. [DOI] [PubMed] [Google Scholar]
- 15. Poole JE, Gleva MJ, Mela T, et al. REPLACE registry investigators. Complication rates associated with pacemaker or implantable cardioverter‐defibrillator generator replacements and upgrade procedures: results from the REPLACE registry. Circulation. 2010;122:1553‐1561. [DOI] [PubMed] [Google Scholar]
- 16. de Bie MK, Fouad DA, Borleffs CJ, et al. Trans‐venous lead removal without the use of extraction sheaths, results of >250 removal procedures. Europace. 2012;14:112‐116. [DOI] [PubMed] [Google Scholar]
- 17. Burri H, Combescure C. Management of recalled implantable cardioverter‐defibrillator leads at generator replacement: a decision analysis model for fidelis leads. Europace. 2014;16:1210‐1217. [DOI] [PubMed] [Google Scholar]
- 18. Kleemann T, Becker T, Strauss M, et al. Prevalence of bacterial colonization of generator pockets in implantable cardioverter defibrillator patients without signs of infection undergoing generator replacement or lead revision. Europace. 2010;12:58‐63. [DOI] [PubMed] [Google Scholar]
- 19. Mittal S, Shaw RE, Michel K, et al. Cardiac implantable electronic device infections: incidence, risk factors, and the effect of the AigisRx antibacterial envelope. Heart Rhythm. 2014;11:595‐601. [DOI] [PubMed] [Google Scholar]
- 20. Borleffs CJ, Thijssen J, de Bie MK, et al. Recurrent implantable cardioverter‐defibrillator replacement is associated with an increasing risk of pocket‐related complications. Pacing Clin Electrophysiol. 2010;33:1013‐1019. [DOI] [PubMed] [Google Scholar]
- 21. Sticherling C, Burri H. Introduction of new industry standards for cardiac implantable electronic devices: balancing benefits and unexpected risks. Europace. 2012;14:1081‐1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lin G, Nishimura RA, Connolly HM, Dearani JA, Sundt TM 3rd, Hayes DL. Severe symptomatic tricuspid valve regurgitation due to permanent pacemaker or implantable cardioverter‐defibrillator leads. J Am Coll Cardiol. 2005;45:1672‐1675. [DOI] [PubMed] [Google Scholar]
- 23. Chang JD, Manning WJ, Ebrille E, Zimetbaum PJ. Tricuspid valve dysfunction following pacemaker or cardioverter‐defibrillator implantation. J Am Coll Cardiol. 2017;69:2331‐2341. [DOI] [PubMed] [Google Scholar]
- 24. Janousek J, van Geldorp IE, Krupickova S, et al. Permanent cardiac pacing in children: choosing the optimal pacing site: a multicenter study. Circulation. 2013;127:613‐623. [DOI] [PubMed] [Google Scholar]
- 25. Hay I, Melenovsky V, Fetics BJ, et al. Short‐term effects of right‐left heart sequential cardiac resynchronization in patients with heart failure, chronic atrial fibrillation, and atrioventricular nodal block. Circulation. 2004;110:3404‐3410. [DOI] [PubMed] [Google Scholar]
- 26. van Rees JB, de Bie MK, Thijssen J, Borleffs CJ, Schalij MJ, van Erven L. Implantation‐related complications of implantable cardioverter‐defibrillators and cardiac resynchronization therapy devices: a systematic review of randomized clinical trials. J Am Coll Cardiol. 2011;58:995‐1000. [DOI] [PubMed] [Google Scholar]
- 27. Biffi M, Bertini M, Ziacchi M, Diemberger I, Martignani C, Boriani G. Left ventricular lead stabilization to retain cardiac resynchronization therapy at long term: when is it advisable? Europace. 2014;16:533‐540. [DOI] [PubMed] [Google Scholar]
- 28. Crossley GH, Biffi M, Johnson B, et al. Performance of a novel left ventricular lead with short bipolar spacing for cardiac resynchronization therapy: primary results of the attain Performa quadripolar left ventricular lead study. Heart Rhythm. 2015;12:751‐758. [DOI] [PubMed] [Google Scholar]
- 29. Leyva F, Zegard A, Qiu T, et al. Cardiac resynchronization therapy using quadripolar versus non‐quadripolar left ventricular leads programmed to biventricular pacing with single‐site left ventricular pacing: impact on survival and heart failure hospitalization. J Am Heart Assoc. 2017;6:e007026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yee R, Gadler F, Hussin A, et al. Novel active fixation mechanism permits precise placement of a left ventricular lead: early results from a multicenter clinical study. Heart Rhythm. 2014;11:1150‐1155. [DOI] [PubMed] [Google Scholar]
- 31. Keilegavlen H, Hovstad T, Færestrand S. Active fixation of a thin transvenous left‐ventricular lead by a side helix facilitates targeted and stable placement in cardiac resynchronization therapy. Europace. 2016;18:1235‐1240. [DOI] [PubMed] [Google Scholar]
- 32. Santini M, Di Fusco SA, Santini A, et al. Prevalence and predictor factors of severe venous obstruction after cardiovascular electronic device implantation. Europace. 2016;18:1220‐1226. [DOI] [PubMed] [Google Scholar]
- 33. Boersma L, Barr C, Knops R, et al. Implant and midterm outcomes of the subcutaneous implantable cardioverter‐defibrillator registry: the EFFORTLESS study. J Am Coll Cardiol. 2017;70:830‐841. [DOI] [PubMed] [Google Scholar]
- 34. Tjong FVY, Brouwer TM, Koop B, et al. Acute and 3‐month performance of a communicating leadless antitachycardia pacemaker and subcutaneous implantable defibrillator. J Am Coll Cardiol. 2017;3:1487‐1498. [DOI] [PubMed] [Google Scholar]
- 35. Reddy VY, Miller MA, Neuzil P, et al. Cardiac resynchronization therapy with wireless left ventricular endocardial pacing: the SELECT‐LV study. J Am Coll Cardiol. 2017;69:2119‐2129. [DOI] [PubMed] [Google Scholar]
- 36. Cappato R, Ali H. Sudden cardiac death: new approaches for implantable cardioverter‐defibrillators (ICDs). Int J Cardiol. 2017;237:38‐41. [DOI] [PubMed] [Google Scholar]


