Abstract
Arrhythmogenic right ventricular dysplasia (ARVD) is a rare cardiomyopathy characterized by the progressive replacement of cardiomyocytes by fatty and fibrous tissue in the right ventricle (RV). These infiltrations lead to cardiac electrical instability and ventricular arrhythmia. Current treatment for ARVD is empirical and essentially based on treatment of arrhythmia. Thus, there is no validated treatment that will prevent the deterioration of RV function in patients with ARVD. The aim of the BRAVE study is to evaluate the effect of ramipril, an angiotensin‐converting enzyme inhibitor, on ventricular myocardial remodeling and arrhythmia burden in patients with ARVD. Despite the fact that myocardial fibrosis is one of the structural hallmarks of ARVD, no study has tested an antifibrotic drug in ARVD patients. The trial is a double‐blind, parallel, multicenter, prospective, randomized, phase 4 drug study. Patients will be randomized into 2 groups, ramipril or placebo. The 120 patients (60 per group) will be enrolled by 26 centers in France. Patients will be followed up every 6 months for 3 years. The 2 co–primary endpoints are defined as the difference of telediastolic RV volume measured by magnetic resonance imaging between baseline and 3 years of follow‐up, and the change in arrhythmia burden during the 3 years of follow‐up. A decrease in RV and/or left ventricular deterioration and in arrhythmia burden are expected in ARVD patients treated with ramipril. This reduction will improve quality of life of patients and will reduce the number of hospitalizations and the risk of terminal heart failure.
Keywords: Arrhythmia/All, Cardiomyopathy, Remodeling/Cardiovascular, Sudden Death
1. INTRODUCTION
Arrhythmogenic right ventricular dysplasia (ARVD) is a rare cardiomyopathy with low incidence (2/10 000 person‐years) and prevalence (0.02%–0.1%). This disease is characterized by the progressive replacement of cardiac muscle cells by fatty and fibrous tissue in the right ventricle (RV). Fontaine et al. were the first to demonstrate the RV origin of ventricular arrhythmias in some patients with apparently healthy heart.1 The disease is diagnosed most often in adolescents and young adults. The disease is more common in men than in women (3:1 ratio). The most common location is between the anterior infundibulum, the apex of the RV, and the lower or diaphragmatic aspect of the RV. This dysplasia can lead to a dilatation or aneurysm formation with paradoxical movements. In more advanced cases, almost all of the RV is replaced by fibrous and fatty tissue. The left ventricle (LV) and the septum are usually spared but can be affected in the most extensive cases. Expressivity of ARVD is variable, from mildly symptomatic clinical forms to severe forms. These infiltrations lead to instability of the electrical activity of the heart thus facilitating the reentry phenomena and therefore ventricular tachycardia (VT), severe ventricular arrhythmias, and even sudden cardiac death (SCD). The clinical diagnosis of ARVD is based on a series of clinical criteria established by international consensus.2, 3
The first clinical signs appear before the age of 40 in >80% of cases. This is one of the common causes of SCD (5% of SCD), especially in the young and athletic. ARVD leads to irreversible heart failure (HF) observed in 75% of the patients. About 50% of ARVD cases are hereditary. Genetic transmission is usually autosomal dominant. The main candidate genes of ARVD are the plakophilin 2 gene (PKP2), desmoglein 2 gene (DSG2), desmocollin 2 gene (DSC2), and desmoplakin gene (DSP). These desmosome proteins are important for the mechanical integrity of the cardiomyocytes. These mutations are associated with more significant structural degradation.
Current treatment for ARVD is empirical and essentially based on treatment of arrhythmia and prevention of SCD with β‐blockers, implantable cardioverter‐defibrillators (ICD), and ablation. An ICD is an efficient treatment for prevention of SCD.4 Long‐term follow‐up studies are rare. Magnetic resonance imaging (MRI) and echocardiograms are the best examinations to monitor progression of ARVD.
There is no preventive treatment of the deterioration of RV function in patients with ARVD. Several arguments may be considered for using antifibrotic and vasodilator medications in this pathology:
A recent experimental study demonstrated that decreasing the preload of the RV allowed the stabilization of cardiomyopathy.5 Fabritz et al. conducted a randomized blinded study to test a load‐reduction therapy combining furosemide and nitrates vs water from heterozygous plakoglobin‐deficient mice. The results of this study showed that this load‐reduction therapy prevented enlargement of the RV induced by exercise and that the rate of inducibility of VT was lower in mice receiving load‐reduction therapy.5
La Gerche et al. showed that physical exercises in high barometric conditions accelerate the deterioration of RV function. Increased stress in the wall of the end‐systolic RV is proportional to the intensity of exercise; moreover, athletes have greater structural modification of their RV relative to their LV compared with nonathletes. They thus demonstrated consistency between acute hemodynamic stress factors during exercise and chronic structural changes in the heart that affect both the RV and LV. These changes are more important to the RV than the LV.6
Several studies have shown that angiotensin‐converting enzyme inhibitors (ACEIs) are associated with a lower incidence of ventricular arrhythmias in patients with congestive HF or LV dysfunction.7, 8
Fibrosis is a component of the arrhythmia substrate with adipocyte degeneration. In 1982, Marcus et al. described the first clinical series of ARVD patients with adipose tissue infiltration, aneurysms, and an inconstant amount of intramyocardial fibrosis.9 This tissue replacement by fatty and fibrous tissue accompanied by degenerative changes of myocytes and nuclei was then consistently found in ARVD patients.10, 11, 12, 13 Besides decreasing the blood pressure and attenuating myocardial fibrosis, it is presumed that ramipril has some effects on lipid peroxydation and on regulation of extracellular matrix proteins.14, 15
We brought to the fore that ACEIs might stabilize myocardial deterioration in a preliminary study. We performed dosages of matrix metalloproteinase (MMP)9 and its inhibitors tissue inhibitor of metalloproteinase (TIMP)1 and TIMP2, in 33 patients treated with both β‐blockers and ACEIs and 31 patients treated with β‐blockers without ACEIs. Each patient had blood samples 1 year apart. Turnover of tissue collagen was measured by evolution of MMP9/TIMP1 ratio and MMP9/TIMP2 ratio between the 2 time points (Student test for paired samples). We observed a stabilization of the MMP9/TIMP1 and MMP9/TIMP2 ratio in patients treated with ACEIs (2.54 ± 1.98 vs 3.38 ± 2.71 for MMP9/TIMP1; 6.92 ± 8.15 vs 7.51 ± 6.42 for MMP9/TIMP2; Figure 1A). In contrast, we observed a significant increase of MMP9/TIMP1 ratio (1.86 ± 1.91 vs 3.74 ± 2.36, P < 0.01) and MMP9/TIMP2 ratio (4.36 ± 4.54 vs 6.70 ± 4.33, P = 0.02) in patients treated with β‐blockers without ACEIs (Figure 1B).
Figure 1.
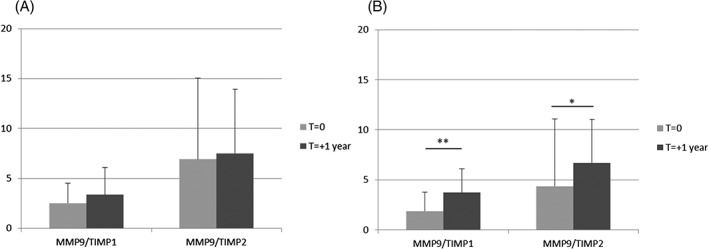
MMP9/TIMP1 and MMP9/TIMP2 ratios (A) in patients treated with β‐blockers with ACEIs and (B) in patients treated with β‐blockers without ACEIs. **P < 0.01; *P = 0.02. Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; MMP, matrix metalloproteinase; TIMP, tissue inhibitor of metalloproteinase
Ramipril was chosen among the ACEIs because there is evidence for abnormal peroxisome proliferator‐activated receptor (PPAR) signaling in ARVD and ramipril that has been shown to restore PPARβ/δ and PPARγ expressions in an experimental model of cardiomyopathy.16, 17 Despite the fact that myocardial fibrosis is one of the structural hallmarks of ARVD, no study has tested an antifibrotic drug in ARVD patients [18].
2. METHODS
Blockade of the Renin‐Angiotensin‐Aldosterone System in Patients With Arrhythmogenic Right Ventricular Dysplasia (BRAVE) will be a national multicenter, prospective, randomized, controlled, double‐blind trial performed in 2 parallel groups. It is a phase 4 drug study. This project aims to evaluate the effect of antifibrotic medication (renin‐angiotensin axis) in patients with ARVD. This is the first randomized controlled trial evaluating the effect of an antifibrotic drug on the RV degradation processes in patients with ARVD. The primary objective is to compare at 3 years of follow‐up the effect of ramipril vs placebo on the reduction of RV deterioration and ventricular arrhythmia in patients with ARVD using MRI and 24‐hour Holter ECG. The second objectives of the study are (1) to compare the effect of ramipril treatment vs placebo at 3 years of follow‐up on morphologic, rhythmic and functional criteria (RV and LV function, arrhythmia onset, functional symptoms), (2) to evaluate whether the treatment effect varies according to the genotype: comparison of RV function at 3 years of follow‐up according to the genotype (no mutation/at least 1 mutation on desmosome genes), and (3) to evaluate the association between fibrosis and inflammation markers and myocardial deterioration at 3 years of follow‐up.
2.1. Patient population
The study will be performed in ARVD patients meeting the criteria of the task force established by the European Society of Cardiology (ESC)/International Society and Federation of Cardiology (ISFC). Patients will be recruited during hospitalization or consultation in the cardiology unit in the 3 French national reference centers and the 22 French competence centers for rare arrhythmias (CARDIOGEN network). The inclusion and exclusion criteria are listed in Table 1.
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria |
|---|
| Patient age > 18 y |
| Diagnosis of ARVD based on Task Force criteria. Two major criteria: 1 morphologic and 1 rhythmic, or 1 major and 2 minor criteria19, 20 |
| Increased RV volume (>100 mL/m2 female, >110 mL/m2 male) |
| Genetic analysis performed for desmosome genes (PKP2, DSG2, DSC2, DSP, JUP) whatever the result, mutation or no mutation |
| LVEF >40% |
| Exclusion criteria |
| Pregnancy |
| No health insurance |
| MRI contraindication (claustrophobia, implantable defibrillator) |
| No genetic analysis for desmosome genes |
| Right HF, defined with symptoms (exertional dyspnea, ankle swelling, epigastric fullness and right upper abdominal discomfort or pain) and signs (raised jugular venous pulse, an accentuated second pulmonary sound, RV gallop, an enlarged tender liver) |
| Ramipril contraindication (hypotension, renal failure) |
| Normal RV volume |
| Heart transplantation |
| Swallowing disorders |
Abbreviations: ARVD, arrhythmogenic right ventricular dysplasia; HF, heart failure; LVEF, left ventricular ejection fraction; MRI, magnetic resonance imaging; RV, right ventricular.
Patients implanted with an ICD for secondary prevention of ventricular arrhythmias during the study will be excluded from the study.
2.2. Study design
Patients will be randomized into 2 groups, ramipril or placebo. The study has a 1:1 ratio of subjects in the 2 groups. It is expected that 26 centers in France will be required to enroll the 120 patients (60 per group). The overall study design is shown in Figures 2 and 3. For key study committees, participating hospitals, and investigators, see Supporting Information, Appendix, in the online version of this article. The study will include 2 groups: experimental group, patients receiving renin‐angiotensin‐aldosterone system (RAAS) inhibitor treatment, ramipril; and control group, patients receiving placebo.
Figure 2.
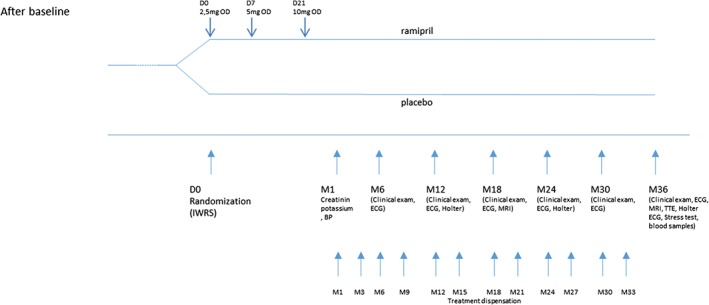
Clinical design of the study from screening to randomization. Abbreviations: ARVD, arrhythmogenic right ventricular dysplasia; BP, blood pressure; ECG, electrocardiogram; ECG‐HA, high‐amplification ECG; IWRS, interactive web response system; LVEF, left ventricular ejection fraction; MRI, magnetic resonance imaging; OD, once a day; TTE, transthoracic echocardiography
Figure 3.
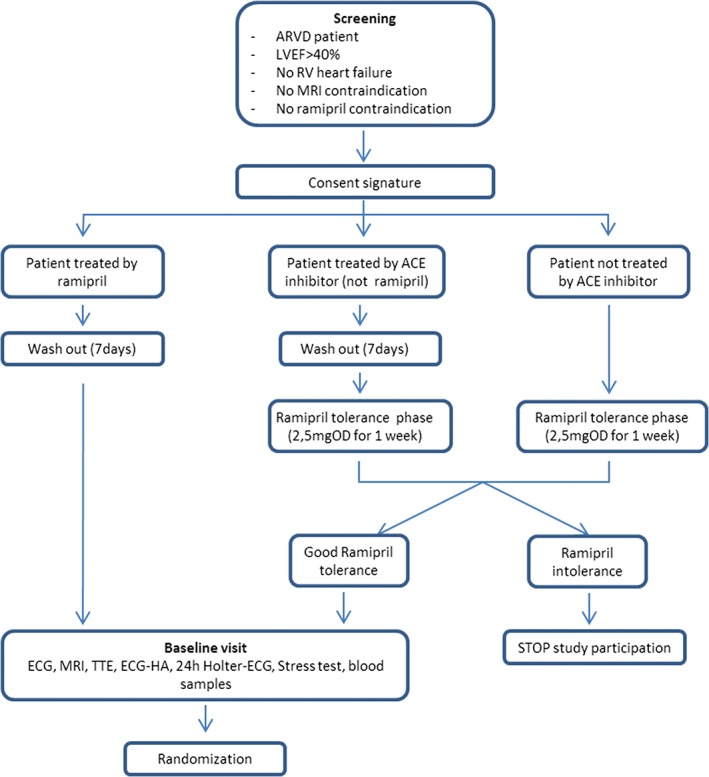
Clinical design of the study from randomization to follow‐up visits. Abbreviations: ACE, angiotensin‐converting enzyme; ECG, electrocardiogram; ECG‐HA, high‐amplification ECG; IWRS, interactive web response system; LVEF, left ventricular ejection fraction; MRI, magnetic resonance imaging; OD, once a day; RV, right ventricular; TTE, transthoracic echocardiography
All randomized subjects will undergo a complete workup (echocardiography, MRI, Holter ECG, stress test, ECG, blood samples) before the beginning of the treatment to confirm the ARVD diagnosis and to evaluate the RV volume and the clinical status of the patient. Patients will be followed up every 6 months for 3 years and any clinical events will be collected. The patient will be questioned at each visit on his sport practice (type of activity, duration, intensity). The activity will be quantified in hours per week. At the last visit (36 months), the complete workup will also be performed (Table 2).
Table 2.
Testing performed at each visit
| Tests | Baseline | Follow‐up: 1, 6, 18, 30 months | Follow‐up: 12, 24 months | Follow‐up: 36 months |
|---|---|---|---|---|
| Clinical exam, arterial blood pressure, Cr, potassium dosages | X | X | X | X |
| ECG, 24‐h Holter ECG | X | — | X | X |
| High‐amplification ECG, TTE, MRI, stress test | X | — | — | X |
| MMP, TIMP, IL dosages | X | — | — | X |
| Observation, adverse‐events collection | — | X | X | X |
Abbreviations: Cr, creatinine; ECG, electrocardiogram; IL, interleukin; MMP, matrix metalloproteinase; MRI, magnetic resonance imaging; TIMP, tissue inhibitor of metalloproteinase; TTE, transthoracic echocardiography.
2.2.1. Randomization method
Centralized randomization will be performed through an interactive web response system (IWRS) stratified by center and plakophilin genotype. The investigator will contact the IWRS after assessing the inclusion and exclusion criteria before the first administration of study drug. Randomization will be balanced between the 2 treatment arms. The IWRS will allocate the treatment group assignment for the subject.
2.2.2. Ramipril tolerance
Based on the Studies of Left Ventricular Dysfunction (SOLVD; enalapril evaluated in 7400 patients), it is expected that 2% to 3% of patients will report side effects (cough, hypotension) and will not continue to participate in the study.19 In consequence, a phase of ramipril tolerance will be planned before the randomization. Each patient will receive ramipril at 2.5 mg once a day for 1 week to identify potential side effects before the baseline visit. Venous blood samples will be taken and potassium and creatinine tests will be performed before and after the ramipril test. Patients will be asked about the occurrence of unusual tiredness. Patients tolerant to ramipril who did not show specific increase in potassium and creatinine, cough, or hypotension will participate in the study.
Patients already treated with an ACEI can participate in the study. A washout phase of 7 days will be completed before the start of the study treatment.
Ramipril dosage will be adjusted in each patient every week until the optimal dosage is attained. The minimal dose tested will be 2.5 mg/d. The optimal dosage corresponds to the maximum dosage tolerated according to current clinical practice. The dose will be adjusted in steps of 2.5 mg every week until the optimal dose. The optimal dose is usually between 5 mg and 10 mg.
2.2.3. Concomitant treatments
There will be no standardization of β‐blocker use because titration of both ramipril and adrenergic blockade requires a specific method. All patients will be treated with β‐blockers. A drug class effect is usually accepted in ARVD patients with no arrhythmias. β‐Blockers appear similar in their clinical effects and therefore, interchangeable. Patients already being treated with angiotensin II receptor blockers or mineralocorticoid antagonists will not be included, or a washout period of 2 weeks will be proposed.
2.3. Endpoints
2.3.1. Primary endpoints
The 2 co‐primary endpoints are (1) the difference of telediastolic RV volume measured by MRI between baseline and 3 years of follow‐up, and (2) the change in arrhythmia burden during the 3 years of follow‐up.
2.3.2. Secondary endpoints
The secondary exploratory endpoints are:
- Efficacy of treatment and progression of ARVD between groups will be considered on morphologic, rhythmic, and functional criteria according to the following hierarchy:
- Morphologic criteria: MRI (baseline and at 3 years of follow‐up): LV function, presence of aneurism, percent of dyskinesia; transthoracic echocardiography: (baseline and at 3 years of follow‐up): size of RV, LV function, aneurism, percent dyskinesia, transesophageal atrial pacing stress echocardiography; ECG (every 6 months): evolution of QRS width (50 mm/s).
- Rhythmic criteria: 24‐hour Holter ECG (every year): number of ventricular extrasystoles, sustained ventricular tachycardia; ECG (every 6 months): PR duration; high amplification ECG (baseline and at 3 years of follow‐up): late potentials; stress test (baseline and at 3 years of follow‐up): number of ventricular extrasystoles .
- Functional criteria: Evolution of functional symptoms at each 6‐month visit: presence/absence of palpitations, ventricular tachycardia, dyspnea, syncope, sudden death, thoracic pain, major adverse cardiac events; number of hospital admissions owing to clinical deterioration during the 3 years of follow‐up.
Comparison of evolution of telediastolic RV volume measured by MRI and arrhythmia burden measured by 24‐hour Holter ECG between baseline and 3 years of follow‐up according to the genotype of desmosome genes.
- Quantification of fibrosis and inflammation (measured at baseline and 3 years of follow‐up):
- Dosage of MMP9 and its inhibitors tissue TIMP1, TIMP2: determination of turnover of collagen with MMP9/TIMP1 ratio and MMP9/TIMP2 ratio
- Dosage of interleukin (IL)6 and IL8
2.4. Statistical analysis
2.4.1. Sample‐size calculation
We considered 2 co‐primary endpoints: symptomatic ventricular arrhythmia and RVSI year‐increase. Based on a 2016 study by Martin et al..., we can expect that 65% of patients in the control group will develop ventricular tachyarrhythmia at the end of the follow‐up.20 We expected 35% of patients in the treated group. With a type I error of 2.5%, 52 patients are needed in each group (power 80%).
Based on a 2010 study by Riley et al, we can expect a right ventricular stroke index (RVSI; systolic RV volume indexed to body surface area) year‐increase of 10 mL/m2 (SD: 8.7 mL/m2) in the control group.21 With a type I error of 2.5% and 59 patients in each group, we will be able to show a diminution of 50% of the RVSI evolution slope (thus, an expected RVSI year‐increase of 5 mL/m2) in the experimental group, with a power of 80%.
We needed a total of 60 patients per group; therefore, 120 patients were recruited.
2.4.2. Descriptive analyses at baseline
For each categorical parameter, total sample size, number of available values and/or number of missing values, together with the observed frequency rate of the pertinent modality presented as a percentage, will be computed for each treatment arm and on the total population.
The following descriptive statistics will be presented for each treatment group and on the total population: total sample size, number of available values, number of missing values, mean and its SE, SD, median, quartiles 1 and 3, minimum, and maximum. Patient characteristics at baseline will be compared between groups using the Fisher exact test for categorical variables and the t test for continuous ones. In case of violation of the normal distribution assumption for continuous baseline characteristics, the Wilcoxon test will be performed. Tests will be 2‐sided and α error risk will be 5%. No substudies are planned.
2.4.3. Primary analysis of the primary evaluation criteria
Arrhythmia burden at the end of follow‐up will be compared between the 2 arms using the Fisher exact test. For the evolution of RSVI, comparison between groups will be performed with a t test after validation of the normal distribution hypothesis with a Q‐Q plot. In case of normality assumption violation, the Wilcoxon test will be performed.
Given the 2 primary endpoints, the α error risk will be corrected for multiple tests, Bonferroni method, and be chosen at 2.5%.
2.4.4. Secondary analyses of the primary evaluation criteria: modelization
Ramipril effect on arrhythmia burden will be studied with a mixed‐effects logistic regression model adjusted for center, stratification variables for randomization, and unbalanced variables despite the randomization.
Ramipril effect on RSVI will be studied with a mixed‐effects linear regression model also including RSVI at baseline, stratification variables for randomization, and unbalanced variables despite the randomization. Statistical tests will be 2‐sided and α error risk will be set at 2.5%.
2.4.5. Secondary evaluation criteria (linked with the secondary endpoints)
The secondary evaluation criteria will be compared between treatment arms using the Fisher exact test for categorical variables and the t test for continuous variables after validation of the normal distribution hypothesis with a Q‐Q plot. In case of normality assumption violation, the Wilcoxon test will be performed. Tests will be 2‐sided with an α error risk at 5%.
3. RESULTS
The study will be funded by a French Health Ministry budget. The first patients should be included in the second quarter of 2018. Given the 1‐year inclusion period and the 3‐year follow‐up period, it is expected that the latest clinical data will be collected in the third quarter of 2022.
4. DISCUSSION
Data to inform prescribing of ACEI treatments for ARVD patients are sparse, and subsequently no consensus exists on the optimal agent(s). Available studies are only based on antiarrhythmic therapies; none target ventricular mechanical complications.22 To our knowledge, the BRAVE study is the first randomized controlled trial for drug therapy in ARVD patients. This study will be prospectively registered, robustly conducted, independently monitored, rigorously analyzed, and transparently reported. We will recruit to target with independent data monitoring, minimal patient or data loss, and achieved comparability at baseline.
Given the pharmacologic properties of ramipril, decrease in RV and/or LV deterioration and in arrhythmia burden are expected in ARVD patients treated with this medication. This beneficial effect is likely to improve the quality of life of patients and reduce the number of hospitalizations as well as the risk of terminal HF.
There is evidence that some ACEIs have additional therapeutic benefits beyond reduction in arterial blood pressure, including oxidative stress–lowering properties or effect on PPARγ pathways. Given the role of adipogenesis and oxidative stress in the pathophysiology of ARVD, future research should further explore the mechanistic actions of ACEIs to establish if other therapeutic benefits exist in ARVD patients. In addition, given the variation in dosing regimens and side‐effect profiles of the ACEI agents prescribed in ARVD, future studies will have to further assess adherence and acceptability of individual agents.
Regarding policy‐making for ACEI prescription, if use of ACEI is shown to be clinically effective in ARVD patients, it is currently likely to be considered cost‐effective for routine use in this patient population. The results of BRAVE will provide evidence regarding whether ACEI is beneficial to RV function (improvement/stabilization) and improves other important parameters including laboratory (collagen turnover) and clinical outcomes (hospitalization rates, arrhythmias). It will clarify whether the benefits of this intervention outweigh the risks. It is therefore hoped that this pivotal trial can provide new findings to allow future consideration of a large randomized controlled trial with mortality outcomes in this important group of patients.
4.1. Study limitations
The BRAVE study has some limitations that should be discussed. It could be argued that the applicability of the results will be limited by the fact that not all patients have the same β‐blocker. However, a drug‐class effect is usually accepted in ARVD patients with no arrhythmias. β‐Blockers appear similar in their clinical effects, and, therefore, interchangeable.
Because ARVD is a rare disease, the recruitment capabilities of BRAVE trial may be a weakness. The already‐operative French network CARDIOGEN, comprising 25 academic centers, will help to ensure the enrollment of the required number of patients. The French CARDIOGEN network belongs to the ERN network GUARD‐HEART (European Reference Network for Rare and Low Prevalence Complex Diseases of the Heart) created this year (http://guardheart.ern-net.eu/). ERN GUARD‐HEART brings together a geographically diverse group of 24 expert healthcare providers from 12 different member states committed to patient‐centered care and efficient practice based on evidence, knowledge, education, and translational research. Some Romanian, Swiss, and Belgian centers of ERN GUARD‐HEART have agreed to participate in the BRAVE study.
Conflicts of interest
The authors declare no potential conflicts of interest.
Supporting information
Appendix S1. Key committees and organizational structure
Morel E, Manati AW, Nony P, et al. Blockade of the renin‐angiotensin‐aldosterone system in patients with arrhythmogenic right ventricular dysplasia: A double‐blind, multicenter, prospective, randomized, genotype‐driven study (BRAVE study). Clin Cardiol. 2018;41:300–306. 10.1002/clc.22884
REFERENCES
- 1. Fontaine G, Fontaliran F, Hébert JL, et al. Arrhythmogenic right ventricular dysplasia. Annu Rev Med. 1999;50:17–35. [DOI] [PubMed] [Google Scholar]
- 2. McKenna WJ, Thiene G, Nava A, et al. Diagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy . Task Force of the Working Group Myocardial and Pericardial Disease of the European Society of Cardiology and of the Scientific Council on Cardiomyopathies of the International Society and Federation of Cardiology. Br Heart J. 1994;71:215–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marcus FI, McKenna WJ, Sherrill D, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation. 2010;121:1533–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yin K, Ding L, Li Y, et al. Long‐term follow‐up of arrhythmogenic right ventricular cardiomyopathy patients with an implantable cardioverter‐defibrillator for prevention of sudden cardiac death. Clin Cardiol. 2017;40:216–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fabritz L, Hoogendijk MG, Scicluna BP, et al. Load‐reducing therapy prevents development of arrhythmogenic right ventricular cardiomyopathy in plakoglobin‐deficient mice. J Am Coll Cardiol. 2011;57:740–750. [DOI] [PubMed] [Google Scholar]
- 6. La Gerche A, Heidbüchel H, Burns AT, et al. Disproportionate exercise load and remodeling of the athlete's right ventricle. Med Sci Sports Exerc. 2011;43:974–981. [DOI] [PubMed] [Google Scholar]
- 7. Campbell RW. ACE inhibitors and arrhythmias. Heart. 1996;76(3 suppl 3):79–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fletcher RD, Cintron GB, Johnson G, et al; The V‐HeFT II VA Cooperative Studies Group . Enalapril decreases prevalence of ventricular tachycardia in patients with chronic congestive heart failure. Circulation. 1993;87(6 suppl):V149–V155. [PubMed] [Google Scholar]
- 9. Marcus FI, Fontaine G, Guiraudon G, et al. Right ventricular dysplasia: a report of 24 adult cases. Circulation. 1982;65:384–398. [DOI] [PubMed] [Google Scholar]
- 10. Thiene G, Nava A, Corrado D, et al. Right ventricular cardiomyopathy and sudden death in young people. N Engl J Med. 1988;318:129–133. [DOI] [PubMed] [Google Scholar]
- 11. Basso C, Thiene G, Corrado D, et al. Arrhythmogenic right ventricular cardiomyopathy: dysplasia, dystrophy or myocarditis? Circulation. 1996;94:983–991. [DOI] [PubMed] [Google Scholar]
- 12. Thiene G, Basso C. Arrhythmogenic right ventricular cardiomyopathy: an update. Cardiovasc Pathol. 2001;10:109–117. [DOI] [PubMed] [Google Scholar]
- 13. d'Amati G, Leone O, di Gioia CR, et al. Arrhythmogenic right ventricular cardiomyopathy: clinicopathologic correlation based on a revised definition of pathologic patterns. Hum Pathol. 2001;32:1078–1086. [DOI] [PubMed] [Google Scholar]
- 14. Shi Q, Abusarah J, Baroudi G, et al. Ramipril attenuates lipid peroxidation and cardiac fibrosis in an experimental model of rheumatoid arthritis. Arthritis Res Ther. 2012;18:14:R223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grimm D, Kromer EP, Böcker W, et al. Regulation of extracellular matrix proteins in pressure‐overload cardiac hypertrophy: effects of angiotensin converting enzyme inhibition. J Hypertens. 1998;16:1345–1355. [DOI] [PubMed] [Google Scholar]
- 16. Cernecka H, Doka G, Srankova J, et al. Ramipril restores PPARβ/δ and PPARγ expressions and reduces cardiac NADPH oxidase but fails to restore cardiac function and accompanied myosin heavy‐chain ratio shift in severe anthracycline‐induced cardiomyopathy in rat. Eur J Pharmacol. 2016;15;791:244–253. [DOI] [PubMed] [Google Scholar]
- 17. Djouadi F, Lecarpentier Y, Hébert JL, et al. A potential link between peroxisome proliferator‐activated receptor signalling and the pathogenesis of arrhythmogenic right ventricular cardiomyopathy. Cardiovasc Res. 2009;84:83–90. [DOI] [PubMed] [Google Scholar]
- 18. Morel E, Drai J, Chevalier P. Angiotensin‐converting enzyme inhibitors can prevent myocardial deterioration. Heart Rhythm, Vol. 14, No. 5, May Supplement 2017; S215. [Google Scholar]
- 19. Yusuf S, Pitt B, Davis CE, et al; SOLVD Investigators . Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293–302. [DOI] [PubMed] [Google Scholar]
- 20. Martin A, Crawford J, Skinner JR, et al; Cardiac Inherited Diseases Group . High arrhythmic burden but low mortality during long‐term follow‐up in arrhythmogenic right ventricular cardiomyopathy. Heart Lung Circ. 2016;25:275–281. [DOI] [PubMed] [Google Scholar]
- 21. Riley MP, Zado E, Bala R, et al. Lack of uniform progression of endocardial scar in patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy and ventricular tachycardia. Circ Arrhythm Electrophysiol. 2010;3:332–338. [DOI] [PubMed] [Google Scholar]
- 22. Corrado D, Wichter T, Link MS, et al. Treatment of arrhythmogenic right ventricular cardiomyopathy/dysplasia: an international task force consensus statement. Eur Heart J. 2015;36:3227–3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Key committees and organizational structure


