Abstract
Background
The association between dyslipidemia, a major risk factor for cardiovascular diseases, and atrial fibrillation (AF) is not clear because of limited evidence.
Hypothesis.
Dyslipidemia may be associated with increased risk of AF in a Chinese population.
Methods
A total of 88 785 participants free from AF at baseline (2006–2007) were identified from the Kailuan Study. Fasting levels of total cholesterol (TC), low‐density lipoprotein cholesterol (LDL‐C), high‐density lipoprotein cholesterol (HDL‐C), and triglycerides (TG) were measured at baseline using standard procedures. The study population was stratified based on quartiles of lipid profile. Incident AF was ascertained from electrocardiograms at biennial follow‐up visits (2008–2015). The associations between incident AF and the different lipid parameters (TC, LDL‐C, HDL‐C, and TG) were assessed by Cox proportional hazards regression analysis.
Results
Over a mean follow‐up period of 7.12 years, 328 subjects developed AF. Higher TC (hazard ratio [HR]: 0.60, 95% confidence interval [CI]: 0.43‐0.84) and LDL‐C (HR: 0.60, 95% CI: 0.43‐0.83) levels were inversely associated with incident AF after multivariable adjustment. HDL‐C and TG levels showed no association with newly developed AF. The results remained consistent after exclusion of individuals with myocardial infarction or cerebral infarction, or those on lipid‐lowering therapy. Both TC/HDL‐C and LDL‐C/HDL‐C ratios were inversely associated with risk of AF (per unit increment, HR: 0.88, 95% CI: 0.79‐0.98 and HR: 0.77, 95% CI: 0.66‐0.91, respectively).
Conclusions
TC and LDL‐C levels were inversely associated with incident AF, whereas no significant association of AF with HDL‐C or TG levels was observed.
Keywords: Atrial Fibrillation, Blood Lipids, Epidemiology, Risk Factors
1. INTRODUCTION
Atrial fibrillation (AF), the most commonly encountered cardiac arrhythmia in clinical practice, is a major cause of ischemic stroke, myocardial infarction, heart failure, and cardiovascular (CV) mortality.1 The morbidity and mortality burden attributable to AF and its associated socioeconomic and healthcare costs is projected to increase steadily in the foreseeable future.2 Multiple risk factors for AF such as increasing age, male sex, and presence of CV diseases have been identified; however, the underlying mechanisms that trigger and sustain AF are not completely understood.3 Recent advances in treatment of AF, such as catheter ablation, have helped in its management. However, a substantial proportion of patients with AF continue to suffer from severe complications.4 Therefore, further investigation and identification of arrhythmogenic factors is a key imperative.
Dyslipidemia, as a major contributor to atherosclerosis and CV diseases, has been implicated as a major risk factor for AF. However, some observational studies have demonstrated an association between low levels of cholesterol and an increased risk of AF, a phenomenon referred to as the cholesterol paradox.5, 6 Moreover, a few longitudinal studies of the association between blood lipids and AF have yielded inconsistent results.7, 8, 9 High levels of total cholesterol (TC) and low‐density lipoprotein cholesterol (LDL‐C), which are regarded as risk factors for atherosclerotic CV diseases, were unexpectedly found to be inversely associated with the risk of AF in several observational studies.5, 6, 7, 9 Moreover, lipid ratios such as TC/high‐density lipoprotein cholesterol (HDL‐C) and LDL‐C/HDL‐C that are considered useful indices for stratification of patients with CV diseases have been seldom investigated in AF risk estimation.7, 10 We therefore conducted a prospective study to investigate the association between lipid profile and incident AF in the general population from the Kailuan Study11 (ChiCTRTNC‐11001489) in China.
2. METHODS
2.1. Study participants
The Kailuan Study is an ongoing prospective cohort study based on the Kailuan community in the Tangshan city in northern China, which represents the Chinese population from a geographic and socioeconomic perspective. Details of the study are described elsewhere.11
Briefly, the baseline (2006–2007) study population, which included 101 510 men and women (age range, 18–98 years), completed structured questionnaires by interviews and underwent clinical examination across 11 subsidiary hospitals responsible for healthcare of this community. The participants were then followed through 2014–2015 with repeat questionnaires and clinical and laboratory examinations every 2 years.
In the current study, participants with missing data pertaining to lipid levels (n = 1440) or electrocardiograms (ECG; n = 530), and those with AF or atrial flutter at baseline (n = 459), were excluded. The present study cohort included individuals who did not meet the exclusion criteria and subsequently received ≥1 annual examination from the baseline examination to the 2014–2015 survey. A total of 88 785 individuals were included in the current analysis. The protocol for this study was in accordance with the guidelines of the Helsinki Declaration II and this study was approved by the Ethics Committee at the Kailuan general hospital. Written informed consent was obtained from all participants prior to their enrollment in the study.
2.2. Ascertainment of AF
AF diagnoses were ascertained based on 12‐lead ECG at biennial follow‐up visits.12 According to the European Society of Cardiology guidelines, a diagnosis of AF was considered when all the following ECG criteria were met: (1) irregular R‐R intervals, (2) absence of repeating P waves, and (3) irregular atrial activity.13 For confirmation of diagnosis in such cases, the ECG was reviewed by 2 cardiologists. The final diagnosis of AF was confirmed only when both cardiologists independently confirmed the same. The incidence time of AF was defined as the time of the first signs of AF.
2.3. Assessment of lipid levels
Overnight fasting venous blood samples were drawn from the antecubital vein using vacuum tubes containing EDTA for storage. Plasma was separated and stored at −80°C for subsequent analyses. TC and triglycerides (TG) were both measured using the enzymatic colorimetric method (Mind Bioengineering Co. Ltd., Shanghai, China); HDL‐C and LDL‐C were measured by direct test method (Mind Bioengineering Co. Ltd., Shanghai, China). Less than 0.1% of measured values were within 5% of the upper limit of detection.
2.4. Assessment of other covariates
Data pertaining to demographic variables, smoking status, income, alcohol intake, education level, physical activity, and information regarding CV diseases were collected from baseline questionnaires and physical examinations in 2006. Detailed procedures on covariate measurements are described elsewhere.14 The diagnosis of diabetic mellitus (DM) was based on documented history of DM, ongoing antidiabetic treatment, or fasting blood glucose levels ≥7.0 mmol/L. Hypertension (HTN) was defined as systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg, with physician diagnosis of HTN, or ongoing antihypertensive treatment. Information on physician‐diagnosed myocardial infarction (MI) and cerebral infarction was collected in the biennial interview. Medical records from a total of 11 hospitals were reviewed annually to further identify potential MI or cerebral infarction events.
2.5. Statistical analysis
Statistical analyses were performed with SAS software, version 9.1 (SAS Institute, Inc., Cary, NC) and SPSS version 13.0 (SPSS Inc., Chicago, IL). Data pertaining to continuous variables are presented as mean ± SD and between‐group differences assessed with 1‐way ANOVA or nonparametric tests. Categorical variables are presented as frequencies and percentages and between‐group differences assessed with the χ2 test. The association of baseline blood lipid levels with incidence of AF was determined by calculation of hazard ratios (HR) and 95% confidence intervals (CI) with use of Cox hazard models, after verification of the proportional hazards assumption with Schoenfeld residuals. Natural cubic spline functions were also used to model the association between risk ratios of AF and lipid profiles after adjusting for potential confounders.15
Initially, we conducted analyses based on quartiles of blood lipid levels. In addition, we investigated lipid levels as continuous variables. Moreover, lipid ratios including TC/LDL‐C, TC/HDL‐C, LDL‐C/HDL‐C, and TG/HDL‐C scaled to 1‐unit increments were included in the analysis. Cox models were initially adjusted for sex and age to evaluate the association between lipid profile and new onset of AF using the lowest quartile of lipids as the reference group. Multivariable Cox models were then adjusted for the following variables: age, sex, education, income, smoking, alcohol intake, systolic blood pressure, diastolic blood pressure, body mass index (BMI), height, physical activity, high‐sensitivity C‐reactive protein (hs‐CRP), serum uric acid, DM, antihypertensive drugs, and history of snoring. Finally, we performed a sensitivity analysis after exclusion of participants with prevalent MI or cerebral infarction or those on lipid‐lowing therapy at baseline and further exclusion of incident MI and cerebral infarction separately. Participants with history of malignancy or BMI <18.5 kg/m2 were also excluded from the sensitivity analysis. We also repeated the main analysis by using the highest quartile as the reference group. All statistical tests were 2‐sided, and P < 0.05 was regarded as significant.
3. RESULTS
3.1. Baseline characteristics
Selected characteristics of the total of 88 785 participants by quartiles of TC are shown in Table 1. Older participants, current daily smokers, and frequent alcohol users had comparatively higher levels of TC. BMI, fasting blood glucose, hs‐CRP, LDL‐C level, HDL‐C level, and serum uric acid level were positively associated with TC levels. Further, individuals with higher TC levels had higher prevalence of DM, HTN, and ischemic CV diseases.
Table 1.
Baseline characteristics of the study population
| Variables | Total | TC | P Value | |||
|---|---|---|---|---|---|---|
| Q1, <166 mg/dL | Q2, 166–190 mg/dL | Q3, 191–216 mg/dL | Q4, >216 mg/dL | |||
| Participants, n | 88 785 | 22 226 | 22 304 | 22 073 | 22 182 | |
| Age, y | 50.83 ± 12.03 | 48.92 ± 13.22 | 50.65 ± 12.27 | 51.27 ± 11.45 | 52.51 ± 10.78 | <0.001 |
| Male sex | 69 832 (78.65) | 17 539 (78.91) | 17 519 (78.55) | 17 344 (78.58) | 17 430 (78.58) | 0.754 |
| BMI, kg/m2 | 25.06 ± 3.47 | 24.70 ± 3.52 | 24.89 ± 3.47 | 25.15 ± 3.41 | 25.52 ± 3.42 | <0.001 |
| Height, cm | 167.38 ± 7.01 | 167.60 ± 6.97 | 167.48 ± 6.99 | 167.40 ± 6.99 | 167.05 ± 7.10 | <0.001 |
| Completed high school | 18 115 (20.40) | 5356 (24.10) | 4557 (20.43) | 3979 (18.03) | 4223 (19.04) | <0.001 |
| Income ≥800¥ | 12 597 (14.19) | 3363 (15.13) | 2977 (13.35) | 2952 (13.37) | 3305 (14.90) | <0.001 |
| Daily smoking | 27 049 (30.47) | 6181 (27.81) | 6573 (29.47) | 6654 (30.15) | 7641 (34.45) | <0.001 |
| Daily alcohol use | 15 754 (17.74) | 3116 (14.02) | 3570 (16.01) | 3980 (18.03) | 5088 (22.94) | <0.001 |
| Vigorous exercise | 13 558 (15.27) | 3065 (13.79) | 3359 (15.06) | 3291 (14.91) | 3843 (17.32) | <0.001 |
| SBP, mm Hg | 130.34 ± 20.68 | 127.67 ± 20.33 | 129.08 ± 20.42 | 130.96 ± 20.50 | 133.65 ± 20.99 | <0.001 |
| DBP, mm Hg | 83.38 ± 11.69 | 81.93 ± 11.50 | 82.74 ± 11.56 | 83.79 ± 11.62 | 85.07 ± 11.84 | <0.001 |
| FBG, mmol/L | 5.45 ± 1.63 | 5.24 ± 1.39 | 5.35 ± 1.47 | 5.46 ± 1.59 | 5.75 ± 1.95 | <0.001 |
| hs‐CRP, mg/L | 0.80 (0.30–2.15) | 0.71 (0.26–2.10) | 0.77 (0.30–2.13) | 0.80 (0.30–2.10) | 0.90 (0.38–2.25) | <0.001 |
| LDL‐C, mg/dL | 90.74 ± 35.44 | 75.00 ± 29.55 | 84.09 ± 29.65 | 93.71 ± 31.39 | 110.22 ± 40.08 | <0.001 |
| HDL‐C, mg/dL | 59.70 ± 15.47 | 54.62 ± 13.79 | 57.87 ± 14.09 | 60.99 ± 14.63 | 65.36 ± 17.10 | <0.001 |
| TG, mg/dL | 147.77 ± 119.92 | 137.18 ± 113.06 | 128.67 ± 91.39 | 144.42 ± 107.29 | 180.92 ± 152.68 | <0.001 |
| SUA, mg/L | 287.87 ± 83.04 | 276.93 ± 79.81 | 283.60 ± 80.69 | 289.02 ± 81.12 | 301.96 ± 88.23 | <0.001 |
| History of snoring | 12 375 (13.94) | 2666 (11.99) | 2874 (12.89) | 3099 (14.04) | 3736 (16.84) | <0.001 |
| DM | 7632 (8.60) | 1495 (6.73) | 1597 (7.16) | 1796 (8.14) | 2744 (12.37) | <0.001 |
| HTN | 37 888 (42.67) | 8448 (38.01) | 8882 (39.82) | 9667 (43.80) | 10 891 (49.10) | <0.001 |
| History of MI | 1041 (1.17) | 226 (1.02) | 243 (1.09) | 236 (1.07) | 336 (1.51) | <0.001 |
| History of cerebral infarction | 1598 (1.80) | 344 (1.55) | 375 (1.68) | 409 (1.85) | 470 (2.12) | <0.001 |
| Antihypertensive drugs | 9600 (10.81) | 1919 (8.63) | 2124 (9.52) | 2394 (10.85) | 3163 (14.26) | <0.001 |
| Lipid‐lowering drugs | 834 (0.94) | 162 (0.73) | 169 (0.76) | 204 (0.92) | 299 (1.35) | <0.001 |
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; DM, diabetes mellitus; FBG, fasting blood glucose; HDL‐C, high density‐lipoprotein cholesterol; hs‐CRP, high‐sensitivity C‐reactive protein; HTN, hypertension; IQR, interquartile range; LDL‐C, low density‐lipoprotein cholesterol; MI, myocardial infarction; Q, quartile; SBP, systolic blood pressure; SUA, serum uric acid; TC, total cholesterol; TG, triglycerides.
Data are presented as n (%), mean ± standard deviation, or median (IQR).
3.2. Blood lipids and incidence of AF
Over a mean follow‐up period of 7.12 years, AF developed in 328 participants; this corresponded to an incidence of 0.52 per 1000 person‐years. The association between blood lipid levels and incident AF is presented in Table 2. In the age‐ and sex‐adjusted models, the HRs and 95% CIs of the top quartile compared with the bottom quartile of lipids were as follows: TC, 0.60 (0.43‐0.84); LDL‐C, 0.60 (0.43‐0.83); HDL‐C, 0.93 (0.67‐1.29); and TG, 0.87 (0.62‐1.21). In the multivariable adjusted models, high TC and LDL‐C levels were consistently associated with a low risk of AF, whereas no significant association of AF with HDL‐C and TG was observed. Further, modeled as a continuous variable, the natural cubic splines supported approximately linear associations of blood lipid parameters with risk of AF (Figure 1). An inverted J‐shaped trend and an inverse trend were observed with respect to the association of TC and LDL‐C with risk of AF, respectively.
Table 2.
HRs (95% CIs) for AF by quartiles of blood lipids
| Quartiles of TC | Q1, <165 mg/dL | Q2, 165–190 mg/dL | Q3, 190–215 mg/dL | Q4, >215 mg/dL | P for Trend |
|---|---|---|---|---|---|
| AF cases | 92 | 91 | 77 | 68 | |
| Person‐years | 159 074 | 159 186 | 157 469 | 156 538 | |
| AF incidencea | 0.57 | 0.57 | 0.49 | 0.43 | |
| Model 1 | 1 (ref) | 0.93 (0.70‐1.24) | 0.80 (0.59‐1.08) | 0.66 (0.48‐0.90) | 0.006 |
| Model 2 | 1 (ref) | 0.93 (0.69‐1.26) | 0.78 (0.57‐1.07) | 0.60 (0.43‐0.84) | 0.001 |
| Sensitivity analysis | |||||
| Model 3 | 1 (ref) | 0.94 (0.69‐1.27) | 0.76 (0.56‐1.05) | 0.59 (0.42‐0.83) | 0.001 |
| Model 4 | 1 (ref) | 0.99 (0.73‐1.35) | 0.80 (0.58‐1.11) | 0.62 (0.44‐0.87) | 0.003 |
| Model 5 | 1 (ref) | 0.99 (0.73‐1.34) | 0.82 (0.60‐1.13) | 0.64 (0.46‐0.89) | 0.004 |
| Quartiles of LDL‐C | Q1, <71 mg/dL | Q2, 71–90 mg/dL | Q3, 90–110 mg/dL | Q4, >110 mg/dL | P for Trend |
|---|---|---|---|---|---|
| AF cases | 111 | 86 | 69 | 62 | |
| Person‐years | 155 898 | 160 511 | 162 232 | 153 625 | |
| AF incidencea | 0.71 | 0.54 | 0.43 | 0.40 | |
| Model 1 | 1 (ref) | 0.93 (0.70‐1.23) | 0.77 (0.57‐1.05) | 0.65 (0.48‐0.89) | 0.004 |
| Model 2 | 1 (ref) | 0.87 (0.65‐1.17) | 0.77 (0.56‐1.04) | 0.60 (0.43‐0.83) | 0.001 |
| Sensitivity analysis | |||||
| Model 3 | 1 (ref) | 0.87 (0.64‐1.18) | 0.77 (0.56‐1.07) | 0.63 (0.45‐0.88) | 0.005 |
| Model 4 | 1 (ref) | 0.85 (0.63‐1.15) | 0.76 (0.55‐1.05) | 0.59 (0.42‐0.82) | 0.002 |
| Model 5 | 1 (ref) | 0.86 (0.64‐1.16) | 0.79 (0.58‐1.08) | 0.59 (0.43‐0.83) | 0.002 |
| Quartiles of HDL‐C | Q1, <50 mg/dL | Q2, 50–58 mg/dL | Q3, 58–68 mg/dL | Q4, >68 mg/dL | P for Trend |
|---|---|---|---|---|---|
| AF cases | 77 | 81 | 87 | 83 | |
| Person‐years | 152 593 | 162 273 | 159 104 | 158 297 | |
| AF incidencea | 0.50 | 0.50 | 0.55 | 0.52 | |
| Model 1 | 1 (ref) | 0.98 (0.71‐1.33) | 1.06 (0.78‐1.44) | 0.82 (0.60‐1.13) | 0.300 |
| Model 2 | 1 (ref) | 1.05 (0.77‐1.45) | 1.09 (0.79‐1.51) | 0.93 (0.67‐1.29) | 0.721 |
| Sensitivity analysis | |||||
| Model 3 | 1 (ref) | 1.07 (0.78‐1.49) | 1.09 (0.79‐1.52) | 0.91 (0.65‐1.28) | 0.614 |
| Model 4 | 1 (ref) | 1.11 (0.80‐1.55) | 1.13 (0.81‐1.58) | 0.96 (0.68‐1.35) | 0.821 |
| Model 5 | 1 (ref) | 1.10 (0.79‐1.52) | 1.13 (0.81‐1.56) | 0.99 (0.71‐1.38) | 0.958 |
| Quartiles of TG | Q1, <80 mg/dL | Q2, 80–112 mg/dL | Q3, 112–172 mg/dL | Q4, >172 mg/dL | P for Trend |
|---|---|---|---|---|---|
| AF cases | 75 | 69 | 91 | 93 | |
| Person‐years | 157 028 | 159 682 | 156 839 | 158 717 | |
| AF incidencea | 0.48 | 0.43 | 0.58 | 0.59 | |
| Model 1 | 1 (ref) | 0.84 (0.60‐1.16) | 1.11 (0.82‐1.50) | 1.21 (0.89‐1.64) | 0.082 |
| Model 2 | 1 (ref) | 0.73 (0.52‐1.03) | 0.86 (0.62‐1.19) | 0.87 (0.62‐1.21) | 0.716 |
| Sensitivity analysis | |||||
| Model 3 | 1 (ref) | 0.70 (0.50‐0.99) | 0.84 (0.60‐1.17) | 0.82 (0.59‐1.15) | 0.513 |
| Model 4 | 1 (ref) | 0.74 (0.52‐1.04) | 0.85 (0.61‐1.19) | 0.86 (0.61‐1.22) | 0.676 |
| Model 5 | 1 (ref) | 0.76 (0.53‐1.05) | 0.84 (0.60‐1.16) | 0.87 (0.62‐1.22) | 0.674 |
Abbreviations: AF, atrial fibrillation; BMI, body mass index; CI, confidence interval; DBP, diastolic blood pressure; DM, diabetes mellitus; HDL‐C, high density‐lipoprotein cholesterol; HR, hazard ratio; hs‐CRP, high‐sensitivity C‐reactive protein; LDL‐C, low density‐lipoprotein cholesterol; MI, myocardial infarction; Q, quartile; ref, reference; SBP, systolic blood pressure; SUA, serum uric acid; TC, total cholesterol; TG, triglycerides.
Model 1 adjusted for sex and age. Model 2 adjusted for sex, age, education, income, smoking, alcohol use, SBP, DBP, BMI, height, physical activity, hs‐CRP, SUA, DM, antihypertensive drugs, snoring. Model 3 adjusted for model 2 and further excluded MI at baseline (eligible participants: 87 744, with 316 cases of incident AF). Model 4 adjusted for model 2 and further excluded cerebral infarction at baseline (eligible participants: 87 187, with 310 cases of incident AF). Model 5 adjusted for model 2 and further excluded lipid‐lowering drugs at baseline (eligible participants: 87 951, with 321 cases of incident AF).
Per 1000 person‐years.
Figure 1.
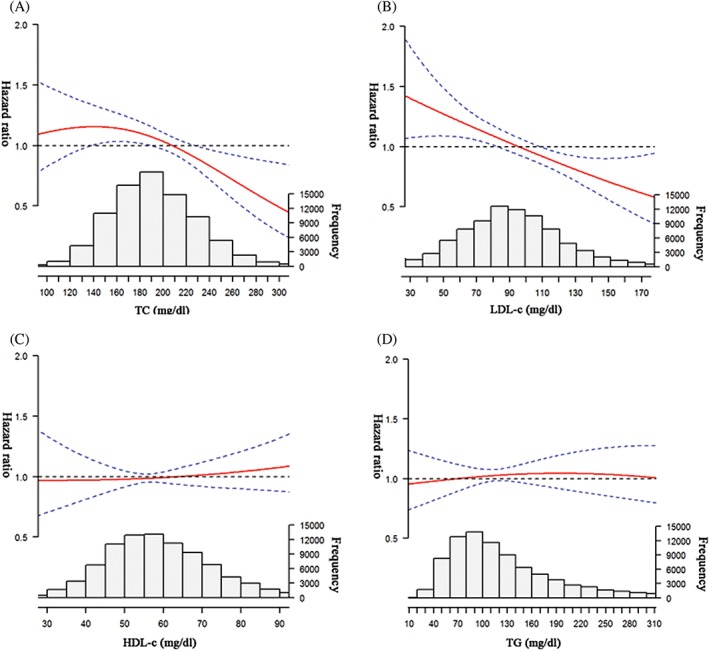
The risk ratio curve (95% CI) for AF by baseline levels of (A) TC, (B) LDL‐C, (C) HDL‐C, and (D) TG adjusted for sex, age, education, smoking, income, alcohol intake, SBP, DBP, BMI, height, physical activity, hs‐CRP, SUA, DM, antihypertensive drugs, and snoring. Abbreviations: AF, atrial fibrillation; BMI, body mass index; CI, confidence interval; DBP, diastolic blood pressure; DM, diabetes mellitus; HDL‐C, high density‐lipoprotein cholesterol; hs‐CRP, high‐sensitivity C‐reactive protein; LDL‐C, low density‐lipoprotein cholesterol; SBP, systolic blood pressure; SUA, serum uric acid; TC, total cholesterol; TG, triglycerides
To assess the possibility that these inverse associations were driven by other risk factors of AF, we excluded participants with prevalent MI (n = 1041), cerebral infarction (n = 1598), and those on lipid‐lowering therapy at baseline (n = 834). In addition, to rule out the confounding effect of incident CV disease, we further excluded incident MI (n = 1017) and cerebral infarction (n = 2483; Table 2 and Supporting Information, Table 1, in the online version of this article). The associations remained consistent in all these sensitivity analyses. To rule out the potential influence of malnourished state in the low TC/LDL‐C group on increasing the risk of AF, we excluded participants with history of malignancy (n = 295) and BMI <18.5 kg/m2 (n = 2024). The results remained unchanged (see Supporting Information, Table 1, in the online version of this article). On repeat analysis using the highest quartile as reference, low TC and LDL‐C significantly increased the risk of AF (Supporting Information, Table 2, in the online version of this article).
3.3. Blood lipid ratios and incident AF
In the secondary analysis, we further investigated the association between incident AF and blood lipid ratios that are frequently used for CV risk stratification. Per‐unit increment in TC/HDL‐C and LDL‐C/HDL‐C ratios decreased the risk of AF by 7% and 17% in the age‐ and sex‐adjusted models. The association became more significant after adjusting for potential confounders. Neither the TC/LDL‐C ratio nor the TG/HDL‐C ratio was associated with incident AF (Table 3).
Table 3.
Blood lipid ratios and risk for development of AF
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| TC/LDL‐C (per‐unit increase) | 1.00 (0.99‐1.02) | 0.900 | 1.00 (0.99‐1.02) | 0.942 |
| TC/HDL‐C (per‐unit increase) | 0.93 (0.84‐1.03) | 0.159 | 0.88 (0.79‐0.98) | 0.020 |
| LDL‐C/HDL‐C (per‐unit increase) | 0.83 (0.72‐0.96) | 0.012 | 0.77 (0.66‐0.91) | 0.001 |
| TG/HDL‐C (per‐unit increase) | 1.00 (0.99‐1.02) | 0.808 | 0.97 (0.92‐1.03) | 0.298 |
Abbreviations: AF, atrial fibrillation; BMI, body mass index; CI, confidence interval; DBP, diastolic blood pressure; DM, diabetes mellitus; HDL‐C, high density‐lipoprotein cholesterol; HR, hazard ratio; hs‐CRP, high‐sensitivity C‐reactive protein; LDL‐C, low density‐lipoprotein cholesterol; SBP, systolic blood pressure; SUA, serum uric acid; TC, total cholesterol; TG, triglycerides.
Model 1 adjusted for sex and age. Model 2 adjusted for sex, age, education, income, smoking, alcohol use, SBP, DBP, BMI, height, physical activity, hs‐CRP, SUA, DM, antihypertensive drugs, snoring.
4. DISCUSSION
In this large community‐based cohort, lower levels of TC and LDL‐C were associated with increased risk of AF, whereas no significant association of incident AF was observed with HDL‐C and TG. Among the various lipid ratios, TC/HDL‐C and LDL‐C/HDL‐C ratios showed an inverse association with new‐onset AF. These associations were independent of other risk factors.
Dyslipidemia, especially high LDL‐C levels and low HDL‐C levels, are well‐established risk factors for CV diseases.16, 17, 18 However, definitive evidence of the association between lipid profile and incident AF is yet to be obtained. Early evidence provided by the Cardiovascular Health Study revealed an association between high TC and reduced risk of AF.6 A pooled analysis of data from the Multi‐Ethnic Study of Atherosclerosis (MESA) study and the Framingham Heart Study showed that HDL‐C and TG instead of TC and LDL‐C were associated with incident AF.8 In contrast, the results from the Atherosclerosis Risk in Communities (ARIC) study were similar to those of our present study, in that the TC and LDL‐C levels were inversely associated with the development of AF, whereas no significant association of incident AF with HDL‐C or TG was observed.9 In the Niigata Preventive Medicine Study, high TC and LDL‐C as well as high HDL‐C were found to be associated with increased risk of AF; however, TG levels and various lipid ratios showed no such association.7 The inconsistent results may be attributable to various factors, such as geographical and ethnic variations, limited confounding factors including obesity and other CV risk factors, and differences with respect to the length of follow‐up.
The potential mechanisms of the inverse association between incident AF and TC and LDL‐C remain unclear. The participants with high TC and LDL‐C levels tended to show more risk factors for AF, including more alcohol consumption, high BMI, and more comorbidities (Table 1). This may explain why the inverse association between TC and risk of AF became more significant when adjusted for these risk factors (Table 2). Thus, the observed association between low TC and increased risk of AF may be driven by other mechanisms instead of the traditional pathways. Based on current studies, several mechanisms may explain the phenomenon. First, blood lipids are known to affect the composition of cell membranes and properties of cell electrophysiology.19, 20 in vitro studies have demonstrated that cholesterol modulates the distribution and function of some ion channels, including those of the Kv1.5 K+ channel, Kir2.1 K+ channel, and Na+ channel, which may be involved in the pathogenesis of AF.21, 22, 23 Second, high levels of cholesterol have an anti‐inflammatory effect in certain circumstances, according to some reports.24, 25 Lipoproteins were found to attenuate the biological response to inflammation by binding lipopolysaccharides in vitro.26 These findings support the possibility that low levels of TC and LDL‐C may contribute to the pathogenesis of AF via enhanced inflammation. Overall, the precise mechanisms that underlie the association between high levels of TC and LDL‐C and increased risk of AF are still not fully illuminated.
4.1. Study limitations
Some limitations of our study should be noted. First, the study population included an occupational population in north China consisting of relatively more men than women; therefore, the results should be treated with caution when extrapolated to the general population. Nevertheless, this geographical confine serves to reduce residual confounding due to the deviation from unmeasured socioeconomic factors. Second, the diagnosis of incident AF based on ECGs recorded at biennial follow‐up may inevitably underestimate AF incidence. In addition, the relative young age of participants may account for the low incidence of AF. Nevertheless, the incidence of AF in the present study, though lower than that in a Western population, was comparable with that reported in recently published studies in China.4, 27 Further, the validity of diagnosis of AF based on standard ECG was well‐accepted in other large cohort studies.7, 8 Other limitations included lack of data on thyroid function as well as data on CV surgical intervention, which may have had a confounding influence on the observed association. Despite these limitations, our study has several strengths, such as the large sample size, the relatively long duration of follow‐up, and inclusion of an extensive spectrum of potential confounders in the analysis.
5. CONCLUSION
In this study, TC and LDL‐C levels, as well as TC/HDL‐C and LDL‐C/HDL‐C ratios, showed an inverse association with incident AF. No significant association was observed between AF risk and HDL‐C and TG levels.
Supporting information
Figure S1. The flowchart of study participants.
Figure S2. Incidence rate of AF (per 1000 person‐years) across age groups.
Table S1. Additional analysis of hazard ratios (95% CIs) for incident AF by quartiles of blood lipids.
Table S2. Hazard Ratios (95% CIs) for AF stratified by quartiles of blood lipids using Q4 as the reference group.
Table S3. The association between incident AF and lipid parameters as continuous variables.
ACKNOWLEDGMENTS
The authors appreciate all of the participants, their families, and the members of the survey team from the Kailuan community.
Author contributions
Xintao Li and Lianjun Gao contributed equally to this work. Xintao Li, Gaolian Jun, and Yunlong Xia designed this study. Xintao Li, Zhao Wang, and Bo Guan wrote the manuscript. Xumin Guan and Binhao Wang conducted the data analysis. Shouling Wu provided the database. Xianjie Xiao, Khalid Bin Waleed, and Clarance Chandran reviewed and edited the article.
Conflicts of interest
The authors declare no potential conflicts of interest.
Li X, Gao L, Wang Z, et al. Lipid profile and incidence of atrial fibrillation: A prospective cohort study in China. Clin Cardiol. 2018;41:314–320. 10.1002/clc.22864
Funding information This study was supported by the National Natural Science Foundation of China (NSFC) Valid: 81570313.
Contributor Information
Shouling Wu, Email: drwusl@163.com.
Yunlong Xia, Email: yunlong_xia@126.com.
REFERENCES
- 1. Stewart S, Hart CL, Hole DJ, et al. A population‐based study of the long‐term risks associated with atrial fibrillation: 20‐year follow‐up of the Renfrew/Paisley study. Am J Med. 2002;113:359–364. [DOI] [PubMed] [Google Scholar]
- 2. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace. 2016;18:1609–1678. [DOI] [PubMed] [Google Scholar]
- 3. Vermond RA, Geelhoed B, Verweij N, et al. Incidence of atrial fibrillation and relationship with cardiovascular events, heart failure, and mortality: a community‐based study from the Netherlands. J Am Coll Cardiol. 2015;66:1000–1007. [DOI] [PubMed] [Google Scholar]
- 4. Guo Y, Tian Y, Wang H, et al. Prevalence, incidence, and lifetime risk of atrial fibrillation in China: new insights into the global burden of atrial fibrillation. Chest. 2015;147:109–119. [DOI] [PubMed] [Google Scholar]
- 5. Psaty BM, Manolio TA, Kuller LH, et al. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96:2455–2461. [DOI] [PubMed] [Google Scholar]
- 6. Annoura M, Ogawa M, Kumagai K, et al. Cholesterol paradox in patients with paroxysmal atrial fibrillation. Cardiology. 1999;92:21–27. [DOI] [PubMed] [Google Scholar]
- 7. Watanabe H, Tanabe N, Yagihara N, et al. Association between lipid profile and risk of atrial fibrillation. Circ J. 2011;75:2767–2774. [DOI] [PubMed] [Google Scholar]
- 8. Alonso A, Yin X, Roetker NS, et al. Blood lipids and the incidence of atrial fibrillation: the Multi‐Ethnic Study of Atherosclerosis and the Framingham Heart Study. J Am Heart Assoc. 2014;3:e001211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lopez FL, Agarwal SK, Maclehose RF, et al. Blood lipid levels, lipid‐lowering medications, and the incidence of atrial fibrillation: the Atherosclerosis Risk in Communities study. Circ Arrhythm Electrophysiol. 2012;5:155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Millán J, Pintó X, Muñoz A, et al. Lipoprotein ratios: physiological significance and clinical usefulness in cardiovascular prevention. Vasc Health Risk Manag. 2009;5:757–765. [PMC free article] [PubMed] [Google Scholar]
- 11. Wang L, Cui L, Wang Y, et al. Resting heart rate and the risk of developing impaired fasting glucose and diabetes: the Kailuan prospective study. Int J Epidemiol. 2015;44:689–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Han X, Yang Y, Chen Y, et al. Association between insomnia and atrial fibrillation in a Chinese population: a cross‐sectional study. Clin Cardiol. 2017;40:765–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ding X, Zheng X, Xing A, et al. High risk factors of atrial fibrillation in type 2 diabetes: results from the Chinese Kailuan study. QJM. 2015;108:885–890. [DOI] [PubMed] [Google Scholar]
- 14. Liu H, Wu S, Li Y, et al. Body mass index and mortality in patients with type 2 diabetes mellitus: a prospective cohort study of 11 449 participants. J Diabetes Complications. 2017;31:328–333. [DOI] [PubMed] [Google Scholar]
- 15. Sun H, Ren X, Chen Z, et al. Association between body mass index and mortality in a prospective cohort of Chinese adults. Medicine (Baltimore). 2016;95:e4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Velagaleti RS, Massaro J, Vasan RS, et al. Relations of lipid concentrations to heart failure incidence: the Framingham Heart Study. Circulation. 2009;120:2345–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Díaz‐Peromingo JA, Albán‐Salgado A, García‐Suárez F, et al. Lipoprotein(a) and lipid profile in patients with atrial fibrillation. Med Sci Monit. 2006;12:CR122–CR125. [PubMed] [Google Scholar]
- 18. Afshar M, Kamstrup PR, Williams K, et al. Estimating the population impact of Lp(a) lowering on the incidence of myocardial infarction and aortic stenosis—brief report. Arterioscler Thromb Vasc Biol. 2016;36:2421–2423. [DOI] [PubMed] [Google Scholar]
- 19. Bastiaanse EM, Höld KM, Van der Laarse A. The effect of membrane cholesterol content on ion transport processes in plasma membranes. Cardiovasc Res. 1997;33:272–283. [DOI] [PubMed] [Google Scholar]
- 20. Yeagle PL. Lipid regulation of cell membrane structure and function. FASEB J. 1989;3:1833–1842. [PubMed] [Google Scholar]
- 21. Balse E, El‐Haou S, Dillanian G, et al. Cholesterol modulates the recruitment of Kv1.5 channels from Rab11‐associated recycling endosome in native atrial myocytes. Proc Natl Acad Sci U S A. 2009;106:14681–14686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Epshtein Y, Chopra AP, Rosenhouse‐Dantsker A, et al. Identification of a C‐terminus domain critical for the sensitivity of Kir2.1 to cholesterol. Proc Natl Acad Sci U S A. 2009;106:8055–8060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lundbaek JA, Birn P, Hansen AJ, et al. Regulation of sodium channel function by bilayer elasticity: the importance of hydrophobic coupling. Effects of micelle‐forming amphiphiles and cholesterol. J Gen Physiol. 2004;123:599–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Folsom AR, Pankow JS, Tracy RP, et al. Association of C‐reactive protein with markers of prevalent atherosclerotic disease. Am J Cardiol. 2001;88:112–117. [DOI] [PubMed] [Google Scholar]
- 25. Saito M, Ishimitsu T, Minami J, et al. Relations of plasma high‐sensitivity C‐reactive protein to traditional cardiovascular risk factors. Atherosclerosis. 2003;167:73–79. [DOI] [PubMed] [Google Scholar]
- 26. Berbée JF, Havekes LM, Rensen PC. Apolipoproteins modulate the inflammatory response to lipopolysaccharide. J Endotoxin Res. 2005;11:97–103. [DOI] [PubMed] [Google Scholar]
- 27. Chien KL, TC Su, Hsu HC, et al. Atrial fibrillation prevalence, incidence and risk of stroke and all‐cause death among Chinese. Int J Cardiol. 2010;139:173–180. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. The flowchart of study participants.
Figure S2. Incidence rate of AF (per 1000 person‐years) across age groups.
Table S1. Additional analysis of hazard ratios (95% CIs) for incident AF by quartiles of blood lipids.
Table S2. Hazard Ratios (95% CIs) for AF stratified by quartiles of blood lipids using Q4 as the reference group.
Table S3. The association between incident AF and lipid parameters as continuous variables.


