Summary
The basal ganglia consist of a variety of subcortical nuclei engaged in motor control and executive functions, such as motor learning, behavioral control, and emotion. The striatum, a major basal ganglia component, is particularly useful for cognitive planning of purposive motor acts owing to its structural features and the neuronal circuitry established with the cerebral cortex. Recent data indicate emergent functions played by the striatum. Indeed, cortico‐striatal circuits carrying motor information are paralleled by circuits originating from associative and limbic territories, which are functionally integrated in the striatum. Functional integration between brain areas is achieved through patterns of coherent activity. Coherence belonging to cortico‐basal ganglia circuits is also present in Parkinson’s disease patients. Excessive synchronization occurring in this pathology is reduced by dopaminergic therapies. The mechanisms through which the dopaminergic effects may be addressed are the object of several ongoing investigations. Overall, the bulk of data reported in recent years has provided new vistas concerning basal ganglia role in the organization and control of movement and behavior, both in physiological and pathological conditions. In this review, basal ganglia functions involved in the organization of main movement categories and behaviors are critically discussed. Comparatively, the multiplicity of Parkinson’s disease symptomatology is also revised.
Keywords: basal ganglia, motor automaticity, Parkinson’s disease, striatum, temporal processing
1. INTRODUCTION
The components of the basal ganglia (BG) system, including the striatum, are bilateral structures that, like the thalamus, serve behavior and movement control through the regulation of cortical output. The BG operate in close relation with the cerebral cortex being part of an extensive loop, the BG‐thalamic‐cortical system, and their association results in several processing circuits between different cortical areas.1, 2 Parallel cortical‐BG‐thalamocortical information flows through two pathways having opposite effects, the direct and indirect pathways,3 whose functioning is crucial for the proper execution of movement. Choosing the contextually appropriate response in the presence of competing alternatives is a critical aspect of motor control.4, 5 Direct and indirect pathways provide the neural mechanism to rapidly switch from a planned to an alternative response.6 The main BG input nucleus, through which the direct and indirect pathways manage cortical information, is the striatum that receives topographical excitatory projections from almost the entire cortical areas. Recent data indicate emergent functions played by the striatum. Indeed, cortico‐striatal circuits carrying motor information are paralleled by circuits originating from associative and limbic territories that are functionally integrated in the striatum. The striatal ability to switch from competing sensory‐motor processes, in response to specific context and situations, is based on its capability in processing, routing, and reverting cortical information.7, 8 Owing to its structural features and the neuronal circuitry established with the cerebral cortex, the striatum is particularly useful for cognitive planning of purposive motor acts. The peculiar striatal regional differentiation, as visible from histochemical evidence, and the circuitry features enable the BG system to operate a functional integration between brain areas.
Overall, the bulk of data reported in recent years has provided new vistas concerning BG role in the organization and control of movement and behavior, both in physiological and pathological conditions. In this review, BG functions involved in the organization of main movement categories and behaviors are critically discussed. Comparatively, the multiplicity of Parkinson’s disease (PD) symptomatology is also revised.
2. ANATOMICAL FEATURES OF BASAL GANGLIA
The striatal route operates a primary functional differentiation by means of three main loops: (i) putamen sensorimotor circuit, with output to the primary motor cortex, supplementary motor area, and premotor cortex; (ii) caudate nucleus associative circuits, with output to the prefrontal cortex; and (iii) ventral striatum limbic circuit, with output to the anterior cingulate cortex and medial prefrontal cortex.9 The BG output structures are the inner segment of the globus pallidus and substantia nigra pars reticulata, which are tonically active, thereby suppressing thalamic activity and ultimately activating cortical motor commands. Through the indirect pathway, cortical inputs are inhibited. It has been suggested that the indirect pathway, passing through the subthalamic nucleus, provides an inhibitory background to impulsive behavior, whereas the direct pathway gives an excitatory input toward specific behaviors.10
The rat striatum is characterized by white matter embedded in grey matter. The striosome and the matrix compartments differentiate the structure of the striatal grey matter.11 Originally described as areas poor in acetylcholine esterase, scattered into the surrounding matrix,12 the striosomes display strong immunoreactivity against enkephalin, substance P, GABA, and neurotensin, whereas the matrix compartment is enriched with parvalbumin and calbindin.13 Most of the neurons composing the striatal grey matter are medium‐sized spiny projection neurons (MSNs). MSNs of the matrix innervate the external and internal segments of globus pallidus, as well as the pars reticulata of the substantia nigra. MSNs located in the striosomal compartment present dendrites that never reach the matrix and project into the substantia nigra pars compacta, preferentially, although collaterals targeting the same routes of the matrix MSNs are also present.12 As a consequence, the striosome compartment is in a strategic position to exert a global control over dopaminergic signaling in the dorsal striatum. In fact, owing to their projections to the pars compacta of the substantia nigra, neurons included in the striosomes may condition striatal dopamine (DA) release.1 Despite the absence of specific studies, peculiar aspects of repetitive behavior appearing in both movement and psychiatric disorders14 may be due to excessive signaling in the striosomal compartment.1
Striatal levels of DA are affected by opioid receptors exerting a general inhibitory effect, depending on their specific striosome/matrix distribution.15 When DA depletion condition occurs striosomal compensatory effects take place increasing opioid activity.16 Moreover, a specific opioid modulation of DA release in dorsal striatum is known.17 Excessive BG opioids transmission seems to be implicated in behavior and movement impairments.18 Differences in striosome and matrix neurons activation have been reported to occur in drug addiction and L‐3,4‐dihydroxyphenylalanine (L‐DOPA) induced dyskinesia, that is, in the presence of DA receptor hyper‐responsivity.1
A regional differentiation in striatal neuronal processing, with respect to mediodorsal striatum associative function, dorsolateral striatum sensorimotor processing, and ventral striatum limbic‐related processing, has been reported.2, 19, 20, 21 In the rat, the striatal white matter is organized in fascicles of fibers running through the entire nucleus. These fibers are topographically arranged and contain projections, not only intrinsic to the striatum, but also directed to the thalamus and brainstem.22 Owing to the presence of a regional differentiation in striatal neuronal processing, the white matter bundles raveling the entire rat striatum are influenced by this regional differentiation in cortico‐striatal inputs.
Hunnicut and co‐workers documented that striatal white matter bundles are formed by both thalamic and cortical afferents.23 The thalamic fibers consist of fasciculated and unfasciculated axons. The former are passing fibers, while the latter make synapses with striatal neurons. The cortical inputs to the striatum have a dual organization: a core projection field of densely packed terminals and a diffuse projection field characterized by converging or sparse thalamic terminals. Data from the above study23 also showed that the striatum is organized into four functional regions. Besides the sensorimotor and limbic functional regions, the third associative region located in the dorsomedial striatum is considered to have a more complex feature characterized by many converging axons originating from several regions. The most caudal part of the striatum is the fourth functional region, integrating multi‐modality sensory inputs controlled by the amygdala. On the other hand, a recent projectome study in MAO A/B KO mice characterized up to 29 small distinct functional striatal domains in which ipsilateral and contra‐lateral cortical information are integrated.24
Interhemispheric and pyramidal cortico‐striatal neurons make synapses with different striatal output neurons involved in the direct and indirect pathways, respectively.25 Cortico‐cortical neurons preferentially innervate the contralateral striatum, whereas pyramidal neurons project only in the ipsilateral striatum. The intrastriatal axon of pyramidal neurons arises as a collateral of the main descending axon as it runs in the fibers bundles. Interhemispheric and pyramidal neurons convey different signals to the striatum. The former are active during the planning phase that preceeds movement, the latter during movement execution.26 In addition, signals provided by pyramidal neurons reach striatal neurons a few milliseconds before those carried by interhemispheric neurons.27
3. THE BASAL GANGLIA FUNCTIONAL MACHINERY
3.1. Cognitive aspects of motor control
Behavior is composed of a set of actions sequentially ordered. A given sequence of motor acts can be successfully and efficiently executed when sensory‐motor associations are acquired, timed, and related to each other, such as to produce a specific outcome. On the contrary, whenever changes in the environment occurr or routine behavior become inappropriate, the repetition of learned and automatized movements can be switched into a cognitively controlled one. The switching between automatic and voluntary controlled movements is a major function of the BG.28
The term ethogram indicates a comprehensive and structured entity characterizing duration, frequency, and latency of behavioral events.29 This definition of ethogram is based on the fact that events are recurrent in time. A set of repeated events may represent the essential substrate for the occurrence of a habit. In fact, behavior is defined as habitual when it occurs repeatedly over time.30 The temporal scale is the “parameter” through which events are related to each other. Stimulus‐response associative mechanisms are subjected to time constraints, before they can be processed to generate an action‐outcome association.31 This processing is the basis of automated behavior.4, 32
Notably, the study of temporal behavior characteristics (T‐pattern) in several animal species, including rodents33, 34 and humans,35 has revealed hidden time‐scale relationships between events. The application of T‐pattern analysis consists in multivariate statistical analysis of behavioral events aimed at the detection of otherwise undetected structures. Behavioral dynamics and the time course of structural relationships among events are dependent on (and they change with) previous experience.36 The striatum is able to process information to build procedural knowledge. This function is based on the ability to chunk individual behavioral components37 and rebuild them into a different timing scale that identifies a new behavioral structure.28 Learning behavior is based on the acquisition of timed relationships connecting various events. Habits are possible when a sequence of events is hierarchically recognizable and reproducible, and the temporal relationship between them is well established.31 This process is based on the appearance of the end‐related signal, developed in striatal activity during learning.38 Whatever the driving force of this end‐related signal, its independence from reward identifies the activity as an “event” useful to build behaviors based on different unit sequences. Based on the acquisition of rules concerning the temporal relationship between actions, the entire sequence of events can be automatically reproduced. Habituation, in other words, is a process through which the association between events (action‐outcome association) is acquired during learning and the reproduction of an actions’ sequence is no longer dependent on external cues.39
The place preferring behavior that rodents exhibit during early learning of a plus‐maze task, shifts into a response strategy as a consequence of the training effect.40 In a similar way, action‐outcome contingency (sensitive to devaluation) passing through the automation process becomes independent from reward, shifting into habitual behavior.38 Accordingly, T‐pattern behavior investigation of rodents subjected to the plus‐maze task revealed a clear behavioral shift after the initial exposure. In the early phase of task execution, a predominance of spatial exploration with a complex manifestation of behavioral elements was evident. During the later phase of the task, a simplification and a re‐organization of behavioral elements occurred, indicating a clear effect of previous experience. The temporal structure of resulting behavior was directed toward a more “egocentric” action plan.36, 38
3.2. Basal ganglia in cognitive motor control
Normal locomotor capabilities depend on the nigrostriatal pathway integrity. DA plays a major role in maintaining BG network stability, this being essential for the selection and process of neuronal activity associated with movement. In physiological condition, BG show a persistent nigral DA neurons firing rate, a steady striatal DA concentration, and continuous striatal DA receptors activation.41 On the other hand, striatal DA shots produced by phasic modulation of nigral neurons firing are the basis of reward‐related behavior.20, 42
BG mediates habit learning, this occurring in a slow and progressive manner, in which stimulus‐response associations are incrementally acquired. Habitual behavior is long‐lasting and context‐dependent. Different regions of the striatum are specialized for habits acquisition and expression, depending on the nature of cortical and DA inputs, as well as on intrinsic circuitry.39, 43
BG mnemonic functions are based on the concept that neural representation of learned information remains in a labile state, subject to external influence briefly after it is first acquired. The subsequent consolidation process would be subjected to DA strengthening so that information storage is first measured by the degree of coupling between the motor response and the “trigger” stimulus (the one raising the re‐action). Building consistent responses to environmental cues implicate the ability to process and collect consistent relationships between stimuli and responses.44
Functionally, the striatum acts as a detector of discontinuously distributed patterns coming from cortical inputs.45 In the rat, at rest, striatal neurons display low spontaneous activity and hyperpolarized membrane potentials. Striatal‐projecting neurons need to simultaneously receive convergent cortical inputs to fire.46 As a result, different BG areas work together to efficiently coordinate behavior and contextual requirements, in other words making decisions.
The dorsomedial striatum mediates goal‐directed behavior requiring conscious efforts while the dorsolateral striatum is involved in executing habitual behaviors.47 In agreement, it has been recently found that the thalamus conveys sensory‐related information to the dorsolateral striatum. On the other hand, the cortico‐striatal inputs contribute to the stimulus‐induced response observed in the dorsolateral striatum.48 This is schematically shown in Figure 1. This framework is crucial when considering that PD patients lose established automatism in executing skilled movements due to the early‐phase disruption of sensorimotor striatum.49, 50 In fact, PD patients often have difficulties in the execution of repetitive internally generated movements while they are able to perform externally paced movements, such as finger tapping. However, repetitive finger tapping performance is improved in the presence of external auditory cues. Additionally, in PD patients, dual‐tasking can exacerbate performing difficulty of simple repetitive movements.6 The activation of neural structures involved in the generation of internally cued movements, such as the putamen, supplementary motor area, and cingulate cortex is impaired in PD patients in off drug treatment and if asked to perform volitional motor tasks.6 Thus, the BG may provide internal timing cues facilitating movement initiation in well‐learned routines, and the switch between learned and automatized motor acts via the supplementary motor area.51 In Parkinsonian patients, the BG‐supplementary motor area system is dysfunctional while the cerebellar‐premotor cortex system seems to be preserved.52 They showed improved ability in performing movement sequences when external cues (auditory and/or visual) are made available, thus compensating internal cueing deficit and reducing the degree of automaticity.6 PD patients engaged in internally driven task showed augmented recruitment of cortico‐cerebellar circuits, as they transitioned from non‐symptomatic to symptomatic stages.53 A possible compensatory recruitment of the cerebellar‐premotor circuit, through disease progression, was hypothesized.53 Accordingly, patients with PD exhibited higher brain activity in the executive area during self‐initiated movements. A lower brain activity in the sensorimotor area during cue‐initiated movements suggests that these patients may use executive control when performing simple motor acts.54 Striatal DA depletion has been shown to improve both the automatisms of internally cued motor skills and the ability to switch from over‐trained behavior to alternative self‐paced response.55 Interestingly, it has recently been shown that stroke patients improved their dual‐task performance impairment by internally directed attentive focus practice.56
Figure 1.
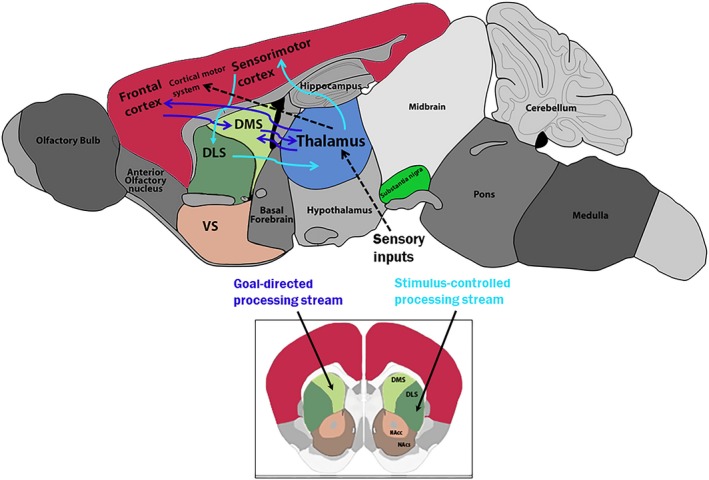
Schematic illustration of the rat brain general flow of basal ganglia circuitry involved in goal‐directed (blue) and stimulus‐controlled (light blue) behaviors. Abbreviation: DMS, DorsoMedial Striatum; DLS, DorsoLateral Striatum; VS, Ventral Striatum; NAcc, Nucleus Accumbens core; NAcs, Nucleus Accumbens shell. (Figure adapted from C. Soares‐Cunha et al. Neurosci Biobehav Rev. 2016;68:370–386).
A first conclusion, which can be drawn from the aforementioned data, encompasses the possibility that the consciously goal‐directed behavior initially undergoes a sensory‐guided motor execution standardization through the habitual sensory‐related process. The final evolution of this process would consist in motor skills automatization, thus switching the behavior contingency dependent on internal cues.8 Considering movements as organized at hierarchical levels, the stereotyped, rhythmic, and goal‐directed movements, we can hypothesize that during the assembling of goal‐directed movements the relation between internally driven actions and current external stimuli is established, accordingly to temporal constraints determined by DA function. This concatenation would permit the replacement of goal‐directed movements with stereotyped (homeostatic) movements, the latter being more manageable by automation process. Thus, motor skills are gained through the repetition of a sequence following a specific pattern of actions. Successful behaviors are obtained when a wide range of selection options (chunking) are available, to choose the most appropriate response to contextual requirements.
3.3. Basal ganglia in stereotyped motor control
Stereotyped movements develop in animal models of PD in the presence of a nearly total DA fibers degeneration, comprehensive of those reaching the ventral striatum.57 Repeated exposure to psychostimulant drugs produces behavioral sensitization in non‐lesioned rats, a phenomenon characterized by spontaneous locomotion enhancement and stereotyped movements.58 On the other hand, behavioral sensitization to psychostimulants may result from: (i) direct pharmacological drugs activity; and (ii) experiences associated with the drugs themselves.58, 59 This hypothesis is surprisingly similar to the suggestion that movement performance per se induces sensitized motor responses in DA‐denervated rats subjected to DA repetitive stimulation.60 Altogether, the above evidence points out that a low DA stimulation can induce repetitive movement impairments, whereas excessive DA stimulation can induce abnormally repetitive behaviors in the form of motor stereotypes.
The neuronal firing pattern in the dorsolateral striatum is specifically correlated with the serial pattern of actions that constitutes syntactic grooming chain. These neurons are differently activated when grooming movements occur in a varied serial order. In particular, neurons coding the grooming syntax are clustered within a restricted area of the anterior dorsolateral striatum.61 A hierarchical organization has been proposed in which the anterior portion of the dorsolateral striatum phasically modulates the circuit generating the grooming syntax chain. Switching actions toward the sensory‐motor drive enable the system to complete each step composing the chain.62, 63 The serial order of action syntax has been proposed to participate in the transition between simple movements and abstract cognition. Disruption of action syntax may be linked to damage of specific anatomic area in the striatopallidal system.61
Therefore, rhythmic movements can be described as those where the action syntax is “recognized” as a consequence of sensorimotor integration in the dorsolateral striatum. Such integration includes the firing tuning of neuronal clusters, functionally selected by external (or internal) stimuli, and temporally associated with a specific motor output. These different events, that is, the sensory information and the motor output, become associated with each other in their temporal occurrence by the DA‐mediated processing. As a result, the motor act is strongly related to the associated stimulus.
It is worth noting that interhemispheric coordination is required for skilled motor performances. Bimanual coordination of skilled finger movements requires intense functional coupling of both cerebral hemispheres motor areas. The interhemispheric integration of the motor commands is particularly important during the early‐phase bimanual task acquisition,64 when motor routines (know‐how) are concatenated. Interhemispheric coordination signals start from striatopallidal pathways and then travel through white matter fibers. DA mediates the temporal gap into which the interhemispheric sensorimotor integration operates. The resulting synchronization of the ipsilateral and the contralateral striatal activity underlies the production of bilateral coordinated movements. The synchronization of ipsilateral activity develops in the dorsomedial striatum, through the action of nigral, thalamic, cortical inputs ending on MSNs. The contralateral synchronization activity is related to the contralateral DA innervation and develops through the striatopallidal white matter fibers.65 As a result, imbalance in interhemispheric functional coupling impairs motor skills execution. Motor symptomatology in PD, behind other reasons, depends on dysfunctional coupling between different striatal areas.23, 65
4. MOTOR SYMPTOMS IN PARKINSON’S DISEASE
In Parkinson’s disease, non‐tremor‐dominant subjects display impaired connectivity in nigro‐pallidal and fronto‐striatal pathways, as compared with tremor‐dominant subjects.66 According to previous findings, non‐tremor‐dominant PD patients showed reduced DA levels in the ventral pallidus, correlated to a reduced connectivity due to nigral damage.66 Interestingly, fMRI studies demonstrated that prefrontal cortex and globus pallidus activation were reduced in non‐tremor compared to tremor‐dominant PD patients and controls.67 On the contrary, in tremor‐dominant PD patients, tremor‐related cerebral activity first arises in the inner segment of the globus pallidus and then propagates to the cerebello‐thalamo‐cortical loop.68, 69, 70 Thus, it seems that a differential effect could be accounted for in the cortico‐subcortical activity and be related to specific symptomatology. An example of coherence belonging to cortical‐BG circuits is also present in PD patients. The excessive activity synchronization occurring in this pathology is reduced by DA therapies.71, 72 Recently, electrophysiological studies on normal subjects and patients, reporting inter‐hemispheric coherence activity during the execution of skilled movements, showed a differential contribution from the two cerebral hemispheres, independently from the dominant hand.73
Bradykinesia and tremor manifestations in PD patients are particularly alleviated by treatment with L‐DOPA.66, 74 Slowness of movement initiation and execution is another cardinal symptom of PD. This impairment is particularly evident in internally generated sequential movements and may benefit from the introduction of external rhythmic cues.75 In line with motor planning deficiency, PD patients are known to exhibit selective deficit in motor timing for sequential movements separated by a supra‐second interval.76 It has been suggested that the dorsal striatum is critical in motor timing control. DA receptors in the striatum, other than in the globus pallidus and substantia nigra reticulata, are more effective in controlling L‐DOPA effect in PD patients. Accordingly, a prominent role of striatum in the interhemispheric coordination, with respect to extra striatal source, was reported.77 Thus, BG‐dependent impairment of motor automaticity results in slowness and high timing variability when performing simple repetitive movements.
Besides the activation of specific cortical areas, such as the supplementary motor area and the lateral premotor cortex, the functional coupling between premotor and sensorimotor areas of the two hemispheres is also important for precise timing and execution of bimanual movements.64 Interestingly, it has been found that cortical enhanced inter‐hemispheric functional coupling occurs during the early‐phase of bimanual coordination learning, but not during sequence repetition or during unimanual sequence learning. Subsequently, BG encoded motor routines may be executed with minimal cortical involvement.64 This may be the underlying ability impairment suffered by PD patients.
BG diseases result in a movement disorders spectrum, characterized by both hypokinetic and hyperkinetic signs. The former are recognizable by bradykinesia and movements deficit, such as in PD patients, the latter are associated with dyskinesia and involuntary movements appearance, such as in Huntington’s disease.78, 79 Movement disorders in psychiatric diseases, such as schizophrenia and addiction, are common,14, 80 suggesting some overlapping pathogenic mechanisms.81 On the other hand, psychiatric symptoms belong to the early appearing movement disorders signs, such as Huntington’s disease or PD.82, 83 In PD patients, postural and locomotor impairments develop when midbrain nigral neurodegeneration induces a near complete DA loss in striatal tissue.84 Similarly, dysfunction in DA signaling underlies a variety of psychiatric diseases, including schizophrenia and drug addiction. The striosomal MSNs located in the lateral striatum are the candidate in regulating DA signaling. In fact, a strict association of opioid and D2‐like DA receptors has been reported.24 Moreover, the MSNs ability to modulate both tonic and phasic DA signals was described.42
5. CONCLUSION
In summary, the cortico‐striatal functioning pattern may be responsible for cognitive planning of movements, allowing optimal synchronization such as to ensure bilateral coordination of neural activities necessary to gain skilled actions. When this function cannot be accomplished, tremor, gait, speech problems (stuttering), tics, or psychiatric disorders will appear. In line with this point of view, recent data showed that a balanced cross‐hemispheric DA release occurs in the dorsomedial striatum only.65 The direct result of such functional arrangement is that, when the striatum is unilaterally denervated, compensatory mechanisms are specifically based on the DA supply to the dorsomedial striatum provided by the contralateral substantia nigra. This allows functional re‐synchronization of the dorsomedial striatal output, but not the dorsolateral one. As a consequence, the main impact of unilateral denervation may result into desynchronization between interhemispheric cortical‐BG‐thalamic‐cortical loops pertaining to different striatal functional areas.
In conclusion, we have reviewed the BG functions involved in the organization of the main movement categories and behaviors. A deep comparison with PD symptomatology in relation to BG dysfunctions has been revised. Our main conclusion is that BG are more than just a switching device. In fact, our analysis suggests that the consequences of bilateral functional coordination loss in neurodegenerative disorders progression, such as PD, deserve careful consideration, assuming that they could emerge and develop unilaterally during the early‐phase of their staging.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
The authors wish to thank Mr. Stefano Amoretti for his help with the literature review.
Florio TM, Scarnati E, Rosa I, et al. The Basal Ganglia: More than just a switching device. CNS Neurosci Ther. 2018;24:677–684. 10.1111/cns.12987
REFERENCES
- 1. Crittenden JR, Graybiel AM. Basal ganglia disorders associated with imbalances in the striatal striosome and matrix compartments. Front Neuroanat. 2011;5:1‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hart G, Leung BK, Balleine BW. Dorsal and ventral streams: the distinct role of striatal subregions in the acquisition and performance of goal‐directed actions. Neurobiol Learn Mem. 2015;108:104‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alexander GE. Functional architecture of basal ganglia circuits: neural substrated of parallel processing. Trends Neurosci. 1990;13:266‐271. [DOI] [PubMed] [Google Scholar]
- 4. Kim HF, Hikosaka O. Parallel basal ganglia circuits for voluntary and automatic behaviour to reach rewards. Brain. 2015;138:1776‐1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Leisman G, Moustafa A, Shafir T. Thinking, walking, talking: integratory motor and cognitive brain function. Front Public Health. 2016;4:1‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wu T, Liu J, Zhang H, Hallett M, Zheng Z, Chan P. Attention to automatic movements in Parkinson’s disease: modified automatic mode in the striatum. Cereb Cortex. 2015;25:3330‐3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stocco A, Lebiere C, Anderson JR. Conditional routing of information to the cortex: a model of the basal ganglia’s role in cognitive coordination. Psychol Rev. 2010;117:541‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Friedman A, Homma D, Gibb LG, et al. A corticostriatal path targeting striosomes controls decision‐making under conflict. Cell. 2015;161:1320‐1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281‐285. [DOI] [PubMed] [Google Scholar]
- 10. Mink JW. Neurobiology of basal ganglia and Tourette syndrome: basal ganglia circuits and thalamocortical outputs. Adv Neurol. 2006;99:89‐98. [PubMed] [Google Scholar]
- 11. Lanciego JL, Luquin N, Obeso JA. Functional neuroanatomy of the basal ganglia. Cold Spring Harb Perspect Med. 2012;2:a009621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Graybiel AM, Ragsdale CW Jr. Histochemically distinct compartments in the striatum of human, monkeys, and cat demonstrated by acetylthiocholinesterase staining. Proc Natl Acad Sci. 1978;75:5723‐5726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gerfen CR. The neostriatal mosaic. I. Compartmental organization of projections from the striatum to the substantia nigra in the rat. J Comp Neurol. 1985;236:454‐476. [DOI] [PubMed] [Google Scholar]
- 14. Peall KJ, Lorentzosb MS, Heyman I, et al. A review of psychiatric co‐morbidity described in genetic and immune mediated movement disorders. Neurosci Biob Rev. 2017;80:23‐35. [DOI] [PubMed] [Google Scholar]
- 15. Samadi P, Bedard PJ, Rouillard C. Opioids and motor complications in Parkinson’s disease. Trends Pharmacol Sci. 2006;27:512‐517. [DOI] [PubMed] [Google Scholar]
- 16. Koizumi H, Morigaki R, Okita S, et al. Response of striosomal opioid signaling to dopamine depletion in 6‐hydroxydopamine‐lesioned rat model of Parkinson’s disease: a potential compensatory role. Front Cell Neurosci. 2013;7:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Campos‐Jurado Y, Martí‐Prats L, Zornoza T, Polache A, Granero L, Cano‐Cebrián MJ. Regional differences in mu‐opioid receptor‐dependent modulation of basal dopamine transmission in rat striatum. Neurosci Lett. 2017;638:102‐108. [DOI] [PubMed] [Google Scholar]
- 18. Davis MI, Crittenden JR, Feng AY, et al. The cannabinoid‐1 receptor is abundantly expressed in striatal striosomes and striosome‐dendron bouquets of the substantia nigra. PLoS ONE. 2018;13:e0191436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pérez‐Díaz F, Díaz E, Sánchez N, Vargas JP, Pearce JM, López JC. Different involvement of medial prefrontal cortex and dorso‐lateral striatum in automatic and controlled processing of a future conditioned stimulus. PLoS ONE. 2017;12:e0189630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Doyon J, Bellec P, Amsel R, et al. Contributions of the basal ganglia and functionally related brain structures to motor learning. Behav Brain Res. 2009;199:61‐75. [DOI] [PubMed] [Google Scholar]
- 21. Balleine BW, O’Doherty JP. Human and rodent homologies in action control: corticostriatal determinants of goal‐directed and habitual action. Neuropsychopharmacology. 2010;35:48‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Coizet V, Heilbronner SR, Carcenac C, et al. Organization of the anterior limb of the internal capsule in the rat. J Neurosci. 2017;37:2539‐2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hunnicutt BJ, Jongbloets BC, Birdsong WT, Gertz KJ. A comprehensive excitatory input map of the striatum reveals novel functional organization. Elife. 2016;5:e19103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hintiryan H, Foster NN, Bowman I, et al. The mouse cortico‐striatal projectome. Nat Neurosci. 2016;19:1100‐1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cowan RL, Wilson J. Spontaneous firing patterns and axonal projections of single corticostriatal neurons in the rat medial agranular cortex. J Neurophysiol. 1994;71:17‐32. [DOI] [PubMed] [Google Scholar]
- 26. Lei W. Evidence for differential cortical input to direct pathway versus indirect pathway striatal projection neurons in rats. J Neurosci. 2004;24:8289‐8299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reiner A, Hart NM, Lei W, Deng Y. Corticostriatal projection neurons – dichotomous types and dichotomous functions. Front Neuroanat. 2010;4:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hikosaka O, Isoda M. Switching from automatic to controlled behavior: cortico‐basal ganglia mechanisms. Trends Cogn Sci. 2010;14:154‐161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sakamoto KQ, Sato K, Ishizuka M, et al. Can ethograms be automatically generated using body acceleration data from free‐ranging birds? PLoS ONE. 2009;4:e5379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Graybiel AM. Basal Ganglia: Habit In: Squire LR (ed). Encyclopedia of Neuroscience. Academic Press, 2009;93‐96. [Google Scholar]
- 31. Dezfouli A, Balleine BW. Actions, action sequences and habits: evidence that goal‐directed and habitual action control are hierarchically organized. PLoS Comput Biol. 2013;9:e1003364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liljeholm M, Dunne S, O’Doherty JP. Differentiating neural systems mediating the acquisition vs expression of goal‐directed and habitual behavioral control. Eur J Neurosci. 2015;41:1358‐1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Casarrubea M, Roy V, Sorbera F, et al. Temporal structure of the rat’s behavior in elevated plus maze test. Behav Brain Res. 2013;237:290‐299. [DOI] [PubMed] [Google Scholar]
- 34. Casarrubea M, Faulisi F, Caternicchia F, et al. Temporal patterns of rat behaviour in the central platform of the elevated plus maze. Comparative analysis between male subjects of strains with different basal levels of emotionality. J Neurosci Methods. 2016;268:155‐162. [DOI] [PubMed] [Google Scholar]
- 35. Magnusson MS. Discovering hidden time patterns in behavior: T‐patterns and their detection. Behav Res Methods Instrum Comput. 2000;32:93‐110. [DOI] [PubMed] [Google Scholar]
- 36. Casarrubea M, Magnusson MS, Roy V, et al. Multivariate temporal pattern analysis applied to the study of rat behavior in the elevated plus maze: methodological and conceptual highlights. J Neurosci Methods. 2014;234:116‐126. [DOI] [PubMed] [Google Scholar]
- 37. Graybiel AM. The basal ganglia and chunking of action repertoires. Neurobiol Learn Mem. 1998;70:119‐136. [DOI] [PubMed] [Google Scholar]
- 38. Desrochers TM, Amemori K, Graybiel AM. Habit learning by naive macaques is marked by response sharpening of striatal neurons representing the cost and outcome of acquired action sequences. Neuron. 2015;87:853‐868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Smith KS, Graybiel AM. Habit formation. Dialogues Clin Neurosci. 2016;18:33‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Poldrack R, Packard MG. Competition among multiple memory systems: converging evidence from animal and human brain studies. Neuropsychologia. 2003;41:245‐251. [DOI] [PubMed] [Google Scholar]
- 41. Cohen MX, Frank MJ. Neurocomputational models of basal ganglia function in learning, memory and choice. Behav Brain Res. 2009;199:141‐156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Marcott PF, Mamaligas AA, Ford CP. Phasic dopamine release drives rapid activation of striatal D2‐receptors. Neuron. 2014;84:164‐176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wu T, Hallett M, Chan P. Motor automaticity in Parkinson’s disease. Neurobiol Dis. 2015;82:226‐234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. White NM. Mnemonic functions of the basal ganglia. Curr Opin Neurobiol. 1997;7:164‐169. [DOI] [PubMed] [Google Scholar]
- 45. Graybiel AM, Aosaki T, Flaherty AW, Kimura M. The basal ganglia and adaptative motor control. Science. 1994;265:1826‐1831. [DOI] [PubMed] [Google Scholar]
- 46. Mahon S, Deniau JM, Charpier S. Various synaptic activities and firing patterns in cortico‐striatal and striatal neurons in vivo. J Physiol Paris. 2003;97:557‐566. [DOI] [PubMed] [Google Scholar]
- 47. Balleine BW, Liljeholm M, Ostlund SB. The integrative function of the basal ganglia in instrumental conditioning. Behav Brain Res. 2009;199:43‐52. [DOI] [PubMed] [Google Scholar]
- 48. Alloway KD, Smith JB, Mowery TM, Watson GDR. Sensory processing in the dorsolateral striatum: the contribution of thalamostriatal pathways. Front Syst Neurosci. 2017;11:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Eller T. Deep brain stimulation for Parkinson’s disease, essential tremor, and dystonia. Dis Mon. 2011;57:638‐646. [DOI] [PubMed] [Google Scholar]
- 50. Francardo V. Modeling Parkinson’s disease and treatment complications in rodents: potentials and pitfalls of the current options. Behav Brain Res. 2017. pii: S0166‐4328(17)31471‐7. [Epub ahead of print]. 10.1016/j.bbr.2017.12.014 [DOI] [PubMed] [Google Scholar]
- 51. Cunnington R, Iansek R, Thickbroom GW, et al. Effects of magnetic stimulation over supplementary motor area on movement in Parkinson’s disease. Brain. 1996;119:815‐822. [DOI] [PubMed] [Google Scholar]
- 52. Haslinger B, Erhard P, Kampfe N, et al. Event‐related functional magnetic resonance imaging in Parkinson’s disease before and after levodopa. Brain. 2001;124:558‐570. [DOI] [PubMed] [Google Scholar]
- 53. Taniwaki T, Okayama A, Yoshiura T, et al. Functional network of the basal ganglia and cerebellar motor loops in vivo: different activation patterns between self‐initiated and externally triggered movements. NeuroImage. 2006;31:745‐753. [DOI] [PubMed] [Google Scholar]
- 54. Shen X, Wong‐Yu ISK, Mak MKY. Effects of exercise on falls, balance, and gait ability in Parkinson’s disease. Neurorehabil Neural Repair. 2016;30:512‐527. [DOI] [PubMed] [Google Scholar]
- 55. Florio TM, Confalone G, Sciarra A, Sotgiu A, Alecci M. Switching ability of over trained movements in a Parkinson’s disease rat model. Behav Brain Res. 2013;250:326‐333. [DOI] [PubMed] [Google Scholar]
- 56. Kal EC, Van Der Kamp J, Houdijk H, Groet E, Van Bennekom CAM. Stay focused! The effects of internal and external focus of attention on movement automaticity in patients with stroke. PLoS ONE. 2015;10:e0136917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. O’Sullivan SS, Wu K, Politis M, et al. Cue‐induced striatal dopamine release in Parkinson’s disease‐associated impulsive‐compulsive behaviours. Brain. 2011;134:969‐978. [DOI] [PubMed] [Google Scholar]
- 58. Chang ST, Tung CS, Lin YL, Chuang CH, Lee AL, Liu YP. Behavioral and cross sensitization after repeated exposure to modafinil and apomorphine in rats. Chin J Physiol. 2010;53:318‐327. [DOI] [PubMed] [Google Scholar]
- 59. Iancu R, Mohapel P, Brundin P, Paul G. Behavioral characterization of a unilateral 6‐OHDA‐lesion model of Parkinson’s disease in mice. Behav Brain Res. 2005;162:1‐10. [DOI] [PubMed] [Google Scholar]
- 60. Frau L, Morelli M, Simola N. Performance of movement in hemiparkinsonian rats influences the modifications induced by dopamine agonists in striatal efferent dynorphinergic neurons. Exp Neurol. 2013;247:663‐672. [DOI] [PubMed] [Google Scholar]
- 61. Cromwell HC, Berridge KC. Implementation of action sequences by a neostriatal site: a lesion mapping study of grooming syntax. J Neurosci. 1996;16:3444‐3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cromwell HC, Atchley RM. Influence of emotional states on inhibitory gating: animals models to clinical neurophysiology. Behav Brain Res. 2015;276:67‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pelosi A, Girault JA, Hervé D. Unilateral lesion of dopamine neurons induces grooming asymmetry in the mouse. PLoS ONE. 2015;10:e0137185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gerloff C, Andres FG. Bimanual coordination and interhemispheric interaction. Acta Psychol. 2002;110:161‐186. [DOI] [PubMed] [Google Scholar]
- 65. Fox ME, Mikhailova MA, Bass CE, et al. Cross‐hemispheric dopamine projections have functional significance. Proc Natl Acad Sci. 2016;113:6985‐6990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Barbagallo G, Caligiuri ME, Arabia G, et al. Structural connectivity differences in motor network between tremor‐dominant and nontremor Parkinson’s disease. Hum Brain Mapp. 2017;38:4716‐4729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Prodoehl J, Planetta PJ, Kurani AS, Comella CL, Corcos DM, Vaillancourt DE. Brain activation differences in tremor and non‐tremor dominant Parkinson’s disease. JAMA Neurol. 2013;70:100‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Dirkx MF, den Ouden H, Aarts E, et al. The cerebral network of Parkinson’s tremor: an effective connectivity fMRI study. J Neurosci. 2016;36:5362‐5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Helmich RC, Janssen MJ, Oyen WJ, Bloem BR, Toni I. Pallidal dysfunction drives a cerebellothalamic circuit into Parkinson tremor. Ann Neurol. 2011;69:269‐281. [DOI] [PubMed] [Google Scholar]
- 70. Helmich RC, Hallett M, Deuschl G, Toni I, Bloem BR. Cerebral causes and consequences of parkinsonian resting tremor: a tale of two circuits? Brain. 2012;135:3206‐3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. George JS, Strunk J, Mak‐Mccully R, Houser M, Poizner H, Aron AR. Dopaminergic therapy in Parkinson’s disease decreases cortical beta band coherence in the resting state and increases cortical beta band power during executive control. Neuroimage Clin. 2013;3:261‐270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sharott A, Vinciati F, Nakamura KC, Magill PJ. A population of indirect pathway striatal projection neurons is selectively entrained to parkinsonian beta oscillations. J Neurosci. 2017;37:9977‐9998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Serrien DJ, Sovijärvi‐Spapé MM. Hemispheric asymmetries and the control of motor sequences. Behav Brain Res. 2015;283:30‐36. [DOI] [PubMed] [Google Scholar]
- 74. Sgroi S, Kaelin‐Lang A, Capper‐Loup C. Spontaneous locomotor activity and L‐DOPA‐induced dyskinesia are not linked in 6‐OHDA parkinsonian rats. Front Behav Neurosci. 2014;8:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Meck WH. Frontal cortex lesions eliminate the clock speed effect of dopaminergic drugs on interval timing. Brain Res. 2006;1108:157‐167. [DOI] [PubMed] [Google Scholar]
- 76. Avanzino L, Pelosin E, Martino D, Abbruzzese G. Motor timing deficits in sequential movements in Parkinson disease are related to action planning: a motor imagery study. PLoS ONE. 2013;8:e75454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zhang W, Zhang L, Liu L, Wang X. Time course study of fractional anisotropy in the substantia nigra of a parkinsonian rat model induced by 6‐OHDA. Behav Brain Res. 2017;328:130‐137. [DOI] [PubMed] [Google Scholar]
- 78. Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci. 2011;34:441‐466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Dickson DW. Neuropathology of Parkinson disease. Parkinsonism Relat Disord. 2018; 46: S30‐S33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Bernard JA, Russell CE, Newberry RE, Goena JRM, Mittald VA. Patients with schizophrenia show aberrant patterns of basal ganglia activation: evidence from ALE meta‐analysis. Neuroimage Clin. 2017;14:450‐463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Patel N, Jankovic J, Hallett M. Sensory aspects of movement disorders. Lancet Neurol. 2014;13:100‐112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Williams‐Gray CH, Worth PF. Parkinson’s disease. Mov Disord. 2016;44:542‐546. [Google Scholar]
- 83. Asakawa T, Fang H, Sugiyama K, et al. Human behavioral assessments in current research of Parkinson’s disease. Neurosci Biobehav Rev. 2016;68:741‐772. [DOI] [PubMed] [Google Scholar]
- 84. Schapira AHV, Chaudhuri KR, Jenner P. Non‐motor features of Parkinson disease. Nat Rev. 2017;18:435‐451. [DOI] [PubMed] [Google Scholar]


