Summary
Objective
Growing evidence has implicated dysfunction of the thalamus and its projection cortical targets in depression. However, the anatomical specificity of thalamo‐cortical connectivity in major depressive disorder (MDD) remains unknown due to the regional heterogeneity of the thalamus and limited methods to examine this.
Methods
Resting‐state fMRI was collected on 70 MDD patients and 70 healthy controls. The thalamus was parcellated based on connectivity with six predefined cortical regions of interest (ROIs). The segmented thalamic nuclei were used as seeds to map connectivity with the rest of the whole brain. The cortical‐to‐thalamus connectivity values and thalamus‐based connectivity maps were compared between groups.
Results
The cortical ROIs demonstrated correlations with spatially distinct zones within the thalamus. We found a trend toward reduced parietal ROI‐to‐thalamus connectivity in MDD. Importantly, MDD patients demonstrated reduced connectivity between prefrontal and parietal thalamus ROIs and bilateral middle frontal gyrus (MFG) and the right posterior default mode network (DMN) and between the prefrontal and motor thalamus ROIs and lateral temporal regions. Conversely, increased connectivity emerged between the motor thalamus ROI and right MFG and right medial frontal gyrus/anterior cingulate; between motor/somatosensory thalamus ROIs and right posterior DMN; between prefrontal/somatosensory thalamus ROIs and cerebellum; and between the parietal thalamus ROI and left insula.
Conclusions
This study is the first to examine the anatomical specificity of thalamo‐cortical connectivity disturbances in MDD. Subjects with MDD demonstrated altered thalamo‐cortical connectivity characterized by a complex pattern of region‐dependent hypo‐ or hyperconnectivity. We therefore speculate that selectively modulating the connectivity of thalamo‐cortical circuitry may be a potential novel therapeutic mechanism for MDD.
Keywords: cortex, functional connectivity, major depressive disorder, resting‐state fMRI, thalamus
1. INTRODUCTION
Major depressive disorder (MDD) has been conceptualized as a brain disorder that affects information exchange across large‐scale neural systems.1, 2, 3 An important focus for examining such large‐scale neural system disturbances is the thalamus as most information input to the cortex is routed through this subcortical region.4, 5, 6 There is considerable evidence from neuroimaging and histological studies implicating dysfunction of the thalamus and its projection cortical targets in the pathophysiology of MDD.7, 8, 9, 10 However, the anatomical specificity of thalamo‐cortical connectivity in MDD remains largely unknown due to the large functional and structural heterogeneity of the thalamus and limited neuroimaging methods for detecting this.
Resting‐state functional magnetic resonance imaging (R‐fMRI), which examines the temporal correlation of spontaneous fluctuations of the blood‐oxygen‐level‐dependent (BOLD) signal across brain regions, provides a powerful tool to systematically characterize thalamo‐cortical connectivity.4, 11 Several R‐fMRI studies reported in the last 10 years have demonstrated abnormalities of functional connectivity in the thalamus of MDD patients. In one of the earlier investigations, MDD patients were found to have reduced thalamic functional connectivity with the cognitive, dorsal aspect of the anterior cingulate, which acts as an intermediary between subcortical regions and the dorsal prefrontal cortex (PFC); in the same group of subjects, such reduced connectivity was normalized following antidepressant treatment.8, 12 Reduced thalamo‐prefrontal connectivity was further associated with treatment resistance.10 Additionally, a recent study has detected lower thalamic connectivity with the left orbitofrontal cortex and right precuneus in MDD patients.13 However, the results are far from consistent. For example, one study14 found increased functional connectivity in the thalamus within the default mode network (DMN), and increased connectivity has also been shown between the thalamus and dorsolateral PFC in MDD patients.15 While differences in methods, sample size, and patient characteristics may contribute to this inconsistency, the regional heterogeneity of the thalamic nuclei may impede conclusive interpretations of thalamo‐cortical abnormalities.
To date, studies have exclusively used the entire thalamus as a region of interest (ROI) to identify thalamic connectivity abnormalities in MDD. Treating the thalamus as a homogeneous structure with a unitary connectivity profile limits potential inferences about the precise locations of thalamo‐cortical connectivity abnormalities. It is well known from human and animal studies that the thalamus is organized into distinct nuclei from which parallel projections reach different regions of the cerebral cortex.5, 16, 17 For example, the anterior/dorsomedial areas of the thalamus connect to the prefrontal cortex, whereas the ventrolateral areas are linked to motor and somatosensory cortices.5 Using R‐fMRI, Zhang et al4 systematically mapped the thalamo‐cortical functional interactions in healthy adults; such patterns of intrinsic functional connectivity correspond well with anatomical parcellation of the thalamus derived from diffusion tensor imaging (DTI) studies.5, 6 The topographical arrangement of reciprocal connections between thalamus and cortex raises the possibility that MDD may be characterized by specific patterns of connectivity disturbance in each cortical territory (or thalamic subdivision). There remains a lack of evidence concerning the anatomical specificity of thalamo‐cortical connectivity disturbances in MDD.
With this knowledge gap in mind, we employed R‐fMRI to examine functional connectivity between the thalamus and cortex in 70 first‐episode, unmedicated MDD patients and 70 healthy controls (HCs), aiming to comprehensively investigate MDD‐related changes in thalamo‐cortical connectivity. On the basis of previous reports,8, 10, 13, 14 we hypothesized that altered thalamic connectivity would be apparent across the prefrontal cortex and default mode network. Given the aforementioned regional heterogeneity of thalamus and its connectivity with widespread cortical regions, altered thalamic connectivity was also expected to be present in other cortical divisions. We were particularly interested in determining whether specific thalamo‐cortical networks are differentially affected in this disorder.
2. METHODS
2.1. Subjects
The study was approved by the Institutional Review Board in Peking University Institute of Mental Health. Voluntary written informed consent was obtained from all subjects prior to participating in this study. No patients who were involuntarily detained in hospital were included. A total of 72 first‐episode drug‐naive MDD patients from psychiatric outpatient or inpatient clinics and 71 HC subjects from the local community were recruited. The diagnosis of MDD was confirmed by a trained psychiatrist using the Mini‐International Neuropsychiatric Interview (MINI) according to DSM‐IV criteria. The inclusion criteria for MDD patients included a current acute major depressive episode, of moderate or greater depressive severity as defined by a score of at least 18 points on the 17‐item version of the Hamilton Rating Scale for Depression (HRSD). Exclusion criteria for the MDD patients included any previous or current use of psychotropic medications, a concurrent comorbid Axis I disorder or Axis II personality disorder, or intellectual disability.
The HCs were required to have no lifetime psychiatric disorder or history of use of psychotropic medications and no history of any psychiatric disorder among their first‐degree relatives. All HCs had a HRSD score of less than 7. An additional questionnaire was administered to ensure that all HCs had no experiences in the prior 6 months that might affect mood, such as examinations, unemployment, or family bereavement. Other exclusion criteria for both MDD and HC groups included: age less than 18 years or more than 60 years, unstable medical condition, neurological illness, substance dependence/abuse, acutely suicidal, or any contraindications to MRI scanning.
2.2. MRI acquisition
Images were acquired with a Siemens 3‐Tesla scanner. The resting‐state functional images were obtained using echo‐planar imaging sequence: repetition time (TR)/echo time (TE), 2000 ms/30 ms; 90° flip angle; matrix, 64 × 64; thickness/gap, 4.0 mm/0.8 mm; and 30 slices. In addition, T1‐weighted images were obtained using magnetization‐prepared rapidly acquired gradient‐echo (MPRAGE) sequence: TR/TE, 2300 ms/3.01 ms; matrix, 256 × 256; and 9° flip angle. The subjects were asked to keep their eyes closed without falling asleep and to move as little as possible. Through confirmation immediately after scanning, no subjects reported discomfort or to have fallen asleep during scanning, and no subjects showed obvious structural damage based on conventional MRI scans.
2.3. Data preprocessing
The R‐fMRI images were preprocessed with Data Processing Assistant for Resting‐State fMRI (DPARSF, http://rfmri.org/DPARSF) based on Statistical Parametric Mapping (SPM12, http://www.fil.ion.ucl.ac.uk/spm). After removal of the first ten volumes, the remaining 200 volumes were corrected for different signal acquisition times. The functional volumes were motion‐corrected using a six‐parameter rigid‐body transformation. Then, the nuisance signals (including Friston 24‐parameter model of head‐motion parameters, cerebrospinal fluid signal, white matter signal, global mean signal, and linear trend)18, 19 were regressed out. Derived images were normalized to Montreal Neurological Institute (MNI) space (3 mm3 isotropic) using Diffeomorphic Anatomical Registration using Exponentiated Lie algebra (DARTEL) tool.20 Then, the transformed images were band‐pass filtered (0.01‐0.1 Hz).
Given a possible confounding effect of micromovements on iFC (Power et al), we calculated the framewise displacement (FD) values for each subject using the Jenkinson formula, which reflect the temporal derivative of the movement parameters.21, 22 One control subject and two patients and who had mean FD >0.2 mm or translation >2 mm or rotation >2 degree were excluded; data from the remaining 70 patients and 70 controls were used for further analysis. There were no significant differences in the mean values of FD between MDD patients and HCs (t = −0.992, P = 0.323).
2.4. Functional connectivity analysis
As aforementioned, previous studies have exclusively used the whole thalamus as a seed to identify thalamic connectivity abnormalities in MDD8, 10, 13; such an approach treats the thalamus as a homogenous structure and limits the examination of any anatomical specificity of thalamo‐cortical connectivity abnormalities. To overcome this limitation, our study conducted a two‐step analysis. The first step was to define subregions of the thalamus by taking cortical ROIs as seeds and compute their functional connectivity within the thalamus. The second step was to map thalamic connectivity with the rest of the whole brain using the thalamic subregions as determined in the first step as seeds.
The detailed analysis was performed as a previous study.23 First, the cortex was segmented into large, anatomically nonoverlapping cortical ROIs, including prefrontal, motor, somatosensory, parietal, temporal, and occipital cortex (Figure 1A, template file was acquired by personal communication with Dr. Woodward). Then, to locate the specific connectivity for each cortical seed within the thalamus, we performed partial correlation analysis six times between the averaged BOLD time series of each cortical ROI and thalamus at the voxel level of the thalamus, with other cortical ROIs used as covariates. The thalamus was masked to include only voxels within the Harvard‐Oxford thalamus atlas (thresholded at 10%; http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Atlases). We used partial correlation analyses to characterize functional connectivity based on the consideration that partial correlation could eliminate shared variances among time courses of all cortical ROIs, thereby mitigating the confounding effects of cortico‐cortical interactions on thalamo‐cortical correlations.4 Based on correlation maps averaged across all subjects, the thalamus was parcellated using the “winner take all” strategy, which ensures that each thalamic voxel was assigned to a cortical ROI that had maximum correlation with it.24 We computed the mean correlation values of each cortical ROI and its associated thalamic nucleus, which generated six thalamo‐cortical connectivity values for each subject. These values were entered into independent‐sample t tests to examine differences between MDD patients and HCs, with age, gender, education level, and mean FD values as covariates.
Figure 1.
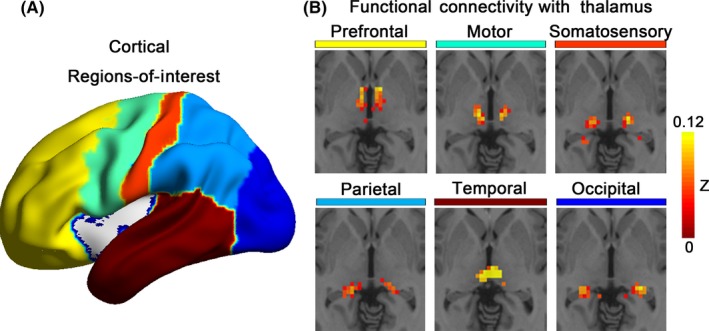
Functional connectivity between thalamus and cortex. (A) The bilateral cortex was divided into six nonoverlapping regions of interest, including the prefrontal, motor, somatosensory, parietal, temporal, and occipital cortex, which were used as seeds to examine the functional connectivity with thalamus; (B) Each cortical area showed specific correlations with distinct nuclei of the thalamus
We then extracted the mean BOLD time courses of each functionally segmented thalamic subregion and computed their correlations with the rest of the whole brain. This step generated six voxel‐wise thalamic connectivity maps for each subject. One‐sample t tests were performed to examine the within‐group patterns of functional connectivity of each thalamic subregion. Independent‐sample t tests were performed to examine between‐group differences in thalamic connectivity, with age, gender, education level, and mean FD values as covariates. We used Gaussian random field theory to correct for multiple testing with a cluster threshold of P < 0.05 and z > 2.9. According to Eklund et al and Chen et al, correction with loose threshold leads to unacceptable family‐wise error rate. Here, we performed one‐tailed GRF correction for each tail, with voxel P < 0.0005 (Z > 3.29) and cluster P < 0.025. With this setup, we can achieve a two‐tailed voxel P < 0.001 and cluster P < 0.05 and well control the family‐wise error rate under 5%.25, 26
2.5. Correlations between connectivity changes and clinical variables
We performed correlation analyses between the mean values of thalamo‐cortical connectivity showing between‐group differences and clinical variables (including illness duration, total HRSD, and its five factors, that is, anxiety/somatization, weight loss, cognitive disturbance, psychomotor retardation, and sleep disturbance). Correlations with P < 0.007(0.05/7) were considered significant which were corrected by Bonferroni method.
3. RESULTS
3.1. Sample characteristics
Demographic and clinical data are presented in Table 1. There were no significant differences between MDD patients and HCs in terms of age, gender, and education level. MDD patients showed significantly higher symptom scores than healthy controls (P < 0.001).
Table 1.
Demographic (a) and clinical (b)characteristics
| Characteristics | MDD patients (n = 70) | Healthy controls (n = 70) | χ2/t | P |
|---|---|---|---|---|
| a. Demographic characteristics | ||||
| Gender (male/female) | 28/42 | 31/39 | 0.264 | 0.732a |
| Age (y) | 32 ± 8.4 | 32 ± 9.3 | −0.019 | 0.985b |
| Education (y) | 13.7 ± 2.9 | 15.1 ± 2.2 | −3.304 | 0.001b |
| Duration of depressive episode (mo) | 6.1 ± 5.4 | – | ||
| b. Clinical characteristics | ||||
| Total HDRS score | 25.6 ± 4.8 | 1.1 ± 1 | 42.058 | <0.001b |
| Anxiety | 7.6 ± 2.3 | 0.5 ± 0.5 | 25.684 | <0.001b |
| Weight loss | 0.8 ± 0.8 | 0 ± 0 | 8.475 | <0.001b |
| Cognitive disturbance | 4.9 ± 2.1 | 0 ± 0 | 19.784 | <0.001b |
| Retardation | 8.5 ± 2 | 0.2 ± 0.4 | 34.129 | <0.001b |
| Sleep disturbance | 3.8 ± 1.4 | 0.4 ± 0.5 | 19.532 | <0.001b |
Unless otherwise indicated, data are expressed as mean values ± SD.
P value obtained by chi‐squared test.
P values obtained by two‐sample t test.
3.2. Thalamo‐cortical connectivity: cortical ROI‐to‐thalamus
As shown in Figure 1B, correlations between each cortical subdivision and thalamic nuclei in our sample were distinct, with substantial correspondence with previous studies.4, 11, 23, 24 Specifically, the prefrontal cortical subdivision strongly correlated with the anterior/mediodorsal portions of the thalamus. The motor and somatosensory ROIs showed strong interactions with the ventrolateral and posterior/ventrolateral thalamic regions, respectively. The temporal cortical ROI correlated strongly with the posterior/medial portions of the thalamus, which presumptively corresponds to the medial geniculate nucleus. The parietal and occipital cortical subdivisions were robustly connected to the posterior/lateral portions of the thalamus, which appears consistent with the lateral pulvinar, lateral geniculate, and superior colliculus. After use of the “winner take all” strategy, the thalamus was segmented into spatially distinct, nonoverlapping nuclei. Although no significant between‐group differences were found in the connectivity values between each cortical ROI and its corresponding thalamic region, we found a trend toward decreased parietal thalamus connectivity in MDD patients compared with HCs (t = −1.92, P = 0.056).
3.3. Thalamo‐cortical connectivity: thalamus ROI‐based whole brain analyses
The thalamic clusters obtained by the above analyses were used as seeds to localize thalamic connectivity with the rest of the brain in the MDD patients. As shown in Figure S1, we found positive connectivity between the prefrontal thalamus ROI and the well‐defined DMN regions, including the medial prefrontal cortex, posterior cingulate cortex/precuneus, and inferior parietal lobe. Both the motor and somatosensory thalamus ROIs showed positive connectivity with the primary and secondary motor and somatosensory cortex, striatum, lateral temporal cortex, as well as cerebellum. Positive connectivity was also found between the somatosensory thalamus ROI and the occipital cortex, while negative connectivity was found between the motor thalamus ROI and regions of the DMN. We observed positive connectivity between the temporal thalamus ROI and the medial prefrontal cortex, anterior cingulate, inferior frontal gyrus/insula, and temporal cortex. The occipital cortex was found to be negatively correlated with widespread brain regions, with some positive connectivity with the calcarine, fusiform gyrus, and cerebellum. The patterns of thalamo‐cortical connectivity were largely similar between the MDD patients and HCs.
There were, however, qualitative differences in the connectivity patterns of the PFC, motor, somatosensory, and parietal thalamic ROIs between the two groups. As shown in Table 2 and Figure 2, MDD patients demonstrated reduced connectivity between the prefrontal and parietal thalamus ROIs and the bilateral middle frontal gyrus (MFG) and the right posterior default mode network (DMN) and between the prefrontal and somatosensory thalamus ROIs and right middle temporal gyrus and left superior temporal gyrus, respectively. Conversely, increased connectivity was found between the motor thalamus ROI and the right middle frontal gyrus, right medial frontal gyrus/anterior cingulate and between both the motor and somatosensory thalamic ROIs and right posterior DMN regions. Increased connectivity also emerged between both the prefrontal and somatosensory thalamus ROIs and cerebellum and between the parietal thalamus ROI and the left insula. We did not found significant group differences with respect to connectivity of the temporal and occipital thalamic ROIs. No correlations between functional connectivity and clinical variables met the threshold of alpha levels <0.005.
Table 2.
Thalamo‐cortical functional connectivity changes in MDD
| MNI | |||||
|---|---|---|---|---|---|
| Thalamus ROI | Contrast | Brain region | Voxels | (x, y, z) | t |
| Prefrontal | HCs > MDD | R. middle frontal gyrus | 94 | 27, 21, 45 | −3.944 |
| R. middle temporal gyrus | 160 | 57, −6, −21 | −4.259 | ||
| R. inferior parietal lobule | 363 | 54, −48, 21 | −4.593 | ||
| R. precuneus | 474 | 6, −54, 33 | −4.466 | ||
| HCs < MDD | R. cerebellum_6 | 95 | 27, −54, −30 | 3.535 | |
| Motor | HCs < MDD | R. middle frontal gyrus | 132 | 45, 15, 42 | 3.482 |
| R. medial frontal gyrus/anterior cingulate | 72 | 3, 51, 12 | 3.874 | ||
| R. inferior parietal lobule | 195 | 54, −54, 30 | 4.171 | ||
| R. precuneus | 222 | 9, −72, 30 | 3.850 | ||
| Somatosensory | HCs > MDD | L. cerebellum_crus 1 | 81 | −39 −69 −36 | 4.524 |
| HCs < MDD | R. Precuneus | 127/84 | 3, −72, 45/−18, −57, 21 | 4.441/3.861 | |
| L. superior temporal gyrus | 112 | −63, −30, 18 | −3.897 | ||
| Parietal | HCs > MDD | L. middle frontal gyrus | 73 | −27, 30, 39 | −4.045 |
| L. inferior parietal lobule | 61 | 57, −57, 33 | −3.468 | ||
| L. insula | 66 | −39, 3, −3 | 4.039 |
L, left; MDD, Major depressive disorder; MNI, Montreal Neurological Institute; R, right; ROI, region of interest.
Figure 2.
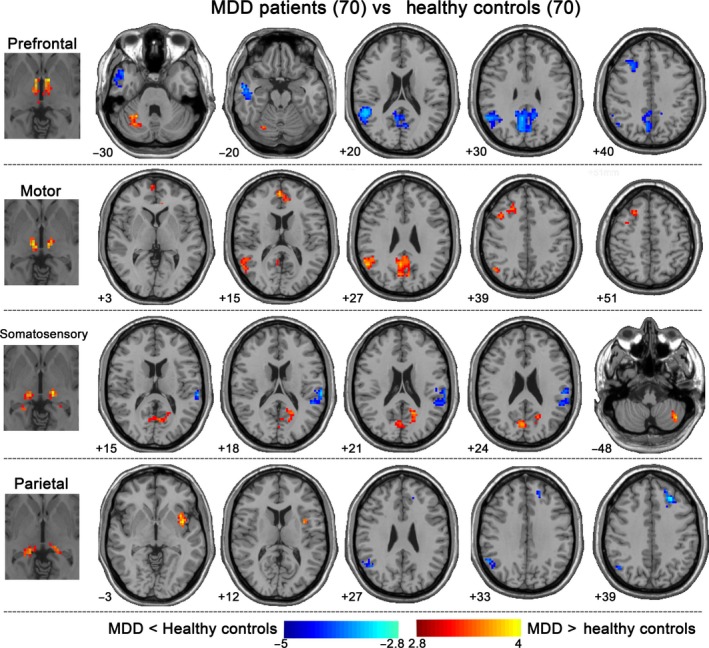
Thalamo‐cortical functional connectivity changes in MDD. Patients demonstrated reduced connectivity between prefrontal/parietal thalamus ROIs and bilateral MFG and right posterior DMN and between prefrontal/motor thalamus ROIs and lateral temporal regions. Conversely, increased connectivity emerged between motor thalamus ROI and right MFG and right medial frontal gyrus/anterior cingulate; between motor/somatosensory thalamus ROIs and right posterior DMN; between prefrontal/somatosensory thalamus ROIs and cerebellum; and between parietal thalamus ROI and left insula. All statistical maps were corrected for multiple comparisons using Gaussian random field theory with a cluster threshold of P < 0.05 and z > 2.9. MFG, middle frontal gyrus; DMN, default mode network; MDD, Major depressive disorder
4. DISCUSSION
This study is the first to examine the anatomical specificity of thalamo‐cortical connectivity disturbances in MDD. We found MDD‐related abnormalities in the connectivity patterns of the prefrontal, motor, somatosensory, and parietal thalamic subdivisions, with both hyperconnectivity and hypoconnectivity. Besides the prefrontal and DMN regions hypothesized, we also found altered thalamic connectivity with regions within other cortical territories. Specifically, reduced connectivity was found between the prefrontal and parietal thalamus ROIs and bilateral middle frontal gyrus and right posterior DMN regions and between the prefrontal and motor thalamus ROIs and lateral temporal regions. Conversely, increased connectivity emerged between the motor thalamus ROI and the right MFG and right medial frontal gyrus/anterior cingulate and between the motor and somatosensory thalamus ROIs and the right posterior DMN. Increased connectivity also presented between the prefrontal/somatosensory thalamus ROIs and the cerebellum and between the parietal thalamus ROI and the left insula.
The finding of decreased connectivity between the prefrontal/parietal thalamus nuclei and the middle frontal gyrus and posterior regions of the DMN is the first important finding of our study. Communication between the mediodorsal thalamus and the PFC modulates emotion and cognition. Reciprocal connections between the mediodorsal thalamus and PFC influence the limbic system by integrating emotionally relevant stimuli to the frontal lobes.9 Inhibition of the mediodorsal thalamus disrupts thalamo‐PFC connectivity and cognition.27 The mediodorsal nucleus of the thalamus in particular has been implicated in MDD. MDD patients have been found to have increased mediodorsal thalamus but reduced dorsal PFC activity and reduced connectivity between the medial thalamic and the dorsal PFC at rest or in response to negative affective stimuli.8 Reduced thalamic‐PFC structural connectivity observed in MDD patients may provide an anatomical basis for the current findings.28, 29 The weakened thalamus connectivity with the PFC may be associated with abnormal norepinephrine transmission, based on recent findings of elevated norepinephrine transporter availability in the mediodorsal thalamus and significant association with impaired attention in MDD.30 Collectively, the previous and current findings provide strong support for impaired connectivity of the thalamic‐PFC circuits in MDD, which may constitute an important neural basis of emotional and cognitive symptoms in MDD.
The posterior regions of the DMN, particularly the posterior cingulate and inferior parietal lobe, are involved in autobiographical memory retrieval,31, 32 the dysfunction of which has been considered as the main cause for excessive negative ruminations observed in MDD.33 Autobiographical memory disturbances have been proposed as a novel therapeutic target for MDD.34 Relevant to our findings, a prior study has revealed dissociation between anterior and posterior functional connectivity in resting‐state DMNs of young adults with MDD.35 Increased connectivity in anterior regions of the DMN was associated with rumination, whereas decreased connectivity in posterior regions of the DMN was associated with autobiographical memory deficits. The current results extend the dysconnectivity of posterior DMN regions to the thalamus, which may indicate that modulating thalamus‐DMN connectivity through targeting the thalamus may be a novel pathway to improve the autobiographical memory dysfunction of MDD.
In contrast to the prefrontal thalamus ROI, the motor and somatosensory thalamus subdivisions demonstrated connectivity strengthening with the PFC and/or posterior DMN regions in MDD patients. Besides the frequently studied thalamic‐PFC connectivity, the thalamus was also strongly connected with sensorimotor regions. It has been shown that tetanization of the thalamic nuclei can induce changes in the efficiency of the excitatory/inhibitory connections in the neuronal networks of the motor cortex.36 Brain hyperactivity has been commonly viewed as either an attempt to compensate for either neuronal deficits or insufficient performance or nonselective recruitment occurring when appropriate regions are less accessible.37 We therefore speculated that increased connectivity in the motor and somatosensory thalamus subdivisions with the middle frontal gyrus, medial frontal gyrus/anterior cingulate, and/or posterior DMN regions may be interpreted as a compensatory effort, a nonselective recruitment, or some combination of these two possibilities, to reduce connectivity between prefrontal/parietal thalamus nuclei and these cortical regions. The exact mechanism remains to be clarified in more specific designs.
The reduced connectivity between the prefrontal and motor thalamus ROIs and the lateral temporal regions in MDD patients is also an important finding given the critical role of the lateral temporal cortex in social cognition.38, 39 MDD is characterized by a severe disruption in social cognition, including impaired theory of mind and empathy.40, 41 More serious than emotional symptoms, impaired social cognition is a poorly controlled and highly relevant dimension of MDD.41 Congruent with social cognition disturbances, studies have shown structural and functional deficits of the lateral temporal regions in MDD, such as reduced gray matter volume42 and reduced responsiveness to positive social stimuli (social interaction, faces, sexual images) in concert with a disturbed neural network involving the prefrontal and parietal cortices, insula, and the hippocampus, which has been found to remit following antidepressant treatment.40 Reduced resting‐state functional connectivity in the left temporal pole and lateral STG has been reported to be a component of disturbed fronto‐temporal and limbic networks in MDD. This evidence raises the possibility that the thalamo‐temporal dysconnectivity may result from pathology of the temporal cortex. In the current context of a clear need for improved treatment of social cognitive dysfunction in MDD,40 our finding provides an important indication that strengthening the thalamo‐temporal connectivity by modulating thalamic activity may also present a potential therapeutic pathway.
The cerebellum is now recognized to modulate cognition and emotion by reciprocal connections with cerebral structures via the thalamus in feedforward loops43 and has been implicated in MDD and traumatic experience.10 It has been shown that MDD has a complex pattern of altered cerebellar‐cerebral functional connectivity characterized by hypoconnectivity with the PFC and DMN and hyperconnectivity with the visual recognition network and parahippocampal gyrus.44 Some environmental factors may lead to MDD vulnerability through alterations of the neural oscillatory activity of the cerebellum during resting state.45 Increased activation in the cerebellum of adolescents with MDD during rewarded sustained attention has been suggested to be a compensatory mechanism for the underactivation of fronto‐striato‐thalamic circuit.46 We therefore speculate that increased cerebellar connectivity between the PFC and motor thalamus nuclei in MDD patients may be a compensatory mechanism for the decreased thalamus connectivity and/or an independent abnormality associated with cognitive and emotional dysfunction.
Given its widespread connections with the frontal, parietal, temporal, and limbic areas, the insula is regarded as an integrative center for emotional, visceromotor, autonomic, and interoceptive information.47, 48 It has been theorized there is an amplification of interoceptive signals within the insula in MDD.49 Consistently, increased insular activity has been shown in acute and remitted MDD patients while viewing negative faces.50, 51 Our findings of increased thalamus‐insula connectivity at rest may therefore signify abnormally enhanced interoceptive processing for negative information, which subsequently contributes to the persistently and pervasive negative processing seen in MDD patients, such as rumination and somatic preoccupations.
4.1. Limitations
Several issues need to be further addressed. Studies showing altered thalamo‐frontal structural connections may provide an anatomical basis for the current findings.28, 29 However, strong functional connections commonly exist between regions with no direct structural connection, rendering the straightforward inference of functional connectivity from structural connectivity impractical.52 Future studies using a multimodal imaging data will help to clarify the contributions of direct/indirect structural connections to thalamo‐cortical functional connectivity in MDD. Moreover, growing evidence has suggested disrupted neural plasticity and cellular resilience, including altered HPA axis and glutamate neurotransmission and impaired neuroprotective signaling in MDD.53 So, how can we reconcile hypothesized upstream cellular‐level disruption with currently observed system‐level thalamo‐cortical abnormalities? More translational evidence from animal experiments in conjunction with human neuroimaging examining the interrelationship between thalamus and cortex will help to answer this question. Clarifying the potential specificity of our findings to MDD is another important research question, given that thalamo‐cortical connectivity alterations have also been detected in a range of other mental disorders, such as schizophrenia and bipolar disorder.23, 54 Importantly, the thalamus may be a potential target for MDD treatment given the central role of the thalamus in large‐scale brain connectivity. Indeed, the network dynamics of thalamo‐cortical circuits have been recently proposed to be a promising pathway for brain stimulation treatment of MDD.55, 56 Further studies modulating thalamic activity by focused brain stimulation technologies will help to test its effectiveness.
5. CONCLUSION
We found abnormalities in resting‐state functional connectivity between the thalamus and cortex in MDD, which were characterized by a complex pattern of region‐dependent (cortical or thalamic subdivisions) hypo‐ or hyperconnectivity. The present investigation clarifies the anatomical specificity of thalamo‐cortical network abnormalities in MDD. Given the limited available clinical tools for assessing complex clinical presentations of MDD in our study, future studies using more specific assessments of neural functions related to the thalamo‐cortical networks will help to clarify the functional consequences of thalamo‐cortical disturbances. In summary, our study suggests that selectively modulating the activity/connectivity of thalamus nuclei may be a potential novel therapeutic mechanism for MDD.
Supporting information
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (No. 81630031), Beijing Municipal Science and Technology Project (Z171100000117016), The Capital Foundation of Medicine Research and Development (2016‐1‐4111), National Key Basic R & D Program of China (973 Program) (2013CB531305), and National Key basic R&D Program (2015BAI13B01).
Kong Q‐M, Qiao H, Liu C‐Z, et al. Aberrant intrinsic functional connectivity in thalamo‐cortical networks in major depressive disorder. CNS Neurosci Ther. 2018;24:1063–1072. 10.1111/cns.12831
Qing‐Mei Kong, Hong Qiao and Chao‐Zhong Liu equally contributed to this study.
Contributor Information
Chao‐Gan Yan, Email: ycg.yan@gmail.com.
Tian‐Mei Si, Email: si.tian-mei@163.com.
REFERENCES
- 1. Sacchet MD, Ho TC, Connolly CG, et al. Large‐scale hypoconnectivity between resting‐state functional networks in unmedicated adolescent major depressive disorder. Neuropsychopharmacology. 2016;41:2951‐2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kaiser RH, Andrews‐Hanna JR, Wager TD, Pizzagalli DA. Large‐scale network dysfunction in major depressive disorder: a meta‐analysis of resting‐state functional connectivity. JAMA Psychiatry. 2015;72:603‐611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Williams LM. Defining biotypes for depression and anxiety based on large‐scale circuit dysfunction: a theoretical review of the evidence and future directions for clinical translation. Depress Anxiety. 2017;34:9‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang D, Snyder AZ, Shimony JS, Fox MD, Raichle ME. Noninvasive functional and structural connectivity mapping of the human thalamocortical system. Cereb Cortex. 2010;20:1187‐1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Behrens TE, Johansen‐Berg H, Woolrich MW, et al. Non‐invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci. 2003;6:750‐757. [DOI] [PubMed] [Google Scholar]
- 6. Johansen‐Berg H, Behrens TE, Sillery E, et al. Functional‐anatomical validation and individual variation of diffusion tractography‐based segmentation of the human thalamus. Cereb Cortex. 2005;15:31‐39. [DOI] [PubMed] [Google Scholar]
- 7. Young KA, Holcomb LA, Yazdani U, Hicks PB, German DC. Elevated neuron number in the limbic thalamus in major depression. Am J Psychiatry. 2004;161:1270‐1277. [DOI] [PubMed] [Google Scholar]
- 8. Anand A, Li Y, Wang Y, et al. Activity and connectivity of brain mood regulating circuit in depression: a functional magnetic resonance study. Biol Psychiatry. 2005;57:1079‐1088. [DOI] [PubMed] [Google Scholar]
- 9. Giguere M, Goldman‐Rakic PS. Mediodorsal nucleus: areal, laminar, and tangential distribution of afferents and efferents in the frontal lobe of rhesus monkeys. J Comp Neurol. 1988;277:195‐213. [DOI] [PubMed] [Google Scholar]
- 10. Lui S, Wu Q, Qiu L, et al. Resting‐state functional connectivity in treatment‐resistant depression. Am J Psychiatry. 2011;168:642‐648. [DOI] [PubMed] [Google Scholar]
- 11. Woodward ND, Heckers S. Mapping thalamocortical functional connectivity in chronic and early stages of psychotic disorders. Biol Psychiatry. 2016;79:1016‐1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Anand A, Li Y, Wang Y, et al. Antidepressant effect on connectivity of the mood‐regulating circuit: an FMRI study. Neuropsychopharmacology. 2005;30:1334‐1344. [DOI] [PubMed] [Google Scholar]
- 13. Tadayonnejad R, Yang S, Kumar A, Ajilore O. Clinical, cognitive, and functional connectivity correlations of resting‐state intrinsic brain activity alterations in unmedicated depression. J Affect Disord. 2015;172:241‐250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Greicius MD, Flores BH, Menon V, et al. Resting‐state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62:429‐437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ye T, Peng J, Nie B, et al. Altered functional connectivity of the dorsolateral prefrontal cortex in first‐episode patients with major depressive disorder. Eur J Radiol. 2012;81:4035‐4040. [DOI] [PubMed] [Google Scholar]
- 16. Sherman SM. Thalamus plays a central role in ongoing cortical functioning. Nat Neurosci. 2016;19:533‐541. [DOI] [PubMed] [Google Scholar]
- 17. Sherman SM, Haber SN. The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat. 2003;26:317‐330. [DOI] [PubMed] [Google Scholar]
- 18. Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R. Movement‐related effects in fMRI time‐series. Magn Reson Med. 1996;35:346‐355. [DOI] [PubMed] [Google Scholar]
- 19. Yan CG, Cheung B, Kelly C, et al. A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. NeuroImage. 2013;76:183‐201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Klein A, Andersson J, Ardekani BA, et al. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. NeuroImage. 2009;46:786‐802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825‐841. [DOI] [PubMed] [Google Scholar]
- 22. Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59:2142‐2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Woodward ND, Karbasforoushan H, Heckers S. Thalamocortical dysconnectivity in schizophrenia. Am J Psychiatry. 2012;169:1092‐1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fair DA, Bathula D, Mills KL, et al. Maturing thalamocortical functional connectivity across development. Front Syst Neurosci. 2010;4:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Eklund A, Nichols TE, Knutsson H. Cluster failure: why fMRI inferences for spatial extent have inflated false‐positive rates. Proc Natl Acad Sci USA. 2016;13:7900‐7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen X, Lu B, Yan CG. Reproducibility of R‐fMRI metrics on the impact of different strategies for multiple comparison correction and sample sizes. Hum Brain Mapp. 2018;39:300‐318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Parnaudeau S, O'Neill PK, Bolkan SS, et al. Inhibition of mediodorsal thalamus disrupts thalamofrontal connectivity and cognition. Neuron. 2013;77:1151‐1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Myung W, Han CE, Fava M, et al. Reduced frontal‐subcortical white matter connectivity in association with suicidal ideation in major depressive disorder. Transl Psychiatry. 2016;6:e835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jia Z, Wang Y, Huang X, et al. Impaired frontothalamic circuitry in suicidal patients with depression revealed by diffusion tensor imaging at 3.0 T. J Psychiatry Neurosci. 2014;39:170‐177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moriguchi S, Yamada M, Takano H, et al. Norepinephrine transporter in major depressive disorder: a PET study. Am J Psychiatry. 2017;174:36‐41. [DOI] [PubMed] [Google Scholar]
- 31. Buckner RL, Andrews‐Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann NY Acad Sci. 2008;1124:1‐38. [DOI] [PubMed] [Google Scholar]
- 32. Spreng RN, Mar RA, Kim AS. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta‐analysis. J Cogn Neurosci. 2009;21:489‐510. [DOI] [PubMed] [Google Scholar]
- 33. Sumner JA, Mineka S, Adam EK, et al. Testing the CaR‐FA‐X model: investigating the mechanisms underlying reduced autobiographical memory specificity in individuals with and without a history of depression. J Abnorm Psychol. 2014;123:471‐486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Köhler CA, Carvalho AF, Alves GS, McIntyre RS, Hyphantis TN, Cammarota M. Autobiographical memory disturbances in depression: a novel therapeutic target? Neural Plast. 2015;2015:759139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhu X, Wang X, Xiao J, et al. Evidence of a dissociation pattern in resting‐state default mode network connectivity in first‐episode treatment‐naive major depression patients. Biol Psychiatry. 2012;71:611‐617. [DOI] [PubMed] [Google Scholar]
- 36. Sil'kis IG. Long‐term changes in the efficiency of the excitatory and inhibitory connections in the neuronal micronetworks of the motor cortex induced by tetanization of the thalamic nuclei and the sensory cortex. Zh Vyssh Nerv Deiat Im I P Pavlova, 1995;45:932‐947. [PubMed] [Google Scholar]
- 37. Logan JM, Sanders AL, Snyder AZ, Morris JC, Buckner RL. Under‐recruitment and nonselective recruitment: dissociable neural mechanisms associated with aging. Neuron. 2002;33:827‐840. [DOI] [PubMed] [Google Scholar]
- 38. Gallagher HL, Frith CD. Functional imaging of ‘theory of mind’. Trends Cogn Sci. 2003;7:77‐83. [DOI] [PubMed] [Google Scholar]
- 39. Beauchamp MS. The social mysteries of the superior temporal sulcus. Trends Cogn Sci. 2015;19:489‐490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Millan MJ, Agid Y, Brüne M, et al. Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat Rev Drug Discov. 2012;11:141‐168. [DOI] [PubMed] [Google Scholar]
- 41. Cusi AM, Nazarov A, Holshausen K, Macqueen GM, McKinnon MC. Systematic review of the neural basis of social cognition in patients with mood disorders. J Psychiatry Neurosci. 2012;37:154‐169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Takahashi T, Yücel M, Lorenzetti V, et al. An MRI study of the superior temporal subregions in patients with current and past major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:98‐103. [DOI] [PubMed] [Google Scholar]
- 43. Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. 2010;46:831‐844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Guo W, Liu F, Xue Z, et al. Abnormal resting‐state cerebellar‐cerebral functional connectivity in treatment‐resistant depression and treatment sensitive depression. Prog Neuropsychopharmacol Biol Psychiatry. 2013;44:51‐57. [DOI] [PubMed] [Google Scholar]
- 45. Córdova‐Palomera A, Tornador C, Falcón C, et al. Environmental factors linked to depression vulnerability are associated with altered cerebellar resting‐state synchronization. Sci Rep. 2016;6:37384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chantiluke K, Halari R, Simic M, et al. Fronto‐striato‐cerebellar dysregulation in adolescents with depression during motivated attention. Biol Psychiatry. 2012;71:59‐67. [DOI] [PubMed] [Google Scholar]
- 47. Harshaw C. Interoceptive dysfunction: toward an integrated framework for understanding somatic and affective disturbance in depression. Psychol Bull. 2015;141:311‐363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655‐667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Paulus MP, Stein MB. Interoception in anxiety and depression. Brain Struct Funct. 2010;214:451‐463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Surguladze SA, El‐Hage W, Dalgleish T, Radua J, Gohier B, Phillips ML. Depression is associated with increased sensitivity to signals of disgust: a functional magnetic resonance imaging study. J Psychiatr Res. 2010;44:894‐902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Guo W, Liu F, Xiao C, et al. Decreased insular connectivity in drug‐naive major depressive disorder at rest. J Affect Disord. 2015;179:31‐37. [DOI] [PubMed] [Google Scholar]
- 52. Honey CJ, Sporns O, Cammoun L, et al. Predicting human resting‐state functional connectivity from structural connectivity. Proc Natl Acad Sci USA. 2009;106:2035‐2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Krishnan V, Nestler EJ. Linking molecules to mood: new insight into the biology of depression. Am J Psychiatry. 2010;167:1305‐1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Anticevic A, Cole MW, Repovs G, et al. Characterizing thalamo‐cortical disturbances in schizophrenia and bipolar illness. Cereb Cortex. 2014;24:3116‐3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Frohlich F. Endogenous and exogenous electric fields as modifiers of brain activity: rational design of noninvasive brain stimulation with transcranial alternating current stimulation. Dialogues Clin Neurosci. 2014;16:93‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Frohlich F, Sellers KK, Cordle AL. Targeting the neurophysiology of cognitive systems with transcranial alternating current stimulation. Expert Rev Neurother 2015;15:145e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


