Abstract
Background
Low QRS voltage has been shown to be associated with increased mortality in the general population and in a small pilot study the combined QRS voltage of ECG leads I and II was found to be associated with in‐hospital mortality.
Hypothesis
Confirm that low QRS voltage predicts the in‐hospital mortality of acutely ill patients, and compare QRS voltage with other predictors of mortality that can be easily, quickly and cheaply obtained at the bedside.
Methods
Prospective observational study of vital signs, QRS voltage and simple tools used to assess mental, functional and nutritional status at the bedside in unselected acutely ill patients admitted to a resource‐poor hospital in sub‐Saharan Africa.
Results
Out of 1486 patients, 77 died (5.2%) in hospital. A combined lead I + II voltage <1.8 mV was present in 789 (53.1%) of patients, and significantly associated with in‐hospital mortality (odds ratio 3.6, 95% CI 2.0‐6.5, χ 2 21.2, P < 0.00001). On logistic regression impaired mobility, the National Early Warning Score, male gender and lead I + II voltage were the only independent predictors of mortality. None of the 445 patients who were mobile on admission with a lead I + II voltage ≥ 1.8 mV died in hospital.
Conclusions
Low QRS voltage, male gender, NEWS, and impaired mobility were independent predictors of in‐hospital mortality in the study population. These four variables, which are easily obtained at the bedside, could potentially provide a rapid, easy, and cheap risk stratification system.
Keywords: acute medicine, early warning scores, ECG, electrocardiogram, QRS voltage, risk stratification, vital signs
1. INTRODUCTION
Although electrocardiogram (ECG) changes are of great diagnostic value and have been used to predict the outcome of cardiac conditions,1, 2, 3, 4 there have only been a few reports of their prognostic value in acutely ill medical patients.5 In a cohort of nearly 10 000 acutely ill patients admitted to an Irish hospital an abnormal ECG was a powerful predictor of in‐hospital mortality: only four of 4177 acutely ill patients with a normal ECG died within 24 hours.6 This observation is of limited value in emergency situations in low‐resource settings, as it requires the immediate availability of a clinician capable of reading a 12 lead ECG, or an ECG machine with well validated interpretation software. ECG dispersion mapping provides a prognostic information that is easy to interpret, but requires specialized equipment.7 There are, however, ECG measurements that nearly all modern machines generate automatically (eg, heart rate, axis, and PR, QRS, QTc, intervals) that can be easily interpreted: QRS voltage amplitude is also a simple measure that is easy to do.
There are multiple causes of low QRS voltage on the ECG, and often it is unexplained and considered to be a normal variant. In addition to cardiac and pericardial disease low QRS voltage has also been observed in many non‐cardiac conditions such as pleural effusions, emphysema, pulmonary infiltrations, and hypothyroidism.8 In a study of 6440 individuals free of cardiovascular disease low QRS voltage was found in 1.4%, and was more common in the elderly, in women, non‐Hispanic blacks and those with pulmonary disease, and malignancies: during a median follow‐up of 14.1 years those with low QRS voltage were twice as likely to die.9
QRS amplitude can vary in the leads V1 to V6 because of artifact and errors in chest lead placement. Therefore, the combined amplitude of limb lead I and II has been recommended as the preferred method of measuring and monitoring QRS voltage.10, 11 We recently reported the results of a small pilot study that showed an association between the combined amplitudes of the QRS complexes of ECG lead I and II and in‐hospital mortality.12 As far as we know this was the first report of low QRS voltage in unselected acutely ill medical patients. In this larger study of 1486 patients we confirmed that low QRS voltage predicts the in‐hospital mortality of acutely ill patients, and compared it to other predictors, all of which can be easily, quickly, and cheaply obtained at the bedside.
2. METHODS
2.1. Study design
Observational study carried out as part of an audit in an ongoing quality improvement project.
2.2. Setting
The 46 bed medical ward of St. Joseph's Kitovu Health Care Complex, 220 bedded healthcare facility located near Masaka, Uganda, 140 km from the capital city of Kampala. Together with the 330 bed Masaka Regional Referral Government Hospital, it serves Masaka Municipality (population of 79 200), and Masaka District with a rural population of 804 300. The hospital has no intensive care or renal dialysis unit, and cannot provide artificial ventilation.
From August 10, 2016 to January 15, 2018 two nurses, employed 12 hours per day for 7 days a week, entered each patient's clinical status and vital signs twice daily into a clinical data management and decision support system (Rapid Electronic Assessment Data System [READS], Tapa Healthcare DAC). In addition an ECG was performed on as many patients as possible within 12 hours of admission to hospital using a reusable ECG Belt and a portable mobile ECG device (LevMed Mobile ECG Kit, LevMed Ltd., Tilburg, Netherlands). The amplitude of lead I and II in all ECGs was measured from the lowest negative deflection (ie, the Q wave) to the highest positive deflection (ie, the R wave), regardless of the ECG baseline. All readings were made by JK without knowledge of the patient's identity, clinical condition or outcome. ECG axis, PR, QRS, QT, and QTc intervals were all provided automatically by the ECG device software.
Vital signs were entered into READS at the bedside immediately after their measurement; all data entries were automatically time and date stamped. Patient disposition (ie, discharge or death) was also subsequently recorded into READS. All the READS data sets were complete. The National Early Warning Score (NEWS),13 a well validated predictor of imminent mortality,14 was calculated from the heart rate, respiratory rate, systolic blood pressure, level of consciousness,15 temperature, oxygen saturations, inspired oxygen entered into the READS database. Nutritional status was assessed by measuring the mid‐upper arm circumference.16 Impaired mobility on presentation, defined as lack of a stable independent gait when first assessed, was also recorded.17
2.3. Participants
All patients, aged 16 years or older, who were admitted to the medical ward.
2.4. Outcomes
All the patients who died in the hospital.
2.5. Potential predictors of in‐hospital mortality examined
The variables examined were those that could be easily obtained without clinical experience, training or expense and included age, gender, length of stay, admission NEWS, and all the NEWS parameters assessed on admission (ie, heart rate, respiratory rate, temperature, systolic blood pressure, oxygen saturation, use of supplemental oxygen, and alertness), impaired mobility on presentation, mid‐upper arm circumference, the heart rate recorded by ECG, ECG axis, PR, QRS, QT and QTc intervals, and amplitude of Leads I and II.
2.6. Statistical methods
all calculations were performed using Epi‐Info version 6.0 (Center for Disease Control and Prevention, USA) and logistic regression analysis using Logistic software.18 The P value for statistical significance was 0.05 and was tested using Student's t‐test and χ 2 analysis that applied Yates continuity correction. The value with the highest χ 2 was used as the “cut‐off” to convert a continuous variable into a categorical variable.
2.7. Ethical approval
Ethical approval of the study was obtained from the Ethics Committee Kitovu Hospital, which conformed to the principles outlined in the Declaration of Helsinki.19 Since no interventions were additional to the usual standard of care the need for written consent was waived. The study is reported in accordance with the STROBE statement.20
3. RESULTS
3.1. Participants
During the study period 2110 eligible patients were admitted to the hospital: 1486 (70%) had an ECG performed, 664 (45%) were men, and 77 (5.2%) died in hospital. Patients who did not have an ECG performed were older (52.9 SD 23.1 vs 49.6 SD 21.8 years, P = 0.002), had a higher NEWS on admission (4.4 SD 3.3 vs 3.5 SD 2.9, P < 0.0001), the same length of stay (73.6 SD 60.7 vs 77.3 SD 56.3 hours, P = 0.18), and a higher in‐hospital mortality (ie, 10.6% vs 5.3%, P < 0.0001).
3.2. Mortality outcomes associated with continuous variables
Patients who died in hospital were the same age as survivors, but had a longer length of hospital stay, and on admission had a higher heart rate, temperature, respiratory rate and NEWS, and a lower systolic blood pressure, oxygen saturation, and mid‐upper arm circumference (Table 1). Patients who died had a longer QTc interval, and lower amplitude of lead I, lead II, and combined amplitude of lead I plus lead II. There was no difference in ECG axis, PR, QRS, and QT intervals between survivors and decedents (Table 1).
Table 1.
Continuous variables in survivors and decedents
| Variable | Survived to discharge (n = 1409) | Died in hospital (n = 77) | P |
|---|---|---|---|
| Age (y) | 49.4 SD 21.7 | 53.2 SD 21.9 | 0.13 |
| Length of stay (h) | 76.4 SD 54.4 | 93.0 SD 82.6 | 0.01 |
| NEWS on admission | 3.3 SD 2.7 | 7.3 SD 3.3 | <0.000001 |
| Heart rate on admission (bpm) | 85.7 SD 17.4 | 97.2 SD 24.7 | <0.000001 |
| Temperature on admission (°C) | 36.8 SD 0.8 | 37.0 SD 1.9 | 0.007 |
| Respiratory rate on admission (bpm) | 21.3 SD 5.3 | 26.9 SD 8.7 | <0.000001 |
| Systolic blood pressure on admission (mmHg) | 114.5 SD 24.0 | 108.0 SD 30.9 | 0.02 |
| Oxygen saturation (%) | 95.6 SD 5.0 | 90.3 SD 11.7 | <0.000001 |
| Mid‐upper arm circumference (cm) | 26.1 SD 4.3 | 23.6 SD 3.4 | <0.000001 |
| PR interval (ms) | 160.6 SD 32.6 | 160.4 SD 33.2 | 0.96 |
| QRS interval (ms) | 94.0 SD 18.1 | 94.2 SD 20.8 | 0.90 |
| QT interval (ms) | 380.5 SD 50.1 | 371.8 SD 70.0 | 0.15 |
| QTc interval (ms) | 446.9 SD 46.4 | 466.0 SD 56.6 | 0.0006 |
| Axis (°) | 36.2 SD 37.7 | 34.5 SD 38.8 | 0.70 |
| Lead I amplitude (mV) | 0.77 SD 0.34 | 0.59 SD 0.31 | 0.000005 |
| Lead II amplitude (mV) | 0.99 SD 0.42 | 0.80 SD 0.51 | 0.0004 |
| Lead I + II amplitude (mV) | 1.76 SD 0.63 | 1.39 SD 0.74 | 0.000001 |
Abbreviation: NEWS, National Early Warning Score.
3.3. Mortality outcomes associated with categorical variables
Patients who were alert on admission and/or did not require supplemental oxygen were less likely to die, and patients with impaired mobility on presentation as well as men were more likely to die. A mid‐upper arm circumference less than 20 cm, a combined voltage of lead I + lead II < 1.8 mV and a QTc interval > 450 ms were also associated with in‐hospital mortality (Table 2). A reduced lead I + II amplitude and an increased QTc interval were both more prevalent in older patients, whereas in‐hospital mortality only increased slightly in the elderly (Figure 1). Patients with a mid‐upper arm circumference < 20 cm were older (56.1 SD 22.7 vs 49.2 SD 21.7 years, P = 0.009), and also more likely to have a lead I + II amplitude < 1.8 mV (odds ratio 3.54, 95% CI 1.92‐6.63, Chi‐square 19.57, P = 0.00001).
Table 2.
Categorical variables available at the bedside: The cut‐offs for lead I + II voltage, QTc interval and mid‐upper arm circumference were those values with the highest χ 2
| Variable | Number (%) | In‐hospital mortality (%) | Odds ratio | 95% CI | χ 2 | P |
|---|---|---|---|---|---|---|
| Impaired mobility on presentation | 656 (44.1%) | 68 (10.4%) | 10.55 | (5.01‐22.99) | 62.37 | <0.00001 |
| Alert | 1424 (95.8%) | 60 (4.2%) | 0.12 | (0.06‐0.23) | 60.48 | <0.00001 |
| On room air only | 1427 (96.0%) | 62 (4.3%) | 0.13 | (0.07‐0.27) | 47.00 | <0.00001 |
| Lead I + II voltage < 1.8 mV | 789 (53.1%) | 61 (7.7%) | 3.57 | (1.97‐6.54) | 21.16 | <0.00001 |
| QTc interval > 450 ms | 611 (41.1%) | 48 (7.9%) | 2.48 | (1.50‐4.11) | 14.07 | 0.0002 |
| Mid‐upper arm circumference (cm) | 72 (4.8%) | 11 (15.3%) | 3.68 | (1.73‐7.68) | 13.61 | 0.0002 |
| Male gender | 664 (44.7%) | 50 (7.5%) | 2.40 | (1.44‐4.00) | 12.6 | 0.0004 |
| Total | 1486 (100.0%) | 77 (5.2%) | — | — | — | — |
Figure 1.
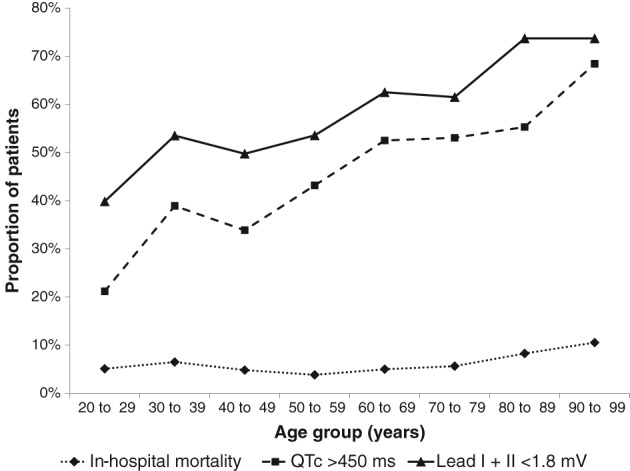
Prevalence of combined voltage lead I + II < 1.8 mV, QTc > 450 ms, and in‐hospital mortality according to age group
3.4. Independence of mortality predictors
All the mortality predictors were tested for independence by logistic regression.
NEWS eliminated all its component parameters as predictors of mortality, including mental alertness and the use of supplemental oxygen. Therefore, the only independent predictors identified were male gender, impaired mobility, NEWS, and the combined amplitude of lead I + lead II (Table 3).
Table 3.
Logistic regression: Only four variables were independent predictors of in‐hospital mortality
| Variable | Coefficient | Odd ratio | (95%CI) | P |
|---|---|---|---|---|
| Constant | −7.0469 SE 0.5165 | — | — | <0.00001 |
| Impaired mobility on presentation | 1.8935 SE 0.3732 | 6.64 | (3.20‐13.80) | <0.00001 |
| NEWS on admission | 0.3286 SE 0.0410 | 1.39 | (1.28‐1.51) | <0.00001 |
| Lead I + II < 1.8 mV | 0.8279 SE 0.3098 | 2.29 | (1.25‐4.20) | 0.0075 |
| Male gender | 1.0556 SE 0.2694 | 2.87 | (1.69‐4.87) | 0.0001 |
Abbreviation: NEWS, National Early Warning Score.
3.5. Clinical relevance
All patients were stratified according to their NEWS and mobility on admission, and combined amplitude of lead I + II (Figure 2). A NEWS “cut‐off” ≥3 was selected as NEWS awards 3 points to altered level of consciousness. The highest mortality was for non‐alert patients regardless of their ECG findings. The alert immobile patients with NEWS ≥ 3 who had a lead I + II voltage < 1.8 mV were three times more likely to die than those with a voltage ≥ 1.8 mV. Out of the 111 immobile patients who had a NEWS < 3 and a combined amplitude ≥ 1.8 mV two died, both from cryptococcal meningitis, one 11 days and the other 14 days after admission. None of the 445 patients who had a stable independent gait on admission and combined amplitude of lead I and II ≥ 1.8 mV died in hospital.
Figure 2.
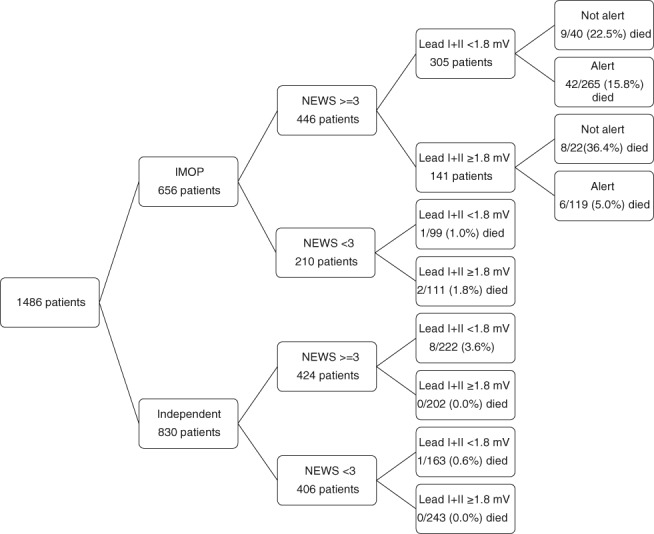
Risk stratification of patients according to mobility, NEWS, QRS voltage, and alertness
4. DISCUSSION
4.1. Major findings
This study found that low QRS voltage, along with male gender, NEWS and impaired mobility, are independent predictors of in‐hospital mortality. If confirmed, these findings could be used to provide a rapid and easy risk stratification system that only requires the patients' mobility, vital signs, and an easily identified ECG finding. QRS voltage may be particularly useful in recognizing mobile patients who are at risk.
Our results confirm that mobility is a powerful predictor of mortality.21 We cannot explain why men were more than twice as likely to die as women: apart from men being younger (47.5 SD 22.1 vs 51.3 SD 21.4 years, P = 0.001) there were no other differences between the sexes (ie, mental status, mobility, NEWS etc were the same).
Low QRS voltage was present in more than half the patients, and was more common in older patients. In this population there was no difference in the ages of those who died in hospital and survivors and, unlike the prevalence of low QRS amplitude there was no significant increase in mortality with age. This suggests that in the study population low QRS voltage reflects the reduced physiological reserve associated with sickness in the elderly better than chronological age.
The mechanisms that determine QRS amplitude are hotly debated. Madias has written extensively on the topic and believes that it is greatly influenced by water content of the organs and tissues surrounding heart,22 and changes in leads I + II are the best way to detect this.10 When water content is increased, electrical resistance is also increased and the QRS voltage will be lowered. Conversely, if the water content is decreased, resistance will fall and the QRS amplitude will rise. This explains why low QRS voltage has been reported in patients with peripheral edema from any cause, including cor pulmonale, perioperative fluid load administration, chronic renal failure, congestive heart failure, and hepatic cirrhosis. Patients with kwashiorkor or hypoalbuminemia from any cause invariably have edema and a low QRS voltage,8 and we also found a low QRS voltage was strongly associated with poor nutrition as reflected by a low mid‐upper arm circumference.
Many of our patients had pneumonia and sepsis23: changes in QRS voltage have been reported in sepsis24 and, although not noted by the authors, changes in lead I and II amplitude during the course of pneumonia can be clearly seen in the ECGs displayed in two published reports of ECG changes in pneumonia.25, 26 Sepsis mortality in the developed world has steadily declined,27 possibly as a result of protocols that promote resuscitation with intravenous fluids.28, 29 However, trials of fluid resuscitation in resource‐limited environments have given conflicting results30: a possible explanation may be that a reliable and practical method of assessing fluid status is not available in these settings, so that some patients may have been overloaded and others under‐loaded with fluid. Changes in QRS amplitude have been used to monitor the response to diuretics in heart failure, renal failure and other conditions11: it is possible that these changes might also be used to guide fluid resuscitation in septic patients. It is probable that patients with low QRS voltages have an increased water content of the organs and tissues surrounding heart, so that fluid resuscitation in these patients should be cautious. Conversely patients with normal QRS voltage are more likely to be hypovolemic and to benefit from rapidly administered intravenous fluids.
4.2. Limitations
Since this study examined a relatively young population of patients in a low‐resource setting in sub‐Saharan Africa, its results may not be applicable elsewhere. Moreover, it is possible that in a larger study more variables, such as prolonged QTc, might have been found to be independent predictors of mortality. Although we tried to perform an ECG on every patient admitted, ECGs were not performed on 30% of them: these patients were sicker and almost twice as likely to die, and many had died before there was an opportunity to perform an ECG. The other major weakness of this study was that all the ECGs were read by only one person, so that inter‐rater reliability could not be tested. However, measuring the amplitude of lead I and II is easy, and not open to subjective interpretation. Moreover, the reusable belt and ECG system used prevented wrong lead placement.31
5. CONCLUSION
Our findings suggest that QRS amplitude could be used as part of a simple risk stratification system, which may be particularly useful in recognizing mobile patients with normal vital signs who are at risk. In addition to risk stratification, low QRS voltage may have other useful clinical applications. It may reflect reduced physiological reserve better than chronological age, and should signal the need for further diagnostic testing, especially for cardiac and pericardial disease. Serial measurements might also indicate fluid status and be useful for monitoring intravenous fluid administration.
ACKNOWLEDGMENTS
The authors wish to acknowledge and thank LevMed Ltd. (www.levmed.net) who provided the LevMed Reusable ECG Belt and LevMed Mobile ECG Kit at no charge, and Tapa Healthcare DAC (Dundalk, Ireland) for the complimentary use of their Rapid Electronic Assessment Data System (READS). None of these sponsors played any part in the design or execution of the study, and all other costs were borne by the authors.
All costs were borne by the authors. John Kellett is a major shareholder, director, and chief medical officer of Tapa Healthcare DAC.
Author's contributions
Martin Opio and John Kellett designed the study and analyzed and interpreted the data. John Kellett read all the ECGS. Patient data was collected and ECG performed as part of routine care by the other members of the Kitovu Hospital Study Group.
Conflict of interest
The authors declare no potential conflict of interests.
Kellett J, Opio MO, Kitovu Hospital Study Group :. QRS voltage is a predictor of in‐hospital mortality of acutely ill medical patients. Clin Cardiol. 2018;41:1069–1074. 10.1002/clc.23030
REFERENCES
- 1. De Bacquer D, De Backer G, Kornitzer M, Blackburn H. Prognostic value of ECG findings for total, cardiovascular disease, and coronary heart disease death in men and women. Heart. 1998;80:570‐577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Greenland P, Xie X, Liu K, et al. Impact of minor electrocardiographic ST segment and/or T‐wave abnormalities on cardiovascular mortality during long‐term follow‐up. Am J Cardiol. 2003;91:1068‐1074. [DOI] [PubMed] [Google Scholar]
- 3. Auer R, Bauer DC, Marques‐Vidal P, et al. Health ABC StudyAssociation of major and minor ECG abnormalities with coronary heart disease events. JAMA. 2012;307:1497‐1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Groot A, Bots ML, Rutten FH, den Ruijter HM, Numans ME, Vaartjes I. Measurement of ECG abnormalities and cardiovascular risk classification: a cohort study of primary care patients in the Netherlands. Br J Gen Pract. 2015;65:e1‐e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tan SY, Sungar GW, Myers J, Sandri M, Froelicher V. A simplified clinical electrocardiogram score for the prediction of cardiovascular mortality. Clin Cardiol. 2009;32:82‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kellett J, Deane B. The simple clinical score predicts mortality for days after admission to an acute medical unit. Q J Med. 2006;99:771‐781. [DOI] [PubMed] [Google Scholar]
- 7. Kellett J, Clifford M. The prediction of death up to 100 days after admission to hospital for acute medical illness ‐ the comparison of two ECG interpretation methods with ECG‐dispersion mapping. Acute Med. 2015;14(4):151‐158. [PubMed] [Google Scholar]
- 8. Madias JE. Low QRS voltage and its causes. J Electrocardiol. 2008;41:498‐500. [DOI] [PubMed] [Google Scholar]
- 9. Usoro AO, Bradford N, Shah AJ, Soliman EZ. Risk of mortality in individuals with low QRS voltage and free of cardiovascular disease. Am J Cardiol. 2014;113:1514‐1517. [DOI] [PubMed] [Google Scholar]
- 10. Madias JE. Superiority of the limb leads over the precordial leads on the 12‐lead ECG in monitoring fluctuating fluid overload in a patient with congestive heart failure. J Electrocardiol. 2007;40:395‐399. [DOI] [PubMed] [Google Scholar]
- 11. Lumlertgul S, Chenthanakij B, Madias JE. ECG leads I and II to evaluate diuresis of patients with congestive heart failure admitted to the hospital via the emergency department. PACE. 2009;32:64‐71. [DOI] [PubMed] [Google Scholar]
- 12. Opio MO, Kellett J, Kitovu Hospital Study Group . The association between a simple measure of QRS voltage and the in‐hospital mortality of acutely ill medical patients. Eur J Intern Med. 2017;39:e9. [DOI] [PubMed] [Google Scholar]
- 13. Royal College of Physicians . National EarlyWarning Score (NEWS): Standardising the Assessment of Acute Illness Severity in the NHS. Report of a Working Party. London: RCP; 2012. www.rcplondon.ac.uk/resources/nationalearlywarningscore-news. [Google Scholar]
- 14. Smith GB, Prytherch DR, Meredith P, Schmidt PE, Featherstone PI. The ability of the National Early Warning Score (NEWS) to discriminate patients at risk of early cardiac arrest, unanticipated intensive care unit admission, and death. Resuscitation. 2013;84:465‐470. [DOI] [PubMed] [Google Scholar]
- 15. McNarry AF, Goldhill DR. Simple bedside assessment of level of consciousness: comparison of two simple assessment scales with the Glasgow coma scale. Anaesthesia. 2004;59:34‐37. [DOI] [PubMed] [Google Scholar]
- 16. Opio MO, Namujwiga T, Nakitende I, Kellett J, Brabrand M, On behalf of the Kitovu hospital study group . The prediction of in‐hospital mortality by mid‐upper arm circumference: a prospective observational study of the association between mid‐upper arm circumference and the outcome of acutely ill medical patients admitted to a resource‐poor hospital in sub‐Saharan Africa. Clin Med. 2018;18:123‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kellett J, Clifford M, Ridley A, Murray A, Gleeson M. A four item scale based on gait for the immediate global assessment of acutely ill medical patients – one look is more than 1000 words. Eur Geriatr Med. 2014;5:92‐96. [Google Scholar]
- 18. Dallal GE. LOGISTIC: a logistic regression program for the IBM PC. Am Stat. 1988;42:272. [Google Scholar]
- 19. World Medical Association . World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191‐2194. [DOI] [PubMed] [Google Scholar]
- 20. Vandenbroucke JP, von Elm E, Altman DG, et al. for the STROBE InitiativeStrengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLoS Med. 2007;4(10):e297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brabrand M, Kellett J, Opio M, Cooksley T, Nickel CH. Should impaired mobility on presentation be a vital sign? Acta Anaesthesiol Scand. 2018;62:945‐952. 10.1111/aas.13098. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 22. Madias JE. On the mechanism of augmentation of electrocardiogram QRS complexes in patients with congestive heart failure responding to dieresis. J Electrocardiol. 2005;38:54‐57. [DOI] [PubMed] [Google Scholar]
- 23. Nabayigga B, Kellett J, Opio MO. The alertness, gait and mortality of severely ill patients at two months after admission to a resource poor sub‐Saharan hospital—why is post‐discharge surveillance not routine everywhere? Eur J Intern Med. 2016;28:25‐31. [DOI] [PubMed] [Google Scholar]
- 24. Madias JE, Bazaz R, Agarwal H, Win M, Medepalli L. Anasarca‐mediated attenuation of the amplitude of electrocardiogram complexes: a description of a heretofore unrecognized phenomenon. J Am Coll Cardiol. 2001;38:756‐764. [DOI] [PubMed] [Google Scholar]
- 25. Stein PD, Matta F, Ekkah M, et al. Electrocardiogram in pneumonia. Am J Cardiol. 2012;110:1836‐1840. [DOI] [PubMed] [Google Scholar]
- 26. Master AM, Romanoff A, Jaffe H. Electrocardiographic changes in pneumonia. Am Heart J. 1931;6:696‐709. [Google Scholar]
- 27. Kaukonen K‐M, Bailey M, Suzuki S, Pilcher D, Bellomo R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000‐2012. JAMA. 2014;311(13):1308‐1316. [DOI] [PubMed] [Google Scholar]
- 28. Rivers E, Nguyen B, Havstad S, et al. Early Goal‐Directed Therapy Collaborative Group; For the early goal‐directed therapy collaborative group. Early goal‐directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368‐1377. [DOI] [PubMed] [Google Scholar]
- 29. Levy MM, Rhodes A, Phillips GS, et al. Surviving sepsis campaign: association between performance metrics and outcomes in a 7.5‐year study. Crit Care Med. 2015;43(1):3‐12. [DOI] [PubMed] [Google Scholar]
- 30. Andrews B, Semler MW, Muchemwa L, et al. Effect of an early resuscitation protocol on in‐hospital mortality among adults with sepsis and hypotension. A randomized clinical trial. JAMA. 2017;318(13):1233‐1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Opio MO, Kellett J, Kitovu Hospital Study Group . An observational study of the quality of ECGs recorded by inexperienced staff in a resource‐poor African hospital using a reusable ECG belt linked to an internet ECG device. Eur J Intern Med. 2017;42:e17‐e18. [DOI] [PubMed] [Google Scholar]


