Abstract
Background
Antithrombotic management of patients with atrial fibrillation (AF) requiring percutaneous coronary intervention (PCI) is highly variable; limited evidence‐based guidelines exist to influence practice.
Hypothesis
Patient characteristics and availability of novel drugs may have contributed to practice variability.
Methods
We undertook an international multicenter retrospective registry of AF patients treated with PCI. The primary measures of interest were antiplatelet and OAC prescriptions at discharge. We compared temporal trends between Prior (2010–2012) and Recent (2013–2015) cohorts and investigated variables associated with OAC prescription.
Results
We identified 488 cases (140 Prior, 348 Recent). Median CHADS2 and HAS‐BLED scores were 2 (IQR, 1–3) and 2 (IQR, 2–3). Clinical characteristics were similar between cohorts, with high (85%) prevalence of ACS. More patients in the Recent cohort, compared with Prior, received OAC (56.9% vs 44.3%; P = 0.01) and NOAC (27.3% vs 3.6%; P < 0.01) at baseline. Triple therapy at discharge was not different between the cohorts. Clinical presentation with ACS and consequent use of potent P2Y12 inhibitors were associated with reduced odds of OAC prescription at discharge (OR: 0.57, P = 0.045 and OR: 0.38, P = 0.023, respectively).
Conclusions
Despite little change over time in clinical characteristics of AF patients undergoing PCI, significantly more patients received OAC at presentation. However, triple therapy was not more frequent in the Recent cohort, and ACS presentation was associated with lack of OAC at discharge. We underscore the need for trial evidence and use of updated guidelines to assist clinicians in balancing ischemic and bleeding risks.
Keywords: Atrial Fibrillation, Drug‐Eluting Stents, Guidelines, Oral Anticoagulants
1. INTRODUCTION
Atrial fibrillation (AF) is a prevalent cardiac condition, affecting ~33 million people worldwide. There is a higher burden of disease in developed countries,1, 2 and AF carries a significant cardio‐embolic stroke and mortality risk.2 AF patients frequently present with comorbid cardiovascular disease or associated risk factors,3, 4, 5 are responsible for increased healthcare costs, and experience diminished quality of life.6 Lifelong oral anticoagulation (OAC) with either vitamin K antagonists (VKA) or non‐VKA oral anticoagulants (NOAC) is the mainstay of therapy, with the goal of reducing the risk of stroke.2, 7
Up to 20% to 30% of AF/atrial flutter (AF/AFL) patients present with clinically significant coronary artery disease,8 and many will require percutaneous coronary intervention (PCI) with stent implantation.9 Although PCI patients are typically managed with dual antiplatelet therapy (DAPT), consisting of combination therapy with aspirin (acetylsalicylic acid) and a P2Y12‐receptor inhibitor,10 antiplatelet therapy alone has been shown to be inadequate for stroke prevention in AF/AFL.11, 12 Yet, combining OAC and DAPT is associated with increased bleeding.9, 13, 14 Until recently,2, 10, 15, 16, 17 physicians were left to balance these risks themselves with little guidance from the literature, which, anecdotally, has led to important practice variability that has yet to be formally quantified.
We therefore sought to determine the extent of practice variability in a real‐world international multicenter cohort of AF/AFL patients receiving coronary stents, as well as the impact of the introduction of both NOAC and newer P2Y12 inhibitors on that variability prior to the recent publication of international guidelines.2, 17
2. METHODS
In TALENT‐AF (The internAtionaL stENT – Atrial Fibrillation study) multicenter registry, we collected unselected AF/AFL patients treated with PCI and implantation of ≥1 coronary stent at one of 4 international academic teaching centers (Canada, 1; Portugal, 1; Italy, 2) from 2010 to 2015. Inclusion criteria were (1) past history of AF/AFL, or AF/AFL at admission not reversing to sinus rhythm in the first 48 hours; and (2) successful stent implantation. Institutional catheterization laboratory databases were cross‐checked with administrative data confirming the attribution of International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) diagnosis codes 427.3 (atrial fibrillation and flutter) and 36.06 or 36.07 (insertion of coronary artery stent) to ensure complete capture of all relevant cases. Only patients with additional non‐AF/AFL indications for or with an absolute contraindication to OAC were excluded from the analysis.
This manuscript complies with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines,18 and the study is registered in the Research Registry (http://www.researchregistry.com) with the unique identifying number researchregistry3510.
The primary measures of interest were the rates and type of antiplatelet and OAC prescriptions at hospital discharge. Clinical outcomes were not explored in this study.
The principal analysis consisted of an evaluation of temporal trends in prescription patterns. The study population was divided into Prior (2010–2012) and Recent (2013–2015) cohorts for comparison, as 2013 corresponded to the first full calendar year that the novel antithrombotic agents were available in all 3 countries.
Secondary analyses included both univariate and multivariable logistic regression using a backward covariate selection algorithm to explore the impact of baseline patient characteristics, as well as clinical presentation (acute coronary syndrome [ACS] or non‐ACS), on discharge treatment choice.
In addition, an exploratory analysis was performed using the clinical information available in the cohort to determine what the rates of guideline‐recommended antithrombotic therapies would be for this cohort according to the 2016 European Society of Cardiology (ESC) atrial fibrillation guidelines.2 The CHA2DS2‐VASC score was calculated for each patient in the cohort. Patients with estimated glomerular filtration rate (eGFR) >30 mL/min with a guideline indication for OAC were assigned NOAC therapy in accordance with the recommendation to prefer NOAC therapy over VKA, whereas those with a eGFR <30 mL/min were assigned VKA.2 These projected rates were then compared with the actual discharge prescribing patterns observed in the Recent cohort.
2.1. Statistical analysis
Continuous variables were compared by means of unpaired t test and dichotomous variables with a χ2 test. All statistical analyses were performed using SAS software version 9.4 (SAS Institute, Inc., Cary, NC). A 2‐tailed P value <0.05 was considered significant for all analyses.
3. RESULTS
The cohort consisted of a total of 488 patients with AF/AFL across the 4 clinical sites. Clinical and procedural characteristics of patients in the Prior (n = 140) and Recent (n = 348) cohorts at the time of PCI are detailed in Table 1. Overall, the cohorts were quite similar, with the only differences being a higher prevalence of previous stroke in the Prior cohort (P = 0.03) and a significant 25% absolute increase in the use of drug‐eluting stents (DES) over time (P < 0.01). There was no difference in the rate of ACS presentation between the cohorts. Radial access was used in roughly two‐thirds of patients. The rate of in‐hospital mortality was 2% overall.
Table 1.
Clinical and procedural characteristics and antithrombotic medications, stratified by Prior and Recent cohorts
| Total Cohort, N = 488 | Prior Cohort, n = 140 | Recent Cohort, n = 348 | P Value | |
|---|---|---|---|---|
| Mean age, y | 73.4 ± 9.4 | 73.1 ± 9.3 | 73.5 ± 9.4 | 0.72 |
| Age > 75 y | 225 (46.1) | 67 (47.8) | 158 (45.4) | 0.69 |
| Male sex | 336 (68.8) | 97 (69.2) | 239 (68.7) | 0.91 |
| DM | 198 (40.5) | 51 (36.4) | 147 (42.2) | 0.26 |
| HTN | 362 (74.2) | 97 (69.2) | 265 (76.1) | 0.13 |
| Stroke | 50 (10.2) | 21 (15.0) | 29 (8.3) | 0.03 |
| HF | 122 (25.0) | 31 (22.1) | 91 (26.1) | 0.42 |
| Bleeding history | 28 (5.7) | 7 (5.0) | 21 (6.0) | 0.83 |
| BMI, kg/m2 | 27.1 ± 7.36 | 26.1 ± 8.3 | 27.4 ± 6.9 | 0.07 |
| eGFR, mL/min | 68.4 ± 36.2 | 68.5 ± 37.0 | 68.4 ± 36.0 | 0.98 |
| <30 mL/min | 50 (10) | 16 (11) | 34 (9.8) | 0.48 |
| Bleeding scores | ||||
| CHADS2 | 2 (1–3) | 2 (1–3) | 2 (1–3) | 0.89 |
| CHA2DS2‐VASc | 4 (3–5) | 4 (3–5) | 4 (3–5) | 0.76 |
| HAS‐BLED | 2 (2–3) | 2 (2–3) | 2 (2–3) | 0.77 |
| ACS presentation | 414 (84.8) | 126 (90.0) | 288 (82.7) | 0.05 |
| Femoral access | 174 (35.6) | 54 (38.6) | 120 (34.5) | 0.24 |
| DES use | 285 (58.4) | 57 (40.7) | 228 (65.5) | <0.01 |
| Baseline medications | ||||
| ASA | 310 (63.5) | 98 (70.0) | 212 (60.1) | 0.12 |
| P2Y12 | 45 (9.2) | 5 (3.6) | 40 (11.5) | 0.02 |
| Prasugrel/ticagrelor | 8 (1.6) | 1 (0.7) | 7 (2.0) | 0.32 |
| DAPT | 38 (7.8) | 5 (3.6) | 33 (9.5) | 0.03 |
| OAC | 260 (53.1) | 62 (44.3) | 198 (56.9) | 0.01 |
| VKA | 160 (32.8) | 57 (40.7) | 103 (29.6) | 0.02 |
| NOAC | 100 (20.1) | 5 (3.6) | 95 (27.3) | <0.01 |
| Dual therapy | 142 (29.1) | 40 (28.6) | 102 (29.3) | 0.87 |
| Discharge medications | ||||
| Prasugrel/ticagrelor | 41 (8.4) | 0 | 41 (11.8) | <0.01 |
| DAPT | 479 (98.1) | 140 (100) | 339 (97.4) | 0.06 |
| OAC | 215 (44.1) | 60 (42.9) | 155 (44.5) | 0.74 |
| VKA | 160 (32.8) | 55 (39.3) | 105 (30.2) | 0.02 |
| NOAC | 55 (11.3) | 5 (3.6) | 50 (14.4) | <0.01 |
| Dual therapy | 5 (1.0) | 0 (0.0) | 5 (1.4) | 0.15 |
| Triple therapy | 209 (42.9) | 60 (42.9) | 149 (42.9) | 0.99 |
Abbreviations: ACS, acute coronary syndrome; ASA, acetylsalicylic acid (aspirin); BMI, body mass index; CHADS2, congestive HF, HTN, age ≥ 75 y, DM, stroke/TIA/TE; CHA2DS2‐VASc, congestive HF, HTN, age ≥ 75 y, DM, stroke/TIA, vascular disease, age 65–74 y, sex category (female); DAPT, dual antiplatelet therapy; DES, drug‐eluting stent; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HAS‐BLED, HTN, abnormal renal/liver function, stroke, bleeding, labile INR, elderly, drugs or alcohol; HF, heart failure; HTN, hypertension; INR, international normalized ratio; IQR, interquartile range; NOAC, non‐VKA oral anticoagulant; OAC, oral anticoagulant; SD, standard deviation; TE, thromboembolism; TIA, transient ischemic attack; VKA, vitamin K antagonist.
Data are presented as n (%), mean ± SD, or median (IQR).
Figure 1.
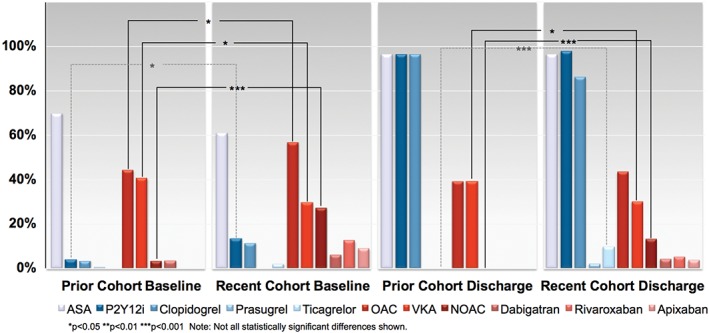
Baseline and discharge antithrombotic use, stratified by Prior and Recent cohorts. Abbreviations: ASA, acetylsalicylic acid (aspirin); NOAC, non‐VKA oral anticoagulant; OAC, oral anticoagulant; P2Y12i, P2Y12 inhibitor; VKA, vitamin K antagonist
Antithrombotic therapy prescriptions both prior to PCI and at discharge in the Prior and Recent cohorts are shown in Table 1 and Figure. Baseline prescriptions changed significantly over time, with a significantly increased rate of both P2Y12‐inhibitor and OAC use in the Recent cohort (P < 0.05 for both comparisons). An increase in the rate of OAC use at baseline was also observed despite a significant reduction in the rate of VKA use over time (P < 0.05) due to significant uptake of NOAC therapy over time (P < 0.01).
Discharge antiplatelet prescriptions patterns showed a significant increase in the use of ticagrelor in AF/AFL patients over time (P < 0.01; Figure). Overall, the rate of OAC use at discharge was not significantly different between the cohorts, but there was a significant reduction in the rate of VKA prescription (P < 0.05) and significant increase in use of NOAC (P < 0.01) over time, similar to the observed changes in baseline treatment.
With respect to impact of baseline patient characteristics on treatment choice, stratification of study population in ACS and non‐ACS patients is presented in Table 2. There was a statistically significant higher use of OAC and of NOAC both at baseline and discharge in non‐ACS compared with ACS patients in the unadjusted analysis. Overall, the strongest independent predictor of NOAC prescription at discharge remained the use of OAC at baseline (odds ratio [OR]: 2.24, 95% confidence interval [CI]: 1.11–4.52, P = 0.024), whereas ACS presentation and the use of newer P2Y12 inhibitors (ticagrelor or prasugrel vs clopidogrel) at discharge were independently associated with the lack of OAC at discharge (OR: 0.57, 95% CI: 0.33–0.99, P = 0.045 and OR: 0.38, 95% CI: 0.16–0.88, P = 0.023, respectively). However, only 8 patients out of 209 discharged on triple therapy (5%) received a novel P2Y12 inhibitor (8 ticagrelor; 0 prasugrel) in our cohort. Variables independently associated with OAC prescription at discharge included baseline OAC (OR: 4.12, 95% CI: 2.74–6.18, P < 0.01), use of DES (OR: 1.67, 95% CI: 1.11–2.51, P = 0.014), and CHA2DS2‐VASC score (OR: 1.16 for each point, 95% CI: 1.02–1.32, P = 0.022).
Table 2.
Clinical and procedural characteristics and antithrombotic medications, stratified by ACS and non‐ACS presentation
| ACS, n = 414 | Non‐ACS, n = 74 | P Value | |
|---|---|---|---|
| Mean age, y | 73.6 ± 9.4 | 72.0 ± 9.0 | 0.18 |
| Age > 75 y | 193 (46.6) | 32 (43.2) | 0.62 |
| Male sex | 280 (67.6) | 56 (75.7) | 0.22 |
| DM | 172 (41.6) | 26 (35.1) | 0.37 |
| HTN | 305 (73.7) | 57 (77.0) | 0.67 |
| Stroke | 44 (10.7) | 6 (8.1) | 0.68 |
| HF | 102 (24.6) | 20 (27.0) | 0.66 |
| Bleeding history | 22 (5.3) | 6 (8.1) | 0.41 |
| BMI, kg/m2 | 26.1 ± 8.15 | 27.1 ± 7.22 | 0.62 |
| eGFR, mL/min | 67.0 ± 34.1 | 68.7 ± 36.6 | 0.72 |
| <30 mL/min | 42 (10.1) | 7 (9.5) | 0.99 |
| Bleeding scores | |||
| CHADS2 | 2 (1–3) | 2 (1–3) | 0.89 |
| CHA2DS2‐VASc | 4 (3–5) | 4 (3–5) | 0.76 |
| HAS‐BLED | 2 (2–3) | 2 (2–3) | 0.77 |
| Femoral access | 132 (31.9) | 24 (32.4) | 0.93 |
| DES use | 246 (59.4) | 40 (54.1) | 0.53 |
| Baseline medications | |||
| ASA | 264 (63.7) | 46 (62.2) | 0.38 |
| P2Y12 | 43 (10.4) | 10 (13.7) | 0.41 |
| Prasugrel/ticagrelor | 7 (1.7) | 1 (1.4) | 0.84 |
| DAPT | 33 (8.0) | 5 (6.8) | 0.72 |
| OAC | 213 (51.5) | 48 (64.9) | 0.03 |
| VKA | 135 (32.6) | 25 (33.8) | 0.37 |
| NOAC | 78 (18.8) | 22 (29.7) | 0.03 |
| Dual therapy | 114 (27.5) | 28 (37.8) | 0.07 |
| Discharge medications | |||
| Prasugrel/ticagrelor | 39 (9.4) | 2 (2.7) | 0.06 |
| DAPT | 409 (98.8) | 70 (94.6) | 0.01 |
| OAC | 172 (41.5) | 43 (58.1) | 0.01 |
| VKA | 131 (31.6) | 29 (39.2) | 0.62 |
| NOAC | 41 (9.9) | 14 (18.9) | 0.02 |
| Dual therapy | 3 (0.7) | 2 (2.7) | 0.12 |
| Triple therapy | 169 (40.8) | 40 (54.1) | 0.03 |
Abbreviations: ACS, acute coronary syndrome; ASA, acetylsalicylic acid (aspirin); BMI, body mass index; CHADS2, congestive HF, HTN, age ≥ 75 y, DM, stroke/TIA/TE; CHA2DS2‐VASc, congestive HF, HTN, age ≥ 75 y, DM, stroke/TIA, vascular disease, age 65–74 y, sex category (female); DAPT, dual antiplatelet therapy; DES, drug‐eluting stent; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HAS‐BLED, HTN, abnormal renal/liver function, stroke, bleeding, labile INR, elderly, drugs or alcohol; HF, heart failure; HTN, hypertension; INR, international normalized ratio; IQR, interquartile range; NOAC, non‐VKA oral anticoagulant; OAC, oral anticoagulant; SD, standard deviation; TE, thromboembolism; TIA, transient ischemic attack; VKA, vitamin K antagonist.
Data are presented as n (%), mean ± SD, or median (IQR).
Real‐world and ESC‐recommended OAC rates are presented in Table 3. Perfect adoption of ESC 2016 guidelines would mean a 2‐fold increase in the rate of OAC and a 3‐fold increase in the rate of NOAC use over the rates observed in our cohort prior to the publication of the guidelines. The rate of VKA was projected to plummet to ~10%. All these differences are statistically significant.
Table 3.
Observed and expecteda incidence of OAC prescription at discharge in the entire cohort
| Total cohort “Observed,” N = 488 | Guidelines‐Recommended “Expected,” N = 488 | P Value | |
|---|---|---|---|
| No anticoagulation | 228 (46.5) | 9 (1.8) | <0.01 |
| Anticoagulation | 260 (53.5) | 479 (97.8) | |
| NOAC | 100 (31) | 431 (90) | <0.01 |
| VKA | 160 (69) | 48 (10) |
Abbreviations: ESC, European Society of Cardiology; NOAC, non‐VKA oral anticoagulant; OAC, oral anticoagulant; VKA, vitamin K antagonist.
Data are presented as n (%).
Adherence to ESC 2016 guidelines.
4. DISCUSSION
This multicenter international retrospective registry of AF/AFL patients receiving coronary stent implantation revealed a number of important findings. First, the clinical characteristics of patients with AF/AFL receiving coronary stents have remained largely stable over time. Second, despite this, baseline clopidogrel use increased significantly, as did use of OAC due to significant uptake of NOAC therapy at baseline. Third, there was also significant uptake of NOAC at discharge at the expense of VKA prescription rates that declined significantly over time. Fourth, we identified clinical predictors of OAC prescription at discharge following PCI. Finally, these changes occurred despite background increases in the rate of DES use and in the rate of novel P2Y12‐inhibitor prescription.
The reasons for practice variability with regard to antithrombotic therapy for AF/AFL patients following PCI in the absence of guidance from professional bodies or definitive trials remain speculative, but they include a perceived higher individual risk of coronary events due to stent‐, anatomy‐, or patient‐related factors in certain cases, perceived or real elevated stroke risk (CHA2DS2‐VASC score), perceived bleeding risk, or all three.9 The independent effect of clinical presentation on discharge prescription choice might be evidence of such influences on clinical decision‐making, consistent with the findings from the Berlin AFibACS Registry, in which only 49.9% of patients with stent received OAC at discharge,19 as many physicians might have been uncomfortable both combining OAC with novel P2Y12 inhibitors20, 21, 22 or forgoing their benefit in ACS patients. To this point, it is also noteworthy that prospective trial data regarding the association of OAC with newer P2Y12 inhibitors are limited to minority (~5%) subgroups of PIONEER‐AF PCI (An Open‐Label, Randomized, Controlled, Multicenter Study Exploring Two Treatment Strategies of Rivaroxaban and a Dose‐Adjusted Oral Vitamin K Antagonist Treatment Strategy in Subjects With Atrial Fibrillation Who Undergo Percutaneous Coronary Intervention)15 and RE‐DUAL PCI (Evaluation of Dual Therapy With Dabigatran vs Triple Therapy With Warfarin in Patients With AF That Undergo a PCI With Stenting),16 both of which were only published after the period of study. Interestingly, in our ACS cohort, only 5% of patients received ticagrelor‐based triple therapy. In the nationwide Danish cohort of AF patients, the risk of bleeding was higher with triple therapy than with any other treatment approach.13 This risk may be reflected in the choice of many physicians not to administer triple therapy to AF/AFL patients with recent ACS and coronary stents. However, underuse of guideline‐recommended antithrombotic drugs, either OAC or antiplatelet therapy, in such patients is associated with an increased risk of death and major cardiovascular events.23
As the risk of bleeding with so‐called triple therapy after PCI in patients with AF/AFL remains elevated compared with either DAPT or OAC alone,13, 24 international guidelines have recently sought to provide guidance on this very subject.2, 10, 17, 25, 26 To this point, it is crucial to ensure adequate early follow‐up of this patient population to comply with the recommended timeline for downgrading triple therapy. Our exploratory analysis suggests a dramatic treatment gap between real‐world practice immediately prior to the publication of recent guidelines and expert statements and what recommended therapy would now be, suggesting the need for follow‐up studies to gauge the rate of uptake of the new recommendations, as well as the impact of the recent PIONEER AF‐PCI15 and RE‐DUAL PCI16 studies.
4.1. Study limitations
Given the retrospective nature of this analysis, a number of limitations must be considered. First, this registry relied on abstracting data from patients' medical records, raising the possibility of ascertainment bias. As the temporal nature of AF/AFL (paroxysmal vs permanent) should not be relevant to the decision as to whether to anticoagulate, our data lack the granularity to stratify for this consideration. As such, we cannot rule out that clinicians might nonetheless be influenced by these factors. Because of imbalances in terms of the number of cases contributed by each center, we also opted not to compare practice patterns between institutions. However, clinicians practicing in different countries might well be influenced differentially by the different guidelines available.2, 25, 26 Furthermore, even though all participating centers are tertiary academic referral centers, which might explain the high rate of ACS presentation in our cohort, another possibility is a lack of robustness in the operationalization of our ACS definition, particularly for patients without myocardial infarction. Finally, for similar reasons, many of the patients treated with PCI at the participating centers are not followed clinically in those centers, limiting our ability to comment on clinical outcomes beyond the index hospitalization.
5. CONCLUSION
Despite little change over time in the clinical characteristics of AF/AFL patients receiving coronary stents and a concomitant increase in the use of both DES and novel P2Y12 inhibitors over the same period, patients are increasingly treated with NOAC both at presentation and discharge. However, this observed increase is dwarfed by the change in clinical practice that would be necessary to comply with the most recent ESC guideline recommendations, suggesting the need for follow‐up quality‐of‐care surveillance.
Conflicts of interest
The authors have received nonfinancial support from Bayer Pharma AG for investigator meetings within the context of the Thrombosis Academy for Learning Education and Network Training (TALENT) program. The sponsor had no role in study design, data collection, data analysis, the writing of the manuscript, or the submission process. The authors declare no other potential conflicts of interest.
Potter BJ, Andò G, Cimmino G, et al. Time trends in antithrombotic management of patients with atrial fibrillation treated with coronary stents: Results from TALENT‐AF (The internAtionaL stENT – Atrial Fibrillation study) multicenter registry. Clin Cardiol. 2018;41:470–475. 10.1002/clc.22898
Funding information Bayer HealthCare
Presented in part as an abstract at the ACC.17 Congress (Washington, DC, March 17–19, 2017), presentation no. 1252‐303.
REFERENCES
- 1. Rahman F, Kwan GF, Benjamin EJ. Global epidemiology of atrial fibrillation. Nat Rev Cardiol. 2014;11:639–654. [DOI] [PubMed] [Google Scholar]
- 2. Kirchhof P, Benussi S, Kotecha D, et al; ESC Scientific Document Group . 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. [DOI] [PubMed] [Google Scholar]
- 3. Andò G, Capranzano P. Non‐vitamin K antagonist oral anticoagulants in atrial fibrillation patients with chronic kidney disease: a systematic review and network meta‐analysis. Int J Cardiol. 2017;231:162–169. [DOI] [PubMed] [Google Scholar]
- 4. Myserlis PG, Malli A, Kalaitzoglou DK, et al. Atrial fibrillation and cognitive function in patients with heart failure: a systematic review and meta‐analysis. Heart Fail Rev. 2017;22:1–11. [DOI] [PubMed] [Google Scholar]
- 5. Odutayo A, Wong CX, Williams R, et al. Prognostic importance of atrial fibrillation timing and pattern in adults with congestive heart failure: a systematic review and meta‐analysis. J Card Fail. 2017;23:56–62. [DOI] [PubMed] [Google Scholar]
- 6. Hong HJ, Kim YD, Cha MJ, et al. Early neurological outcomes according to CHADS2 score in stroke patients with nonvalvular atrial fibrillation. Eur J Neurol. 2012;19:284–290. [DOI] [PubMed] [Google Scholar]
- 7. Sterne JA, Bodalia PN, Bryden PA, et al. Oral anticoagulants for primary prevention, treatment and secondary prevention of venous thromboembolic disease, and for prevention of stroke in atrial fibrillation: systematic review, network meta‐analysis and cost‐effectiveness analysis. Health Technol Assess. 2017;21:1–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kralev S, Schneider K, Lang S, et al. Incidence and severity of coronary artery disease in patients with atrial fibrillation undergoing first‐time coronary angiography. PLoS One. 2011;6:e24964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Capodanno D, Angiolillo DJ. Management of antiplatelet and anticoagulant therapy in patients with atrial fibrillation in the setting of acute coronary syndromes or percutaneous coronary interventions. Circ Cardiovasc Interv. 2014;7:113–124. [DOI] [PubMed] [Google Scholar]
- 10. Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio‐Thoracic Surgery (EACTS). Eur Heart J. 2018;39:213–260. [DOI] [PubMed] [Google Scholar]
- 11. Hart RG, Pearce LA, Aguilar MI. Meta‐analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–867. [DOI] [PubMed] [Google Scholar]
- 12. Connolly S, Pogue J, Hart R, et al; ACTIVE Writing Group of the ACTIVE Investigators . Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial Fibrillation Clopidogrel Trial With Irbesartan for Prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet. 2006;367:1903–1912. [DOI] [PubMed] [Google Scholar]
- 13. Lamberts M, Olesen JB, Ruwald MH, et al. Bleeding after initiation of multiple antithrombotic drugs, including triple therapy, in atrial fibrillation patients following myocardial infarction and coronary intervention: a nationwide cohort study. Circulation. 2012;126:1185–1193. [DOI] [PubMed] [Google Scholar]
- 14. Lamberts M, Gislason GH, Olesen JB, et al. Oral anticoagulation and antiplatelets in atrial fibrillation patients after myocardial infarction and coronary intervention. J Am Coll Cardiol. 2013;62:981–989. [DOI] [PubMed] [Google Scholar]
- 15. Gibson CM, Mehran R, Bode C, et al. Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N Engl J Med. 2016;375:2423–2434. [DOI] [PubMed] [Google Scholar]
- 16. Cannon CP, Bhatt DL, Oldgren J, et al; REDUAL‐PCI Steering Committee and Investigators . Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med. 2017;377:1513–1524. [DOI] [PubMed] [Google Scholar]
- 17. Macle L, Cairns J, Leblanc K, et al; CCS Atrial Fibrillation Guidelines Committee . 2016 Focused Update of the Canadian Cardiovascular Society Guidelines for the Management of Atrial Fibrillation [published correction appears in Can J Cardiol. 2017;33:552–553]. Can J Cardiol. 2016;32:1170–1185. [DOI] [PubMed] [Google Scholar]
- 18. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4:e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maier B, Hegenbarth C, Theres H, et al; AFibACS Registry . Antithrombotic therapy in patients with atrial fibrillation and acute coronary syndrome in the real world: data from the Berlin AFibACS Registry. Cardiol J. 2014;21:465–473. [DOI] [PubMed] [Google Scholar]
- 20. Sarafoff N, Martischnig A, Wealer J, et al. Triple therapy with aspirin, prasugrel, and vitamin K antagonists in patients with drug‐eluting stent implantation and an indication for oral anticoagulation. J Am Coll Cardiol. 2013;61:2060–2066. [DOI] [PubMed] [Google Scholar]
- 21. Jackson LR 2nd, Ju C, Zettler M, et al. Outcomes of patients with acute myocardial infarction undergoing percutaneous coronary intervention receiving an oral anticoagulant and dual antiplatelet therapy: a comparison of clopidogrel versus prasugrel from the TRANSLATE‐ACS Study. JACC Cardiovasc Interv. 2015;8:1880–1889. [DOI] [PubMed] [Google Scholar]
- 22. Fu A, Singh K, Abunassar J, et al; CAPITAL Investigators . Ticagrelor in triple antithrombotic therapy: predictors of ischemic and bleeding complications. Clin Cardiol. 2016;39:19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ancedy Y, Lecoq C, Saint Etienne C, et al. Antithrombotic management in patients with atrial fibrillation undergoing coronary stent implantation: what is the impact of guideline adherence? Int J Cardiol. 2016;203:987–994. [DOI] [PubMed] [Google Scholar]
- 24. Dewilde WJ, Oirbans T, Verheugt FW, et al; WOEST Study Investigators . Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open‐label, randomised, controlled trial. Lancet. 2013;381:1107–1115. [DOI] [PubMed] [Google Scholar]
- 25. Angiolillo DJ, Goodman SG, Bhatt DL, et al. Antithrombotic therapy in patients with atrial fibrillation undergoing percutaneous coronary intervention: a North American Perspective–2016 Update. Circ Cardiovasc Interv. 2016;9:e004395. [DOI] [PubMed] [Google Scholar]
- 26. Andrade JG, Macle L, Nattel S, et al. Contemporary atrial fibrillation management: a comparison of the current AHA/ACC/HRS, CCS, and ESC Guidelines. Can J Cardiol. 2017;33:965–976. [DOI] [PubMed] [Google Scholar]


