Abstract
Background
The de Winter electrocardiogram (EKG) pattern is a novel sign that indicates left anterior descending coronary artery (LAD) occlusion in patients with chest pain. This study aimed to assess the prevalence and clinical characteristics of patients with this pattern.
Hypothesis
The de Winter EKG pattern is an special anterior ST‐segment elevation myocardial infarction (STEMI) equivalents without obvious ST‐segment elevation.
Methods
This retrospective study included all patients with anterior myocardial infarction admitted between January 2011 and December 2017. Patients were categorized into two groups: those with the de Winter EKG pattern and those with typical STEMI.
Results
Of 441 patients, 15 (3.4%) with anterior myocardial infarction had the de Winter EKG pattern. Similar to those with typical STEMI, the majority of patients with the de Winter EKG pattern had ST‐segment elevation, pathologic Q wave, and absence of R wave at follow‐up. The median time from recognition of this pattern until its evolution was 114 minutes. The ST‐segment in leads V3R to V5R and leads V7 to V9 were normal or slightly depressed when a typical de Winter EKG pattern was noted in leads V1 to V6. The culprit lesion was mainly in the proximal LAD or the diagonal branch. Patients with this EKG pattern responded poorly to thrombolytic therapy.
Conclusions
We believe that the de Winter EKG pattern may be a sign of ischemia and presents at the early stage of STEMI rather than being an independent pattern. In patients with this pattern, a percutaneous coronary intervention rather than follow‐up and thrombolytic strategy should be performed.
Keywords: de Winter, electrocardiogram, percutaneous coronary intervention, STEMI
1. INTRODUCTION
Electrocardiogram (EKG) is an essential tool for the diagnosis of acute myocardial ischemia and the evaluation of evolving myocardial infarction in the emergency and cardiology departments. EKG can distinguish between patients with an ST‐segment elevation myocardial infarction (STEMI) and those with a non‐STEMI, which can guide management along with biomarkers of myocardial necrosis. A well‐trained physician can directly interpret EKG results to establish a guided treatment for patients with chest pain. Although several patients with acute myocardial infarction caused by occlusion of the epicardial coronary artery do not present with ST‐elevation on EKG, they may present with other EKG abnormalities known as STEMI equivalents. The de Winter sign is a STEMI equivalent that was first reported in 2008. It is characterized by an upsloping ST‐segment depression at the J‐point in leads V1 to V6 that continues into a tall positive symmetrical T wave, which signifies occlusion of the proximal left anterior descending coronary artery (LAD). 1 Since the prevalence and clinical characteristics of this particular EKG pattern are not fully clarified, this retrospective analysis aimed to compare patients with the de Winter sign with those having typical STEMI changes.
2. METHODS
2.1. Study setting and population
The medical records of all patients with anterior myocardial infarction who were treated at Yongchuan Hospital of Chongqing Medical University Hospital between January 2011 and December 2017 were retrospectively reviewed. EKG was the main focus of this study. Data from EKGs that were conducted at the first medical contact (FMC) in the emergency and cardiology departments were independently evaluated by two cardiologists who were experienced in the interpretation of EKG changes. Patients with an upsloping ST‐segment depression in leads V1 to V6 with a positive symmetrical T wave on EKG were included in the subject group, regardless of the timing of the EKG. Conversely, those with typical STEMI changes and without the former EKG pattern were included in the control group. Demographic data, risk factors, admission characteristics, EKG changes, and angiographic findings of all patients were collected. This study was approved by the appropriate ethics committee and was therefore performed in accordance with the ethical standards laid down in the Declaration of Helsinki and its later amendments. The requirement of informed consent was waived due to this study's retrospective design.
2.2. Data analysis
Data are expressed as a number (percentage) or median (interquartile range). The Mann‐Whitney U test was used to compare median values. Normality was determined using the Shapiro‐Wilks test. Normally distributed continuous variables were compared using the Student's t test. All categorical variables were depicted using frequency distributions, and the χ 2 test was used for comparisons. Two‐sided P values of <0.05 were considered statistically significant. All statistical analyses were performed using SPSS software version 19.0 (SPSS Inc., Chicago, Illinois).
3. RESULTS
Among 441 patients with anterior STEMI, 15 (3.4%) patients had the de Winter EKG pattern (study group). The ST‐segment showed a > 1 mm upsloping ST‐segment depression at the J‐point in the precordial leads that continued into tall, peaked symmetrical T waves and slight ST‐segment elevation in the aVR lead. The QRS complexes were normal or with right bundle branch block in 14 patients and one patient, respectively. In leads V7 to V9 and RV3 to RV5, the ST‐segment was usually normal or slightly depressed (Figure 1). The incidence of this particular EKG pattern in this study (3.4%) was higher than that in the de Winter study (2%) (P = 0.042).
Figure 1.
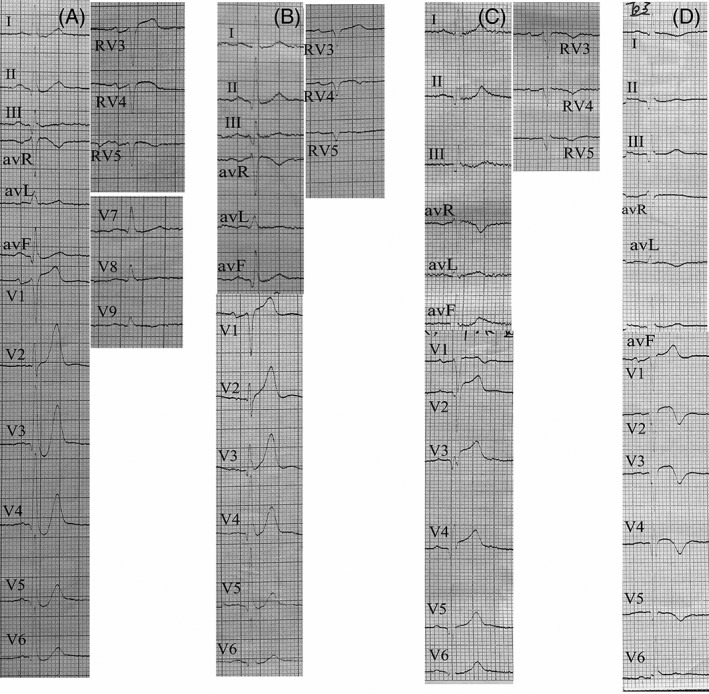
Twelve‐lead electrocardiogram of a patient with de winter electrocardiogram pattern. A, Electrocardiogram (EKG) at first medical contact showed de Winter EKG pattern in the precordial leads. B, Repeat EKG performed 30 min after the first medical contact EKG showed that the J‐point of the ST‐segment returned to baseline. C, Another EKG completed 184 min after the first medical contact EKG showed a typical anterior ST‐segment elevation myocardial infarction. D, 18 h later, T wave inversion was observed
As acute myocardial infarction evolved, the majority of patients (13/15) with the de Winter EKG pattern showed ST‐segment elevation, development of pathologic Q wave, and loss of R wave within a short period. The median time from the recognition of the de Winter EKG pattern to its evolution was 114 minutes (range, 30‐461 minutes). These changes were consistent with those observed in typical STEMI EKGs and mainly occurred in leads V1 to V4. The evolution of this EKG pattern was observed in one patient (Figure 2). Tall symmetrical T waves in the precordial leads were observed in the early phase, followed by an upsloping ST‐segment depression at the J‐point. As ischemia continued, ST‐segment elevation finally appeared. Regarding the remaining two patients in the study group, one patient had an R wave loss without typical ST‐segment elevation, and the other patient exhibited recovery of EKG due to timely reperfusion. Further, the de Winter EKG pattern was recognized in most study group patients (14/15) at the FMC. For the remaining patient (1/15), the EKG pattern was identified during hospitalization for STEMI re‐occlusion following thrombolytic therapy.
Figure 2.
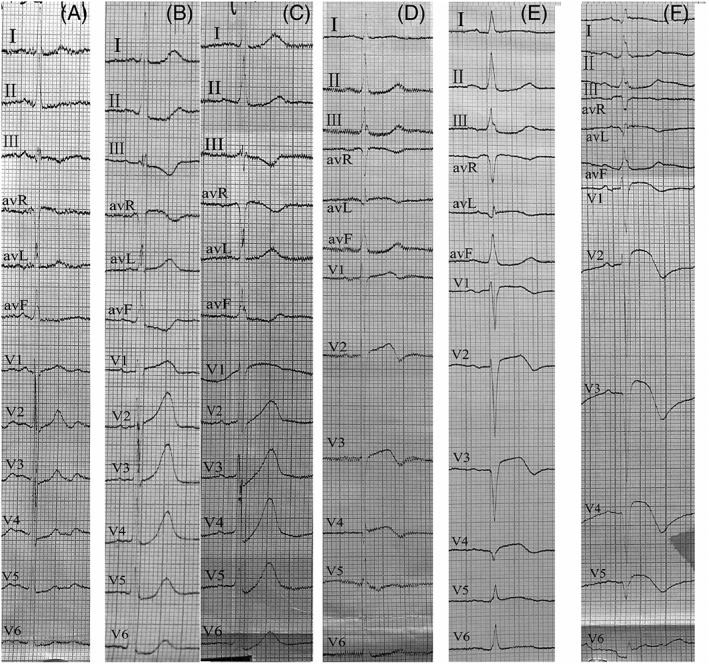
Electrocardiograms of a 71‐year‐old woman. A, The patient was admitted for stable angina. Initial electrocardiogram (EKG) showed slight depression of the ST‐segment in leads V2‐V5. B, An EKG taken 10 min after she felt persistent chest pain showed prominent and symmetric T waves in leads V2 to V4. C. Forty minutes later, the de Winter EKG pattern was observed in the precordial leads. D, 6.3 h later. E, 9 h later. F, 39.3 h later. Finally, the ST‐segment elevation, Q wave formation, and loss of R waves were observed in the anterior wall, which fulfilled the characteristics of the STEMI EKG process
Of the 15 patients in the study group, two patients refused reperfusion treatment and were placed on conservative treatment, and 13 patients received reperfusion therapy following recognition of typical STEMI changes. Of these 13 patients, nine underwent emergency coronary angiography and received drug‐eluting stents in the LAD, except for one patient who received percutaneous transluminal coronary angioplasty (PTCA) in the diagonal branch. This patient was diagnosed with high lateral myocardial necrosis as their EKG showed that the ST‐segment was elevated in the I and aVL leads, in addition to the de Winter EKG pattern in leads V1 to V5. Further, angiography revealed an occlusion in the first diagonal branch. However, the de Winter EKG pattern in leads V1 to V5 was resolved following PTCA of the diagonal branch (Figure 3). Four patients received thrombolytic therapy following the recognition of an ST‐segment elevation. Two out of four patients underwent rescue percutaneous coronary intervention (PCI) following failed thrombolysis, while the other two patients had successful thrombolysis; however, one of the patients experienced re‐occlusion afterward. All patients who underwent angiography had a diffuse multi‐vessel disease, with the most serious stenosis detected in the LAD and the least serious stenosis in the right circumflex artery (RCX).
Figure 3.
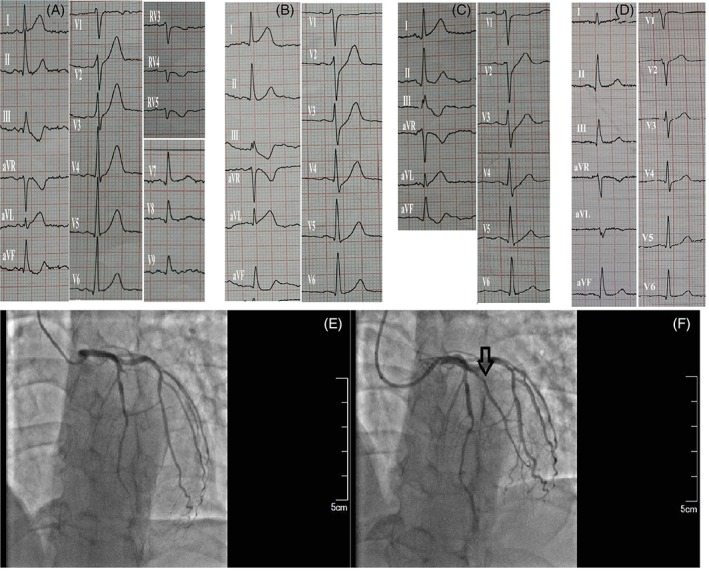
Electrocardiographic and angiographic imaging of a 51‐year‐old male patient. A, At the first medical contact, an initial electrocardiogram (EKG) showed clinical signs of high lateral myocardial infarction for approximately 33 min. The ST‐segment was elevated in the I and aVL leads and a tall symmetric T wave was observed in leads V2‐V5. B, Ninety‐three minutes later, the de Winter EKG sign was observed in leads V2‐V5. E, Angiogram revealed a complete diagonal branch occlusion (arrow). D and F, Blood flow was restored after percutaneous transluminal coronary angioplasty (arrow) and the EKG changes resolved in leads V2‐V5
Complications of patients in the study group were as follows: one patient had severe gastrointestinal bleeding, one patient had stent thrombosis, one patient suddenly died, and one patient experienced electrical storm. Characteristics of the de Winter EKG pattern study group are shown in Table 1.
Table 1.
Characteristics of the de winter electrocardiograph group
| Characteristics | Patients with anterior MI and the de winter electrocardiograph pattern (n = 15) |
|---|---|
| Electrocardiogram | |
| Sinus rhythm | 15 (100) |
| Heart rate(min−1) (median [IQR]) | 75 (52‐108) |
| Conduction n (%) | 11 (73) |
| PR interval, ms | 151.5 ± 22.9 |
| QRS duration, ms | 94.8 ± 14.0 |
| QTc interval, ms | 418.2 ± 18.2 |
| Time from the onset of symptoms to EKG recording; n, min (median) | 15, 108 (18‐302) |
| Time from EKG recording to reperfusion; n (%), min (median) | 12 (80%),182 (15‐420) |
| Time from the de winter EKG pattern to ST‐segment elevation/Q wave appearance; n (%), min (median) | 13 (86.7%), 114 (30‐461) |
| Treatment | |
| Thrombolysis therapy | 4 (27) |
| Medical therapy | 2 (14) |
| Primary PCI | 9 (60) |
| Multi‐vessel diseasea | 11 (100) |
| Pre‐PCI TIMI flow 0‐1 | 8 (72.7) |
| Collateral circulation | 0 (0) |
Abbreviations: IQR, interquartile range; MI, myocardial infarction; PCI, percutaneous coronary intervention; TIMI, thrombolysis in myocardial infarction.
Data available in 11 cases.
Compared to patients with typical anterior STEMI (control group), patients in the study group were younger and had a higher incidence of hypertriglyceridemia, high total cholesterol, and high serum chloride concentration (Table 2).
Table 2.
Baseline characteristics of patients treated with primary percutaneous coronary intervention for anterior myocardial infarction
| Patients with anterior MI and the de winter EKG pattern (n = 15) | Patients with typical anterior STEMI (n = 426) | P value | |
|---|---|---|---|
| Age, years | 60.3 ± 13.1 | 66.7 ± 11.6 | 0.033 |
| Sex, n (%) | |||
| Male | 13 (87) | 298 (70) | 0.161 |
| Female | 2 (13) | 128 (30) | |
| History, n (%) | |||
| Family history of CAD | 2 (13) | 9 (2) | 0.004 |
| Cigarette smoker | 11 (73) | 243 (57) | 0.219 |
| Hypertension | 6 (40) | 183 (43) | 0.819 |
| Diabetes | 2 (13) | 68 (16) | 0.768 |
| Hypercholesterolemia | 5 (33) | 119 (28) | 0.676 |
| Heart failure | 0 (0) | 17 (4) | 0.446 |
| Myocardial infarction | 0 (0) | 30 (7) | 0.294 |
| UCG, n (%) | 7 (47) | 209 (49) | |
| EF | 62.0 ± 13.0 | 53.7 ± 11.7 | 0.068 |
| LV | 50.1 ± 4.8 | 49.2 ± 6.7 | 0.721 |
| LA | 29.0 ± 4.1 | 32.8 ± 5.6 | 0.036 |
| RV | 19.3 ± 1.9 | 19.6 ± 2.5 | 0.606 |
| RA | 32.4 ± 2.7 | 33.2 ± 3.8 | 0.561 |
| HbA1c, n (%) | 10 (67) | 251 (59) | |
| 5.8 ± 0.6 | 6.3 ± 1.6 | 0.489 | |
| Blood lipid, n (%) | 15 (100) | 379 (89) | |
| TC | 5.1 ± 1.3 | 4.5 ± 1.2 | 0.047 |
| LDL | 3.2 ± 1.0 | 2.70 ± 1.0 | 0.075 |
| HDL | 1.0 ± 0.3 | 1.1 ± 0.4 | 0.378 |
| TG | 2.3 ± 1.7 | 1.6 ± 1.3 | 0.01 |
| Fasting blood glucose, n (%) | 14 (93) | 378 (89) | |
| 7.4 ± 1.6 | 7.7 ± 4.0 | 0.255 | |
| Renal function, n (%) | 14 (93) | 409 (96) | |
| UA | 355.9 ± 59.0 | 345.9 ± 109.8 | 0.386 |
| CR | 78.9 ± 16.8 | 82.5 ± 42.7 | 0.533 |
| Blood routine, n (%) | 15 (100) | 405 (95) | |
| WBC | 12.1 ± 4.1 | 11.0 ± 5.4 | 0.136 |
| Hb | 138.6 ± 11.2 | 132.3 ± 21.0 | 0.195 |
| PLT | 225.3 ± 58.2 | 199.6 ± 104.1 | 0.053 |
| Electrolytes, n (%) | 14 (93) | 158 (37) | |
| K, mmol/L | 4.0 ± 0.5 | 3.8 ± 0.5 | 0.26 |
| Na, mmol/L | 140.6 ± 2.7 | 138.7 ± 4.0 | 0.083 |
| Cl, mmol/L | 105.8 ± 3.0 | 103.3 ± 4.4 | 0.038 |
| Stay in hospital‐days | 9.5 ± 5.6 | 9.4 ± 6.2 | 0.954 |
| Cost‐CNY | 47 436.4 ± 32 067.7 | 52 293.9 ± 22 526.9 | 0.455 |
| Clinical adverse events n (%) | 5 (33) | 128 (30) | 0.757 |
Abbreviations: CAD, coronary artery disease; Cl, chloride; CNY, China Yuan; CR, creatinine; EF, ejection fraction; Hb, hemoglobin; HbA1c, glycosylated hemoglobin A1c; HDL, high density lipoprotein; K, potassium; LA, left atrium; LDL, low density lipoprotein; LV, left ventricular; MI, myocardial infarction; Na, sodium; PLT, platelets; RA, right atrium; RV, right ventricular; STEMI, ST‐segment elevation myocardial infarction; TC, total cholesterol; TG, hypertriglyceridemia; UA, uric acid; UCG, ultrasonic cardiogram; WBC, white blood cell.
4. DISCUSSION
Although the de Winter EKG pattern was initially recorded by Dressler in 1947, EKG changes in a large sample of patients were reported in detail by de Winter in 2009.2, 3 That was the first time that these specific EKG changes were linked to findings from coronary angiography, which revealed that the culprit lesion was in the proximal LAD. This specific EKG pattern was considered static and persistent from the time of initial recording at symptom presentation up to instant revascularization.1, 3, 4 This pattern's evolution may not be identified for many reasons, such as a short reperfusion time due to skillful EKG interpretation, correct diagnosis, immediate PCI therapy, and/or minor loss of the myocardium. However, in our study, almost all patients had dramatic changes in leads V1 to V4, which were consistent with the evolution of a typical STEMI and coincided with the findings of previous case reports.5, 6, 7 The lack of attention and recognition of the de Winter EKG pattern contributed to a significant increase in reperfusion time (182 and 60 minutes in our study and de Winter's research, respectively). Most of our patients received reperfusion therapy after identification of an ST‐segment elevation. Therefore, our patients fully exhibited the diversity of evolution, including ST‐segment elevation, R wave loss, and formation of pathologic Q wave. The entire process of this EKG pattern was observed in one patient. Tall, symmetrical T waves in the precordial leads were observed in the early phase, followed by upsloping ST‐segment depression. Thus, we believe that the de Winter EKG pattern is merely an early stage manifestation of the evolution of STEMI that appears following the occurrence of hyperacute T waves.
Both de Winter's patients and our patients who received primary PCI exhibited resolved EKG changes. In addition, one patient had high lateral myocardial necrosis and the de Winter EKG pattern in leads V1 to V5. Since the area of myocardial necrosis is known to be surrounded by an area of ischemia, it is reasonable to speculate that this patient's anterior wall was under ischemic conditions. Moreover, the de Winter EKG pattern resolved following PTCA surgery on the first diagonal branch; hence, we concluded that the ischemia of the anterior wall caused the de Winter EKG pattern in leads V1 to V5. This latter finding indicated that this specific EKG pattern may be a sign of ischemia rather than necrosis.
To our knowledge, this study is the first to report patients with the de winter EKG pattern who received thrombolytic therapy following the identification of an ST‐segment elevation. However, two patients failed thrombolysis and one patient had re‐occlusion following successful thrombolysis, which suggested that patients with this EKG pattern respond poorly to thrombolytic therapy and that PCI therapy may be superior to thrombolytic therapy, even in patients with subsequent ST‐segment elevation. This is further supported by the guideline that recommends that suspicion of an ongoing myocardial ischemia is an indication for a primary PCI strategy, even in patients without a diagnosis of an ST‐segment elevation.8
In our study, patients with the de Winter EKG pattern developed various clinical adverse events. This may be partly due to the absence of a typical ST‐segment elevation or missed diagnosis of this EKG pattern by the medical staff, which may have led to failure of providing timely reperfusion treatment and subsequently, a large area of myocardial injury and poor prognosis.
In de Winter's research, 67% of patients had single LAD lesions. Conversely, in our study, all patients who underwent angiography had a diffuse multi‐vessel disease, with the most serious stenosis detected in the LAD and the least in the RCX. The reason for this finding may be that all of our male patients were heavy smokers, and it was previously reported that patients with coronary artery disease who were smokers had diffuse and more severe lesions in the coronary artery than those who were non‐smokers.9, 10, 11
Of the 15 patients in the study group, 6 patients had the right‐sided and posterior precordial leads recorded, which has not been previously reported. Our results revealed that the ST‐segment was normal or slightly depressed in the V3R to V5R and V7 to V9 leads. Posterior infarction can also present with an anterior ST‐segment depression; however, in this study, posterior infarction was not detected, even after the left circumflex artery (LCX) and the right coronary artery were stenosed. The ST‐segment depression in leads V7 to V9 resolved following reperfusion therapy of the LAD or the first diagonal branch, which suggested that the EKG changes in leads V7 to V9 were not caused by lesions in the LCX or RCX.
In de Winter's research, 2% of patients with LAD occlusion demonstrated the de Winter EKG pattern, whereas in our study, 3.4% of patients with anterior myocardial infarctions that were caused by an occlusion in the LAD or its branches exhibited the characteristic EKG sign. Similar to de Winter's research, 14 of the 15 patients with this EKG pattern in our study were diagnosed at the FMC, while the remaining patient was diagnosed during myocardial infarction. This suggests that the difference in the incidence of this EKG pattern between our patients and de Winter's patients was likely due to the emergence of the de Winter EKG pattern being identified at both the FMC and during myocardial infarction.
The lack of activation of the sarcolemmal adenosine triphosphate (ATP) sensitive potassium (KATP) channels was believed to cause the absence of ST‐segment elevation in patients.1 Because in knockout animal models of acute ischemia, the ST‐segment was not elevated, which was consistent with de Winter's findings on the staticity of this EKG pattern.12 However, unlike former animal experiments, our results revealed that almost all patients with this EKG pattern developed an ST‐segment elevation if they did not receive primary PCI. Therefore, the function of KATP channels in patients with the de Winter EKG pattern needs further exploration. In the hyperacute phase of STEMI, tall, symmetrical T waves are caused by subendocardial ischemia. In the acute phase of STEMI, ST‐segment elevation is caused by transmural ischemia. Therefore, there is the possibility that a critical time of ischemia exists when the ischemia expanded from the subendocardium to the epicardium at the point that the ST‐segment change was initial and subtle (Figure 1B).13, 14 In isolated canine ventricular wedge preparations subjected to 0‐flow ischemia, intramural unipolar electrograms that were positioned transmurally (endocardium, M3, M2, M1, and epicardium) showed the characteristic de Winter EKG pattern in the mid‐myocardium.15 The different sensitivities of ischemia between those in the epicardium and those in the endocardium, especially in the cells at the junction between the mid‐myocardium and endocardium, may play an important role in the development of this EKG pattern. The mid‐myocardium's responsiveness to oxygen deprivation may be the main reason for the occurrence of the de Winter EKG pattern.
Further, some patients with non‐cardiac diseases present with the de Winter EKG pattern. A possible cause of depression of the anterior ST‐segment is tachycardia. Since tachycardia can cause upsloping ST‐segment depression as well as elevation in troponin levels as result of ischemia, it may be difficult to distinguish between the ischemia causing the de Winter EKG pattern and that causing tachycardia.16 In our study, patients with the de Winter EKG pattern had a normal heart rate (mean, 75 beats/min), which suggests that this EKG pattern needs to be recognized as a manifestation of ischemia in patients with chest pain symptoms.
5. LIMIATATIONS
Our study had a few limitations. First, this was a single center, retrospective study with a small sample size, which may have led to an inaccurate assessment of the incidence of the de Winter EKG pattern, despite reporting that this specific EKG pattern can occur in the event of the disease and over the course of STEMI. Second, almost all of our patients received reperfusion therapy following identification of ST‐segment elevation, which may have caused the significant increase in reperfusion time and subsequently, an increase in the number of clinical adverse events. Other techniques can be utilized to assess chest pain in patients. Transthoracic echocardiography may provide evidence of focal wall motion abnormalities and facilitate triage in patients with EKG findings that are difficult to interpret.17 Third, the mechanism of this EKG pattern is merely a speculation that needs further exploration, especially at the molecular and iron‐channel levels, in order to clarify the reason for its main development in the anterior wall.
6. CONCLUSIONS
In conclusion, we believe that the de Winter EKG pattern is a sign of ischemia. In almost all patients with this EKG pattern who did not receive primary PCI treatment, the pattern will evolve into STEMI that mainly occurred in the anterior wall. Moreover, the infarct‐related arteries were the LAD and its branches. The ST‐segment was normal or slightly depressed in the right‐sided and posterior precordial leads when the precordial leads showed an ST‐segment depression at the J‐point with upsloping ST‐segments continuing into tall, symmetrical T waves.
ACKNOWLEDGMENTS
Aihua Wang should be treated as joint first author. We thank Professor Liao Ronghong, Vincent Lim, and Wang Liya for their exceptional help with the paper.
Conflict of interest
The authors declare no potential conflict of interests.
Xu J, Wang A, Liu L, Chen Z. The de winter electrocardiogram pattern is a transient electrocardiographic phenomenon that presents at the early stage of ST‐segment elevation myocardial infarction. Clin Cardiol. 2018;41:1177–1184. 10.1002/clc.23002
REFERENCES
- 1. de Winter RJ, Verouden NJ, Wellens HJ, Wilde AA, Interventional Cardiology Group of the Academic Medical Center . A new ECG sign of proximal LAD occlusion. N Engl J Med. 2008;359:2071‐2073. [DOI] [PubMed] [Google Scholar]
- 2. Dressler W, Roesler H. High T waves in the earliest stage of myocardial infarction. Am Heart J. 1974;34:627‐645. [DOI] [PubMed] [Google Scholar]
- 3. Verouden NJ, Koch KT, Peters RJ, et al. Persistent precordial “hyperacute” T‐waves signify proximal left anterior descending artery occlusion. Heart. 2009;95:1701‐1706. [DOI] [PubMed] [Google Scholar]
- 4. de Winter RW, Adams R, Verouden NJ, et al. Precordial junctional ST‐segment depression with tall symmetric T‐waves signifying proximal LAD occlusion, case reports of STEMI equivalence. J Electrocardiol. 2016;49:76‐80. [DOI] [PubMed] [Google Scholar]
- 5. Goktas MU, Sogut O, Yigit M, Kaplan O. A novel electrocardiographic sign of an ST‐segment elevation myocardial infarction‐equivalent: de winter syndrome. Cardiol Res. 2017;8:165‐168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goebel M, Bledsoe J, Orford JL, et al. A new ST‐segment elevation myocardialinfarction equivalent pattern? Prominent T wave and J‐point depression in the precordial leads associated with ST‐segment elevation in lead aVr. Am J Emerg Med. 2014;32:287.e5‐287.e8. [DOI] [PubMed] [Google Scholar]
- 7. Sheng F, He M, Zhang M, Shen GY. A STEMI equivalent of de winter sign missed by an emergency physician. J Electrocardiol. 2016;49:620‐622. [Google Scholar]
- 8. Ibanez B, James S, Agewall S, et al. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST‐segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2017, 2018;39:119‐177. [DOI] [PubMed] [Google Scholar]
- 9. Doyle JT, Dawber TR, Kannel WB, et al. The relationship of cigarette smoking to coronary heart disease; the second report of the combined experience of the Albany, NY and Framingham, Mass. Studies. JAMA. 1964;190:886‐890. [PubMed] [Google Scholar]
- 10. Wang XL, Tam C, McCredie RM, et al. Determinants of severity of coronary artery disease in Australian men and women. Circulation. 1994;89:1974‐1981. [DOI] [PubMed] [Google Scholar]
- 11. Waters D, Lespérance J, Gladstone P, et al. Effects of cigarette smoking on the angiographic evolution of coronary atherosclerosis. A Canadian coronary atherosclerosis intervention trial (CCAIT) substudy. CCAIT study group. Circulation. 1994;94:614‐621. [DOI] [PubMed] [Google Scholar]
- 12. Li RA, Leppo M, Miki T, Seino S, Marban E. Molecular basis of electrocardiographic ST‐segment elevation. Circ Res. 2000;87:837‐839. [DOI] [PubMed] [Google Scholar]
- 13. Birnbaum Y. Bayés de Luna a, Fiol M, et al. common pitfalls in the interpretation of electrocardiograms from patients with acute coronary syndromes with narrow QRS: a consensus report. J Electrocardiol. 2012;45:463‐475. [DOI] [PubMed] [Google Scholar]
- 14. Gorgels AP. ST‐elevation and non‐ST‐elevation acute coronary syndromes: should the guidelines be changed? J Electrocardiol. 2013;46:318‐323. [DOI] [PubMed] [Google Scholar]
- 15. Di Diego JM, Antzelevitch C. Acute myocardial ischemia: cellular mechanisms underlying ST segment elevation. J Electrocardiol. 2014;47:486‐490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Agewall S, Giannitsis E, Jernberg T. Troponin elevation in coronary vs. non‐coronary disease. Eur Heart J. 2011;32:404‐411. [DOI] [PubMed] [Google Scholar]
- 17. American College of Emergency Physicians , Society for Cardiovascular Angiography and Interventions , O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:e78‐e140. [DOI] [PubMed] [Google Scholar]


