Summary
Objective
To investigate changes in the functional connectivity (FC) pattern in the posterior cingulate cortex (PCC) of Parkinson's disease (PD) patients with mild cognitive impairment and dementia by employing resting‐state functional magnetic resonance imaging (RS‐fMRI).
Methods
Twenty‐seven PD patients with different cognitive status and 9 healthy control subjects (control group) were enrolled for RS‐fMRI. The RS‐fMRI data were analyzed with DPARSF and REST software. Regions with changed functional connectivity were determined by the seed‐based voxelwise method and compared between groups. Correlation between the intensity of FC and the MoCA scores of PD group was analyzed.
Results
Parametric maps showed statistical increases in PCC functional connectivity in PD‐MCI patients and decreases in PCC connectivity in PDD patients. The latter group of patients also showed evidence for increased connectivity between prefrontal cortices and posterior cerebellum. A significant positive correlation was found between the MoCA scores and the strength of PCC connectivity in the angular gyrus and posterior cerebellum and a negative correlation between MoCA scores and PCC connectivity in all other brain regions.
Conclusion
When patients transition from PD‐NCI to PD‐MCI, there appears to be an increase in functional connectivity in the PCC, suggesting an expansion of the cortical network. Another new network (a compensatory prefrontal cortical‐cerebellar loop) later develops during the transition from PD‐MCI to PDD.
Keywords: dementia, functional connectivity, mild cognitive impairment, Parkinson's disease, posterior cingulate cortex, resting‐state functional magnetic resonance imaging
1. INTRODUCTION
Parkinson's disease (PD), a progressive neurodegenerative disease, is characterized by primary motor symptoms of tremor, rigidity, bradykinesia, and postural instability.1 Moreover, various nonmotor symptoms, such as cognitive disorders, depression, and anxiety, also seriously affect the quality of life of patients with PD.2 A community‐based study shows that 80% of PD patients who have survived for 20 years will develop PD with dementia (PDD),3 which gravely affects the prognosis of PD patients and increases the caregivers’ burden and overall healthcare costs.4
PD with mild cognitive impairment (PD‐MCI), a well‐defined “transition state” between PD with no cognitive impairment (PD‐NCI) and PDD, is an early manifestation of dementia and indicates an increasing risk of PDD.5 Approximately 25% of newly diagnosed patients with PD satisfy the clinical criteria of PD‐MCI, which affects a number of cognitive capacities, such as memory, visual‐spatial function, and attention/executive ability.6 In a 3‐year follow‐up study, more than 25% of PD‐MCI patients, in comparison with less than 1% of PD‐NCI patients, developed dementia.5 Hence, early screening of PD‐MCI patients and early intervention will one day be important for delaying the progression to PDD.
Resting‐state functional MRI (RS‐fMRI), a new noninvasive method for assessing functional brain connectivity at rest, has located a series of intrinsic connectivity networks (ICNs) in the brain, including the default mode network (DMN), the dorsal attention network (DAN), and the frontoparietal networks (FPN). All these networks are dynamically interrelated and vital for cognitive processing.7 Using RS‐FMRI, Hacker et al have found that striatal functional connectivity in the Parkinson's disease group was markedly altered.8 Other previous studies have documented a functional disruption of the DMN in nondemented patients with PD, specifically between posterior cingulate cortex (PCC), medial prefrontal cortex, and inferior parietal nodes, suggesting that a dysfunction in the DMN connectivity may promote the development of cognitive decline in PD.9 Gorges et al have reported that along with PD‐related pathological progression, functional disruptions within the DMN seem to be strongly associated with cognitive impairment.10 During cognitive processing, PCC, which plays a crucial role in DMN, is functionally related to ventral anterior cingulate cortex (vACC) and the DMN‐related brain regions.11 Previous studies have found white matter lesions12 or reduced metabolism13 in the PCC of PDD patients when compared with nondemented PD patients. Therefore, changes in the functional connectivity (FC) in the PCC might be implicated in the cognitive impairment of patients with PD. To our best knowledge, resting‐state functional connectivity in PD patients with cognitive impairment (PD‐CI) has been scarcely investigated in the available literature. Thus, the current case‐control study attempted to explore the patterns of FC in the PCC of PD‐MCI and PDD patients by RS‐fMRI.
2. METHODS
2.1. Participants
Twenty‐seven patients with PD, who were diagnosed according to the UK Parkinson's Disease Society Brain Bank Criteria, were enrolled from The Affiliated Union Hospital of Fujian Medical University and granted their informed consent. Nine healthy controls (HC) were recruited and matched for age, sex, and length of education. Exclusion criteria were other brain disorders, other types of dementia (ie, Alzheimer's disease, frontotemporal dementia, dementia with Lewy body, and vascular dementia), cognitive impairment secondary to other disorders (ie, low levels of vitamin B12 or folic acid, abnormalities in thyroid function), abnormal MRI findings (ie, tumor, hydrocephalus, or severe vascular lesions), severe systemic disease, major psychiatric illness, prior cerebral surgery, positive VDRL test, and acritochromacy. The study was approved by the Ethical Committee for Medical Research of Fujian Union Hospital (Fuzhou, China) and recorded in China Clinical Trial Registry (ChiCTR‐ROC‐17011740).
2.2. Neuropsychological measurements
A senior neuropsychologist administered the tests to HC subjects and patients who were in the “on” medication state. The motor state was evaluated using the Hoehn and Yahr scale and the motor section of the unified Parkinson's Disease Rating Scale (UPDRS‐III). The depression severity in PD patients and controls was assessed with Hamilton Depression Scale (HAMD) to eliminate subjects with severe depression (HAMD scores >20). Drug intake was recorded, and dopaminergic treatment was calculated as levodopa equivalents. Levodopa equivalent daily dose (LEDD) in patients with PD was calculated as advised by Tomlinson et al14.
Overall cognitive function was evaluated with the Mini‐Mental State Examination (MMSE) and Montreal Cognitive Assessment (MoCA). Five different cognitive domains (attention and working memory, executive functions, language, memory, and visuospatial function) were assessed using a series of neuropsychological tests as suggested by the Movement Disorder Society Criteria.15 The diagnosis and differential diagnosis of patients as PD‐MCI or PDD were made accordingly.15, 16 PD‐MCI patients were diagnosed by the level I category guidelines of the Movement Disorder Society,15 and patients not satisfying the criteria for PD‐MCI or PDD were diagnosed as PD‐NCI.
2.3. MRI acquisition
MRI images were obtained on a 3.0 T GE Medical System scanner equipped with an 8‐channel parallel head coil. During the resting‐state scan, participants lay on the back with their heads held in a foam padding and rubber earplugs to reduce noise. They were instructed to breathe calmly and not to think or fall asleep. T1 fluid‐attenuated inversion recovery sequence (repetition time [TR] = 24 ms; echo time [TE] = 6 ms; flip angle [FA] = 35%; matrix = 256 × 256; field of view [FOV] = 220 × 220 mm; slice thickness = 0.9 mm) was used to acquire axial anatomical images for registration and normalization of the functional images. During an 8‐minute scanning session, 240 BOLD echo‐planar RS‐fMRI images were obtained for each subject with a gradient‐recalled echo‐planar imaging pulse sequence (TR = 2000 ms; TE = 35 ms; FA = 80°; matrix = 64 × 64, FOV = 240 × 240 mm; thickness/gap = 5/0 mm; in‐plane resolution = 64 × 64 mm; slice numbers = 43).
2.4. Preprocessing of fMRI data
The resting‐state network data were analyzed with a MATLAB toolbox, named Data Processing Assistant for Resting‐State fMRI Basic Edition (DPARSF_3.0; http://www.restfmri.net). All software programs were run on a Statistical Parametric Mapping platform (SPM8; http://www.fil.ion.ucl.ac.uk/spm) and REST software (http://www.restfmri.net).
All DICOM files obtained from the scanner were converted into Neuroimaging Informatics Technology Initiative (NIfTI) file format. For each participant, the first 10 time points were discarded for signal equilibrium and participants’ adaptation to the scanning noise (each subject had 230 useful sets of volumes). Next, functional images received the following preprocessing steps: slice timing, motion correction, spatial standardization to the Montreal Neurological Institute (MNI) EPI template (resampling voxel size = 3 × 3 × 3 mm3) in SPM8, and spatial smoothing with an 8‐mm full‐width at half‐maximum (FWHM) Gaussian kernel. Linear trend removal and temporal band‐pass filtering (0.01‐0.08 Hz) were performed on the time series of each voxel. Further analysis only included subjects with less than 1.5‐mm maximum displacement in the x‐, y‐, or z‐plane, and less than 1.5° of angular rotation on each axis.
2.5. Functional connectivity and statistical analysis
Seed‐based correlation was analyzed to identify increased or decreased cerebellar FC with REST software. Regions of interest (ROIs) were set at the bilateral PCC according to the automated anatomical labeling (AAL) template. The averaged time course was extracted from the bilateral PCC, and the correlation was then analyzed in a voxelwise manner to generate the FC in the PCC by computing the temporal cross‐correlation between the mean time series of each ROI and the time series of each voxel within the brain. Finally, the correlation coefficient map was converted into Z‐scores by Fisher's r‐to‐z transform to improve the normality, thus acquiring the entire brain Z‐score map of each subject.
One‐sample t tests were respectively performed on the Z‐score map of each of the four groups. Within‐group multiple comparisons were used to identify the FC networks in PCC of the four groups, with age and gender as covariates. Pearson's correlation was used to calculate the correlation between the intensity of FC in the PCC and the MoCA scores of PD groups. Significant differences were set at a corrected significance level of P < 0.05. The threshold correction was performed with AlphaSim program in REST software.
Statistical Package for Social Sciences (SPSS, version 19.0) was used for statistical analysis. One‐way analysis of variance (ANOVA) was adopted for comparisons between demographic factors across groups, the independent‐samples t test for comparisons between groups, and Pearson's chi‐squared test for comparisons between categorical variables (hand dominance and sex). The statistical significance threshold was set at P < 0.05.
3. RESULTS
3.1. Demographics and global cognition
A total of 36 subjects were enrolled in this study, including nine PD‐NCI patients, nine PD‐MCI patients, nine PDD patients, and nine matched healthy subjects. Their demographic and clinical features are summarized in Table 1. The patients with PD bore a great similarity in disease duration, sex ratio, length of education, severity of depressive symptoms, handedness, UPDRS‐III motor score, and PD medications. Compared with healthy controls and the PD‐NCI group, the PD‐MCI group performed more poorly in MMSE (P < 0.05) and MoCA tests (P < 0.01), and the PDD group had lower MMSE (P < 0.01) and MoCA scores (P < 0.01).
Table 1.
Demographic and global neuropsychological data of study subjects
| HC | PD‐NCI | PD‐MCI | PDD | P‐value ANOVA | |
|---|---|---|---|---|---|
| n | 9 | 9 | 9 | 9 | ‐ |
| Age (years) | 70.0 ± 5.9 | 69.6 ± 7.8 | 68.4 ± 5.9 | 74.0 ± 5.1 | 0.274 |
| Gender (female:male) | 7/2 | 7/2 | 9/0 | 7/2 | 0.551 |
| Handedness (R: L) | 9/0 | 9/0 | 9/0 | 9/0 | 1.000 |
| Education (years) | 7.67 ± 3.70 | 8.44 ± 2.19 | 6.33 ± 2.18 | 7.78 ± 6.94 | 0.547 |
| Disease duration (years) | ‐ | 4.06 ± 2.56 | 4.67 ± 2.35 | 4.44 ± 1.94 | 0.850 |
| UPDRS‐III | ‐ | 28.9 ± 10.5 | 33.1 ± 11.6 | 37.0 ± 12.1 | 0.338 |
| H &Y score | ‐ | 2.11 ± 0.82 | 2.17 ± 0.66 | 3.17 ± 0.90a , c | 0.884 |
| LEDD (mg) | ‐ | 630 ± 270 | 718 ± 372 | 721 ± 182 | 0.747 |
| MMSE | 29.1 ± 0.9 | 29.3 ± 1.3 | 27.2 ± 1.9a | 19.2 ± 3.5b , d | 0.000 |
| MoCA | 26.7 ± 1.2 | 27.0 ± 0.9 | 21.9 ± 2.4b | 14.1 ± 3.1b , d | 0.000 |
All values were expressed as mean ± SD.
UPDRS, Unified Parkinson's Disease Rating Scale; H &Y, Hoehn & Yahr; LEDD, levodopa equivalent daily dose; MMSE, Mini‐Mental State Examination; MoCA, Montreal Cognitive Assessment; HC, healthy control; PD‐NCI, PD with no cognitive impairment; PD‐MCI, PD with mild cognitive impairment; PDD, PD with dementia; ANOVA, one‐way analysis of variance.
Indicates group differences at a significance level of P < 0.05 when compared with HC group/PD‐NCI group.
Indicates group differences at a significance level of P < 0.01 when compared with HC group/PD‐NCI group.
Indicates group differences at a significance level of P < 0.05 when compared with PD‐MCI group.
Indicates group differences at a significance level of P < 0.01 when compared with PD‐MCI group.
3.2. Single domains cognition
Compared with the PD‐NCI group, the PD‐MCI group fared poorly in the assessment of memory with WAIS‐IV Logical Memory subtest, the evaluation of visuospatial function with Benton's Judgment of Line Orientation and Hooper Visual Organization test, and the assessment of language with WAIS‐IV Similarities test (P < 0.05) (Table 2).
Table 2.
Single cognitive domains study in PD‐NCI and PD‐MCI group
| Cognitive domains | Neuropsychological test | PD‐NCI (n = 9) | PD‐MCI (n = 9) | P‐value |
|---|---|---|---|---|
| Attention & working memory assessment | Digit span backward | 5.00 ± 1.32 | 4.33 ± 0.50 | 0.176 |
| Stroop color test | 22.9 ± 7.8 | 27.0 ± 9.6 | 0.334 | |
| Executive functions | Semantic verbal fluency (animal naming task) | 16.2 ± 3.7 | 12.1 ± 2.2 | 0.011a |
| Ten points Clock‐drawing test | 9.44 ± 0.73 | 8.56 ± 1.51 | 0.131 | |
| Language | WAIS‐IV Similarities test | 6.89 ± 1.97 | 9.78 ± 1.72 | 0.004b |
| Boston Naming Test | 25.9 ± 2.7 | 20.8 ± 3.9 | 0.005b | |
| Memory | Hopkins Verbal Learning Test (immediate recall) | 6.63 ± 1.50 | 6.86 ± 1.56 | 0.762 |
| Hopkins Verbal Learning Test (delayed recall) | 7.78 ± 1.39 | 7.33 ± 1.87 | 0.576 | |
| Hopkins Verbal Learning Test (recognition) | 19.4 ± 4.4 | 19.4 ± 3.00 | 1.000 | |
| WAIS‐IV Logical Memory test | 22.1 ± 2.6 | 13.2 ± 4.0 | 0.000b | |
| Visuospatial function | Hooper Visual Organization test | 19.4 ± 4.4 | 14.3 ± 5.3 | 0.039a |
| Benton's Judgment of Line Orientation | 28.5 ± 1.00 | 24.7 ± 4.7 | 0.032b |
All values were expressed as mean ± SD.
Indicates group differences at a significance level of P < 0.05 when compared with PD‐NCI group.
Indicates group differences at a significance level of P < 0.01 when compared with PD‐NCI group.
3.3. PCC connectivity: within‐group analyses
Between‐group comparison showed different patterns of functional connectivity in the PCC of the HC group and PD groups. P < 0.05 was set as the threshold for display (cluster size: >54 voxels; AlphaSim corrected). Blue and green areas represent regions showing significantly decreased connectivity. The color bar indicates the T value from two‐sample t test analysis between the two groups.
3.3.1. PCC connectivity between the PD‐NCI group and the HC group
Compared with the HC group, the PD‐NCI group reported markedly decreased connectivity between the PCC and several brain areas, including the left superior frontal gyrus, right angular gyrus, superior parietal gyrus. No regions were found showing increased connectivity to the PCC of the PD‐NCI group (Table 3A and Figure 1A).
Table 3.
Difference of PCC FC in patients between HC group, PD‐NCI group, PD‐MCI group, and PDD group, and the correlation between PCC connectivity and MoCA scores in PD‐MCI group and PD‐NCI group
| Brain region (AAL) | Cluster size (voxels) | Coordinates MNI | T value | |||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| A | ||||||
| PD‐NCI group vs HC group | Left Superior Frontal Gyrus | 54 | −27 | 63 | 9 | −3.3465 |
| Right Angular Gyrus | 52 | 51 | −48 | 42 | −5.2663 | |
| Left Superior Parietal Gyrus | 36 | −39 | −63 | 60 | −5.1522 | |
| B | ||||||
| PD‐MCI group vs PD‐NCI group | Left Middle Frontal Gyrus | 252 | −39 | 21 | 45 | 4.3907 |
| Right Middle Frontal Gyrus | 87 | 39 | 24 | 48 | 5.3799 | |
| Medial Superior Frontal Gyrus | 43 | 3 | 45 | 45 | 3.9279 | |
| Left Middle Temporal Gyrus | 63 | −57 | −24 | −15 | 3.7534 | |
| Left Precuneus | 105 | −6 | −63 | 30 | 4.7026 | |
| Posterior Lobe of Cerebellum | 46 | −12 | −57 | −42 | 4.1632 | |
| C | ||||||
| PDD group vs PD‐NCI group | Anterior cingulate and paracingulate gyri | 32 | 0 | 48 | 3 | −4.4104 |
| Left Middle frontal gyrus | 101 | −30 | 18 | 63 | 5.9691 | |
| Superior Frontal Gyrus | 68 | −30 | 18 | 63 | 5.9691 | |
| D | ||||||
| PDD group vs PD‐MCI group | Left caudate | 36 | −15 | 15 | 3 | −6.1061 |
| Right thalamus | 24 | 15 | −27 | 6 | −4.6346 | |
| Precuneus | 9 | 15 | −27 | 6 | −4.6346 | |
| Middle Frontal Gyrus | 110 | 42 | 27 | 30 | −4.0794 | |
| Right Angular Gyrus | 85 | 45 | −60 | 39 | −5.2748 | |
| E | ||||||
| Correlation between PCC connectivity and MoCA scores in PD‐MCI group and PD‐NCI group | Primary visual cortex | 61 | −3 | −96 | 6 | −0.5984 |
| Cuneus | 54 | 6 | 36 | 3 | −0.5710 | |
| Lingual gyrus | 174 | −45 | −81 | 9 | −0.5271 | |
| Precuneus | 44 | 12 | −54 | 69 | −0.7692 | |
| Angular Gyrus | 35 | 51 | −54 | 48 | 0.4851 | |
| Posterior cerebellum | 114 | 15 | −69 | −30 | 0.7561 | |
Figure 1.
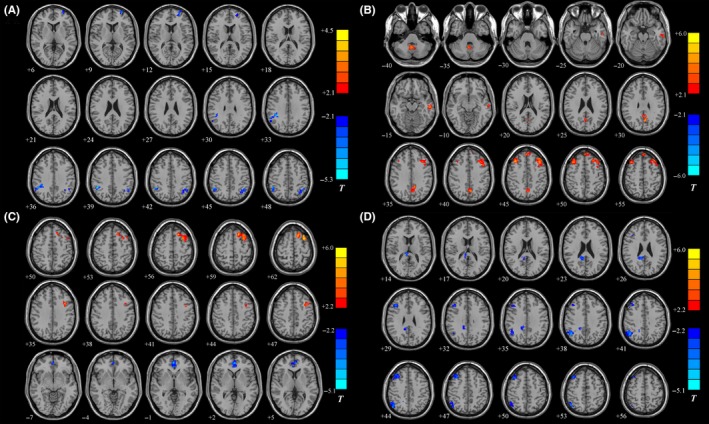
Statistical parametric map showing the significant differences in the PCC FC between different groups. A, The PD‐NCI group vs the HC group. B, The PD‐MCI group vs the PD‐NCI group. C, The PDD group vs the PD‐NCI group. D, The PDD group vs the PD‐MCI group. Blue and green regions indicate decreased connectivity to the PCC of the former group. Red and yellow areas represent regions showing significantly increased connectivity to the PCC of the former group. P < 0.05 was set as the threshold for display (cluster size: >54 voxels; AlphaSim corrected)
3.3.2. PCC connectivity between the PD‐MCI group and the PD‐NCI group
Compared with the PD‐NCI group, the PD‐MCI group showed increased PCC functional connectivity to the left middle frontal gyrus, right middle frontal gyrus, medial superior frontal gyrus, left middle temporal gyrus, left precuneus, and posterior lobe of cerebellum. No regions were found showing markedly decreased connectivity to the PCC of the PD‐MCI group (Table 3B and Figure 1B).
3.3.3. PCC connectivity between the PDD group and the PD‐NCI group
Compared with the PD‐NCI group, the PDD group showed an increased PCC functional connectivity to the left middle frontal gyrus and superior frontal gyrus. Anterior cingulate and paracingulate gyri demonstrated a noticeably decreased connectivity to the PCC of the PDD group (Table 3C and Figure 1C).
3.3.4. PCC connectivity between the PDD group and the PD‐MCI group
Compared with the PD‐MCI group, the PDD group showed increased PCC functional connectivity to the left caudate, right thalamus, precuneus, middle frontal gyrus, and right angular gyrus. No regions indicated a markedly decreased connectivity to the PCC of the PD‐MCI group (Table 3D and Figure 1D).
3.4. Correlation between PCC connectivity and MoCA scores in the PD‐MCI group and the PD‐NCI group
A positive correlation was found between the MoCA scores and the strength of PCC connectivity with the angular gyrus and posterior cerebellum, while a negative correlation was evident between the scores and the strength of functional connectivity of PCC with the primary visual cortex, cuneus, lingual gyrus, and precuneus. (Table 3E and Figure 2A,B).
Figure 2.
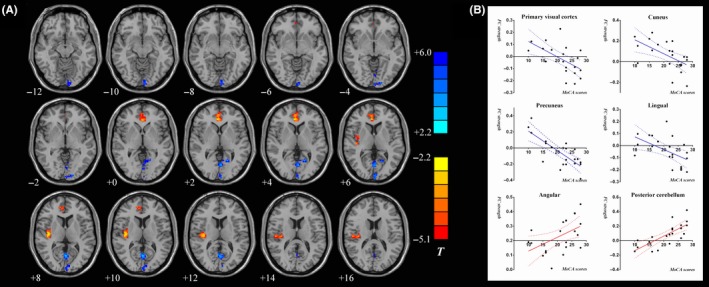
Correlation analysis between the strength of FC in the PCC and the MoCA scores in the PD‐MCI group and the PD‐NCI group. A, Statistical parametric map showing the correlations analysis in the PD‐MCI group and the PD‐NCI group. Red and yellow areas represent regions showing significantly positive correlation between the PCC FC strength and MoCA scores. Blue and green regions indicate negative correlation between the PCC FC strength and MoCA scores. B, Pearson's correlation analysis results between the strength of FC in the PCC and the MoCA scores in PD‐NCI and PD‐MCI patients. The statistical significance threshold was set at P < 0.05
4. DISCUSSION
In present study, we found that the presence of cognitive impairment in PD was associated with abnormal resting‐state FC connectivity patterns in the PCC in PD‐MCI and PDD patients and that an increased FC strength between the PCC and certain regions served as a compensation for cognitive dysfunction in PD‐MCI patients. On the other hand, PD‐MCI patients had impairments in a range of cognitive domains including executive functions, visuospatial functions, language, and memory function. We will elaborate our findings from the following aspects: (i) increased PCC connectivity in PD‐MCI, (ii) decreased PCC connectivity in PDD, (iii) a compensatory prefrontal cortical‐cerebellar loop in PD with cognitive impairment (PD‐CI), and (iv) neuropsychology abnormalities in PD‐MCI.
4.1. Increased functional connectivity in PD‐MCI patients
With the application of RS‐fMRI, studies have found that PD‐NCI patients, who transition more quickly into PD‐MCI (<1 year), reported decreased resting‐state functional connectivity mainly between the medial prefrontal cortex and the posterior cingulate cortex and between the parieto‐occipital areas and the caudate.17 Another study has found markedly reduced FC in the PCC of the right parahippocampus of the PDD patients and documented that the FC strength of the PCC with the right MTL in PD was noticeably correlated with MoCA scores by insights from both functional and anatomical connections (DTI).18 However, the dynamic changes with the progression of cognitive impairment were not observed. The current study assessed resting‐state functional connectivity of the posterior cingulate cortex in PD patients with different cognitive status using seed‐based correlation analyses. We found that when patients transitioned from PD‐NCI to PD‐MCI, increased FC strength with PCC was observed in regions such as middle frontal gyrus, posterior cerebellum lobe, middle temporal gyrus, and left precuneus (Figure 3B), while no regions showing increased FC strength with the PCC were observed in PD‐NCI patients when compared with the NC group (Figure 3A). Similarly, Gorges et al have demonstrated increased correlations (hyperconnectivity) in different ICNs (DMN, left and right frontoparietal control, basal ganglia‐thalamic, etc.) of PD patients without cognitive deficits.10
Figure 3.
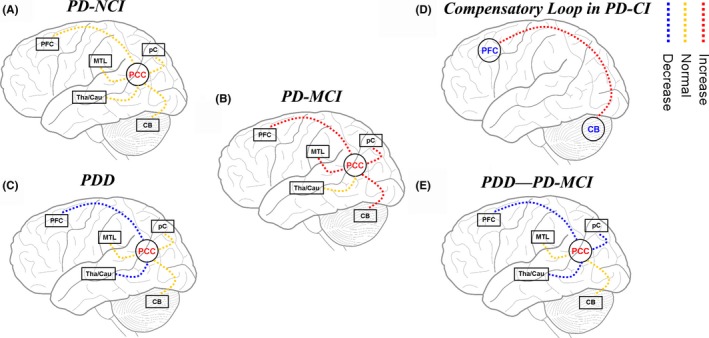
The node pattern and the progression of FC in the PCC of PD patients with different cognitive status: A, Normal PCC connectivity in PD‐NCI patients when compared with the NC group; B, increased PCC connectivity in PD‐MCI group when compared with the PD‐NCI group; C, decreased PCC connectivity in PDD patients when compared with the PD‐NCI group; D, a compensatory prefrontal cortical‐cerebellar loop in PD‐CI; E, decreased PCC connectivity in PDD patients when compared with the PD‐MCI group
The prefrontal cortex has been shown to play essential roles in executive functions.19 The temporal lobe, which includes the middle temporal gyrus and hippocampus as its constituents, is mainly involved in visual perception, sensory information processing, memory, speech comprehension, and emotional activity. It also serves as a common semantic network of words and images, from the upper left occipital brain back through the medial temporal cortex to the inferior frontal gyrus.20 The relationship between the cerebellum and cognitive function is also widely acknowledged. The cerebellum participates in advanced cognitive function mainly through the cortex‐thalamus‐cerebellar loops, of which the posterior lobe is involved in memory and executive function.21 The increased functional connectivity in the PCC with such regions reported in PD‐MCI patients may not participate in the poor performance of PD‐MCI patients when they were compared with PD‐NCI patients. According to the functional imaging of healthy subjects, precuneus plays a central role in a series of highly integrated tasks including visuospatial imagery, episodic memory retrieval, and self‐processing operations, namely first‐person perspective taking and an experience of agency.22 Subregions with different functions in the angular gyrus (AnG) help to restore episodic and semantic memories.23 The increased FC observed in those regions may be a resource recruitment as an initial response to the mild cognitive dysfunction in PD patients.
4.2. Decreased functional connectivity in PDD patients
With the progression of cognitive dysfunction, this increased FC observed in the PD‐MCI patients was gradually lost in the PDD group. Moreover, a decreased functional connectivity between the PCC and the caudate or thalamus was observed (Figure 3C,E). Similarly, Gorges et al's study also revealed reduced intrinsic FC within the DMN and within the dorsal attention network in PD patients with cognitive impairment.10 Structural magnetic resonance imaging studies have demonstrated that the atrophy of the frontal lobe and cholinergic structure, and the related cognitive performance of the frontal lobe are considered to be predictors of dementia in PD‐MCI patients, presenting a unique cognitive contour model and neuroanatomical basis for progressive PD‐MCI.24 This speculation has gained support from DTI studies of PD‐MCI,25 and the current study confirms it from the perspective of functional connection. The thalamus, as the core structure of the brain, includes the primary relay nucleus and connects anatomically to the subcortical, cortical, and cerebral structure and regions in different ways,26 acting as the “classification center” of information and engaging in the process of episodic memory and executive function, including information processing speed, directional attention, and working memory.27 Taken together, the lost compensatory role of FC strength of PCC observed in PD‐MCI patients and decreased functional connectivity between the PCC and the caudate or thalamus are associated with the progression from PD‐MCI to PDD.
4.3. Compensatory prefrontal cortical‐cerebellar loop in PD‐CI patients
Compared with the NC group, the PD‐NCI group reported markedly decreased connectivity between PCC with the left superior frontal gyrus, right angular gyrus, superior parietal gyrus, and no increased connectivity between PCC and other cerebral regions, indicating that the decreased FC strength between the PCC of the PD‐MCI patients with such regions is related to motor dysfunction. Through a comparison of PD‐MCI group with PD‐NCI group, between which the motor dysfunction was well‐matched, we found increased FC strength between the PCC of the PD‐MCI patients and regions such as middle frontal gyrus, middle temporal lobe, precuneus, and posterior cerebellum lobe. The relationship between the cerebellum and cognitive function has been largely acknowledged. The cerebellum participates in advanced cognitive function mainly through the cortex‐thalamus‐cerebellar loops, in which the posterior lobe is involved in memory and executive function. Changes in the cortical‐thalamic‐cerebellar loop contribute to the development of cognitive disorders.21 Compared with other regional activity measurements, the detection of abnormal functional connectivity in the cerebellum can be a more sensitive and preferred index for functional disturbance in aMCI patients.28 In our study, the FC strength of the PCC with the thalamus did not decrease/increase in PD‐MCI patients, indicating that it may be retained in PD‐MCI, instead of being a compensatory node. Because of this, we speculate that the “prefrontal cortical‐cerebellar loop” may act as a compensatory loop in PD‐MCI patients, and degeneration of functional connectivity in this loop may be an important mechanism for the progression of cognitive dysfunction (Figure 3D).
As revealed in the comparison of single cognitive domain tests, we found that except for Hopkins Verbal Learning Test for episodic memory, including immediate and delayed free recall and recognition of word lists and pictures, PD‐MCI patients scored poorly in tests for executive function (semantic verbal fluency), language (Boston Naming Test), logical memory, and visuospatial function. Meanwhile, the decreased FC strength between the PCC of the PDD group and regions including the middle frontal gyrus, right thalamus, and angular gyrus is related to impaired episodic memory, which was not observed in the PD‐MCI group, indicating that episodic memory may be retained in PD‐MCI patients and damaged in PDD patients. A recent study has claimed that a decline in episodic memory may not be adequate to assess cognitive decline in PD patients and that PD‐MCI patients may display an early decline in working memory and visuospatial processing before a clinical declaration of PDD.29 Therefore, functional connectivity between the PCC and other regions such as middle frontal gyrus, right thalamus, and angular gyrus is also involved in the progression of PD‐CI.
4.4. Neuropsychology abnormalities in PD‐MCI patients
MoCA test has been recommended for screening PD‐MCI patients, with an excellent sensitivity of 89‐93.1% and a good specificity of 84%.30 MoCA score <26 is proposed as level I criterion for PD‐MCI diagnosis by Movement Disorder Society Task Force.15 However, MoCA is believed to be limited in diagnostic accuracy for PD‐MCI.31 Therefore, we further detected cognitive function of multiple cognitive domains in the PD‐MCI and PD‐NCI group using neuropsychological test battery recommended by Movement Disorder Society Task Force.15 In line with MoCA evaluation results, the PD‐MCI group showed impairments in the aforementioned cognitive domains, among which the logical memory function was significantly damaged. This is consistent with the findings of previous studies which document that PD patients demonstrate cognitive decline in the related cerebral regions.6, 32, 33
5. CONCLUSION
In summary, resting‐state functional connectivity in the PCC is associated with cognitive performance in PD patients. Increased FC strength between the PCC and certain cerebral regions is involved in compensation for PD‐MCI, and reduced FC strength is associated with progression from PD‐MCI to PDD and aggravates the cognitive performance. The prefrontal cortical‐cerebellar loop may act as a compensatory loop in PD‐CI. Our findings may provide a new perspective into the neural mechanisms underlying the development of cognitive impairment in PD.
5.1. Limitations and outlook
However, our research has some limitations. First, only a small number of subjects were enrolled in the current study, and the diagnosis of PC‐MCI patients only satisfied the level I diagnostic criterion. Second, due to motor dysfunction and poor cooperation, comprehensive cognitive function assessment was not carried out in PDD patients and so no more detailed and comprehensive understanding of the characteristics and progression of cognitive function of PD patients was obtained. Third, the potential impact of different medication protocols was not ruled out in our study. However, no significant difference in the amount of levodopa was found between the PD groups. Future studies will recruit more PD patients with a follow‐up design to verify our hypotheses. We are going to explore imaging markers of PD‐MCI combining different imaging techniques such as RS‐fMRI and DTI method.
CONFLICT OF INTEREST
On behalf of all authors, the corresponding author states that there is no conflict of interest.
ETHICAL STANDARDS
The study protocol was approved by the ethical committee for medical research of Fujian Union Hospital (Fuzhou, China) and registered in China Clinical Trial Registry (ChiCTR‐ROC‐17011740). The study was conducted according to the Declaration of Helsinki. Every patient provided written informed consent before entering the study.
ACKNOWLEDGMENTS
We would like to thank Professor Hongzhi Huang from School of Foreign Languages of Fujian Medical University for his kind proofreading and polishing this manuscript.
Zhan Z‐W, Lin L‐Z, Yu E‐H, et al. Abnormal resting‐state functional connectivity in posterior cingulate cortex of Parkinson's disease with mild cognitive impairment and dementia. CNS Neurosci Ther. 2018;24:897–905. 10.1111/cns.12838
Funding information
This study was supported by grants from National Natural Science Foundation of China (No. 81571257; No. 81771179), Fujian Provincial Natural Science Foundation (No. 2015J01398), and Young and Middle‐aged Talent Training Key Project in Health System of Fujian Province, China (2014‐ZQN‐ZD‐11), Fujian Provincial Natural Science Foundation Youth Project (No. 2015J05154).
REFERENCES
- 1. Jankovic J. Parkinson's disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79:368‐376. [DOI] [PubMed] [Google Scholar]
- 2. Chaudhuri KR, Healy DG, Schapira AH. Non‐motor symptoms of Parkinson's disease: diagnosis and management. Lancet Neurol. 2006;5:235‐245. [DOI] [PubMed] [Google Scholar]
- 3. Hely MA, Reid WG, Adena MA, et al. The Sydney multicenter study of Parkinson's disease: the inevitability of dementia at 20 years. Mov Disord. 2008;23:837‐844. [DOI] [PubMed] [Google Scholar]
- 4. Aarsland D, Bronnick K, Ehrt U, et al. Neuropsychiatric symptoms in patients with Parkinson's disease and dementia: frequency, profile and associated care giver stress. J Neurol Neurosurg Psychiatry. 2007;78:36‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Williams‐Gray CH, Foltynie T, Brayne CE, et al. Evolution of cognitive dysfunction in an incident Parkinson's disease cohort. Brain. 2007;130:1787‐1798. [DOI] [PubMed] [Google Scholar]
- 6. Aarsland D, Bronnick K, Williams‐Gray C, et al. Mild cognitive impairment in Parkinson disease: a multicenter pooled analysis. Neurology. 2010;75:1062‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349‐2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hacker CD, Perlmutter JS, Criswell SR, et al. Resting state functional connectivity of the striatum in Parkinson's disease. Brain. 2012;135:3699‐3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Disbrow EA, Carmichael O, He J, et al. Resting state functional connectivity is associated with cognitive dysfunction in non‐demented people with Parkinson's disease. J Parkinsons Dis. 2014;4:453‐465. [DOI] [PubMed] [Google Scholar]
- 10. Gorges M, Muller HP, Lule D, et al. To rise and to fall: functional connectivity in cognitively normal and cognitively impaired patients with Parkinson's disease. Neurobiol Aging. 2015;36:1727‐1735. [DOI] [PubMed] [Google Scholar]
- 11. Fransson P, Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: evidence from a partial correlation network analysis. NeuroImage. 2008;42:1178‐1184. [DOI] [PubMed] [Google Scholar]
- 12. Matsui H, Nishinaka K, Oda M, et al. Dementia in Parkinson's disease: diffusion tensor imaging. Acta Neurol Scand. 2007;116:177‐181. [DOI] [PubMed] [Google Scholar]
- 13. Griffith HR, den Hollander JA, Okonkwo OC, et al. Brain N‐acetylaspartate is reduced in Parkinson disease with dementia. Alzheimer Dis Assoc Disord. 2008;22:54‐60. [DOI] [PubMed] [Google Scholar]
- 14. Tomlinson CL, Stowe R, Patel S, et al. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord. 2010;25:2649‐2653. [DOI] [PubMed] [Google Scholar]
- 15. Litvan I, Goldman JG, Troster AI, et al. Diagnostic criteria for mild cognitive impairment in Parkinson's disease: Movement Disorder Society Task Force guidelines. Mov Disord. 2012;27:349‐356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Emre M, Aarsland D, Brown R, et al. Clinical diagnostic criteria for dementia associated with Parkinson's disease. Mov Disord. 2007;22:1689‐1707; quiz 1837. [DOI] [PubMed] [Google Scholar]
- 17. Shin NY, Shin YS. Different functional and microstructural changes depending on duration of mild cognitive impairment in Parkinson disease. AJNR Am J Neuroradiol. 2016;37:897‐903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen B, Fan GG, Liu H, Wang S. Changes in anatomical and functional connectivity of Parkinson's disease patients according to cognitive status. Eur J Radiol. 2015;84:1318‐1324. [DOI] [PubMed] [Google Scholar]
- 19. Funahashi S, Andreau JM. Prefrontal cortex and neural mechanisms of executive function. J Physiol Paris. 2013;107:471‐482. [DOI] [PubMed] [Google Scholar]
- 20. Vandenberghe R, Price C, Wise R, et al. Functional anatomy of a common semantic system for words and pictures. Nature. 1996;383:254‐256. [DOI] [PubMed] [Google Scholar]
- 21. Buckner RL. The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron. 2013;80:807‐815. [DOI] [PubMed] [Google Scholar]
- 22. Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564‐583. [DOI] [PubMed] [Google Scholar]
- 23. Bonnici HM, Richter FR, Yazar Y, Simons JS. Multimodal feature integration in the angular gyrus during episodic and semantic retrieval. J Neurosci. 2016;36:5462‐5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee JE, Cho KH, Song SK, et al. Exploratory analysis of neuropsychological and neuroanatomical correlates of progressive mild cognitive impairment in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2014;85:7‐16. [DOI] [PubMed] [Google Scholar]
- 25. Agosta F, Canu E, Stefanova E, et al. Mild cognitive impairment in Parkinson's disease is associated with a distributed pattern of brain white matter damage. Hum Brain Mapp. 2014;35:1921‐1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang D, Snyder AZ, Shimony JS, et al. Noninvasive functional and structural connectivity mapping of the human thalamocortical system. Cereb Cortex. 2010;20:1187‐1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fama R, Sullivan EV. Thalamic structures and associated cognitive functions: relations with age and aging. Neurosci Biobehav Rev. 2015;54:29‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bai F, Liao W, Watson DR, et al. Mapping the altered patterns of cerebellar resting‐state function in longitudinal amnestic mild cognitive impairment patients. J Alzheimers Dis. 2011;23:87‐99. [DOI] [PubMed] [Google Scholar]
- 29. Johnson DK, Langford Z, Garnier‐Villarreal M, et al. Onset of mild cognitive impairment in Parkinson disease. Alzheimer Dis Assoc Disord. 2016;30:127‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kandiah N, Zhang A, Cenina AR, et al. Montreal Cognitive Assessment for the screening and prediction of cognitive decline in early Parkinson's disease. Parkinsonism Relat Disord. 2014;20:1145‐1148. [DOI] [PubMed] [Google Scholar]
- 31. Chou KL, Lenhart A, Koeppe RA, Bohnen NI. Abnormal MoCA and normal range MMSE scores in Parkinson disease without dementia: cognitive and neurochemical correlates. Parkinsonism Relat Disord. 2014;20:1076‐1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Muslimovic D, Post B, Speelman JD, Schmand B. Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology. 2005;65:1239‐1245. [DOI] [PubMed] [Google Scholar]
- 33. Lucas‐Jimenez O, Ojeda N, Pena J, et al. Altered functional connectivity in the default mode network is associated with cognitive impairment and brain anatomical changes in Parkinson's disease. Parkinsonism Relat Disord. 2016;33:58‐64. [DOI] [PubMed] [Google Scholar]


