Abstract
Background
Late gadolinium enhancement (LGE) assessed with cardiovascular magnetic resonance (CMR) correlates with ventricular arrhythmias and survival in patients with structural heart disease. Whether some LGE characteristics may specifically improve prediction of arrhythmic outcomes is unknown.
Hypothesis
We sought to evaluate scar characteristics assessed with CMR to predict implantable cardioverter‐defibrillator (ICD) interventions in dilated cardiomyopathy of different etiology.
Methods
96 consecutive patients evaluated with CMR received an ICD. Biventricular volumes, ejection fraction, and myocardial LGE were evaluated. LGE was defined as “complex” (Cx‐LGE) in presence of ≥1 of the following: ischemic pattern, involving ≥2 different coronary territories; epicardial pattern; global endocardial pattern; and presence of ≥2 different patterns. The primary endpoint was occurrence of any appropriate ICD intervention. A composite secondary endpoint of cardiovascular death, cardiac transplantation, or ventricular assist device implantation was also considered.
Results
During a median follow‐up of 75 months, 30 and 25 patients reached the primary and secondary endpoints, respectively. Cx‐LGE was correlated with a worse primary endpoint survival (log‐rank P < 0.001). Cx‐LGE and right ventricular end‐diastolic volume were independently associated with the primary endpoint (HR: 3.22, 95% CI: 1.56–6.65, P = 0.002; and HR: 1.06, 95% CI: 1.00–1.12, P = 0.045, respectively), but not with the secondary endpoint.
Conclusions
Cx‐LGE identified at CMR imaging seems promising as an independent and specific prognostic factor of ventricular arrhythmias requiring ICD therapy in dilated cardiomyopathy of different etiologies.
Keywords: cardiovascular magnetic resonance, ICD therapy, myocardial fibrosis, sudden cardiac death
1. INTRODUCTION
Sudden cardiac death (SCD) and malignant ventricular arrhythmias (VA) constitute a major cause of death in patients affected by dilated cardiomyopathy (DCM) of various etiologies.
The implantable cardioverter‐defibrillator (ICD) has been shown to be effective in detecting and treating such arrhythmias. Indeed, in various clinical settings, ICD patients have displayed marked reductions in mortality, ranging from 23% in the Sudden Cardiac Death in Heart Failure Trial (SCD‐HeFT)1 to 59% in the Multicenter Automatic Defibrillator Implantation Trial (MADIT‐I).2
However, SCD risk stratification is still suboptimal, particularly in primary prevention. In a MADIT‐II post hoc analysis, only 35% of the patients who received an ICD required antiarrhythmic therapy during a 3‐year follow‐up,3 and in multicenter randomized trials4 the number of implantations needed to prevent a major arrhythmic event has been seen to range from 3 to 11. In this category, candidacy for primary‐prevention ICD implantation is based essentially on etiology, left ventricular ejection fraction (LVEF), and New York Heart Association (NYHA) functional class.5
Concerning DCM of nonischemic etiology (NIDCM), the recently published results from the Danish Study to Assess the Efficacy of ICDs in Patients With Non‐ischemic Systolic Heart Failure on Mortality (DANISH) show that ICD implantation for primary prevention based solely on LVEF may not result in a clinical benefit on survival.6 Therefore, new powerful predictors of spontaneous arrhythmias are required to tailor indications for implantation; this would improve both treatment and cost‐effectiveness.
Pathophysiologic studies and clinical evidence support the reentrant nature of VA in structural heart disease. Several studies have shown that myocardial scarring, fibrosis, and bordering transition tissue make up most of the arrhythmogenic substrate in ischemic cardiomyopathy (IDCM)7, 8 and in >80% of NIDCM.
Cardiovascular magnetic resonance (CMR) by means of late gadolinium enhancement (LGE) imaging is currently considered the reference technique for detecting, quantifying, and describing myocardial fibrosis and displays high precision and reproducibility.9
The aim of our investigation was to verify the presence of CMR predictors of VA and hard clinical events in DCM patients receiving an ICD. In particular, we sought to investigate the prediction power and specificity of a subgroup of LGE patterns that might be associated with a high arrhythmic burden.
2. METHODS
We retrospectively considered all consecutive patients evaluated with CMR and subsequently scheduled for ICD implantation at our center between June 2006 and June 2010, whose clinical data had been previously collected in the SCARFEAR (cardiovaSCular mAgnetic Resonance predictors oF AppropriatE ImplAntable CardioverteR defibrillator therapy delivery) registry, active at our center since 2006. Inclusion criteria were: (1) age > 14 years; (2) class I or IIa indication for ICD implantation for primary or secondary prevention of SCD in ischemic (IDCM) and NIDCM, according to guidelines5; and (3) CMR comprehensive of LGE imaging performed ≤6 months before implantation.
Secondary‐prevention implantations were included to evaluate the independency between CMR data and clinical VA in predicting appropriate ICD therapy.
DCM was defined by means of echocardiographic examination as a left ventricular end‐diastolic volume (LVEDV‐echo) >75 mL/m2; depressed global LV function was defined as an LVEF (LVEF‐echo) <55%10. Etiology was considered nonischemic in absence of a history of myocardial infarction together with no or minimal coronary artery disease on coronary angiography (absence of stenosis >50% on epicardial vessels).
Hypertrophic cardiomyopathy, congenital heart disease, and arrhythmogenic right ventricular cardiomyopathy were excluded by means of echocardiography and anamnestic data.
Prospective data collection was approved by our hospital institutional review board, and all patients gave their written informed consent. All patients received a commercially available ICD device.
Antitachycardia programming for primary prevention was left to clinical judgment, and in general included a single therapeutic window with 1 burst and subsequent shocks, usually with a threshold of 188 beats per minute; in secondary prevention, a VT treatment window was generally programmed with a lower threshold below the minimal frequency of the clinical tachycardia(s).
All patients were followed up clinically and by device interrogation every 6 months. Programming modifications were allowed in accordance with clinical judgment. The following prespecified events were recorded: (1) appropriate antitachycardia ICD therapy, (2) ventricular assist device (VAD) implantation, (3) cardiac transplantation, and (4) death from any cause.
All detected and treated arrhythmias were reviewed by 2 experienced electrophysiologists, particularly to exclude inappropriate therapy administration.
The primary endpoint was the occurrence of a sustained VA requiring an ICD therapy (either antitachycardia pacing or direct current shock). The secondary endpoint was a composite of major adverse cardiac events (MACE) including death from any cause, heart transplantation, or VAD implantation.
2.1. CMR acquisition protocol
Magnetic resonance imaging examinations were performed on a 1.5 T clinical scanner (Avanto; Siemens, Erlangen, Germany) using a phased array receiver coil. The study protocol included the acquisition of steady‐state free precession cine images (typical sequence setup: echo time/repetition time, 1.6/3.2 ms; flip angle, 65°; pixel size, 2.4 × 1.4 mm; generalized autocalibrating partial parallel acquisition [GRAPPA] parallel imaging) in the standard long‐axis planes and in sequential short‐axis planes (slice thickness, 8 mm; gap, 2 mm) covering the entire ventricles from the atrioventricular ring to the apex. Starting from 10 minutes after intravenous injection of gadolinium‐DTPA (Magnevist; Bayer‐Schering, Berlin, Germany; 0.15 mmol/kg) or gadobutrol (Gadovist, Bayer‐Schering; 0.15 mmol/kg), acquisition of LGE images was performed, in planes matched to the orientation of the cine images. LGE images were acquired by means of a segmented inversion‐recovery fast gradient echo sequence (typical sequence setup: echo time/repetition time, 1.4/5.4 ms; flip angle, 10°; slice thickness, 8 mm; gap, 2 mm; typical spatial resolution, 2.2 × 1.5 mm; inversion times adjusted to null normal myocardium11). A minimum set including all short‐axis views was also acquired by means of a segmented phase‐sensitive inversion recovery technique.
2.2. Image postprocessing
Two blinded observers evaluated CMR images. The endocardial and epicardial borders of the myocardium were planimetered on the short‐axis cine images; ventricle volumes and ejection fraction were subsequently obtained by summing endocardial discs, corrected for atrioventricular valve systolic motion, as required by the dedicated software (Argus Viewer Syngo MR software; Siemens). Absolute values of ventricular volumes were preferred instead of the indexed ones, to represent more closely the critical mass to sustain ventricular reentry.12 The presence of pericardial effusion and ventricular aneurysm was also considered.
As previously described,13 myocardial late‐enhancement presence was determined on inversion‐recovery images and defined as the presence of areas of high signal intensity visually detected, in ≥2 perpendicular views; questionable cases were further discriminated by phase‐sensitive inversion recovery images analysis, determination of signal intensity of the region of interest in relation to remote normal nulled myocardium, or in‐plane matching with precontrast imaging, as required. In the case of disagreement, a third opinion was required.
In the case of LGE positivity, the pattern was described in accordance with previously published criteria,14 including subendocardial ischemic pattern, midwall distribution, epicardial distribution, right ventricular involvement, and global endocardial distribution. To reflect usual clinical practice, it was preemptively established that LGE analysis should not require specialized software.
We defined LGE as “complex” (Cx‐LGE) based on a few particular LGE settings that might represent a vulnerable substrate for VA occurrence. Specifically, we speculated that multifocality and a higher LGE burden, alone or variably combined, would represent the key characteristics of an arrhythmogenic substrate. Therefore, we included into this definition the patients showing ≥1 of the following LGE settings: ischemic pattern, involving ≥2 different coronary territories (subtype 1); epicardial distribution (subtype 2); global endocardial distribution (subtype 3); and coexistence of ≥2 different LGE patterns (subtype 4). Of note, any midwall distribution at the junctions between RV free walls and interventricular septum coexisting with midwall septal LGE was not considered sufficient to satisfy Cx‐LGE criteria. Typical short‐axis LGE subtypes are reported in the Supporting Information, Figure, in the online version of this article.
2.3. Statistical analysis
A retrospective analysis of prospectively collected data was performed by means of Stata software version 12.0 (StataCorp LP, College Station, TX).
Continuous data are shown as mean ± SD, and categorical data as absolute and relative frequencies. A χ2 test was used to compare categorical variables between groups, and the t test for unpaired data was used for continuous data. Primary‐ and secondary‐endpoint survival rates were evaluated by plotting Kaplan–Meier curves, and subgroup survival rates were compared by the log‐rank test. Continuous data were dichotomized as a function of their median values.
For each variable deemed clinically relevant, a univariate hazard ratio (HR) with a 95% confidence interval (CI) was calculated by Cox regression method. After checking for collinearity, significant univariate predictors were included in a multivariable Cox regression model. The reference for continuous variables was 10 units.
Any 2‐tailed P value <0.05 was considered statistically significant.
3. RESULTS
A total of 96 patients were enrolled. Baseline clinical characteristics are reported in Table 1.
Table 1.
Baseline population characteristics
| Overall (N = 96) | |
|---|---|
| Age, y, mean (min–max) | 59 (30–83) |
| Male sex | 75 (78.1) |
| Ischemic etiology | 53 (55.2) |
| LBBB | 49 (51.0) |
| Permanent AF | 10 (10.4) |
| LVEF, % | 27.7 ± 5.1 |
| MR grade (echo) | |
| Absent/trivial | 30 (31.3) |
| Mild | 34 (35.4) |
| Moderate | 24 (25.0) |
| Severe | 8 (8.3) |
| NYHA class | |
| I | 8 (8.3) |
| II | 28 (29.2) |
| III | 54 (56.3) |
| IV | 6 (6.2) |
| Secondary prevention | 12 (12.5) |
| Implanted ICD device | |
| Single chamber | 51 (53.1) |
| Medtronic Virtuoso VR | 16 |
| Medtronic Virtuoso II VR | 13 |
| Medtronic Maximo | 9 |
| Medtronic Onyx | 7 |
| Sorin Ovatio | 3 |
| Sorin Paradym | 3 |
| Dual chamber | 2 (2.1) |
| Medtronic Virtuoso DR | 2 |
| CRT | 43 (44.8) |
| Medtronic Concerto | 20 |
| Medtronic Consulta | 18 |
| Medtronic Insync Maximo | 2 |
| Medtronic Insync Sentry | 2 |
| Medtronic Protecta | 1 |
Abbreviations: AF, atrial fibrillation; CRT, cardiac resynchronization therapy; echo, echocardiography; ICD, implantable cardioverter‐defibrillator; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; max, maximum; min, minimum; MR, mitral regurgitation; NYHA New York Heart Association; SD, standard deviation.
Data are presented as n (%) or mean ± SD unless otherwise noted.
Clinical indications for CMR were evaluation of etiology of DCM in 48 patients, assessment of viability in 41, exclusion of arrhythmogenic cardiomyopathy in 3, suspected endoventricular thrombus on echocardiography in 2, and stress MR and suspected infiltrative cardiomyopathy in 1 patient each.
LGE was highly prevalent in the population studied (96.2% if etiology was ischemic, and 76.7% in nonischemic DCM). Cx‐LGE was present in 21 ischemic DCM patients (39.6%) and in 11 nonischemic DCM patients (25.6%). LGE characteristics and the distribution of Cx‐LGE subtypes are shown in Table 2.
Table 2.
Characteristics of LGE by etiology
| Ischemic Etiology, n = 53 | Nonischemic Etiology, n = 43 | |
|---|---|---|
| LGE positive | 51 (95.7) | 38 (77.6) |
| Ischemic pattern | 48 (90.5) | 1 (2.3) |
| Nonischemic pattern | 3 (5.7) | 31 (81.6) |
| Cx‐LGE | 21 (39.6) | 11 (25.6) |
| Subtype 1a | 16 (34.0) | 0 (0.0) |
| Subtype 2b | 0 (0.0) | 6 (11.3) |
| Subtype 3c | 0 (0.0) | 4 (7.5) |
| Subtype 4d | 5 (9.4) | 3 (7.0) |
Abbreviations: Cx‐LGE, complex late gadolinium enhancement; LGE, late gadolinium enhancement.
Data are presented as n (%).
≥1 pattern can coexist in the same patient; patterns are calculated for LGE‐positive patients and Cx‐LGE for overall patients.
Subtype 1: Ischemic pattern, involving ≥2 different coronary territories.
Subtype 2: Epicardial distribution.
Subtype 3: Global endocardial distribution.
Subtype 4: Coexistence of ≥2 different patterns.
The median ICD follow‐up was 75 months (interquartile range, 34–86 months).
3.1. Primary endpoint
During the observation period, the primary endpoint was reached in 30 patients (31.3%). On the basis of detection criteria and arrhythmia cycle length, these were automatically classified by the device as ventricular fibrillation in 15 cases and ventricular tachycardia in 15. Initial therapy was a direct current shock in 8 cases and antitachycardia pacing in 22.
Baseline characteristics were similar in patients experiencing ≥1 ICD intervention, compared with event‐free subjects (Table 3), including the proportion of implantations for secondary prevention (12.5% vs 13.3%; P = 0.865).
Table 3.
Baseline clinical and CMR characteristics with respect to endpoints
| Overall, N = 96 | Sustained VA Requiring ICD Therapy | MACE | |||||
|---|---|---|---|---|---|---|---|
| ICD Rx, n = 30 | No ICD Rx, n = 66 | P Value | MACE, n = 25 | No MACE, n = 71 | P Value | ||
| Age, y (min–max) | 59 (30–83) | 60 (51–68) | 60 (51–68) | 0.414 | 58 (45–69) | 59 (51–67) | 0.351 |
| Male sex | 74 (77.1) | 24 (80.0) | 50 (76.0) | 0.844 | 20 (80.0) | 54 (76.0) | 0.899 |
| NYHA class III/IV | 60 (62.5) | 16 (53.3) | 36 (54.5) | 0.104 | 18 (72.0) | 42 (59.1) | 0.367 |
| Secondary prevention | 12 (12.5) | 4 (13.3) | 8 (12.0) | 0.867 | 5 (20.0) | 7 (9.9) | 0.333 |
| LVEF–echo (%) | 27.7 ± 5.1 | 28.1 ± 5.0 | 27.4 ± 5.2 | 0.541 | 26.7 ± 5.4 | 25.0 ± 5.0 | 0.270 |
| MR grade 3–4 | 32 (33.3) | 8 (26.6) | 24 (36.0) | 0.484 | 13 (52.0) | 19 (26.7) | 0.039 |
| LVEDV‐CMR, mL | 278 ± 88 | 279 ± 85 | 277 ± 90 | 0.942 | 318 ± 114 | 264 ± 72 | 0.007 |
| LVESV‐CMR, mL | 207 ± 76 | 204 ± 76 | 208 ± 76 | 0.846 | 244 ± 96 | 194 ± 63 | 0.04 |
| LVEF‐CMR, % | 26.7 ± 7.6 | 28.1 ± 8.4 | 26.1 ± 7.2 | 0.224 | 24.7 ± 8.4 | 27.4 ± 7.3 | 0.126 |
| RVEDV‐CMR, mL | 127 ± 54 | 135 ± 61 | 124 ± 50 | 0.333 | 152 ± 74 | 119 ± 41 | 0.007 |
| RVESV‐CMR, mL | 67 ± 50 | 66 ± 47 | 69 ± 55 | 0.782 | 95 ± 67 | 58 ± 37 | 0.001 |
| RVEF‐CMR, % | 51.2 ± 16.1 | 50.6 ± 29.3 | 52.4 ± 18.8 | 0.622 | 41.7 ± 18.3 | 54.6 ± 13.9 | <0.001 |
| LGE positivity | 84 (87.5) | 29 (96.7) | 55 (83.3) | 0.151 | 24 (96.0) | 60 (84.5) | 0.253 |
| Cx‐LGE | 32 (33.3) | 17 (56.7) | 15 (22.7) | 0.002 | 8 (32.0) | 24 (33.8) | 0.935 |
Abbreviations: CMR, cardiovascular magnetic resonance; Cx‐LGE, complex late gadolinium enhancement; echo, echocardiography; ICD, implantable cardioverter‐defibrillator; LGE, late gadolinium enhancement; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end‐systolic volume; MACE, major adverse cardiac events; max, maximum; min, minimum; MR, mitral regurgitation; NYHA, New York Heart Association; RVEDV, right ventricular end‐diastolic volume; RVEF, right ventricular ejection fraction; RVESV, right ventricular end‐systolic volume; Rx, prescription; SD, standard deviation; VA, ventricular arrhythmia.
Data are expressed as n (%) or mean ± SD, unless otherwise noted.
All but 1 of the subjects who received an appropriate ICD therapy had LGE, as well as the majority of patients who received no therapies (96.7% vs 83.3%; P = 0.151). Cx‐LGE was the only CMR characteristic that was significantly associated with the primary endpoint (56.7% vs 22.7%; P = 0.002). In the subgroup of patients featuring a primarily ischemic LGE (n = 46 [47.9%]), the incidence of the primary endpoint was higher in the Cx‐LGE group, although not statistically significant (28.6% vs 50%; P = 0.21).
Patients with a right ventricular end‐diastolic volume measured with CMR (RVEDV‐CMR) higher than the median value or Cx‐LGE showed a lower primary endpoint survival (Figure A,B), though statistical significance was reached only for Cx‐LGE (P < 0.001).
The results of the Cox univariate and multivariate analyses are reported in Table 4. Only Cx‐LGE and RVEDV‐CMR proved to be independently associated with the primary endpoint (HR: 3.22, 95% CI: 1.56–6.65, P = 0.002; and HR: 1.06, 95% CI: 1.00–1.12, P = 0.045, respectively).
Table 4.
Univariate and multivariate analyses for the primary endpoint
| Sustained VA Requiring ICD Therapy | ||||||
|---|---|---|---|---|---|---|
| Univariate | Multivariate | |||||
| Variables | HR | 95% CI | P Value | HR | 95% CI | P Value |
| Sex | 1.43 | 0.59–3.50 | 0.425 | — | — | — |
| NYHA class III/IV | 0.68 | 0.33–1.39 | 0.293 | — | — | — |
| Secondary prevention | 1.22 | 0.43–3.49 | 0.711 | — | — | — |
| AF | 0.65 | 0.16–2.70 | 0.553 | — | — | — |
| LBBB | 0.46 | 0.22–0.97 | 0.041 | 0.68 | 0.31–1.52 | 0.348 |
| MR grade 3–4 | 1.00 | 0.45–2.23 | 1.00 | — | — | — |
| LVEF‐CMR (10% step) | 1.04 | 0.65–1.67 | 0.862 | — | — | — |
| LVEDV‐CMR (10‐mL step) | 1.03 | 0.98–1.08 | 0.216 | — | — | — |
| RVEF‐CMR (10% step) | 0.90 | 0.71–1.13 | 0.365 | — | — | — |
| RVEDV‐CMR (10‐mL step) | 1.07 | 1.01–1.14 | 0.034 | 1.06 | 1.00–1.12 | 0.045 |
| LV aneurysm | 1.02 | 0.46–2.29 | 0.956 | — | — | — |
| LGE positivity | 5.79 | 0.80–42.14 | 0.084 | — | — | — |
| Ischemic LGE | 1.18 | 0.58–2.43 | 0.651 | — | — | — |
| Cx‐LGE | 3.27 | 1.59–6.74 | 0.001 | 3.22 | 1.56–6.65 | 0.002 |
Abbreviations: AF, atrial fibrillation; CI, confidence interval; CMR, cardiovascular magnetic resonance; Cx‐LGE, complex late gadolinium enhancement; HR, hazard ratio; ICD, implantable cardioverter‐defibrillator; LBBB, left bundle branch block; LGE, late gadolinium enhancement; LV, left ventricular; LVEDV‐CMR, left ventricular end‐diastolic volume measured with CMR; LVEF‐CMR, left ventricular ejection fraction measured with CMR; MR, mitral regurgitation; NYHA, New York Heart Association; RVEDV‐CMR, right ventricular end‐diastolic volume measured with CMR; RVEF‐CMR, right ventricular ejection fraction measured with CMR; VA, ventricular arrhythmia.
3.2. Secondary endpoint
During the observation period, 25 patients (26.0%) experienced a MACE. Fourteen patients died, 8 underwent heart transplantation, and 3 received a left ventricular assist device (LVAD).
Baseline clinical and echocardiographic characteristics were similar in the MACE and event‐free groups, except for the presence of a higher grade of mitral regurgitation in the former. Conversely, CMR‐derived ventricular volumes and ejection fractions differed significantly, with the MACE group showing more severe dilation of both ventricles and a lower RVEF (Table 3). In terms of LGE presence or complexity, no differences were observed.
Table 5 shows the results of univariate and multivariate predictivity analyses with regard to the secondary endpoint. CMR‐derived diastolic ventricular volumes and RVEF were confirmed to be associated with a higher MACE hazard on univariate analysis; on multivariate analysis, however, only LVEDV‐CMR and RVEF‐CMR remained statistically significant (HR: 1.05, 95% CI: 1.00–1.11 per 10‐mL volume increment, P = 0.05; and HR: 0.69, 95% CI: 0.50–0.96 per 10% ejection fraction decrement, P = 0.027, respectively). Cx‐LGE or RVEDV‐CMR did not engender a higher risk of MACE.
Table 5.
Univariate and multivariate analyses for the secondary endpoint
| MACE | ||||||
|---|---|---|---|---|---|---|
| Univariate | Multivariate | |||||
| Variables | HR | 95% CI | P Value | HR | 95% CI | P Value |
| Male sex | 1.33 | 0.50–3.52 | 0.571 | — | — | — |
| NYHA class III/IV | 1.53 | 0.64–3.66 | 0.337 | — | — | — |
| Secondary prevention | 2.02 | 0.76–5.37 | 0.162 | — | — | — |
| AF | 1.25 | 0.38–4.15 | 0.719 | — | — | — |
| LBBB | 0.91 | 0.42–1.99 | 0.816 | — | — | — |
| MR grade 3–4 | 2.80 | 1.28–6.14 | 0.010 | 1.72 | 0.75–3.93 | 0.204 |
| LVEF‐CMR (10% step) | 0.59 | 0.33–1.04 | 0.068 | — | — | — |
| LVEDV‐CMR (10‐mL step) | 1.08 | 1.03–1.13 | 0.001 | 1.05 | 1.00–1.11 | 0.050 |
| RVEF‐CMR (10% step) | 0.63 | 0.50–0.80 | <0.001 | 0.69 | 0.50–0.96 | 0.027 |
| RVEDV‐CMR (10‐mL step) | 1.10 | 1.04–1.16 | <0.001 | 1.01 | 0.92–1.10 | 0.903 |
| LGE positivity | 4.46 | 0.61–32.73 | 0.143 | — | — | — |
| Ischemic LGE | 1.20 | 0.54–2.63 | 0.659 | — | — | — |
| Cx‐LGE | 1.09 | 0.47–2.52 | 0.846 | — | — | — |
Abbreviations: AF, atrial fibrillation; CI, confidence interval; CMR, cardiovascular magnetic resonance; Cx‐LGE, complex late gadolinium enhancement; HR, hazard ratio; LBBB, left bundle branch block; LGE, late gadolinium enhancement; LVEDV‐CMR, left ventricular end‐diastolic volume measured with CMR; LVEF‐CMR, left ventricular ejection fraction measured with CMR; MACE, major adverse cardiac events; MR, mitral regurgitation; NYHA, New York Heart Association; RVEDV‐CMR, right ventricular end‐diastolic volume measured with CMR; RVEF‐CMR, right ventricular ejection fraction measured with CMR.
Significant Kaplan–Meier survival estimates of secondary endpoint–free survival are reported in the Figure (C,D). A baseline RVEF‐CMR below the median value of the population showed a significant worse survival (P < 0.001), whereas the group with LVEDV‐CMR above the median value showed a nonsignificant worse survival (P = 0.231). Survival with respect to Cx‐LGE was similar (P = 0.846).
Figure 1.
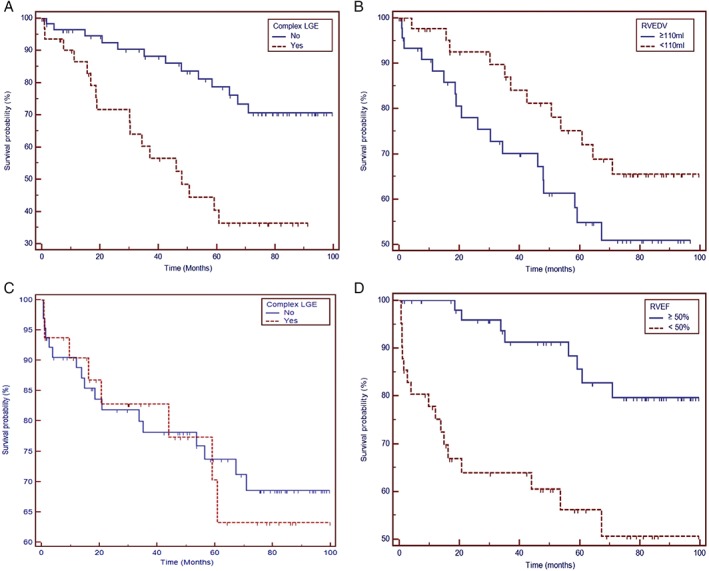
(A) Kaplan–Meier survival estimates of primary endpoint–free survival in relation to Cx‐LGE. (B) Survival curves from primary endpoint in relation to RVEDV‐CMR. (C) Secondary endpoint–free survival in relation to Cx‐LGE. (D) Secondary endpoint–free survival in relation to RVEF‐CMR. Abbreviations: CMR, cardiovascular magnetic resonance; Cx‐LGE, complex late gadolinium enhancement; RVEDV‐CMR, right ventricular end‐diastolic volume measured with CMR; RVEF, right ventricular ejection fraction
4. DISCUSSION
The main finding of our study is that some LGE settings, which we grouped under the definition of Cx‐LGE, seem to be independently associated with ICD therapy in DCM patients of any etiology. The second main finding is that Cx‐LGE seems to have no predictive power with regard to clinical endpoints other than ICD therapies. It follows that Cx‐LGE may be promising as a pure arrhythmic prognostic indicator.
Because the presence of ischemic LGE in IDCM is the rule, studies investigating IDCM patients tried to discriminate high‐risk patients by means of infarct tissue quantification15 and heterogeneity, initially described as a “gray area” of LGE,16 by means of postprocessing with specialized software.
Concerning NIDCM, the majority of studies considered the presence of LGE as a potential marker of adverse clinical and arrhythmic outcome17; this evidence is of outmost importance to suggest the use of LGE to assess prognosis in DCM.
Nevertheless, it is our opinion that a crucial question remained unresolved: whether delayed‐enhancement CMR imaging is helpful in predicting specifically ventricular arrhythmic events, whatever the general prognosis of the patient.
Our results demonstrate that a more detailed LGE classification seems to have this potentiality. We decided to consider etiologic and pathophysiologic criteria to define Cx‐LGE; indeed, we speculated that LGE distribution, multifocality, and a higher LGE burden, alone or variably combined, would represent the key characteristics of an arrhythmogenic substrate.
In this view, Cx‐LGE subtypes can be explained as follows: (1) subtype 1 and 4 might represent LGE of a significant extension, multifocal, and/or with more extended border tissue (ie, “gray area”); (2) subtype 2 is mainly associated with healed or chronic myocarditis, which is frequently characterized by random and multifocal fibrosis, as described in pathological specimens,18 and other rare diseases (sarcoidosis, Fabry disease, Chagas disease) are correlated with VA and SCD and frequently present an epicardial LGE13; and (3) in subtype 3, the extension of LGE has been mainly considered.
Though apparently intricate, this definition has a few advantages, mainly: (1) no need for specialized sequences for acquisition; (2) immediate identification; (3) no extra postprocessing time; (4) no specific software application required.
Our cohort included severe patients; indeed, LGE positivity was much more prevalent irrespective to the observed outcome. For this reason, LGE positivity per se seems to be unable to allow further clinical or arrhythmic stratification in this kind of patients. Albeit lacking statistical significance, probably due to small sample size, the observed distribution of primary endpoint in IDCM patients seems to confirm the general results of the study.
Recent literature data confirm the presence of midwall LGE as a negative prognostic marker for patients with DCM. Puntmann et al19 found midwall LGE as a negative prognostic marker in heart failure and DCM, both for survival and for VA; this appears to be nonspecific in discriminating arrhythmic endpoints and clinical ones. In this context Cx‐LGE is an attempt to overcome the problem, as a specific arrhythmic marker. In another recent work by Halliday and colleagues,20 a different population was considered, having required for inclusion a LVEF of ≥40%. Importantly, midwall LGE was defined as midmyocardial or subepicardial, thus including a substantial quote of Cx‐LGE pattern; patients with midwall LGE showed a significantly worse prognosis concerning a composite endpoint of cardiovascular death, transplantation, or hospitalization, confirming this to be a less specific marker for a pure arrhythmic endpoint.
Concerning the RV, systolic dysfunction and dilation are known to confer a worse prognosis in both ischemic and idiopathic DCM.21, 22 To the best of our knowledge, evidence on the role of the RV in DCM risk stratification for VA and SCD is lacking. Only a recent longitudinal cohort study found a trend toward a long‐term increase of VA and SCD in DCM patients with persistent RV dysfunction, but sample size was low (52 patients with long‐term RV dysfunction over an initial cohort of 512 patients) and RV examination was performed by echocardiography only.23 Data from our series show that RV dilation is independently and specifically associated with higher risk of ICD therapy, although survival analysis is not statistically significant, possibly due to limited cases enrolled in the study. However, the relevant role of the RV in determining hard clinical events limits the specificity of RVEDV in predicting VAs.
In our cohort, left bundle branch block showed a likely protective effect in univariate analysis. This observation can be explained by the high quota of cardiac resynchronization therapy patients enrolled, where cardiac resynchronization could have positively affected outcome, at least for nonarrhythmic events.
Arrhythmic recurrence is generally higher in patients with an ICD for secondary prevention as opposed to primary prevention. This was not seen in our population, possibly due to particular severity of the primary‐prevention group.
In this study, the secondary endpoint was considered mainly to assess the specificity of risk factors; interestingly, as a side observation, the value of CMR in predicting a worse prognosis could still be appreciated, albeit baseline characteristics were that of advanced heart failure.
Cx‐LGE proved to be influential in predicting hard clinical events, reinforcing its specificity for VA prediction. Moreover, divergence of survival curves for the primary endpoint suggests that Cx‐LGE might confer a higher arrhythmic risk throughout the clinical evolution of the cardiac condition.
4.1. Study limitations
Our study has some limitations. First, this is a retrospective, single‐center, registry‐based analysis, thus not all confounding factors and interactions may have been taken into account. Second, our tertiary referral institute is provided with LVAD and heart transplantation programs; therefore, our DCM population may have been significantly more compromised with respect to the general DCM population, so results should be extrapolated with caution. In particular, as the considered endpoints were not independent, an early MACE occurrence in particularly severe patients precluded further observation for ICD therapies. Third, only a minority of patients receiving an ICD at our institution underwent the CMR study protocol, in a nonrandomized fashion; although CMR results were not taken into consideration in establishing an ICD indication, a selection bias cannot be excluded. Fourth, ICD programming was not standardized by the protocol; in particular, activation/nonactivation of a VT treatment window and different settings of detection criteria may have influenced fulfillment of the primary endpoint. Finally, LGE quantification was not evaluated in our patients.
5. CONCLUSION
Proarrhythmic substrate imaging constitutes a step toward a more specific prediction of arrhythmic events. We propose Cx‐LGE as an easily determined risk factor for VA and SCD in DCM of any etiology with ICD implantation indication, particularly if to determine ICD indication, especially in patients with borderline classical criteria.
Candidacy for ICD implantation and for more intensive therapeutic programs also could also be considered in the light of CMR results; in this regard, our proposed Cx‐LGE definition should be considered preliminary and will hopefully be refined in future studies.
Patients affected by a cardiomyopathy with mild to moderate functional compromise, who do not currently fulfill ICD implantation criteria, are still at considerable risk of arrhythmic events; testing Cx‐LGE prospectively in future SCD prevention trials in this population might be highly desirable.
Supporting information
Figure S1 Supplementary figure online: Complex LGE subtypes. Subtype 1: a 57 years old male with ischemic dilated cardiomyopathy, transmural inferior and inferoseptal ischemic scar and a subendocardial anterolateral scar. Subtype 2: a 67 years old female with dilated cardiomyopathy, normal coronary arteries, syncopal recurrent ventricular tachycardia, and extensive subepicardial LGE. Subtype 3: a 60 years old male affected by stable Churg‐Strauss vasculitis, dilated cardiomyopathy, normal coronary arteries and global endocardial LGE. Subtype 4: a 61 years old male with silent myocardial infarction, normal coronary arteries, severe tricuspid regurgitation, transmural infero‐lateral and lateral ischemic scar (pattern 1) and extensive right ventricular LGE (pattern 2).
ACKNOWLEDGMENTS
The authors thank Medtronic Italia for technical support, in the persons of Elisa Bancalà, Davide Ottolina, and Silvia Bisetti.
Conflicts of interest
The authors declare no potential conflicts of interest.
REFERENCES
- 1. Bardy GH, Lee KL, Mark DB, et al; SCD‐HeFT Investigators . Amiodarone or an implantable cardioverter‐defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. [DOI] [PubMed] [Google Scholar]
- 2. Moss AJ, Hall WJ, Cannom DS, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. N Engl J Med. 1996;335:1933–1940. [DOI] [PubMed] [Google Scholar]
- 3. Moss AJ, Greenberg H, Case RB, et al; MADIT‐II Research Group . Long‐term clinical course of patients after termination of ventricular tachyarrhythmia by an implanted defibrillator. Circulation. 2004;110:3760–3765. [DOI] [PubMed] [Google Scholar]
- 4. Camm J, Klein H, Nisam S. The cost of implantable defibrillators: perceptions and reality. Eur Heart J. 2007;28:392–397. [DOI] [PubMed] [Google Scholar]
- 5. Zipes DP, Camm AJ, Borggrefe M, et al. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Europace. 2006;8:746–837. [DOI] [PubMed] [Google Scholar]
- 6. Køber L, Thune JJ, Nielsen JC, et al; DANISH Investigators . Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med. 2016;375:1221–1230. [DOI] [PubMed] [Google Scholar]
- 7. De Bakker JM, van Capelle FJ, Janse MJ, et al. Reentry as a cause of ventricular tachycardia in patients with chronic ischemic heart disease: electrophysiologic and anatomic correlation. Circulation. 1988;77:589–606. [DOI] [PubMed] [Google Scholar]
- 8. Haqqani HM, Marchlinski FE. Electrophysiologic substrate underlying postinfarction ventricular tachycardia: characterization and role in catheter ablation. Heart Rhythm. 2009;6(8 suppl):S70–S76. [DOI] [PubMed] [Google Scholar]
- 9. Mahrholdt H, Wagner A, Holly TA, et al. Reproducibility of chronic infarct size measurement by contrast‐enhanced magnetic resonance imaging. Circulation. 2002;106:2322–2327. [DOI] [PubMed] [Google Scholar]
- 10. Lang RM, Bierig M, Devereux RB, et al; American Society of Echocardiography's Nomenclature and Standards Committee; Task Force on Chamber Quantification; American College of Cardiology Echocardiography Committee; American Heart Association; European Association of Echocardiography, European Society of Cardiology . Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7:79–108. [DOI] [PubMed] [Google Scholar]
- 11. Simonetti OP, Kim RJ, Fieno DS, et al. An improved MR imaging technique for the visualization of myocardial infarction. Radiology. 2001;218:215–223. [DOI] [PubMed] [Google Scholar]
- 12. Kumar S, Tedrow UB, Stevenson WG. Entrainment mapping. Card Electrophysiol Clin. 2017;9:55–69. [DOI] [PubMed] [Google Scholar]
- 13. Kim RJ, Shah DJ, Judd RM. How we perform delayed enhancement imaging [published correction appears in J Cardiovasc Magn Reson 2003;5:613–615]. J Cardiovasc Magn Reson. 2003;5:613–615. [DOI] [PubMed] [Google Scholar]
- 14. Mahrholdt H, Wagner A, Judd RM, et al. Delayed enhancement cardiovascular magnetic resonance assessment of non‐ischaemic cardiomyopathies. Eur Heart J. 2005;26:1461–1474. [DOI] [PubMed] [Google Scholar]
- 15. Scott PA, Morgan JM, Carroll N, et al. The extent of left ventricular scar quantified by late gadolinium enhancement MRI is associated with spontaneous ventricular arrhythmias in patients with coronary artery disease and implantable cardioverter‐defibrillators. Circ Arrhythm Electrophysiol. 2011;4:324–330. [DOI] [PubMed] [Google Scholar]
- 16. Roes SD, Borleffs CJ, van der Geest RJ, et al. Infarct tissue heterogeneity assessed with contrast‐enhanced MRI predicts spontaneous ventricular arrhythmia in patients with ischemic cardiomyopathy and implantable cardioverter‐defibrillator. Circ Cardiovasc Imaging. 2009;2:183–190. [DOI] [PubMed] [Google Scholar]
- 17. Gulati A, Jabbour A, Ismail TF, et al. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy [published correction appears in JAMA 2013;310:99] . JAMA. 2013;309:896–908. [DOI] [PubMed] [Google Scholar]
- 18. Burke AP, Tavora F. Lymphocytic and autoimmune myocarditis In: Burke AP, Tavora F, eds. Practical Cardiovascular Pathology: An Atlas. 1st ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2011:241–248. [Google Scholar]
- 19. Puntmann VO, Carr‐White G, Jabbour A, et al; International T1 Multicentre CMR Outcome Study . T1‐Mapping and outcome in nonischemic cardiomyopathy: all‐cause mortality and heart failure. JACC Cardiovasc Imaging. 2016;9:40–50. [DOI] [PubMed] [Google Scholar]
- 20. Halliday BP, Gulati A, Ali A, et al. Association between midwall late gadolinium enhancement and sudden cardiac death in patients with dilated cardiomyopathy and mild and moderate left ventricular systolic dysfunction. Circulation. 2017;135:2106–2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Polak JF, Holman BL, Wynne J, et al. Right ventricular ejection fraction: an indicator of increased mortality in patients with congestive heart failure associated with coronary artery disease. J Am Coll Cardiol. 1983;2:217–224. [DOI] [PubMed] [Google Scholar]
- 22. Juillière Y, Barbier G, Feldmann L, et al. Additional predictive value of both left and right ventricular ejection fractions on long‐term survival in idiopathic dilated cardiomyopathy. Eur Heart J. 1997;18:276–280. [DOI] [PubMed] [Google Scholar]
- 23. Merlo M, Gobbo M, Stolfo D, et al. The prognostic impact of the evolution of RV function in idiopathic DCM. JACC Cardiovasc Imaging. 2016;9:1034–1042. [DOI] [PubMed] [Google Scholar]
Pedretti S, Vargiu S, Baroni M, et al. Complexity of scar and ventricular arrhythmias in dilated cardiomyopathy of any etiology: Long‐term data from the SCARFEAR (Cardiovascular Magnetic Resonance Predictors of Appropriate Implantable Cardioverter‐Defibrillator Therapy Delivery) Registry. Clin Cardiol. 2018;41:494–501. 10.1002/clc.22911
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Supplementary figure online: Complex LGE subtypes. Subtype 1: a 57 years old male with ischemic dilated cardiomyopathy, transmural inferior and inferoseptal ischemic scar and a subendocardial anterolateral scar. Subtype 2: a 67 years old female with dilated cardiomyopathy, normal coronary arteries, syncopal recurrent ventricular tachycardia, and extensive subepicardial LGE. Subtype 3: a 60 years old male affected by stable Churg‐Strauss vasculitis, dilated cardiomyopathy, normal coronary arteries and global endocardial LGE. Subtype 4: a 61 years old male with silent myocardial infarction, normal coronary arteries, severe tricuspid regurgitation, transmural infero‐lateral and lateral ischemic scar (pattern 1) and extensive right ventricular LGE (pattern 2).


