Summary
Aim
The effects of sevoflurane on microglia/macrophages, promoting or suppressing their activation, remains controversy. We aimed to determine whether sevoflurane preconditioning can protect brain via changing microglia/macrophage dynamics and phagocytosis profile after ischemia.
Methods
The impact of sevoflurane preconditioning was evaluated on microglia/macrophage migration, phagocytosis and proliferation altogether from day 1 to day 7 after transient middle cerebral arterial occlusion (tMCAO) in rats.
Results
Sevoflurane preconditioning was identified to accelerate microglia/macrophage migrating to and invasion in the ischemic core from day 1 to day 5 after damage. Significant accumulation of amoeboid and phagocytic microglia/macrophages was observed in sevoflurane group from day 3 to day 5 after ischemia injury. In addition, sevoflurane pretreatment also promoted the proliferation of microglia/macrophage (Iba1+/Ki67+) dramatically in ischemic core on day 3 postinsult.
Conclusions
Our current study has identified the impact of sevoflurane preconditioning on microglia/macrophage dynamics, including its migration, phagocytosis, and proliferation at early stage after brain ischemia and reperfusion. Sevoflurane might enhance microglia/macrophage activation and promote brain repair. These results could help to approach more relevant microglia/macrophage cell‐based strategy for human stroke therapy.
Keywords: ischemia and reperfusion, microglia/macrophage, migration, phagocytosis, proliferation, sevoflurane
1. INTRODUCTION
Amounts of preclinical researches have demonstrated the protective effects of sevoflurane, one of the commonly used inhalational anesthetics, against brain ischemia in vivo and in vitro.1, 2, 3 However, the neuroprotective effect of sevoflurane on the central nervous system (CNS) resident microglia and circulating‐derived macrophages, the first line of cerebral defense against stroke, remains controversial. Some studies suggested that sevoflurane preconditioning provided antiinflammatory profile by inhibiting microglia/macrophage activation and decreasing inflammatory cytokines after focal cerebral ischemia.4, 5 But a growing body of evidences revealed that the microglia/macrophage exerted dual functions to either expand ischemic damage by releasing proinflammatory neurotoxins, or support brain recovery by eliminating cellular debris and secreting trophic factors after stroke.6, 7, 8 The neural survival has been aided by the presence of microglia/macrophages in vivo and in vitro.9, 10
In our previous study, we found that sevoflurane preconditioning conferred neurogenesis against cerebral ischemia and reperfusion (I/R) injury, and the neuroprotective effects were diminished by minocycline, a microglia/macrophage antagonist.11 In view of the paradoxical profiles of microglia/macrophages, we aimed to further explore the impact of sevoflurane preconditioning on microglia/macrophage migration, phagocytosis and proliferation altogether till 7 days after transient middle cerebral arterial occlusion (tMCAO). The results might provide potential microglia/macrophage cell‐based therapeutic strategy for cerebral ischemia.
2. MATERIAL AND METHODS
2.1. Animals and sevoflurane preconditioning
The adult male Sprague‐Dawley rats (280‐320 g) were provided by SLAC Experimental Animal Co. Ltd (Shanghai, China) and randomly allocated into sham group, control group (ischemic exposure only), or Sevo group (sevoflurane preconditioning + ischemic exposure). In the Sevo group, rats were administered with 1.2% sevoflurane (Baxter) + 98% O2 in a sealed chamber for 60 minutes on 4 consecutive days as described before.5, 11 The rats in the control group were exposed to 98% O2 instead as previously described. Twenty‐four hours after 4‐day pretreatment, the rats were subjected to tMCAO. Neither sevoflurane nor ischemia was exposed to the rats in the sham group. Based on our previous study, the successful rate of tMCAO modeling was 0.86.11 The reasons of modeling failure included hemorrhage, no infarct, and no reperfusion. During the prolonged survival interval (till 7 days after I/R injury), 10 rats died in control (7 rats) and Sevo (3 rats) group, and were replaced to ensure 8 rats at each time point per group. Ninety‐two rats were totally used in this study. All procedures were approved by the Committee of Animal Research, Fudan University, followed the ARRIVE guidelines and the Guide of Care and Use of Laboratory Animals.
2.2. Transient middle cerebral artery occlusion (tMCAO)
Zea Longa's modified MCAO model was adopted to mimic transient focal brain ischemia and reperfusion.12 After the rats were anesthetized with 400 mg/kg chloral hydrate (intraperitoneal injection), the right common carotid artery and external carotid artery were isolated and ligated. A 0.38 ± 0.02 mm diameter monofilament (the Cinontech CO. Ltd, Beijing, China) was gently inserted from the right external carotid artery to the right middle cerebral artery and advanced along the internal carotid artery until occluding the origin of the middle cerebral artery. After 90 minutes occlusion, the reperfusion was established by withdrawing the monofilament. Then the rats were sutured and placed back to cages with free access to water and food. During the surgical procedure, the animals were warmed with a heating pad to maintain body temperature at 37 ± 0.2°C.
2.3. Floating brain sections preparation and immunostaining
The rats were sacrificed under deep anesthesia with chloral hydrate. Then the brain tissues were fixed with 4% PFA, dehydrated with 20% sucrose in 4% PFA, and 30% sucrose in 0.1 mol/L PB for 24 hours. The dehydrated brain tissues were embedded in OCT compound (SAKURA, Japan) for 1 hour at −25°C, and sectioned coronally with 45 μm thickness by cryostat (CM1850, Leica, Germany). The floating sections were preserved in cryoprotectant buffer (30% sucrose + 30% glycol in 0.1 mol/L PB) for later use. Before immunostaining, the sections were rinsed with 0.01 mol/L PBS for 6 times and heated with antigen retrieval buffer (Beyotime, Shanghai, China) at 99°C for 10 minutes. Then the sections were washed with 0.01 mol/L PBS for 5 times at room temperature and blocked by 0.5% Triton X‐100 (Sangon Biotech, Shanghai, China) and 10% donkey serum (Jackson ImmunoResearch Laboratories, Inc. USA) in 0.01 mol/L PBS for 2 hours. The sections were incubated with primary antibodies, including goat anti‐Iba1 (1:200, Abcam), mouse anti‐MAP2 (1:1000, Sigma), rabbit anti‐Ki67 (1:300, Sigma) at 4°C overnight and secondary antibodies (1:1000, ThermoFisher, USA) for 2 hours at room temperature. DAPI (1:10000, Abcam) was used for nuclei counting. At each time point, we have 2 rats in the sham group, and 8 rats in both Sevo and control group.
Neurons and microglia/macrophages were labeled with MAP2 and Iba1 immunofluorescent biomarkers, respectively. Because of its sensitivity to neuronal injury, MAP2 was used to outline the ischemic boundary in this study.13, 14 We defined MAP2 negative (MAP2‐) parts as infarct regions and MAP2 positive (MAP+) parts as noninfarct regions (including penumbra and normal parenchyma).15, 16
2.4. Image analysis
All images were scanned with Leica SP8 confocal microscopes (Leica Microsystems), and Z‐stacked with LAS X software. Cellular morphology and cell counting were analyzed by LAS X and Image J (Fiji edition) in 3D mode, respectively. Integrated optical density (IOD) of the whole infarct region, including cortex and striatum, was analyzed by Image Pro Plus software. We measured 3 times for each stitching section per group and calculated the mean values as the final IOD number. Eight coronal sections per group were analyzed by 20 μm‐thick Z‐stacks (1 μm per step) with 63× oil‐immersion. For the stitching pictures, 20 μm‐thick Z‐stacks (5 μm per step) with 20× oil‐immersion were utilized for analysis.
2.5. Statistical analysis
GraphPad Prism 6 or SPSS 16 was used for statistical analysis. The data with normal distribution were presented as mean ± SEM and analyzed by Student's t test or one‐way ANOVA, followed by Games‐Howell post hoc analysis. P < .05 was considered significantly different.
3. RESULTS
3.1. Sevoflurane preconditioning accelerated microglia/macrophage migration after cerebral I/R injury
In this study, we observed and compared the microglia/macrophage migrating processes with or without sevoflurane preconditioning from day 1 till day 7 after focal I/R injury. The stitching pictures with 20× oil‐immersion showed microglia/macrophage migrating toward ischemic zones from an overall perspective after brain I/R injury (Figure 1). A rapid response of microglia/macrophages was observed on day 1 after brain ischemia. The Iba1 positive (Iba1+) microglia/macrophages were much brighter and more intensive in the Sevo group compared with those of the control group (Figure 1A,E). On day 3 after I/R, a large number of Iba1+ microglia/macrophages accumulated in the MAP2− ischemic core in the Sevo group. However, fewer Iba1+ cells were observed in the control group (Figure 1B,F). Our data showed that the ischemic regions were covered with amounts of Iba1+ microglia/macrophages in both groups on day 5 and day 7 after injury, which indicated the peak of neural inflammatory responses against stroke (Figure 1C,D,G,H).
Figure 1.
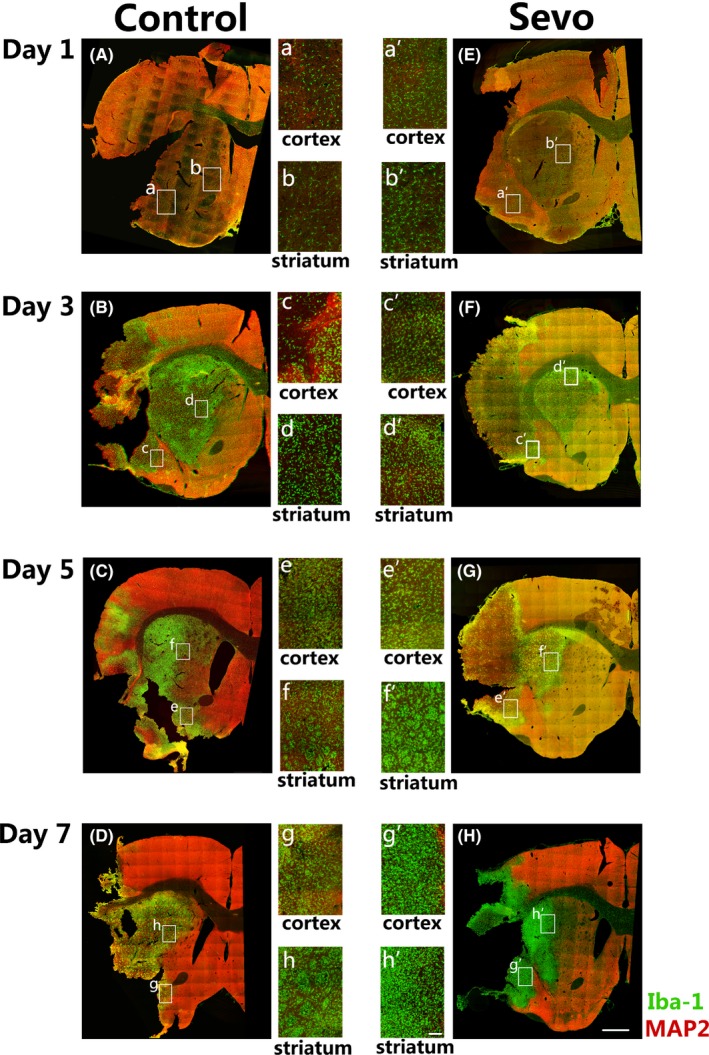
Sevoflurane preconditioning accelerated microglia/macrophage migration after cerebral I/R injury. A‐H, Representative stitching images of ipsilateral sections in the Sevo and control group stained with MAP2 and Iba1 from day 1 to day 7 after cerebral I/R injury. Boxed areas indicate magnified infarct cortex and striatum which display in middle panels, respectively. Scale bar = 1 mm. a‐h, High‐resolution images of infarcted cortex and striatum in the control group till day 7 after I/R injury. Scale bar = 100 μm. a'‐h', High‐resolution images of infarcted cortex and striatum in the Sevo group till day 7 after I/R injury. Scale bar = 100 μm
The activation of microglia/macrophages in cortex and striatum were compared between groups till day 7 after ischemia (Figure 1a‐h'). Compared with the control group, there were more Iba1+ microglia/macrophages in the Sevo group accumulated in the infarcted cortex and striatum since day 1 after injury. However, the activated Iba1+ cells in the control group increased in the infarct regions till day 3. The data indicate that sevoflurane preconditioning might accelerate the migration of microglia/macrophages toward infarct regions after I/R injury.
3.2. Sevoflurane preconditioning promoted microglia/macrophage activation after cerebral I/R injury
With the utilization of higher magnification (63× oil‐immersion), morphological changes of the activated microglia/macrophages were clearly compared between 2 groups (Figure 2C). On day 1 after I/R, the number of Iba1+ microglia/macrophages in the infarcted cortex significantly increased in the Sevo group than those in the control group. More ramified Iba1+ cells, with thick branches and big cell bodies, accumulated at the ischemic core in the Sevo group (Figure 2 Ca',2Cb'). In the Sevo group, amounts of Iba1+ microglia/macrophages were observed to present amoeboid morphology without ramified branches and covered the whole infarcted regions since day 3 after ischemia. The amoeboid microglia/macrophages presented bigger cell bodies and more cytoplasm with phagocytic debris engulfment (Figure 3B). However, fewer amoeboid cells in the control group accumulated in the ischemia areas at that time point (Figure 2 Cc',Ce'). On day 7 after I/R, the ischemic regions were covered with abundant amoeboid Iba1+ cells in both groups, but no significant difference existed between 2 groups (Figure 2 Cg',Ch').
Figure 2.
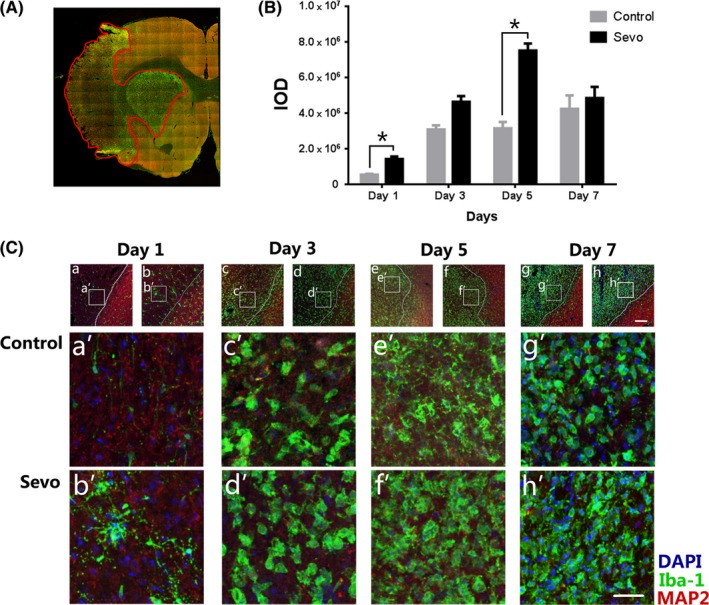
Sevoflurane preconditioning promoted microglia/macrophage activation after cerebral I/R injury. A, The stitching image shows the whole infarct regions for integrated optical density (IOD) calculation. The infarcted boundary is defined by the MAP2 staining. B, Comparison of IOD values of the infarct region between 2 groups. Data were shown as mean ± SEM. *P < .05, n = 8. Ca‐h, Representative images of ipsilateral cortex in the Sevo and control group stained with MAP2, Iba1, and DAPI. Boxed areas indicate infarcted cortex in below panels. Scale bar = 75 μm. Ca'‐h', High‐resolution images of infarcted cortex from day 1 to day 7 after I/R injury. Scale bar = 25 μm
Figure 3.
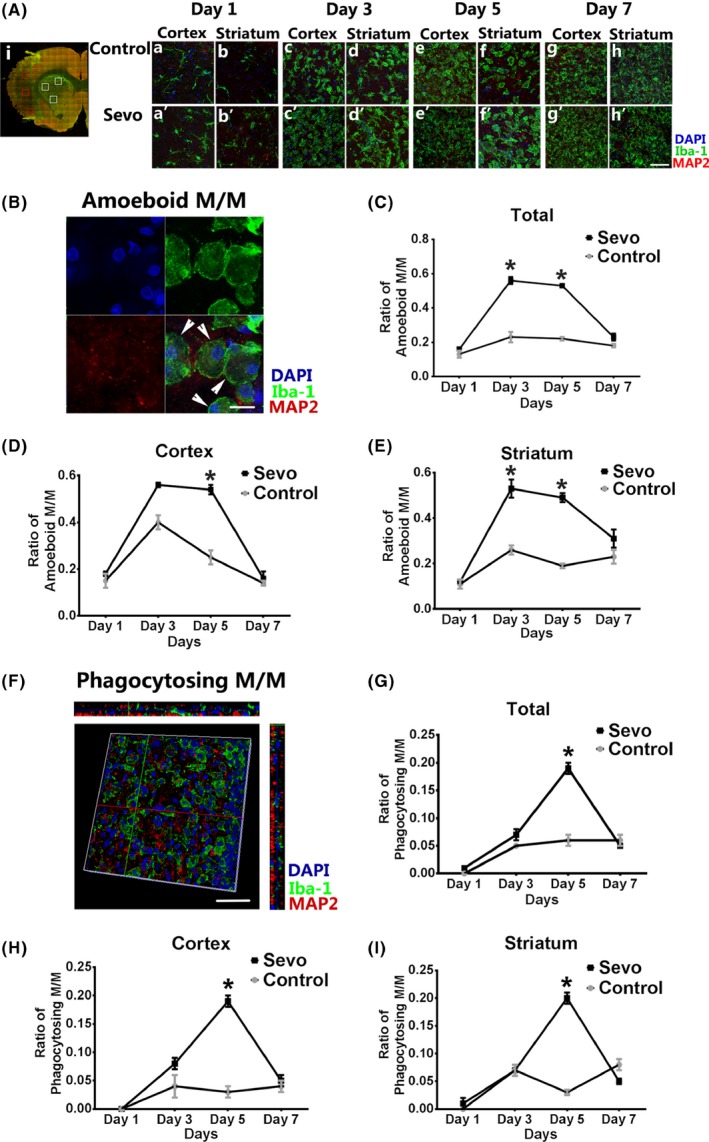
Sevoflurane preconditioning enhanced microglia/macrophage phagocytosis after I/R injury. Ai: Representative stitching image shows the boxed areas for observation and calculation of amoeboid and phagocytosing microglia/macrophages (M/M) after injury. Aa‐h', Comparison of infarcted regions in the Sevo and control group from day 1 to day 7 after ischemia. Scale bar = 50 μm. B, High‐resolution image of amoeboid M/M stained with Iba1, MAP2, and DAPI. Scale bar = 10 μm. C‐E, The ratio of amoeboid M/M was analyzed in the infarcted cortex and striatum from day 1 to day 7. F: 3D reconstructed image of phagocytosing M/M staining with Iba‐1, MAP2, and DAPI. Scale bar = 20 μm. G‐I, The ratio of phagocytosing M/M was analyzed in the infarcted cortex and striatum from day 1 to day 7. Data were shown as mean ± SEM. *P < .05, n = 8
IOD analysis of Iba1+ cells in the whole infarct region was used to compare the activation of microglia/macrophage between 2 groups (Figure 2A). The IOD values of the Sevo group was significantly higher than those of the control group on day 1 (1.42 × 106 ± 0.14 × 106 vs. 5.51 × 105 ± 0.40 × 105, P < .05) and day 5 (7.81 × 106 ± 0.29 × 106 vs. 3.10 × 106 ± 0.31 × 106, P < .05), respectively. No statistical difference was found on day 3 and day 7 (Figure 2B).
3.3. Sevoflurane preconditioning enhanced phagocytic efficiency of the microglia/macrophages
The numbers of amoeboid Iba1+ cells were counted and compared in the ischemic cortex and striatum till day 7 after I/R injury (Figure 3A‐E). On day 3 and day 5, the total number of amoeboid microglia/macrophages in the Sevo group was significantly higher than that of the control group (P < .05, Figure 3C). In the Sevo group, the big and round “amoeboid” cells significantly increased in the ischemic cortex on day 5, and in the ischemic striatum on day 3 and day 5 (Figure 3D,E). The data indicated that sevoflurane preconditioning promoted amoeboid changes of microglia/macrophages for consequent phagocytosis in the ischemic areas.
Costaining with MAP2 and Iba1 was used for further analysis of the microglia/macrophage phagocytosis. The phagocytosing cells were identified as Iba1+ cells with MAP2+ debris engulfment (Figure 3F). Our data showed that the phagocytosing microglia/macrophages of the Sevo group significantly increased in both ischemic cortex and striatum on day 5 after I/R injury (P < .05), compared with those of the control group (Figure 3G‐I), which further demonstrates the promotion of sevoflurane on phagocytic efficiency of microglia/macrophage after I/R injury.
3.4. Sevoflurane preconditioning promoted proliferation of microglia/macrophages after I/R injury
To determine whether sevoflurane preconditioning can promote the microglia/macrophage proliferation after stroke, we took advantage of colabeling with Iba1 and Ki67 to evaluate and compare the microglia/macrophage proliferation between 2 groups (Figure 4). On the first day after injury, more ramified Iba1+ cells were observed in the Sevo group in the ischemic cortex and striatum (Figure 4A). However, the costaining data showed no difference of proliferating cells in both 2 groups (Figure 4C‐E). On day 3 after I/R, a proliferating peak of Ki67+/Iba1+ cells was observed in both infarcted cortex and striatum followed with a decline on day 5 in the Sevo group. The number of proliferating microglia/macrophages in the Sevo group was significantly higher than that in the control group (P < .05) (Figure 4C‐E). After that, no statistical difference was observed in the proliferation between 2 groups. The results demonstrate that sevoflurane preconditioning enhanced the microglia/macrophage proliferation which peaked on day 3 after stroke.
Figure 4.
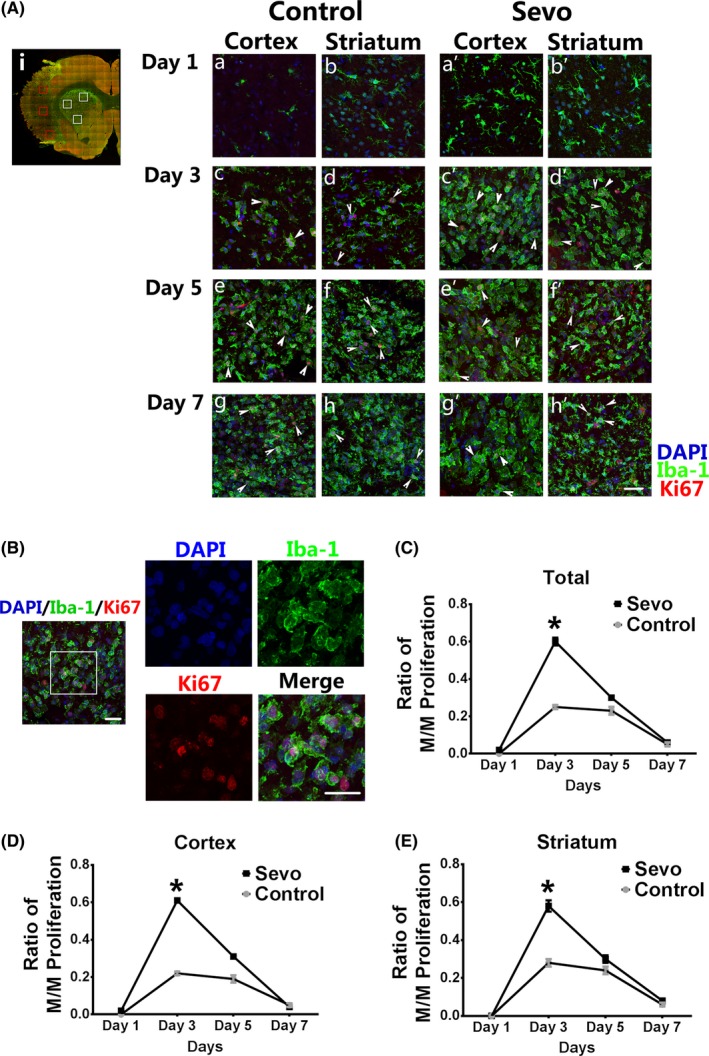
Sevoflurane preconditioning promoted microglia/macrophage proliferation after I/R injury. Ai: Representative stitching image shows the boxed areas for observation and calculation of proliferating microglia/macrophages (M/M) after injury. Aa‐h', Comparison of proliferating M/M stained with Iba1 and Ki67 in the infarcted regions between 2 groups from day 1 to day 7 after ischemia. Scale bar = 25 μm. White arrows show the proliferating M/M with Iba1 and Ki67 costaining. B, Representative confocal image shows the proliferating M/M. The boxed area indicates magnified region of the right panels. Scale bar = 20 μm. C‐E, The ratio of M/M proliferation was analyzed in the infarcted cortex and striatum from day 1 to day 7 after injury. Data were shown as mean ± SEM. *P < .05, n = 8
4. DISCUSSION
Given that there has been much controversy concerning the role of microglia/macrophages in the sevoflurane‐induced neuroprotection,4, 11 the current study directly compared the microglia/macrophage activation, migration, proliferation, and phagocytosis with or without sevoflurane preconditioning after cerebral I/R injury, to provide a better understanding of the effect of sevoflurane on microglia/macrophage dynamics against stroke.
Here, we used the stitching pictures with 20× oil‐immersion to investigate microglia/macrophage migration after ischemic stroke from an overall perspective. Our data indicate that sevoflurane preconditioning‐induced quicker response of microglia/macrophage under pathological conditioning, by accelerating their migration toward the infarcted cortex and striatum at the first day after I/R injury. On the contrary, an apparent delay in migration (till day 3 after I/R) was observed in the microglia/macrophages without sevoflurane pretreatment.
Consistent with the previous studies,17, 18 the reactive microglia/macrophages with sevoflurane administration were observed to exhibit ramified morphology as a rapid responding,19 accumulated in and around the lesion sites since day 3 after stroke. Li XQ et al.20 pointed out that sevoflurane provided protection by inhibiting microglia recruitment after spinal cord I/R injury. While sevoflurane was reported to exacerbate neuroinflammation via microglia activation in the developing mouse brain.21 These paradoxical results can be explained by the difference of experimental model and animal ages. In our previous studies, we found that the reactive microglia with sevoflurane preconditioning was significantly reduced in the penumbra area at 48 hours after reperfusion,5 while apparently accumulated in infarcted cortex with ramified morphology on the first day after injury.11 In the present study, the contradictory results were well explained by the dynamic observation of microglia/macrophage migration with the advantage of the stitching pictures. Our data demonstrated that sevoflurane preconditioning accelerated microglia/macrophage migrating from healthy area and ischemic penumbra toward infarct core after cerebral I/R injury.
When challenged with excitotoxicity or inflammation, microglia/macrophages boost their phagocytic efficiency with different strategies to maintain the cerebral homeostasis.9, 22 The phagocytosing microglia/macrophages with “amoeboid” morphology have been confirmed to promote neural survival by clearing potential toxic debris and reducing subsequent secondary brain damage.22, 23, 24 The “eat‐me” and “find‐me” signals might induce microglia/macrophage migrating to and phagocytosing apoptotic and necrotic neurons.25 In the current study, upon sevoflurane preconditioning, the ramified microglia/macrophages rapidly transformed to amoeboid morphology and accumulated in the infarcted cortex and striatum since day 3 after I/R injury, indicating that sevoflurane enhanced phagocytic efficiency by increasing the phagocytic capacity per cell. Compared with those of the control group, the phagocytosing cells, defined as Iba1+ cells engulfed with MAP2+ debris, significantly increased in the infarct areas in the Sevo group on day 5 after injury, which supports that more phagocytic cells were recruited by sevoflurane preconditioning. Our data with costaining of Iba1 and Ki67 (a classical proliferative biomarker26) further confirmed that sevoflurane preconditioning promoted microglia/macrophage proliferation in the challenge of I/R injury, consistent with the previous study of Allan S.M. et al27 Altogether, the present study demonstrated that sevoflurane preconditioning helped to boost phagocytic efficiency of microglia/macrophages by recruiting more phagocytic cells, increasing the phagocytic capacity per cell, and/or increasing the number of microglia/macrophages.
There are some limitations in the current study. No microglia‐specific biomarker has been obtained to distinguish from macrophages and other myeloid‐derived cells. Iba1, an ionized calcium binding adaptor molecule‐1, is one of the most useful biomarkers for observing the physiological and pathological morphology of microglial cells.28, 29 However, in the pathological state, Iba1 shows limited specificity for microglial staining and binds peripheral macrophages infiltrating the ischemic brain through broken blood‐brain barriers. The instability of CD45 levels of injured brain also poses a hurdle for tracking resident microglia and peripheral macrophages.30, 31 Therefore, we could not identify the temporospatial dynamics of microglial cells and macrophages, respectively. Furthermore, the current study is limited in a descriptive observation of microglia/macrophage dynamics after I/R injury. Many researchers have indicated the potential pathways or receptors involved in microglia/macrophage activation, such as notch‐1 signaling,32 pattern recognition receptors(PRRs),33 toll‐like receptor 2.34 The data of Hu X et al16 suggest that the microglia/macrophage polarization might play a key role in ischemic injury expansion. Yang Q et al35 pointed out that the activation of the notch signaling pathway may be involved in sevoflurane preconditioning‐induced neuroprotection in rat brain. However, no mechanism has been reported on how sevoflurane preconditioning boosts microglia/macrophage to be activated. The underlying mechanisms need to be explored in both wildtype and transgenic animals with microglia‐specific fluorescent biomarker in vivo and in vitro, which might distinguish the effects of sevoflurane preconditioning on CNS‐resident microglia and circulating‐derived macrophages.
In conclusion, the current study confirmed that sevoflurane preconditioning could promote microglia/macrophage migration, phagocytosis, and proliferation altogether till 7 days after cerebral I/R injury, which might be the potential neuroprotective mechanism of sevoflurane preconditioning. The observation of microglia/macrophage dynamics after ischemia might provide glia‐based therapeutic strategy for human stroke.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
This work was supported by National Natural Science Foundation of China to Qiong Yu (grant number 81200937).
Dang D‐D, Saiyin H, Yu Q, Liang W‐M. Effects of sevoflurane preconditioning on microglia/macrophage dynamics and phagocytosis profile against cerebral ischemia in rats. CNS Neurosci Ther. 2018;24:564–571. 10.1111/cns.12823
Contributor Information
Qiong Yu, Email: yu_qiong816@sina.com.
Wei‐Min Liang, Email: chiefliang@sina.cn.
REFERENCES
- 1. Yu Q, Wang H, Chen J, Gao Y, Liang W. Neuroprotections and mechanisms of inhalational anesthetics against brain ischemia. Fron Biosci. 2010;E2:1275‐1298. [DOI] [PubMed] [Google Scholar]
- 2. Wang H, Shi H, Yu Q, Chen J, Zhang F, Gao Y. Sevoflurane preconditioning confers neuroprotection via anti‐apoptosis effects. Acta Neurochir. 2016;121:55‐61. [DOI] [PubMed] [Google Scholar]
- 3. Zhang Y, Tian SY, Li YW, et al. Sevoflurane preconditioning improving cerebral focal ischemia‐reperfusion damage in a rat model via PI3K/Akt signaling pathway. Gene. 2015;569:60‐65. [DOI] [PubMed] [Google Scholar]
- 4. Wang H, Lu S, Yu Q, et al. Sevoflurane preconditioning confers neuroprotection via anti‐inflammatory effects. Fron Biosci. 2011;E3:604‐615. [DOI] [PubMed] [Google Scholar]
- 5. Yu Q, Chu M, Wang H, et al. Sevoflurane preconditioning protects blood‐brain‐barrier against brain ischemia. Fron Biosci. 2011;E3:978‐988. [DOI] [PubMed] [Google Scholar]
- 6. Patel AR, Ritzel R, McCullough LD, Liu F. Microglia and ischemic stroke: a double‐edged sword. Int J Physiol Pathophysiol Pharmacol. 2013;5:73‐90. [PMC free article] [PubMed] [Google Scholar]
- 7. Morgan SC, Taylor DL, Pocock JM. Microglia release activators of neuronal proliferation mediated by activation of mitogen‐activated protein kinase, phosphatidylinositol‐3‐kinase/Akt and delta–Notch signaling cascades. J Neurochem. 2004;90:89‐101. [DOI] [PubMed] [Google Scholar]
- 8. Ma Y, Wang J, Wang Y, Yang GY. The biphasic function of microglia in ischemic stroke. Prog Neurobiol. 2016;1417:1‐25. [DOI] [PubMed] [Google Scholar]
- 9. Aldskogius H. Microglia in neuroregeneration. Microsc Res Tech. 2001;54:40‐46. [DOI] [PubMed] [Google Scholar]
- 10. Polazzi E, Gianni T, Contestabile A. Microglial cells protect cerebellar granule neurons from apoptosis: evidence for reciprocal signalling. Glia. 2001;36:271‐280. [DOI] [PubMed] [Google Scholar]
- 11. Li L, Saiyin H, Xie J, et al. Sevoflurane preconditioning induced endogenous neurogenesis against ischemic brain injury by promoting microglial activation. Oncotarget. 2017;8:28544‐28557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Longa EZWP, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84‐91. [DOI] [PubMed] [Google Scholar]
- 13. Huh JW, Raghupathi R, Laurer HL, Helfaer MA, Saatman KE. Transient loss of microtubule‐associated protein 2 immunoreactivity after moderate brain injury in mice. J Neurotrauma. 2003;20:975‐984. [DOI] [PubMed] [Google Scholar]
- 14. Hicks RR, Smith DH, McIntosh TK. Temporal response and effects of excitatory amino acid antagonism on microtubule‐associated protein 2 immunoreactivity following experimental brain injury in rats. Brain Res. 1995;678:151‐160. [DOI] [PubMed] [Google Scholar]
- 15. Dawson DA, Hallenbeck JM. Acute focal ischemia‐induced alterations in MAP2 immunostaining: description of temporal changes and utilization as a marker for volumetric assessment of acute brain injury. J Cereb Blood Flow Metab. 1996;16:170‐174. [DOI] [PubMed] [Google Scholar]
- 16. Hu X, Li P, Guo Y, et al. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke. 2012;43:3063‐3070. [DOI] [PubMed] [Google Scholar]
- 17. Grønberg NV, Johansen FF, Kristiansen U, Hasseldam H. Leukocyte infiltration in experimental stroke. J Neuroinflammation. 2013;10:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang G, Zhang J, Hu X, et al. Microglia/macrophage polarization dynamics in white matter after traumatic brain injury. J Cereb Blood Flow Metab. 2013;33:1864‐1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Davalos D, Grutzendler J, Yang G, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752‐758. [DOI] [PubMed] [Google Scholar]
- 20. Li XQ, Cao XZ, Wang J, Fang B, Tan WF, Ma H. Sevoflurane preconditioning ameliorates neuronal deficits by inhibiting microglial MMP‐9 expression after spinal cord ischemia/reperfusion in rats. Mol Brain. 2014;7:1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shen X, Dong Y, Xu Z, et al. Selective anesthesia‐induced neuroinflammation in developing mouse brain and cognitive impairment. Anesthesiology. 2014;118:502‐515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Neumann H, Kotter MR, Franklin RJ. Debris clearance by microglia: an essential link between degeneration and regeneration. Brain. 2009;132:288‐295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Perego C, Fumagalli S, De Simoni MG. Temporal pattern of expression and colocalization of microglia/macrophage phenotype markers following brain ischemic injury in mice. J Neuroinflammation. 2011;8:1‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hidetaka T, Tomita M, Tanahashi N, et al. Hydrogen peroxide enhances phagocytic activity of ameboid microglia. Neurosci Lett. 1998;240:5‐8. [DOI] [PubMed] [Google Scholar]
- 25. Neher JJ, Neniskyte U, Zhao JW, Bal‐Price A, Tolkovsky AM, Brown GC. Inhibition of microglial phagocytosis is sufficient to prevent inflammatory neuronal death. J Immunol. 2011;186:4973‐4983. [DOI] [PubMed] [Google Scholar]
- 26. Lopez F, Belloc F, Lacombe F. Dumain P. Reiffers J, Bernard, P. Boisseau MR. Modalities of synthesis of Ki67 antigen during the stimulation of lymphocytes. Cytometry. 1991;12:42‐9. [DOI] [PubMed] [Google Scholar]
- 27. Denes A, Vidyasagar R, Feng J, et al. Proliferating resident microglia after focal cerebral ischaemia in mice. J Cereb Blood Flow Metab. 2007;27:1941‐1953. [DOI] [PubMed] [Google Scholar]
- 28. Ritzel RM, Patel AR, Grenier JM, et al. Functional differences between microglia and monocytes after ischemic stroke. J Neuroinflammation. 2015;12:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Imai Y, Ibata I, Ito D, Ohsawa K, Kohsaka S. A novel gene iba1 in the major histocompatibility complex class III region encoding an EF hand protein expressed in a monocytic lineage. Biochem Bioph Res Co. 1996;224:855‐862. [DOI] [PubMed] [Google Scholar]
- 30. Ponomarev E, Shriver L, Maresz K, Dittel B. Microglial cell activation and proliferation precedes the onset of CNS autoimmunity. J Neurosci Res. 2005;81:374‐389. [DOI] [PubMed] [Google Scholar]
- 31. Melief J, Koning N, Schuurman K, et al. Phenotyping primary human microglia: tight regulation of LPS responsiveness. Glia. 2012;60:1506‐1517. [DOI] [PubMed] [Google Scholar]
- 32. Yao L, Kan EM, Kaur C, et al. Notch‐1 signaling regulates microglia activation via NF‐kappaB pathway after hypoxic exposure in vivo and in vitro. PLoS ONE. 2013;8:e78439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Saijo K, Crotti A, Glass CK. Regulation of microglia activation and deactivation by nuclear receptors. Glia. 2013;61:104‐111. [DOI] [PubMed] [Google Scholar]
- 34. Stirling DP, Cummins K, Mishra M, Teo W, Yong VW, Stys P. Toll‐like receptor 2‐mediated alternative activation of microglia is protective after spinal cord injury. Brain. 2014;137:707‐723. [DOI] [PubMed] [Google Scholar]
- 35. Yang Q, Yan W, Li X, et al. Activation of canonical notch signaling pathway is involved in the ischemic tolerance induced by sevoflurane preconditioning in mice. Anesthesiology. 2012;117:996‐1005. [DOI] [PubMed] [Google Scholar]


