Abstract
Clinical trials have demonstrated the benefits of cardiac implantable electrical devices, which include pacemakers, implantable cardioverter‐defibrillators (ICDs), and cardiac resynchronization therapy (CRT), with respect to key clinical outcomes and survival. Women more often require permanent pacing for sick sinus syndrome, whereas atrioventricular block is more common in men. Women appear to have a higher incidence of complications with pacemaker implantation, as well as with ICD and CRT implantation. The indications for ICDs and CRT do not have any distinctions based on sex, and outcomes are comparable in men and women. In fact, women often seem to have better outcomes with CRT compared with men. Despite the demonstrated benefits of these devices, ICDs and CRT are underutilized in women. In this review, we explore sex differences in utilization, outcomes, and complications with pacemakers, ICDs, and CRT.
Keywords: Cardiac Resynchronization Therapy, Gender, Implantable Cardioverter‐Defibrillators, Pacemakers, Sex
1. INTRODUCTION
Cardiac implantable electrical devices, a term that includes pacemakers, implantable cardioverter‐defibrillators (ICDs), and cardiac resynchronization therapy (CRT), have had a major impact on symptoms, morbidity, mortality, and quality of life in patients who meet indications for these devices. In this review, we will focus on sex‐based differences in the utilization, outcomes, and complications of these devices.
2. PACEMAKERS
2.1. Indications and utilization
The main indications for pacemaker implantation in women have been shown to be sick sinus syndrome (SSS) and atrial fibrillation (AF) with bradyarrhythmias, as reported in a retrospective study by Nowak et al.,1 among 17 826 patients (47.2% women) in Germany undergoing primary pacemaker implantation. The main indication in men was atrioventricular block. Women tend to be older than men at the time of initial pacemaker implantation, and indeed, the mean age of women in the Nowak study was 77.3 ± 10.2 years, vs 74.0 ± 10.4 years in men (P < 0.01). In patients age > 80 years, men received more dual‐chamber pacemakers than did women.1
Studies addressing the influence of sex on the selection of cardiac pacemakers have reached varying conclusions. Roeters Van Lennep et al2 analyzed data from a pacemaker registry (N = 33 564; 41.4% women) and concluded that there was no significant difference in the selection of pacemakers based on sex. On the other hand, Schüppel et al3 found that women were less likely to receive dual‐chamber pacemakers than were men. In patients age ≥ 80 years, women predominantly received single‐chamber ventricular devices, whereas men received more dual‐chamber devices. Guha et al4 analyzed 328 670 patients admitted for SSS (54.2% women) and found that women had lower rates of pacemaker implantation compared with men.
Essebag et al5 found that the use of amiodarone in the treatment of patients with new‐onset AF significantly increased the risk of bradyarrhythmias requiring pacemaker insertion in women (hazard ratio [HR]: 4.69, 95% confidence interval [CI]: 1.99‐11.05, P = 0.02) but not in men. This finding was independent of weight, basal metabolic index, or amiodarone dose.
For pacemakers as well as for other implantable devices, cosmetic considerations may make submammary implantation an attractive option. Especially in younger women, a discussion about location of device is warranted, with the involvement of a plastic or breast surgeon as needed.
2.2. Outcomes
A 30‐year follow‐up study analyzing the prognostic importance of baseline patient characteristics influencing the survival of patients with permanent pacemakers concluded that women survived longer than men despite a higher mean age at the time of implantation (Figure 1).6 In general, quality of life (QOL) improves in patients after pacemaker implantation. The Canadian Trial of Physiological Pacing (CTOPP)7 did not find any sex differences in QOL after pacemaker implantation, whereas the Mode Selection Trial (MOST) found higher QOL scores and improved functional status in men compared with women.8
Figure 1.

Kaplan–Meier analysis of survival after permanent pacemaker implantation stratified by sex. The overall median survival time after pacemaker implantation was significantly higher in women, at 118 months in women vs 92 months in men (P < 0.0001). Reproduced with permission from Brunner et al6
2.3. Complications
Irrespective of age or type of pacemaker implantation, periprocedural complications such as pneumothorax and pocket hematoma are more common in women.1 Although the reasons for the higher complication rates in women are not well understood, smaller body habitus may be a contributing factor. Hospitalizations for cardiac device–related infections have been reported to be more common in men.9 On the other hand, Debski et al10 did not find any sex difference in the incidence of cardiac‐device infections.
2.4. Leadless pacemakers
Leadless pacemakers currently are available as single‐chamber ventricular pacemakers. They are an attractive option for patients who do not need atrial leads, such as those with a rare need for pacing or those with permanent AF. When compared with transvenous single‐chamber ventricular pacemakers, leadless ventricular pacemakers avoid problems with pocket‐ and lead‐related complications. However, clinical trials have shown a higher rate of cardiac perforation and pericardial effusion with leadless pacemakers.11 At the present time, there are no data on any sex differences in outcomes or complications specifically in leadless pacemakers.
2.5. Summary
The main differences between men and women with respect to permanent pacemakers include differences in common indications (atrioventricular block in men and SSS in women), better survival in women after implantation, but a higher rate of complications in women. Studies have shown lower implantation rates in women despite comparable indications. The approach to pacemaker implantation in patients should be sex‐neutral, but perhaps with particular attention to potential vascular and other complications in women.
3. CARDIAC RESYNCHRONIZATION THERAPY
3.1. Indications and utilization
Current class I 2013 American College of Cardiology Foundation/American Heart Association (ACCF/AHA) guideline indications for CRT include left ventricular ejection fraction (LVEF) ≤35%, left bundle branch block (LBBB) with QRS duration (QRSd) ≥150 ms, and New York Heart Association (NYHA) class II, III, or ambulatory IV symptoms on guideline‐directed medical therapy.12 There are class IIa recommendations for less extreme QRS prolongation and/or non‐LBBB morphology. Women are more likely to have nonischemic cardiomyopathy, for which CRT has yielded better results compared with ischemic cardiomyopathy. The Registry to Improve the Use of Evidence‐Based Heart Failure Therapies in the Outpatient Setting (IMPROVE HF) prospective cohort study showed that CRT was underutilized in patients who met indications by current guidelines, but there was no sex difference in utilization.13
3.2. Outcomes
Women have been underrepresented in clinical trials of CRT, despite the fact that the prevalence of heart failure (HF) is similar in men and women (Table 1). In the Multicenter InSync Randomized Clinical Evaluation (MIRACLE) study, women receiving CRT experienced longer times to first HF‐related hospitalization or death (HR: 0.157).14 In the Cardiac Resynchronization–Heart Failure (CARE‐HF) and Resynchronization for Ambulatory Heart Failure (RAFT) trials, there was a significant and comparable mortality benefit in both men and women.15, 16 In the Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) trial, there was a significant reduction in death and hospitalization in the trial as a whole.17 In subgroup analysis, the trends were the same for men and women, although the CIs for women were wide and crossed 1.0.
Table 1.
Major CRT trials with outcomes
| Trial | Total No. of Subjects (% Female) | Inclusion Criteria | Randomization | HR for Events (95% CI) |
|---|---|---|---|---|
| COMPANION16 | 1520 (33) | LVEF ≤35%, NYHA class III–IV, QRSd ≥120 ms | OMT vs OMT + CRT‐D | HR for death: men, 0.63 (0.4‐0.9); women, 0.58 (0.25‐1.13) |
| CARE‐HF14 | 813 (27) | LVEF ≤35%, NYHA class III–IV, QRSd ≥120 ms, LVEDD ≥30 mm | OMT vs OMT + CRT | HR for death or cardiac hospitalization: men, 0.62 (0.49‐0.79); women, 0.64 (0.42‐0.97) |
| MADIT‐CRT17 | 1820 (25) | LVEF ≤30%, NYHA class I–II, QRSd ≥130 ms | ICD vs CRT‐D | HR for HF event or death: men, 0.76 (0.59‐0.97); women, 0.37 (0.22‐0.61) |
| RAFT15 | 1798 (17) | LVEF ≤30%, NYHA class II–III, QRSd ≥120 ms | ICD vs CRT‐D | HR death or HF admission: men, 0.82 (0.7‐0.95); women, 0.52 (0.35‐0.85). Difference between men and women is not significant (P = 0.09). |
| MIRACLE13 | 453 (32) | LVEF ≤35%, NYHA class III–IV, QRSd ≥130 ms | Placebo vs CRT | Women, but not men, with CRT experienced longer times to first HF hospitalization or death (HR in women: 0.157). |
Abbreviations: CARE‐HF, Cardiac Resynchronization–Heart Failure; CI, confidence interval; COMPANION, Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure; CRT, cardiac resynchronization therapy; CRT‐D, cardiac resynchronization therapy with defibrillator; HF, heart failure; HR, hazard ratio; ICD, implantable cardioverter‐defibrillator; LVEDD, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; MADIT‐CRT, Multicenter Automatic Defibrillator Implantation Trial With Cardiac Resynchronization Therapy; MIRACLE, Multicenter InSync Randomized Clinical Evaluation; NYHA, New York Heart Association; OMT, optimal medical therapy; QRSd, QRS duration; RAFT, Resynchronization for Ambulatory Heart Failure; REVERSE, Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction.
Reproduced with permission and modified from Tompkins et al.34
The Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy (MADIT‐CRT trial)18 enrolled 1820 patients, of whom 453 were women. Eligible patients had NYHA class I and II symptoms, LVEF ≤30%, and QRSd ≥130 ms. Patients were randomized to CRT with defibrillator (CRT‐D) or ICD alone, with the primary outcome measure being HF events or death. The primary endpoint was met in 11% of women with CRT‐D, vs 20% of men (P < 0.01; Figure 2). Women were also found to have significantly greater echocardiographic evidence of reverse cardiac modeling (significant improvement in LVEF, left ventricular end‐systolic volume, and left ventricular end‐diastolic volume) than men. The investigators attributed these sex differences in outcomes partly to differences in baseline characteristics, such as more nonischemic cardiomyopathy (76% vs 36%; P < 0.001) and LBBB morphology (87% vs 65%; P < 0.01) in women than in men, respectively, which are known predictors of better outcomes with CRT.
Figure 2.

Kaplan–Meier estimates of cumulative probability of HF or death stratified by sex and ICD or CRT‐D therapy. Reproduced with permission from Arshad et al.18 Abbreviations: CRT‐D, cardiac resynchronization therapy‐defibrillator; HF, heart failure; ICD, implantable cardioverter‐defibrillator
Given the underrepresentation of women in CRT trials, the class IIa indication for CRT for a QRSd of 120 to 149 ms may be more reflective of the results found in men, who have demonstrated less benefit at this QRSd. With a shorter QRSd at baseline in normal women compared with normal men, women have relatively more dyssynchrony for any particular prolonged QRSd, a factor that might contribute to the better outcomes observed in women with CRT.18
A meta‐analysis of 3 major CRT‐D vs ICD clinical trials (MADIT‐CRT, RAFT, and the Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction Study [REVERSE]) was conducted by the US Food and Drug Administration to assess CRT benefit in women.16, 18, 19, 20 These studies included patients with mild HF alone. There were 22% women among the total of 4076 patients in these trials. Women had more LBBB (85% vs 68%) and less ischemic cardiomyopathy (33% vs 67%) than did men. This analysis found a better outcome in women with LBBB at a QRSd of 130 to 149 ms compared with men with LBBB (Figure 3). In this subgroup, women had a 76% relative reduction in HF or death (HR: 0.24, 95% CI: 0.11‐0.53, P < 0.001) and a 76% relative reduction in death alone (HR: 0.24, 95% CI: 0.06‐0.89, P < 0.03) compared with no significant benefit in men in either of the endpoints. Loring et al21 did a retrospective study in 144 642 CRT‐D recipients and found that LBBB was associated with a 31% reduction in death in women (HR: 0.69, 95% CI: 0.67‐0.71) compared with 16% in men. A possible reason for this difference may be the use of conventional electrocardiographic criteria for the diagnosis of LBBB, leading to more false‐positive LBBB diagnoses in men. Strauss et al. proposed sex‐specific stricter LBBB criteria (QRSd ≥140 ms for men and ≥130 ms for women, along with mid‐QRS notching or slurring in ≥2 contiguous leads),22 the use of which, they proposed, would lead to better identification of patients most likely to benefit from CRT. However, Bertaglia et al23 did an observational study in 335 patients comparing the echocardiographic response to CRT using strict vs conventional LBBB criteria. The authors found no significant difference in the echocardiographic response to CRT between these 2 groups over the course of 12 months. The relatively shorter follow‐up period was a major limitation of this study.
Figure 3.
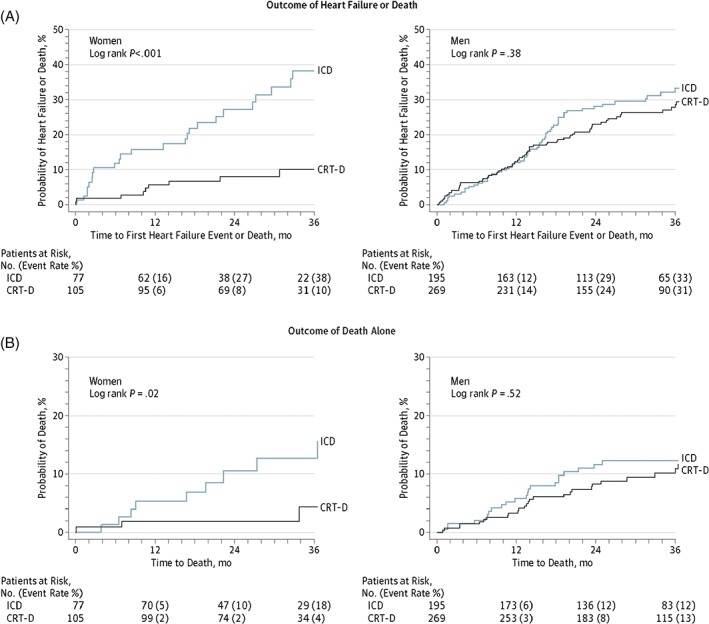
Kaplan–Meier estimates of CRT‐D vs ICD outcomes in LBBB and QRSd of 130 to 149 ms stratified by sex. Reproduced with permission from Zusterzeel et al.20 Abbreviations: CRT‐D, cardiac resynchronization therapy‐defibrillator; ICD, implantable cardioverter‐defibrillator; LBBB, left bundle branch block; QRSd, QRS duration
3.3. Complications
In the MADIT‐CRT trial,18 women had a higher likelihood of all device‐related adverse events compared with men, at 10.5% vs 7.9%, respectively (P = 0.001). Women had more pneumothoraces (3% in women vs 0.73% in men), but men had more lead dislodgements (1.7% in women vs 3.2% in men).
3.4. Summary
Although current guidelines do not have any differences in indications for CRT based on sex, it is clear that, in general, women are more likely to benefit than men. The higher incidence of nonischemic cardiomyopathy in women and the greater relative difference for any prolonged QRSd in women compared with the normal QRSd both contribute to this observation. Although complication rates in women are somewhat higher than in men, the greater expected benefit in women indicates that physicians should be more willing to consider CRT implantation in women with class IIa indications.
4. IMPLANTABLE CARDIOVERTER‐DEFIBRILLATORS
4.1. Indications and utilization
There are no distinctions based on sex for the indications for primary‐ and secondary‐prevention ICDs.12 Although secondary‐prevention ICDs are implanted in patients who have had a potentially life‐threatening ventricular arrhythmia, primary‐prevention ICDs are indicated in patients with ischemic or nonischemic cardiomyopathy and symptomatic HF (except in patients with ischemic cardiomyopathy and LVEF ≤30%). ICDs are also indicated in patients with certain genetic syndromes and a variety of other conditions, but they are outside the scope of this review.
A recent observational study by Hess et al24 examined whether patients with HF and an LVEF ≤35% received counseling about ICD placement. Among >21 000 patients across 236 sites, they found that only one‐fifth of patients who were eligible for ICD placement received counseling. Furthermore, they determined that women were counseled less frequently than men (19.3% vs 24.6%; P < 0.001).
A similar study by Hernandez et al25 involved an observational analysis of >13 000 patients admitted with HF and LVEF of ≤30%. They found that among those patients eligible for ICD therapy, only 35.4% received ICD therapy at discharge. They found a particularly low rate of ICD implantation in women and minorities. After adjusting for confounding variables, they found the adjusted odds ratio of ICD use to be 0.62 (95% CI: 0.56‐0.68) for white women and 0.56 (95% CI: 0.44‐0.71) for black women, compared with white men.
Amit et al26 aimed to compare the indications for ICD implantation as well as outcomes in women with those in men in a prospective study. All patients with ICDs or CRT‐Ds implanted in Israel from July 2010 to February 2013 were included, with a median follow‐up of 1 year. Of the 3544 subjects included, only 17% were women. There was no significant difference between men and women in the cumulative probability of appropriate ICD therapy or death.
The finding that women are less likely than men to receive ICD therapy was further demonstrated by Curtis et al.27 They used diagnostic codes to identify primary‐ and secondary‐prevention cohorts in patients who were eligible for ICD therapy. In their sample of >230 000 Medicare patients, in patients with prior myocardial infarction and either cardiomyopathy or HF (primary‐prevention cohort), they found that men were 3.2× more likely than women to receive ICD therapy. Among patients with a history of cardiac arrest or ventricular tachycardia (VT; secondary‐prevention cohort), men were 2.4× more likely than women to receive therapy.
4.2. Outcomes
The major randomized clinical trials comparing ICDs with medical therapy have shown a significant mortality benefit in men, but the results in women have been equivocal28, 29, 30 (Table 2). It has been difficult to draw firm conclusions, given the low representation of women in these trials and the resulting low statistical power. A meta‐analysis of 5 major randomized clinical trials of ICD therapy found a mortality benefit in men (HR: 0.78, 95% CI: 0.70‐0.87, P < 0.001) but not in women (HR: 1.01, 95% CI: 0.76‐1.33, P = 0.95).31
Table 2.
Major ICD trials and outcomes
| Trial | Total Population | Indication | % Women | Randomization | Mortality |
|---|---|---|---|---|---|
| MADIT I38 | 196 | Primary prevention of SCD | 8 | Antiarrhythmic drug therapy vs ICD | 54% relative reduction in mortality in ICD group; not stratified by sex |
| MADIT II39 | 1232 | Primary prevention of SCD | 16 | ICD vs medical therapy | Men: HR: 0.66, P = 0.011; women: HR: 0.57, P = 0.132 |
| SCD‐HeFT28 | 2521 | Primary prevention of SCD | 23 | ICD vs medical therapy + amiodarone vs medical therapy alone | Men: HR: 0.73, 95% CI: 0.57‐0.93; women: HR: 0.96, 95% CI: 0.58‐1.61 |
| MUSTT30 | 704 | Primary prevention of SCD | 10 | Standard medical therapy vs medical therapy plus EP‐guided therapy | Mortality in EP‐guided therapy group: men, 21%; women, 32% (P = 0.13) |
| DEFINITE29 | 458 | Primary prevention of SCD | 29 | Medical therapy vs ICD | Men: HR: 0.49, 95% CI: 0.27‐0.90, P = 0.019; women: HR: 1.14, 95% CI: 0.50‐2.64, P = 0.754 |
| AVID40 | 1885 | Secondary prevention of SCD | 22 | Antiarrhythmic drug therapy vs ICD | Mortality in women, 15.5%, and men, 14.4%, compared with 24.5% in patients without ICD |
Abbreviations: AVID, Antiarrhythmics Versus Implantable Defibrillators; CI, confidence interval; DEFINITE, Defibrillators in Non‐Ischemic Cardiomyopathy Treatment Evaluation; EP, electrophysiology; HR, hazard ratio; ICD, implantable cardioverter‐defibrillator; MADIT, Multicenter Automatic Defibrillator Implantation Trial; MUSTT, Multicenter Unsustained Tachycardia Trial; SCD, sudden cardiac death; SCD‐HeFT, Sudden Cardiac Death in Heart Failure Trial.
Reproduced with permission and modified from Narasimha et al.40
Barra et al32 performed an observational multicenter cohort study in 5307 patients with ischemic or nonischemic dilated cardiomyopathy with no history of sustained ventricular arrhythmias. They compared survival in patients with CRT‐D with patients with CRT without defibrillator functionality (CRT–pacemaker [CRT‐P]) and stratified the results by sex. At a median follow‐up of 34 months, using inverse probability, a benefit of CRT‐D over CRT‐P was seen in male patients (HR: 0.78, 95% CI: 0.65‐0.94, P = 0.012) but not in women. This study concluded that the addition of a defibrillator to CRT might convey a benefit only in well‐selected male patients. The authors attributed this less beneficial effect of adding an ICD to CRT in women to a lower risk of sudden cardiac death and a higher prevalence of nonischemic cardiomyopathy in women. The authors acknowledged that the study was not powered to detect small differences in women, as they only accounted for 22% of the total study population.
In a large multicenter cohort study, sex differences in primary‐prevention ICD utilization and outcomes in patients with ischemic and nonischemic cardiomyopathy were studied by Providência et al.33 Only 15.1% of the patients in the study were female, and 53.8% of the women received CRT‐D therapy. Fewer appropriate therapies were observed in women (HR: 0.59, 95% CI: 0.45‐0.76, P < 0.001). Although there was no sex difference in mortality in the study overall, lower mortality was observed in women who received CRT‐D (HR: 0.68, 95% CI: 0.47‐0.97, P = 0.034).
Tompkins et al34 evaluated the influence of sex on arrhythmic events or death in subjects from the MADIT‐CRT trial.18 They found that women were 38% less likely to experience VT, ventricular fibrillation (VF), or death compared with men (HR: 0.62, 95% CI: 0.49‐0.79, P < 0.001). In comparison with men, women with ischemic cardiomyopathy were 49% less likely to develop VT/VF (P = 0.003). However, the frequency of VT, VF, or death did not differ significantly between men and women with nonischemic cardiomyopathy (P = 0.063). Interestingly, they demonstrated that women had an increased risk of death after the first appropriate shock compared with men.
As mentioned earlier, the impact of ICD placement on survival in high‐risk individuals has been well documented in the literature. Gopinathannair at el35 evaluated the effect of ICD placement on quality of life (QOL). They followed 1461 patients after ICD placement and evaluated QOL at 1 year. They reported that at baseline, women had a lower QOL; but 1 year after ICD placement, they had similar improvement in QOL scores from baseline compared with men. Thus, not only are there survival benefits for patients who are indicated for ICDs, there is also evidence to suggest QOL improves with ICD placement in both men and women.
4.3. Complications
Peterson et al36 did a retrospective study in 161 470 patients (27% women) undergoing ICD implantation from the National Cardiovascular Data Registry ICD Registry. Women had a higher rate of any adverse events than men (4.4% vs 3.3%; P < 0.001). After adjusting for confounding factors, women still had significantly higher odds of any adverse event (odds ratio: 1.32, 95% CI: 1.24‐1.39). In addition, older women had significantly more complications than did younger women. Hematoma and lead dislodgement were the most common complications. Individual complications predominant in women included cardiac perforation, conduction block, coronary venous dissection, lead dislodgment, hemothorax, pneumothorax, deep phlebitis, and pericardial tamponade.
In a retrospective study37 of 2954 patients (26% women) undergoing ICD implantation for the primary prevention of sudden cardiac death, the rate of device‐related complications was higher in women than in men (10.74% vs 7.52%, respectively; adjusted OR: 1.38, 95% CI: 1.01‐1.90). When analyzing individual complications, women had a significantly higher risk of developing tamponade than did men (1.89% vs 0.47%, respectively; P < 0.001). Other individual complications were similar in both groups, including infection and reimplantation rates.
4.4. Summary
Although the indications for ICD therapy are the same in women and men, women have a lower rate of ICD implantation. There is some indication from clinical trials that the survival benefit from ICDs is greater in men, which may be related to the higher incidence of ischemic cardiomyopathy and VT/VF in men. However, women have been underrepresented in clinical trials of ICD therapy, making it difficult to draw any firm conclusions. At the present time, decisions on ICD implantation should not differ in men and women. As with other implantable devices, however, the complication rate is acknowledged to be somewhat higher in women.
Conflicts of interest
Anne B. Curtis: Abbott: medical advisory board; data monitoring committee for clinical trials; honoraria for speaking; Medtronic, Inc.: data monitoring committee for clinical trial; honoraria for speaking.
Elango K, Curtis AB. Cardiac implantable electrical devices in women. Clin Cardiol. 2018;41:233–239. 10.1002/clc.22903
REFERENCES
- 1. Nowak B, Misselwitz B, Erdogan A, et al. Do gender differences exist in pacemaker implantation?—results of an obligatory external quality control program. Europace. 2010;12:210–215. [DOI] [PubMed] [Google Scholar]
- 2. Roeters Van Lennep JE, Zwinderman AH, Roeters Van Lennep HW, et al. No gender differences in pacemaker selection in patients undergoing their first implantation. Pacing Clin Electrophysiol. 2000;23:1232–1238. [DOI] [PubMed] [Google Scholar]
- 3. Schüppel R, Büchele G, Batz L, et al. Sex differences in selection of pacemakers: retrospective observational study. BMJ. 1998;316:1492–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guha A, Xiang X, Haddad D, et al. Eleven‐year trends of inpatient pacemaker implantation in patients diagnosed with sick sinus syndrome. J Cardiovasc Electrophysiol. 2017;28:933–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Essebag V, Reynolds MR, Hadjis T, et al. Sex differences in the relationship between amiodarone use and the need for permanent pacing in patients with atrial fibrillation. Arch Intern Med. 2007;167:1648–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brunner M, Olschewski M, Geibel A, et al. Long‐term survival after pacemaker implantation: prognostic importance of gender and baseline patient characteristics. Eur Heart J. 2004;25:88–95. [DOI] [PubMed] [Google Scholar]
- 7. Newman D, Lau C, Tang AS, et al. Effect of pacing mode on health‐related quality of life in the Canadian Trial of Physiologic Pacing. Am Heart J. 2003;145:430–437. [DOI] [PubMed] [Google Scholar]
- 8. Fleischmann KE, Orav EJ, Lamas GA, et al. Pacemaker implantation and quality of life in the Mode Selection Trial (MOST). Heart Rhythm. 2006;3:653–659. [DOI] [PubMed] [Google Scholar]
- 9. Sridhar AR, Lavu M, Yarlagadda V, et al. Cardiac implantable electronic device‐related infection and extraction trends in the U.S. Pacing Clin Electrophysiol. 2017;40:286–293. [DOI] [PubMed] [Google Scholar]
- 10. Debski M, Ulman M, Zabek A, et al. Gender differences in dual‐chamber pacemaker implantation indications and long‐term outcomes. Acta Cardiol. 2016;71:41–45. [DOI] [PubMed] [Google Scholar]
- 11. Tjong FV, Reddy VY. Permanent leadless cardiac pacemaker therapy: a comprehensive review [published correction appears in Circulation. 2017;136:e24]. Circulation. 2017;135:1458–1470. [DOI] [PubMed] [Google Scholar]
- 12. Yancy CW, Jessup M, Bozkurt B, et al. 2013. ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. [DOI] [PubMed] [Google Scholar]
- 13. Curtis AB, Yancy CW, Albert NM, et al. Cardiac resynchronization therapy utilization for heart failure: Findings from IMPROVE HF. Am Heart J. 2009;158:956–964. [DOI] [PubMed] [Google Scholar]
- 14. Woo GW, Petersen‐Stejskal S, Johnson JW, et al. Ventricular reverse remodeling and 6‐month outcomes in patients receiving cardiac resynchronization therapy: analysis of the MIRACLE study. J Interv Card Electrophysiol. 2005;12:107–113. [DOI] [PubMed] [Google Scholar]
- 15. Cleland JG, Daubert JC, Erdmann E, et al; CARE‐HF Study Investigators . The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549. [DOI] [PubMed] [Google Scholar]
- 16. Tang AS, Wells GA, Talajic M, et al; Resynchronization‐Defibrillation for Ambulatory Heart Failure Trial Investigators. Cardiac‐resynchronization therapy for mild‐to‐moderate heart failure. N Engl J Med. 2010;363:2385–2395. [DOI] [PubMed] [Google Scholar]
- 17. Bristow MR, Saxon LA, Boehmer J, et al. Cardiac‐resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–2150. [DOI] [PubMed] [Google Scholar]
- 18. Arshad A, Moss AJ, Foster E, et al; MADIT‐CRT Executive Committee . Cardiac resynchronization therapy is more effective in women than in men: the MADIT‐CRT (Multicenter Automatic Defibrillator Implantation Trial With Cardiac Resynchronization Therapy) trial. J Am Coll Cardiol. 2011;57:813–820. [DOI] [PubMed] [Google Scholar]
- 19. Daubert C, Gold MR, Abraham WT, et al. Prevention of disease progression by cardiac resynchronization therapy in patients with asymptomatic or mildly symptomatic left ventricular dysfunction: insights from the European cohort of the REVERSE (Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction) trial. J Am Coll Cardiol. 2009;54:1837–1846. [DOI] [PubMed] [Google Scholar]
- 20. Zusterzeel R, Selzman KA, Sanders WE, et al. Cardiac resynchronization therapy in women: US Food and Drug Administration meta‐analysis of patient‐level data. JAMA Intern Med. 2014;174:1340–1348. [DOI] [PubMed] [Google Scholar]
- 21. Loring Z, Caños DA, Selzman K, et al. Left bundle branch block predicts better survival in women than men receiving cardiac resynchronization therapy: long‐term follow‐up of ~145,000 patients. JACC Heart Fail. 2013;1:237–244. [DOI] [PubMed] [Google Scholar]
- 22. Strauss DG, Selvester RH, Wagner GS. Defining left bundle branch block in the era of cardiac resynchronization therapy. Am J Cardiol. 2011;107:927–934. [DOI] [PubMed] [Google Scholar]
- 23. Bertaglia E, Migliore F, Baritussio A, et al. Stricter criteria for left bundle branch block diagnosis do not improve response to CRT. Pacing Clin Electrophysiol. 2017;40:850–856. [DOI] [PubMed] [Google Scholar]
- 24. Hess PL, Hernandez AF, Bhatt DL, et al. Sex and race/ethnicity differences in implantable cardioverter‐defibrillator counseling and use among patients hospitalized with heart failure: findings from the Get With The Guidelines–Heart Failure Program. Circulation. 2016;134:517–526. [DOI] [PubMed] [Google Scholar]
- 25. Hernandez AF, Fonarow GC, Liang L, et al. Sex and racial differences in the use of implantable cardioverter‐defibrillators among patients hospitalized with heart failure. JAMA. 2007;298:1525–1532. [DOI] [PubMed] [Google Scholar]
- 26. Amit G, Suleiman M, Konstantino Y, et al; Israeli Working Group on Pacing and Electrophysiology. Sex differences in implantable cardioverter‐defibrillator implantation indications and outcomes: lessons from the nationwide Israeli‐ICD registry. Europace. 2014;16:1175–1180. [DOI] [PubMed] [Google Scholar]
- 27. Curtis LH, Al‐Khatib SM, Shea AM, et al. Sex differences in the use of implantable cardioverter‐defibrillators for primary and secondary prevention of sudden cardiac death. JAMA. 2007;298:1517–1524. [DOI] [PubMed] [Google Scholar]
- 28. Bardy GH, Lee KL, Mark DB, et al; Sudden Cardiac Death in Heart Failure Trial (SCD‐HeFT) Investigators . Amiodarone or an implantable cardioverter‐defibrillator for congestive heart failure [published correction appears in N Engl J Med. 2005;352:2146]. N Engl J Med. 2005;352:225–237. [DOI] [PubMed] [Google Scholar]
- 29. Kadish A, Dyer A, Daubert JP, et al; Defibrillators in Non‐Ischemic Cardiomyopathy Treatment Evaluation (DEFINITE) Investigators. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350:2151–2158. [DOI] [PubMed] [Google Scholar]
- 30. Buxton AE, Lee KL, Fisher JD, et al; Multicenter Unsustained Tachycardia Trial Investigators. A randomized study of the prevention of sudden death in patients with coronary artery disease [published correction appears in N Engl J Med. 2000;342:1300]. N Engl J Med. 1999;341:1882–1890. [DOI] [PubMed] [Google Scholar]
- 31. Ghanbari H, Dalloul G, Hasan R, et al. Effectiveness of implantable cardioverter‐defibrillators for the primary prevention of sudden cardiac death in women with advanced heart failure: a meta‐analysis of randomized controlled trials. Arch Intern Med. 2009;169:1500–1506. [DOI] [PubMed] [Google Scholar]
- 32. Barra S, Providência R, Duehmke R, et al; French‐UK‐Sweden CRT Network . Sex‐specific outcomes with addition of defibrillation to resynchronisation therapy in patients with heart failure. Heart. 2017;103:753–760. [DOI] [PubMed] [Google Scholar]
- 33. Providência R, Marijon E, Lambiase PD, et al. Primary‐prevention implantable cardioverter defibrillator (ICD) therapy in women—data from a multicenter French registry. J Am Heart Assoc. 2016;5:e002756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tompkins CM, Kutyifa V, Arshad A, et al. Sex differences in device therapies for ventricular arrhythmias or death in the Multicenter Automatic Defibrillator Implantation Trial With Cardiac Resynchronization Therapy (MADIT‐CRT) trial. J Cardiovasc Electrophysiol. 2015;26:862–871. [DOI] [PubMed] [Google Scholar]
- 35. Gopinathannair R, Lerew DR, Cross NJ, et al. Longitudinal changes in quality of life following ICD implant and the impact of age, gender, and ICD shocks: observations from the INTRINSIC RV trial. J Interv Card Electrophysiol. 2017;48:291–298. [DOI] [PubMed] [Google Scholar]
- 36. Peterson PN, Daugherty SL, Wang Y, et al; National Cardiovascular Data Registry. Gender differences in procedure‐related adverse events in patients receiving implantable cardioverter‐defibrillator therapy. Circulation. 2009;119:1078–1084. [DOI] [PubMed] [Google Scholar]
- 37. Masoudi FA, Go AS, Magid DJ, et al. Age and sex differences in long‐term outcomes following implantable cardioverter‐defibrillator placement in contemporary clinical practice: findings from the Cardiovascular Research Network. J Am Heart Assoc. 2015;4:e002005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Moss AJ, Hall WJ, Cannom DS, et al; Multicenter Automatic Defibrillator Implantation Trial Investigators. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. N Engl J Med. 1996;335:1933–1940. [DOI] [PubMed] [Google Scholar]
- 39. Moss AJ, Zareba W, Hall WJ, et al; Multicenter Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. [DOI] [PubMed] [Google Scholar]
- 40. Narasimha D, Curtis AB. Sex differences in utilisation and response to implantable device therapy. Arrhythm Electrophysiol Rev. 2015;4:129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]


