Summary
Aims
Neuromyelitis optica spectrum disorder (NMOSD) is an inflammatory autoimmune disease of the central nervous system. Increasing evidence indicates that NMOSD is a Th2‐ and Th17‐dominant disease. IL‐25, IL‐31, and IL‐33 are three newly found Th2‐related cytokines, and their roles in the pathogenesis of NMOSD have not been studied. This study aimed to measure the serum levels of IL‐25, IL‐31, and IL‐33 in patients with NMOSD and evaluate their clinical implications.
Methods
Serum was collected from patients with NMOSD (n = 48) and healthy controls (HC, n = 28). Serum level measurements of IL‐25, IL‐31, IL‐33, IL‐17A, and IL‐6 were performed using enzyme‐linked immunoassay (ELISA) method.
Results
The serum levels of IL‐25, IL‐31, and IL‐33 were significantly higher in patients with NMOSD as compared to HC. The serum level of IL‐31 was significantly correlated with IL‐17A (r = 0.382,P = 0.009) in patients with NMOSD; the latter is a critical cytokine in the pathogenesis of NMOSD. The serum level of IL‐33 was higher in patients with characteristic brain lesions than patients without (307 pg/mL vs 166 pg/mL, P = 0.028). Furthermore, the serum level of IL‐33 in the acute phase of the disease was positively correlated with annualized relapse rate (r = 0.364, P = 0.04).
Conclusion
We found higher serum levels of IL‐25, IL‐31, and IL‐33 in patient with NMOSD as compared to healthy controls. The serum level of IL‐33 during acute phase was associated with more past attacks in patients with NMOSD.
Keywords: interleukin ‐31, interleukin ‐33, interleukin‐25, neuromyelitis optica spectrum disorders, Th2 axis
1. INTRODUCTION
Neuromyelitis optica spectrum disorder (NMOSD) is an inflammatory autoimmune disease of the central nervous system (CNS). It is classically characterized by recurrent optic neuritis and transverse myelitis.1 It also includes anti‐aquaporin‐4 (AQP4)‐IgG‐seropositive patients with first‐attack transverse myelitis, optic neuritis, or some brainstem and encephalitic presentations.2, 3
T and B cells and various cytokines have been related to the pathogenesis of NMOSD.4 Studies show that Th17‐ and Th2‐associated cytokines are upregulated in the cerebrospinal fluid or serum of patients with NMOSD.4 Therefore, based on current evidence, it is regarded that NMOSD expresses Th17 and Th2 axes in the development of the disease, whereas MS is primarily a Th1‐dominant disease.5 IL‐17A and IL‐6 are two cytokines of the Th17 axis. It is widely accepted that IL‐17A and IL‐6 play pivotal roles in the development of NMOSD lesions.5, 6
IL‐25, IL‐31 and IL‐33 are newly discovered Th2‐related cytokines.7, 8, 9, 10 They have been proven to be involved in the immunopathology of disorders, including asthma, fulminant hepatitis, atopic dermatitis, Inflammatory Bowel Disease (IBD), and arthritis.8, 9, 10 However, their roles in NMOSD have not been studied.
Therefore, in this study, we had the following aims: (i) to learn the serum levels of IL‐25, IL‐31, and IL‐33 in patients with NMOSD compared to healthy controls (HC); (ii) to investigate the correlation between the serum levels of IL‐25, IL‐31, and IL‐33 and the serum levels of IL‐17A and IL‐6 in patients with NMOSD; and (iii) to evaluate the clinical implications of serum IL‐25, IL‐31, and IL‐33 levels in patients with NMOSD.
2. METHODS
2.1. Study population
The study was carried out at the Shanghai RenJi Hospital, which is a major teaching hospital of the Medical School of Shanghai Jiao Tong University. We recruited consecutive patients admitted to our clinical center from January 2015 to December 2016 with the diagnosis of NMOSD. In this study, the diagnoses of all the patients were reviewed, and only the patients who fulfilled the diagnostic criteria established by Wingerchuket al11 in 2015 were included. We also chose healthy subjects who were matched well with the patients with NMOSD according to the age and sex of the controls.
The study was approved by the Ethics Committee of RenJi Hospital. Informed consent was obtained from all the included patients and healthy controls.
2.2. Clinical data collection
The clinical, laboratory, and radiology records of all the included patients were retrospectively reviewed. The clinical information collected for the patients with NMOSD included age, sex, total disease duration, current disease duration, Expanded Disability Status Scale (EDSS) score, annualized relapse rate (ARR), serum status and titers of anti‐AQP4 antibody, presentation of optic neuritis (ON), myelitis, longitudinally extensive transverse myelitis (LETM), characteristic brain lesions on MR images, and immunomodulatory drugs used at the time of sampling. Total disease duration is defined as the period from the time of the first attack to the time of sampling. Current disease duration refers to the period from the time of the current attack to the time of sampling. The patient was regarded as being in the acute phase of the attack if the current disease duration was less than 30 days. EDSS score was checked at the time of sampling. ARR is calculated using the time interval from the onset of the first attack to the time of sampling. We define the relapse of NMOSD as a clinical exacerbation presenting with new or worsening symptoms accompanied with a change on the neurological examination that correlated with a new or enhancing MRI lesion. The interval should be at least 30 days since the previous relapse.12 Anti‐AQP4 and antimyelin‐oligodendrocyte‐glycoprotein (MOG) antibodies were tested in all patients using a cell‐based assay.
We defined severe ON as a visual function score of 5 or 6 on the EDSS. Characteristic brain lesions of NMOSD refer to the increased signal on T2‐weighted MRI sequences in the following patterns: ➀lesions involving the dorsal medulla (especially the area postrema), ➁periependymal surfaces of the fourth ventricle in the brainstem/cerebellum, ➂lesions involving the hypothalamus, thalamus, or periependymal surfaces of the third ventricle, ➃large, confluent, unilateral, or bilateral subcortical or deep white matter lesions, ➄long (1/2 of the length of the corpus callosum or greater), diffuse, heterogeneous, or edematous corpus callosum lesions, ➅long corticospinal tract lesions, unilateral or bilateral, contiguously involving the internal capsule and cerebral peduncle, or ➆extensive periependymal brain lesions, often with gadolinium enhancement.11
In our center, high‐dose methylprednisolone (MP) pulse therapy is the first‐line of therapy to treat acute disease attacks. If the patients had the contradiction of the use of high‐dose steroids, high‐dose intravenous immunoglobulin (IVIG) would be another option. If the patient's condition does not sufficiently improve or if the neurological symptoms worsen, a second course of high‐dose MP pulse therapy or IVIG would be applied. After the acute attack, the patient would be slowly weaned off of the oral steroid, and long‐term immunosuppressive treatment would be initiated immediately. Azathioprine and mycophenolate mofetil are the first‐line selection of immunosuppressants in our center.
2.3. Blood sampling and the measurement of serum cytokines
Blood samples (3‐5 mL) were drawn from the peripheral veins in a red‐topped serum‐separating tube (BD Vacutainer®, Franklin Lakes, NJ, USA) according to the routine puncture method, and then the serum was collected using a centrifuge and stored at −80°C. Serum level measurement of IL‐25, IL‐31, IL‐33, IL‐17A, and IL‐6 was performed using an enzyme‐linked immunoassay (ELISA) according to the manufacturer's instructions (R&D system, Minneapolis, MN, USA).
2.4. Statistical analysis
Data analysis was performed using SPSS version 18.0 (SPSS Inc., Chicago, Ill., USA). The Kolmogorov‐Smirnov Z test was used to verify the normality of the data. Categorical variables were summarized as counts (percentage) and continuous variables as the means (standard error, SE) or medians (interquartile ranges, IQR), if not distributed normally. Statistical comparisons between the groups of NMOSD and healthy controls (HC) were performed using Student's t test or the Mann‐Whitney U test for continuous variables, as well as the chi‐square and Fisher's exact tests for categorical variables, as deemed appropriate. Correlations between continuous variables were assessed by Pearson's correlation coefficient or Spearman's correlation coefficient. A two‐tailed probability value < 0.05 was considered significant.
3. RESULTS
3.1. Clinical data of patients with NMOSD
A total of 48 patients were enrolled. The mean age was 44.0 years; the female to male ratio was 3:1. The median total disease duration was 348 days (IQR, 94.8‐1715 days). The median current disease duration was 17.5 days (IQR, 9‐32.8 days). The serum anti‐AQP4 antibody was positive in 27 (56.3%) patients, while anti‐MOG antibody was positive in 4 (8.3%) patients, and the remaining 17 (35.4%) patients were negative for both antibodies. Annualized relapse rate (ARR) was 0.7 (IQR, 0.1‐2.0). Twenty‐eight healthy subjects were recruited as controls. The age and sex ratios were not significantly different between the NMOSD group and HC group (Table 1).
Table 1.
Clinical data of patients with neuromyelitis optica spectrum disorders (NMOSD) and healthy controls (HC)
| NMOSD (N = 48) | HC (N = 28) | P value | |
|---|---|---|---|
| Age (mean±SE) | 44.0 ± 1.9 | 44.4 ± 2.4 | 0.889 |
| Sex (Female, %) | 36 (75) | 21 (75) | 1.000 |
| Anti‐AQP4 antibodya (%) | 27 (56.3) | ||
| EDSSb (median, IQR) | 3 (2‐6.75) | ||
| Current disease durationc (median, IQR, day) | 17.5 (9‐32.8) | ||
| Total disease durationd (median, IQR, day) | 348 (94.8‐1715) | ||
| Annualized relapse rate (%) | 0.7 (0.1‐2.0) | ||
| Clinical presentations (%) | |||
| ONe | 31 (64.6) | ||
| Severe ONf | 19 (39.6) | ||
| Myelitis | 39 (81.2) | ||
| LETMg | 30 (62.5) | ||
| Characteristic brain lesions on MR imagesh | 28 (58.3) | ||
Anti‐AQP4 antibody: anti‐aquaporin‐4 antibody.
EDSS: Expanded Disability Status Scale.
Current disease duration refers to the period from the time of current attack to the time of sampling.
Total disease duration is defined as the period from the time of first attack to the time of sampling.
ON: optic neuritis.
severe ON: defined as the score of visual function of EDSS 5 or 6.
LETM: longitudinally extensive transverse myelitis.
Characteristic brain lesions of NMOSD refers to the increased signal on T2‐weighted MRI sequence in the patterns as defined by Wingerchuk et al11 in 2015.
Of all 48 patients with NMOSD, 31 patients had ON; 19 patients had severe ON; 39 patients had myelitis; 30 patients had LETM; and 28 patients had characteristic brain lesions on cranial MR images. Eleven patients presented with an initial NMOSD attack.
At the time of sampling, 13 patients were drug‐naïve, while 35 patients had already been treated with immunomodulatory drugs. Twenty‐three patients had used steroids only. Three patients had used immunosuppressants only. One patient had used IVIG only. Five patients had used both steroids and immunosuppressants. Three patients had used both steroids and IVIG.
3.2. Serum levels of IL‐25, IL‐31, and IL‐33 in patients with NMOSD
The mean serum levels of IL‐25 and IL‐31 were significantly higher in patients with NMOSD compared to HC (IL‐25: 29.8 ± 1.5 pg/mL vs 21.2 ± 1.3 pg/mL, P < 0.001; IL‐31: 107 ± 5.4 pg/mL vs 74.2 ± 5.3 pg/mL, P < 0.001).
The median serum levels of IL‐33 were also significantly higher in patients with NMOSD compared to HC (142 (96.3‐301) pg/mL vs 102.0 (76.8‐150) pg/mL, P = 0.012).
The serum level of IL‐25 was correlated with the serum level of IL‐31 and IL‐33 (r = 0.429, P = 0.002; r = 0.389, P = 0.006, respectively), while the serum levels of IL‐31 and IL‐33 were not correlated with each other in patients with NMOSD.
3.3. Correlation of serum levels of IL‐25, IL‐31, and IL‐33 with serum levels of IL‐17A and IL‐6 in patients with NMOSD
IL‐17A and IL‐6 are widely studied in patients with NMOSD and are regarded as two important cytokines in the pathogenesis of the disease. Therefore, the association between the serum levels of IL‐25, IL‐31, and IL‐33 and the serum levels of IL‐17A and IL‐6 was compared in patients with NMOSD. The results showed that the serum level of IL‐31 was significantly correlated with IL‐17A (r = 0.382, P = 0.009) in patients with NMOSD (Figure 1).
Figure 1.
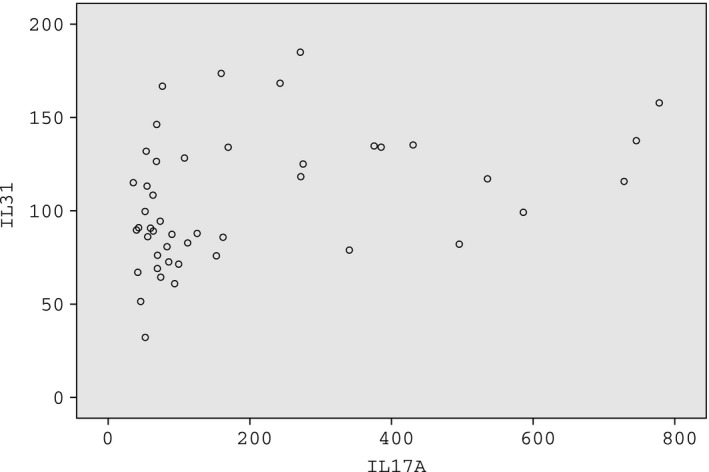
The serum level of IL‐31 was significantly correlated with IL‐17A (r = 0.382, P = 0.009) in patients with neuromyelitis optica spectrum disorders (NMOSD)
3.4. Correlation of serum levels of IL‐25, IL‐31, and IL‐33 with clinical data in patients with NMOSD
There was no significant difference in the serum levels of IL‐25, IL‐31, and IL‐33 between patients with and without ON, patients with and without severe ON, patients with and without myelitis, or patients with and without LETM. However, the serum level of IL‐33 was significantly higher in patients with characteristic brain lesions than patients without these lesions (307 pg/mL vs 166 pg/mL, P = 0.028).
Thirty‐two patients were in the acute phase (within 30 days from the onset of current attack). There was no significant difference in the serum level of IL‐25, IL‐31, and IL‐33 between patients in the acute phase (n = 16) and patients in the nonacute phase (n = 32).
To evaluate the effect of immunomodulatory drugs on the profile of IL‐25, IL‐31, and IL‐33, we compared the serum level of IL‐25, IL‐31, and IL‐33 in patients who had used immunomodulatory drugs (n = 35) and patients who were drug‐naïve (n = 13). No significant difference was found between the two groups.
The serum levels of IL‐25, IL‐31, and IL‐33 had no association with EDSS scores, total disease duration, or current disease duration. There was also no significant correlation between the level of cytokines and anti‐AQP4 antibody titers.
As the cytokine levels in the acute phase can better represent the inflammatory status of the patients, only patients in the acute phase were included in the analysis of the correlation with ARR. Among the 32 patients in the acute phase, only the serum level of IL‐33 was positively correlated with ARR (r = 0.364,P = 0.04; Figure 2).
Figure 2.
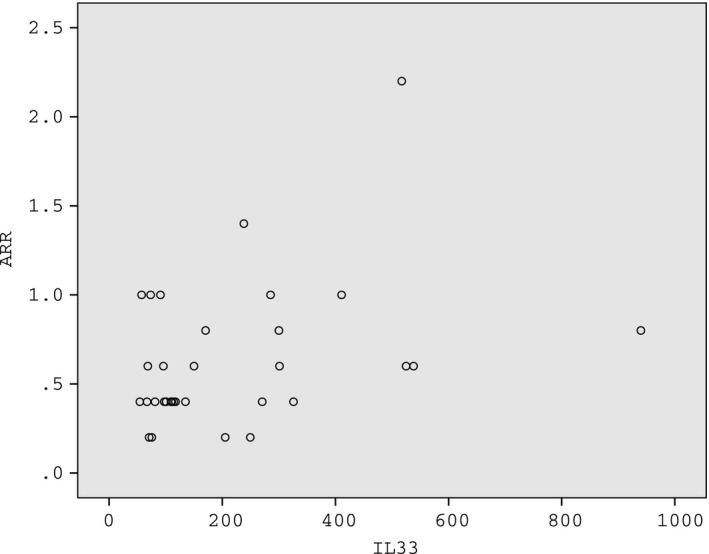
The serum level of IL‐33 in the acute phase was positively correlated with annualized relapse rate (ARR) in patients with neuromyelitis optica spectrum disorders (NMOSD) (r = 0.364, P = 0.04)
4. DISCUSSION
To our knowledge, the present study was the first one to detect the serum levels of IL‐25, IL‐31, and IL‐33 in patients with NMOSD. Our study demonstrated that the serum levels of IL‐25, IL‐31, and IL‐33 were all increased in patients with NMOSD compared to HC. The serum level of IL‐31 was associated with IL‐17A, which was regarded as a critical cytokine in the pathogenesis of NMOSD. Patients with typical brain lesions of NMOSD on the MR images had higher serum levels of IL‐33. Moreover, the serum level of IL‐33 in the acute phase of the disease was positively correlated with ARR, which indicated that a higher serum level of IL‐33 during the acute phase was related to more past relapses in patients with NMOSD.
The discovery of the anti‐AQP4 antibody in 2004 has dramatically changed our understanding of the pathophysiology of NMOSD.2 However, detailed mechanisms about how the autoantibodies are produced and attack the nervous system remain unknown.4 Cytokines and chemokines have been proven to play important roles in the pathogenesis of NMOSD by several recent immunological studies.5 In the current study, we found that the serum levels of three Th2‐related cytokines (IL‐25, IL‐31, and IL‐33) were increased in patients with NMOSD compared to HC.
IL‐25 (also called IL‐17E) is a member of the IL‐17 family. IL‐25 (IL‐17E) is the most distinct one in this family, with only a 16% similarity to IL‐17A (the most studied one).13 Diverse cells, including Th2 cells, eosinophils, basophils, microglia, epithelial cells, endothelial cells, alveolar macrophages, and IgE‐activated mast cells, can produce IL‐25.14 IL‐25 targets haematopoietic cells, such as Th2, Th9, or natural killer T cells; the latter can produce Th2 axis‐related cytokines, such as IL‐4, IL‐5, IL‐9, and IL‐13, under the induction of IL‐25.14 IL‐4 is a key regulator in humoral and adaptive immunity. It can promote the differentiation of B cells into plasma cells.15 IL‐5 has the effect of the stimulation of B‐cell growth and can increase the secretion of immunoglobulins.16 IL‐25 has protective roles against worm or bacterial infection; it might also participate in intestinal inflammation. IL‐25 has been proven to be a pathogenic factor during allergic diseases (such as asthma and atopic dermatitis) due to its induction of Th2 cytokines.7, 8 We found that the serum level of IL‐25 was increased in patients with NMOSD. Thus, we hypothesized that IL‐25 might play a role in the pathogenesis of NMOSD by promoting the production of autoantibodies, such as the anti‐AQP4 antibody.
IL‐31 belongs to the family of IL‐6 cytokines, which also includes IL‐6, IL‐11, and IL‐27.17 IL‐31 is mainly produced by CD4 + T cells, particularly activated Th2 cells.18 The IL‐31 receptor complex has been found to be expressed by various cells, including dorsal root ganglia, activated monocytes, macrophages, basophils, eosinophils, and keratinocytes.19 Recent studies reported that patients with inflammatory bowel disease, asthma, allergic rhinitis, and atopic dermatitis had increased IL‐31 serum levels.9 Moreover, the serum levels IL‐31 had been correlated with disease activity in some of the above‐mentioned diseases.9 In the current study, we found an increased serum level of IL‐31 in patients with NMOSD. We also noted a positive correlation between serum level of IL‐31 and IL‐17A, which is identified as a critical cytokine in the pathogenesis of NMOSD.5, 6 However, there was no correlation between serum level of IL‐31 and disease severity or activity. Further study is needed to reveal the role of IL‐31 in the development of NMOSD and its interaction with IL‐17A.
Recently, IL‐33 was found to be a new member of the IL‐1 cytokine family.20 Through heterotrimeric binding to T1/ST2 and IL‐1RAcP, IL‐33 can induce type‐2 immune responses.21 High expression of IL‐33 has been observed in the lung, gastrointestinal tract, skin, and brain.20 Many immune cells are responsive to IL‐33 and carry cell surface T1/ST2.22 The most well‐described effect of IL‐33 is the initiation of innate and adaptive type‐2 immune responses, which is represented by the production of IL‐4, IL‐5, and IL‐13.22, 23 Additionally, IL‐33 can regulate Th17‐ and Th1‐coordinated inflammation, including neutrophil and antiviral T‐cell responses. The effects of IL‐33 in health and disease are highly tissue‐specific and are influenced by the microenvironment.9 Recent studies proved that the IL‐33:ST2 axis was involved in the immunopathology of diseases, including atopic dermatitis, asthma, inflammatory bowel disease, and arthritis.24, 25, 26 As NMOSD is regarded as a Th17‐ and Th2‐dominant disease, we hypothesized that IL‐33 might also be involved in the pathogenesis of the disease by promoting Th17‐ and Th2‐related immune responses. The results of our study partially verified this hypothesis. In the current study, we revealed a higher serum level of IL‐33 in patients with NMOSD compared to HC; a higher level of serum IL‐33 may suggest the presence of typical brain lesions of NMOSD; moreover, a higher serum level of IL‐33 during an acute phase was related to more past attacks, which may imply a higher disease activity in patients with NMOSD. Therefore, further study is warranted to disclose a detailed mechanism of IL‐33 in the immunopathology of NMOSD. In addition, as predictors of the relapse of NMOSD are currently lacking, IL‐33 might be a promising candidate.
As far as we know, this is the first study to reveal the possible role of the three newly discovered Th2‐related cytokines in the pathogenesis of NMOSD. However, there are several limitations. One limitation in this study is the relatively small number of patients included and the lack of follow‐up; therefore, large‐scale studies should be conducted with longer periods of follow‐up. Second, we only detected the serum level of the cytokines; it would be much better if we could detect the serum and CSF levels simultaneously. Third, we did not compare the serum levels of the cytokines of the same patient before and after immunomodulatory treatment and during the different stages of the disease, which could be different. Finally, underlying mechanisms of the three cytokines in NMOSD pathogenesis should be further studied. Furthermore, it might not be appropriate that MOG‐IgG positive patients were included in the current study as accumulating evidence suggests that anti‐MOG‐related disorders might be their own disease entity.27, 28, 29 However, according to the 2015 diagnostic criteria of NMOSD, MOG‐IgG is not explicitly mentioned as an exclusion criterion; therefore, we still included patients with MOG‐IgG positive who met the 2015 criteria in the current study.
5. CONCLUSION
In conclusion, we found higher serum levels of IL‐25, IL‐31, and IL‐33 in patients with NMOSD compared to HC. A higher level of serum IL‐33 may suggest the presence of typical brain lesions of NMOSD; furthermore, a higher serum level of IL‐33 during an acute phase may imply a higher disease activity in patients with NMOSD. However, further studies are required to investigate the underlying mechanisms of the three cytokines and the immunopathology of NMOSD.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
Serum samples of the patients with NMOSD were stored in and obtained from Renji Biobank, Shanghai Jiao Tong University School of medicine.30 We are grateful to all the staff of Renji Biobank.
Zhang Y, Yao X‐Y, Gao M‐C, et al. Th2 axis‐related cytokines in patients with neuromyelitis optica spectrum disorders. CNS Neurosci Ther. 2018;24:64–69. 10.1111/cns.12774
Funding information
This study was supported by the National Natural Science Foundation of China (No. 81471219, 81230027) and the Program of Shanghai Academic Research Leader (14XD1403400).
The first two authors contributed equally to this work.
REFERENCES
- 1. Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology. 2006;66:1485‐1489. [DOI] [PubMed] [Google Scholar]
- 2. Lennon VA, Wingerchuk DM, Kryzer TJ, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364:2106‐2112. [DOI] [PubMed] [Google Scholar]
- 3. Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG. The spectrum of neuromyelitis optica. Lancet Neurol. 2007;6:805‐815. [DOI] [PubMed] [Google Scholar]
- 4. Uzawa A, Mori M, Arai K, et al. Cytokine and chemokine profiles in neuromyelitis optica: significance of interleukin‐6. Mult Scler. 2010;16:1443‐1452. [DOI] [PubMed] [Google Scholar]
- 5. Uzawa A, Masahiro M, Kuwabara S. Cytokines and chemokines in neuromyelitis optica: pathogenetic and therapeutic implications. Brain Pathol. 2014;24:67‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bukhari W, Barnett MH, Prain K, Broadley SA. Molecular pathogenesis of neuromyelitis optica. Int J Mol Sci. 2012;13:12970‐12993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xu M, Dong C. IL‐25 in allergic inflammation. Immunol Rev. 2017;278:185‐191. [DOI] [PubMed] [Google Scholar]
- 8. Song X, He X, Li X, Qian Y. The roles and functional mechanisms of interleukin‐17 family cytokines in mucosal immunity. Cell Mol Immunol. 2016;13:418‐431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rabenhorst A, Hartmann K. Interleukin‐31: a novel diagnostic marker of allergic diseases. Curr Allergy Asthma Rep. 2014;14:423. [DOI] [PubMed] [Google Scholar]
- 10. Hardman C, Ogg G. Interleukin‐33, friend and foe in type‐2 immune responses. Curr Opin Immunol. 2016;42:16‐24. [DOI] [PubMed] [Google Scholar]
- 11. Wingerchuk DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85:177‐189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kessler RA, Mealy MA, Levy M. Early indicators of relapses vs pseudorelapses in neuromyelitis optica spectrum disorder. Neurol Neuroimmunol Neuroinflamm. 2016;3:e269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Iwakura Y, Ishigame H, Saijo S, Nakae S. Functional specialization of interleukin‐17 family members. Immunity. 2011;34:149‐162. [DOI] [PubMed] [Google Scholar]
- 14. Song X, Qian Y. IL‐17 family cytokines mediated signaling in the pathogenesis of inflammatory diseases. Cell Signal. 2013;25:2335‐2347. [DOI] [PubMed] [Google Scholar]
- 15. Puri RK. Structure and function of interleukin‐4 and its receptor. Cancer Treat Res. 1995;80:143‐185. [DOI] [PubMed] [Google Scholar]
- 16. Takatsu K. Interleukin‐5: an overview. Cancer Treat Res. 1995;80:187‐208. [DOI] [PubMed] [Google Scholar]
- 17. Dillon SR, Sprecher C, Hammond A, et al. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat Immunol. 2004;5:752‐760. [DOI] [PubMed] [Google Scholar]
- 18. Bilsborough J, Leung DY, Maurer M, et al. IL‐31 is associated with cutaneous lymphocyte antigen‐positive skin homing T cells in patients with atopic dermatitis. J Allergy Clin Immunol. 2006;117:418‐425. [DOI] [PubMed] [Google Scholar]
- 19. Cornelissen C, Lüscher‐Firzlaff J, Baron JM, Lüscher B. Signaling by IL‐31 and functional consequences. Eur J Cell Biol. 2012;91:552‐566. [DOI] [PubMed] [Google Scholar]
- 20. Schmitz J, Owyang A, Oldham E, et al. IL‐33, an interleukin‐1‐like cytokine that signals via the IL‐1 receptor‐related protein ST2 and induces T helper type 2‐ associated cytokines. Immunity. 2005;23:479‐490. [DOI] [PubMed] [Google Scholar]
- 21. Liu X, Hammel M, He Y, et al. Structural insights into the interaction of IL‐33 with its receptors. Proc Natl Acad Sci USA. 2013;110:14918‐14923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Neill DR, Wong SH, Bellosi A, et al. Nuocytes represent a new innate effector leukocyte that mediates type‐2 immunity. Nature. 2010;464:1367‐1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kondo Y, Yoshimoto T, Yasuda K, et al. Administration of IL‐33 induces airway hyperresponsiveness and goblet cell hyperplasia in the lungs in the absence of adaptive immune system. Int Immunol. 2008;20:791‐800. [DOI] [PubMed] [Google Scholar]
- 24. Kurowska‐Stolarska M, Kewin P, Murphy G, et al. IL‐33 induces antigen‐specific IL‐5 + T cells and promotes allergic‐induced airway inflammation independent of IL‐4. J Immunol. 2008;181:4780‐4790. [DOI] [PubMed] [Google Scholar]
- 25. Pastorelli L, Garg RR, Hoang SB, et al. Epithelial‐derived IL‐33 and its receptor ST2 are dysregulated in ulcerative colitis and in experimental Th1/Th2 driven enteritis. Proc Natl Acad Sci USA. 2010;107:8017‐8022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xu D, Jiang HR, Kewin P, et al. IL‐33 exacerbates antigen‐induced arthritis by activating mast cells. Proc Natl Acad Sci USA. 2008;105:10913‐10918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jarius S, Ruprecht K, Kleiter I, et al. in cooperation with the Neuromyelitis Optica Study Group (NEMOS) . MOG‐IgG in NMO and related disorders: a multicenter study of 50 patients. Part 1: frequency, syndrome specificity, influence of disease activity, long‐term course, association with AQP4‐IgG, and origin. J Neuroinflammation. 2016;13:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jarius S, Ruprecht K, Kleiter I, et al. in cooperation with the Neuromyelitis Optica Study Group (NEMOS) . MOG‐IgG in NMO and related disorders: a multicenter study of 50 patients. Part 2: epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long‐term outcome. J Neuroinflammation. 2016;13:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cobo‐Calvo Á, Ruiz A, D'Indy H, et al. MOG antibody‐related disorders: common features and uncommon presentations. J Neurol. 2017;264:1945‐1955. [DOI] [PubMed] [Google Scholar]
- 30. Ma Y, Dai H, Wang L, Zhu L, Zou H, Kong X. Consent for use of clinical leftover biosample: a survey among Chinese patients and the general public. PLoS One. 2012;7:e36050. [DOI] [PMC free article] [PubMed] [Google Scholar]


