Summary
Aims
Social isolation increases the onset of Alzheimer's disease (AD). Environmental enrichment, a complicated social and physical construct, plays beneficial effects on brain plasticity and function. This study was designed to determine whether physical enrichment can reduce the deleterious consequences of social isolation on the onset of AD.
Methods
One‐month‐old APPswe/PS1dE9 transgenic AD model mice were singly housed in the enriched physical environment for 8 weeks and then received behavioral tests, neuropathological analyses, and Western blot of the hippocampus.
Results
The enriched physical environment reversed spatial cognitive decline of socially isolated APPswe/PS1dE9 mice. The functional reversal was associated with decreases in cellular apoptosis, synaptic protein loss, inflammation, and glial activation in the hippocampus, without changes in amyloid β neuropathology.
Conclusion
These results suggest that the enriched physical environment may serve as a nonpharmacological intervention for delaying the onset of AD accompanied with social isolation.
Keywords: Alzheimer's disease, amyloid β, cognitive dysfunction, environmental enrichment, hippocampus, neuroinflammation, social isolation
1. INTRODUCTION
Alzheimer's disease (AD) is the most common form of dementia.1 Despite extensive investigation into potential therapies for AD, no effective pharmacological treatment is currently available.2 Nonpharmacological intervention strategy may be a pragmatic approach to the treatment of dementia.3, 4 To achieve this, one needs to identify factors that aggravate or mitigate the onset of AD.
Social isolation (SI) is defined as a state lacking communication with family, acquaintances or friends, and/or willfully avoiding any contact with others.5 SI is a known risk factor of AD that exacerbates cognitive impairments.6, 7, 8, 9 Avoiding SI by participating various social tasks might be therapeutic that activate the brain and slow the progress of dementia.10, 11, 12
Enriched environment (EE) has been proved consistently that reduce memory decline in aged animals via improving hippocampal plasticity and neurogenesis.13, 14, 15 Despite variable outcomes of amyloid β (Aβ) plaque deposition, EE has been shown to mitigate hippocampal‐dependent spatial memory impairment in AD mouse models.16, 17, 18, 19, 20, 21 The experimental paradigm of EE includes housing more animals in a large cage to increase their social interactions and providing physical conditions to facilitate sensory, cognitive, and motor activities.22
In this designed study, we aimed to distinguish the beneficial effects on AD mice whether from physical or social aspect of EE. We also determined whether the enriched physical environment along is sufficient to slow the AD progression under SI condition. To address these issues, one‐month‐old APPswe/PS1dE9 transgenic (APP/PS1) mice, the most extensively used AD model,23 were used in this study. APP/PS1 mice were housed in designed environments for 8 weeks and then compared with the behavioral outcomes, neuropathology, and molecular changes in the hippocampus that affected by the conditions.
2. MATERIALS AND METHODS
2.1. Animal groups
On postnatal day 28, weaned, male APP/PS1 mice from each litter were randomly assigned to four groups: group housing in a standard environment (GR+SE), socially isolated housing in a standard environment (SI+SE), group housing in an enriched environment (GR+EE), and socially isolated housing in an enriched environment (SI+EE). Mice in GR+EE and SI+EE groups were raised in large plastic cages (length: 47 cm; width: 30 cm; height: 23 cm), with a variety of contents, such as toys, wooden houses, tunnels, ladders, running wheels, nesting materials.15 The items were renewed and rearranged per 3‐4 days for ensuring the novelty and spatial variability. Animals in the GR+SE and SI+SE groups were reared in standard plastic cages (length: 31 cm; width: 22 cm; height: 15 cm) without any exposure to stimulation. Four mice in each cage in GR+SE or GR+SE group, while only one mouse per cage was housed in SI+SE or SI+EE group. Mice in each group were maintained in their respective experimental conditions until behavioral experiments were performed 8 weeks later.
Mice were maintained at a constant room temperature (18‐22°C), with a controlled illumination (12:12‐h light/dark cycle), relative humidity of 30%‐50%, and food and water were available ad libitum. All animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Nanjing Medical University.
2.2. Y‐maze test
The Y‐maze test contains two 5‐min stages, training stage and testing stage, with an interval of 2 hours.24 During the first stage, one arm named as the novel arm was blocked by a black baffle, and mouse moved only in the other two arms for 5 minutes. During the second stage, the novel arm was opened, and the mouse could freely move throughout all 3 arms for 5 minutes. The percentage of time spent in the novel arm and the numbers of entries were calculated.
2.3. Morris water maze (MWM)
As previously described,25 the MWM training was conducted over 5 consecutive days, with 4 trials per day. The escape latency and swimming speed were analyzed. On the 6th day, the hidden platform was removed, allowing mice to swim in the pool for 60 s. The percentage of total time spent in the target quadrant and the platform crossing times were counted.
The above activities of mice were captured by a digital video camera connected to a computer‐controlled system (Beijing Sunny Instruments Co. Ltd, Beijing, China). All tests were performed by two independent experimenters who were each blinded to the experimental schedule.
2.4. Section preparation
Following deep anesthesia, mice were transcardially perfused with 0.9% saline by perfusion pump for 5 minutes, followed by 4% paraformaldehyde for 15 minutes. Brains were removed, postfixed overnight at 4°C, and embedded in paraffin following standard procedures. Coronal brain sections containing the hippocampus were serially cut at 5 μm, using a paraffin slicing machine, for immunohistochemistry, Thioflavin‐S staining or Congo red staining.
2.5. Immunohistochemistry
Immunohistochemical staining was performed as previously described.25 Brain sections were incubated with one of the following primary antibodies: rabbit anti‐6E10 (1:1000; Covance, Denver, PA, USA), rabbit anticaspase‐3 (1:1000; CST, Danvers, MA, USA), mouse antiglial fibrillary acidic protein (GFAP) (1:1000; Millipore, Bedford, MA, USA), rabbit anti‐ionized calcium‐binding adaptor molecule 1 (Iba‐1) (1:1000; Wako, Osaka, Japan), or rabbit anti‐NeuN (1:400; Millipore) at 4°C overnight. Following PBS rinsing, sections were incubated with biotinylated‐conjugated goat anti‐mouse or rabbit IgG (1:200, Vector Laboratories, Burlingame, CA, USA) for 1 hour at 37°C and then visualized with DAB (Sigma‐Aldrich, St. Louis, MO, USA).
2.6. Image analysis and cell counting
The images of the hippocampus were captured at × 100 magnification and analyzed using NIH ImageJ software (NIH Image, Bethesda, MD, USA). The area of positive signal for GFAP or Iba‐1 was quantified by the interest grayscale threshold analysis.25 The percentage area of positive signal was then calculated by dividing the area of positive signal to the total area in the region of interest. Caspase‐3–positive apoptotic cells and total hematoxylin‐positive cells in the pyramidal layer of CA1‐3 and the granular layer of dentate gyrus (DG) were also counted, and the results were expressed as the percentage of cellular apoptosis. The neuronal number and area of CA1‐3 pyramidal layer and DG granular cell layer on each section were measured, respectively. Total number of NeuN cells in the pyramidal layer of the CA1‐3 subregions and granule cell layer of the dentate gyrus was quantified using the formula: T = (N × V)/t, where N, V, and t are the cell density, volume of the structure, and thickness of the section, respectively.26 Five sections per mouse, and four mice per group, were averaged to provide a mean value for each group.
2.7. Western blotting
The homogenized protein samples of hippocampus were loaded into 10%‐16% Tris/tricine SDS gels and transferred to PVDF membranes using a Bio‐Rad miniprotein‐III wet transfer unit.25 After blocking for 1 hour in 5% nonfat milk/TBST, the membranes were incubated at 4°C overnight with one of the primary antibodies listed in Table 1. Horseradish peroxidase–conjugated secondary antibodies (Vector Laboratories) were used, and bands were visualized using ECL plus detection system. GAPDH was used as an internal control for protein loading and transfer efficiency.
Table 1.
Antibodies used in the Western blot
| Antibodies | MW (kDa) | Clone | Dilution | Source | Catalog number |
|---|---|---|---|---|---|
| Aβ1‐42 | 87 | Rabbit, monoclonal | 1:1000 | Abcam | ab10148 |
| ADAM10 | 82 | Rabbit, polyclonal | 1:1000 | Millipore | AB19026 |
| APP | 100 | Rabbit, polyclonal | 1:1000 | Sigma | SAB4300464 |
| ASC | 24 | Rabbit, polyclonal | 1:500 | Santa Cruz | sc‐22514‐R |
| BACE1 | 70 | Mouse, monoclonal | 1:1000 | Millipore | MAB5308 |
| Caspase 1 | 20 | Rabbit, polyclonal | 1:1000 | Millipore | 06‐503‐I |
| GAPDH | 37 | Rabbit, polyclonal | 1:800 | Bio world | BS60630 |
| IKKβ | 88 | Rabbit, polyclonal | 1:1000 | Cell Signaling | 8943s |
| IL‐1β | 17 | Rabbit, polyclonal | 1:1000 | Millipore | AB1832P |
| IL‐6 | 22 | Rabbit, polyclonal | 1:1000 | Abcam | ab83339 |
| NLRP3 | 106 | Mouse, polyclonal | 1:1000 | AdipoGen | AG‐20B‐0006 |
| p‐IKKβ | 88 | Rabbit, polyclonal | 1:1000 | Cell Signaling | 2694 |
| p‐P65 | 65 | Rabbit, polyclonal | 1:1000 | Cell Signaling | 3033s |
| P65 | 65 | Rabbit, polyclonal | 1:1000 | Cell Signaling | 8242s |
| Procaspase 1 | 46 | Rabbit, polyclonal | 1:500 | Millipore | 06‐503‐1 |
| PSD95 | 85 | Rabbit, polyclonal | 1:1000 | Abcam | ab18258 |
| Pro‐IL‐1β | 34 | Rabbit, polyclonal | 1:1000 | Millipore | AB1832P |
| PS1 | 45 | Rabbit, polyclonal | 1:1000 | Sigma | PRS4203 |
| SAPP‐α | 98 | Rabbit, monoclonal | 1:800 | IBL | 11088 |
| SYP | 70 | Rabbit, polyclonal | 1:500 | Abcam | ab64581 |
| TNF‐α | 17 | Rabbit, polyclonal | 1:1000 | Abcam | ab9739 |
| 6E10 | 4‐100 | Mouse, monoclonal | 1:1000 | Convance | SIG‐39320‐200 |
2.8. Statistical analysis
Data were expressed as mean ± SEM. Statistical analyses were conducted using SPSS software, version 19.0 (SPSS Inc., Chicago, IL, USA). The MWM platform training data were analyzed by repeated‐measures ANOVA with day of training as the within‐subject variable, and housing (group housing vs isolated housing) and environment (standard environment vs enriched environment) as the between‐subject factors. The other data were analyzed by two‐way ANOVA with housing and environment as factors, followed by Newman‐Keuls post hoc multiple comparison test. A value of P < 0.05 was considered statistically significant.
3. RESULTS
3.1. Physical EE rescued spatial cognitive deficits of socially isolated APP/PS1 mice
The hippocampus‐dependent spatial learning and memory ability were tested by the MWM and Y‐maze. Repeated‐measures ANOVA revealed that SI+SE mice had significantly prolonged escape latency compared with GR+SE mice during the 5 days of training (F 4,70 = 8.172, P = 0.017). However, treatment with EE significantly alleviated the poor spatial learning task induced by SI (F 4,70 = 12.055, P = 0.001, SI+EE mice vs SI+SE mice). Group housing in enriched environments also improved spatial learning performance (F 4,70 = 6.357, P = 0.04, GR+EE mice vs GR+SE mice) (Figure 1A). Swimming speed was not affected by housing (F 4,140 = 0.671, P = 0.426), environment (F 4,140 = 0.363, P = 0.556), or housing × environment (F 4,140 = 0.011, P = 0.917) (Figure 1B).
Figure 1.
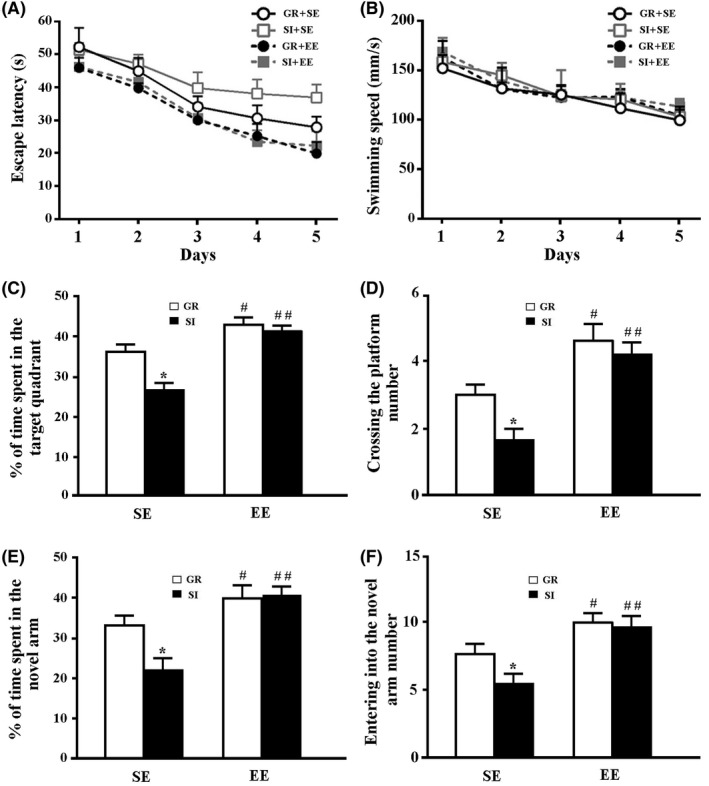
The behavioral analyses. (A) The mean escape latency during the MWM training period. (B) Swimming speed. (C) The percentage of time spent in the target quadrant in the probe test. (D) The number of platform area crossing. (E) The percentage of time spent in the novel arm in the Y‐maze test. (F) The number of entries into the novel arm. Data represent mean±SEM from 8 mice per group. Data in Figure 1A,B were analyzed by repeated‐measures ANOVA. *P < 0.05. Data in other figures were analyzed by ANOVA with post hoc Student‐Newman‐Keuls test. *P < 0.05, GR vs SI; #P < 0.05, ##P < 0.01, EE vs SE
On the 6th day, a probe trial was performed to assess the spatial memory ability. Two‐way ANOVA revealed that the percentage of time spent and numbers of platform area crossings in the target quadrant were significantly decreased by SI (F 1,28 = 8.247, P = 0.011; F 1,28 = 4.862, P = 0.043, respectively) and increased by EE (F 1,28 = 30.892, P < 0.001; F 1,28 = 26.184, P < 0.001, respectively), but were not affected by their interaction (F 1,28 = 4.34, P = 0.053; F 1,28 = 1.262, P = 0.279, respectively). The enriched physical environment arrested SI‐induced spatial memory dysfunctions, revealed by increases in the percentage of time spent and numbers of platform area crossings in the target quadrant (P = 0.0005, P = 0.0016, respectively) (Figure 1C,D).
Similarly, in the Y‐maze test, the percentage of time in the NA and numbers of NA entrances were increased by EE (F 1,28 = 6.887, P = 0.012; F 1,28 = 11.993, P = 0.001, respectively), but not affected by SI (F 1,28 = 2.094, P = 0.156; F 1,28 = 2.438, P = 0.126, respectively) or their interaction (F 1,28 = 2.131, P = 0.152; F 1,28 = 1.687, P = 0.201, respectively). Time stayed in and numbers entered into the NA increased in SI+EE mice, compared to those in SI+SE mice (P = 0.0034, P = 0.0011, respectively; Figure 1E,F).
3.2. Physical EE rescued cellular apoptosis and synaptic loss in the hippocampus of socially isolated APP/PS1 mice
We determined whether EE and/or SI affected the number of hippocampal neurons of APPswe/PS1dE9 transgenic mice. There were no differences in NeuN‐positive neurons in the CA1‐3 pyramidal layer (housing: F 1,12 = 1.25, P = 0.296; environment F 1,12 = 1.255, P = 0.295; interaction F 1,12 = 0.002, P = 0.964) and DG granular cell layer (housing: F 1,12 = 1.070, P = 0.331; environment: F 1,12 = 1.358, P = 0.277; interaction: F 1,12 = 0.275, P = 0.614) among four groups (Figure 2A,B). This result is consistent with the previous studies that apparent neuronal death occurs in the aged but not adult APP/PS1 mice.24, 27
Figure 2.
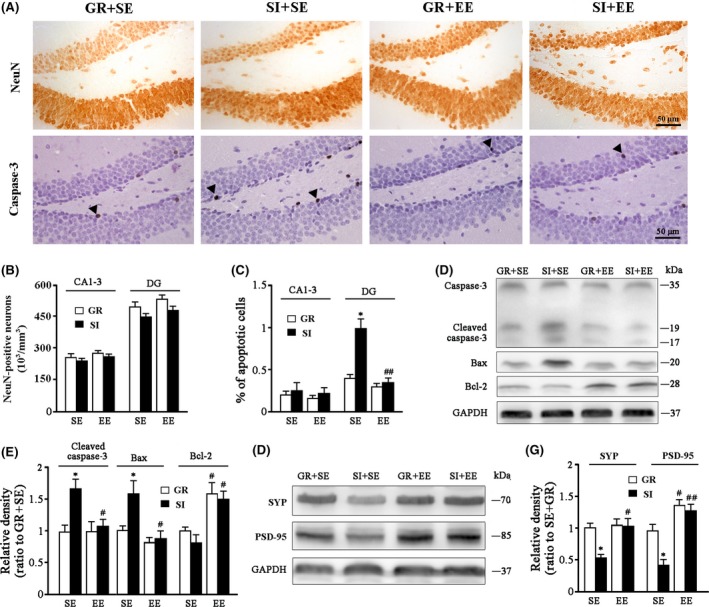
Analyses of cellular apoptosis and synaptic protein expression in the hippocampus. (A) Immunohistochemical staining for NeuN (upper panel) and caspase‐3 (lower panel) in the hippocampus. Arrowheads show caspase‐3–positive apoptotic cells. (B) NeuN‐positive neuronal count in the CA1‐3 and DG. (C) The percentage of apoptotic cells in the CA1‐3 and DG. (D,E) Representative bands of Western blot and densitometry analysis of cleaved caspase‐3, Bax, and Bcl‐2 protein levels. (F,G) Representative bands of Western blot and densitometry analysis of SYP and PSD‐95 protein levels. Data represent mean ± SEM from 4 mice per group. The statistical analysis was performed by ANOVA with post hoc Student‐Newman‐Keuls test. *P < 0.05, GR vs SI; #P < 0.05, ##P < 0.01, EE vs SE
Isolation stress exposure has been shown to cause cellular apoptosis in the hippocampus.28 Thus, we determined whether an enriched physical environment rescued hippocampal neuronal apoptosis of socially isolated APP/PS1 mice. The immunohistochemistry revealed that caspase‐3 labeled cells were mainly located at the subgranular layer of DG in each group (Figure 2A). Quantify data further demonstrated that a marked enhancement of caspase‐3 labeled cells in the DG region, but not the CA1‐3 regions of SI+SE mice (P = 0.0476; P = 0.5981 vs GR+SE mice), indicating a region‐specific feature of cellular apoptosis. The enriched physical environment rescued social isolation‐induced cellular apoptosis in the DG region (P = 0.0044, SI+EE mice vs SI+SE mice) (Figure 2C).
We also examined the protein expression levels of cleaved caspase‐3, a key activator of downstream apoptotic signal transduction pathways, in the hippocampus.29 As expected, cleaved caspase‐3 levels were increased by SI (F 1,12 = 10.13, P = 0.003), whereas decreased by EE (F 1,12 = 6.693, P = 0.013), with a significant interaction effect (F 1,12 = 7.738, P = 0.009). Compared to SI+SE mice, SI+EE mice showed low levels of cleaved caspase‐3 protein (P = 0.024; Figure 2D,E). In addition, SI increased proapoptotic Bax (F 1,12 = 8.124, P = 0.006), but decreased antiapoptotic Bcl‐2 expression (F 1,12 = 5.464, P = 0.035), while EE decreased Bax (F 1,12 = 15.708, P < 0.001), but increased Bcl‐2 expression (F 1,12 = 22.003, P < 0.001), and no significant interaction effects were observed (F 1,12 = 0.559, P = 0.466; F 1,12 = 0.192, P = 0.673, respectively). SI+SE mice showed low levels of Bax and high levels of Bcl‐2 when compared to SI+EE mice (P = 0.0192; P = 0.0137, respectively; Figure 2D,E).
Our previous study showed that SI exacerbates loss of synapse in the hippocampus, contributing to the severe cognitive declines of isolated aged APP/PS1 mice.24 To address whether the enriched physical environment rescues synaptic loss of socially isolated young APP/PS1 mice, we examined expression levels of presynaptic vesicle protein synaptophysin (SYP) and postsynaptic density protein‐95 (PSD‐95) in the hippocampus among different experimental groups. EE increased (F 1,12 = 6.432, P = 0.015; F 1,12 = 42.03, P < 0.001, respectively), whereas SI decreased SYP and PSD‐95 levels (F 1,12 = 5.381, P = 0.028; F 1,12 = 9.878, P = 0.004, respectively), without significant interaction effects (F 1,12 = 4.225, P = 0.072; F 1,12 = 4.465, P = 0.067). SI+EE mice showed high levels of SYP and PSD‐95 compared to SI+SE mice (P = 0.0134; P = 0.0038, respectively; Figure 2F,G).
3.3. Neither physical EE nor SI affected APP processing in the hippocampus of APP/PS1 mice
APP/PS1 mouse model begins to Aβ plaque deposition by 5‐6 months of age.30 In agreement with this, neither Congo red‐positive plaques nor Thioflavin‐S–positive dense core plaques were observed in the hippocampus of 3‐month‐old APP/PS1 mice in each group (Figure 3A). To further evaluate the effect of different environmental exposures on APP production and processing of APP/PS1 mice, the hippocampal expression levels of APP and its nonamyloidogenic peptide soluble amyloid precursor protein α (sAPPα) and amyloidogenic peptide Aβ1‐42 were determined by Western blotting. The two‐way ANOVA revealed no significant effects of housing, environment or their interaction on levels of APP (F 1,12 = 0.27, P = 0.617; F 1,12 = 0.021, P = 0.889; F 1,12 = 0.000, P = 0.988, respectively), sAPPα (F 1,12 = 0.273, P = 0.615; F 1,12 = 0.263, P = 0.622; F 1,12 = 0.000, P = 0.988, respectively) and Aβ1‐42 (F 1,12 = 0.42, P = 0.531; F 1,12 = 0.000, P = 0.988; F 1,12 = 0.016, P = 0.904, respectively; Figure 3B,D). Subsequently, APP hydrolases, including two amyloidogenic enzymes, presenilin 1 (PS1) and β‐site amyloid precursor protein‐cleaving enzyme 1 (BACE1), and a nonamyloidogenic enzyme, a‐disintegrin and metalloproteinase 10 (ADAM10) were detected in the hippocampus of each group.1 No significant effects of housing, environment, or their interaction on ADAM10 (F 1,12 = 2.705, P = 0.126; F 1,12 = 3.493, P = 0.086; F 1,12 = 0.545, P = 0.475, respectively), BACE1 (F 1,12 = 0.682, P = 0.433; F 1,12 = 2.816, P = 0.132; F 1,12 = 0.572, P = 0.471, respectively) and PS1 expression (F 1,12 = 0.23, P = 0.644; F 1,12 = 3.132, P = 0.115; F 1,12 = 0.005, P = 0.947, respectively) were observed (Figure 3B,D). Additionally, we performed Western blot to assess APP, sAPPα, transmembrane peptide C‐terminal fragments (CTFβ),and Aβ1‐40 protein expression levels with 6E10 antibody, and densitometry analysis showed that none of the above parameters displayed difference among the four groups (Figure 3C,E). Taken together, these results suggest that neither enriched physical environment nor isolated housing affects APP production and processing before the onset of Aβ plaque load in the hippocampus of APP/PS1 mice.
Figure 3.
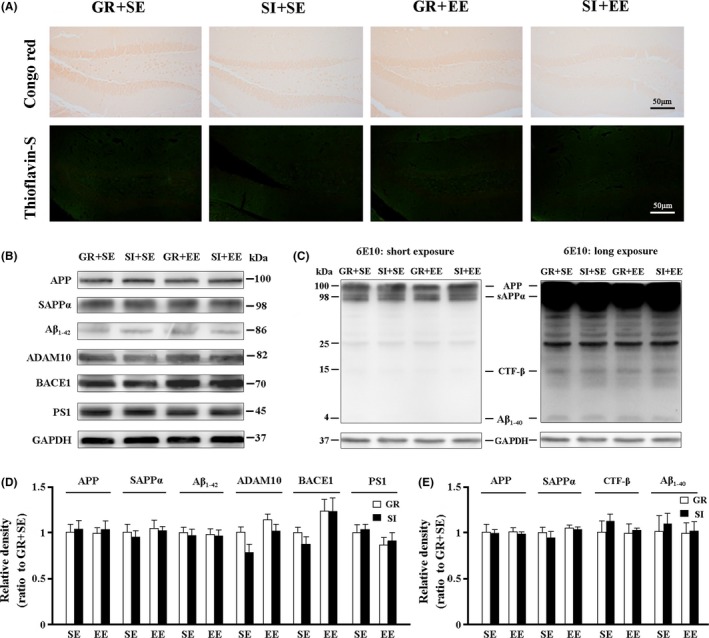
Analyses of Aβ burden and metabolism in the hippocampus. (A) 6‐E10 immunohistochemical staining and Thioflavin‐S staining. Neither 6E10‐immunopositive diffuse plaques nor Thioflavin‐S–positive fibrillar plaques were observed in the hippocampus of all groups. Immunoreactivity for 6‐E10 was present at the somata of CA1 pyramidal neurons and granular DG neurons. (B,D) Representative bands of Western blot and densitometry analysis of APP, and its nonamyloidogenic product sAPPα and amyloidogenic product Aβ1‐42, as well as APP secretases ADAM10, BACE1, and PS1. (C,E) Western blotting using 6‐E10 and densitometry analysis showed expression levels of APP, sAPPα, CTFβ, and Aβ1‐40. Data represent mean ± SEM from 4 mice per group. The statistical analysis was performed by ANOVA with post hoc Student‐Newman‐Keuls test. *P < 0.05, GR vs SI; #P < 0.05, ##P < 0.01, EE vs SE
3.4. Physical EE rescued neuroinflammation, NLRP3 inflammasome signaling pathways and glial activation in the hippocampus of socially isolated APP/PS1 mice.
It has been shown that stress through its interaction with the immune system increases the levels of proinflammatory cytokines and neuroinflammation, contributing to neurodegeneration in AD.31 To further address whether the enriched physical environment restores neuroinflammation caused by SI, expression levels of pro‐interleukin‐1β (pro‐IL‐1β), IL‐1β, IL‐6 and tumor necrosis factor alpha (TNF‐α) in the hippocampus of APP/PS1 mice were determined by Western blotting. Environment, housing, and their interaction had significant effects on the levels of pro‐IL‐1β (F 1,12 = 27.649, P < 0.001; F 1,12 = 27.257, P < 0.001; F 1,12 = 30.405, P < 0.001, respectively), IL‐1β (F 1,12 = 35.981, P < 0.001; F 1,12 = 20.262, P < 0.001; F 1,12 = 13.641, P = 0.001, respectively), IL‐6 (F 1,12 = 47.671, P < 0.001; F 1,12 = 27.712, P < 0.001; F 1,12 = 26.116, P < 0.001, respectively) and TNF‐α (F 1,12 = 52.043, P < 0.001; F 1,12 = 30.534, P < 0.001; F 1,12 = 27.374, P < 0.001, respectively). SI+SE mice showed higher levels of the above parameters than GR+SE mice (P = 0.007; P = 0.009; P = 0.0048; P = 0.0043, respectively). However, they were greatly attenuated by the enriched physical environment (P = 0.0014; P = 0.0037; P = 0.0025; P = 0.0024, respectively, SI+EE mice vs SI+SE mice) (Figure 4A,B).
Figure 4.
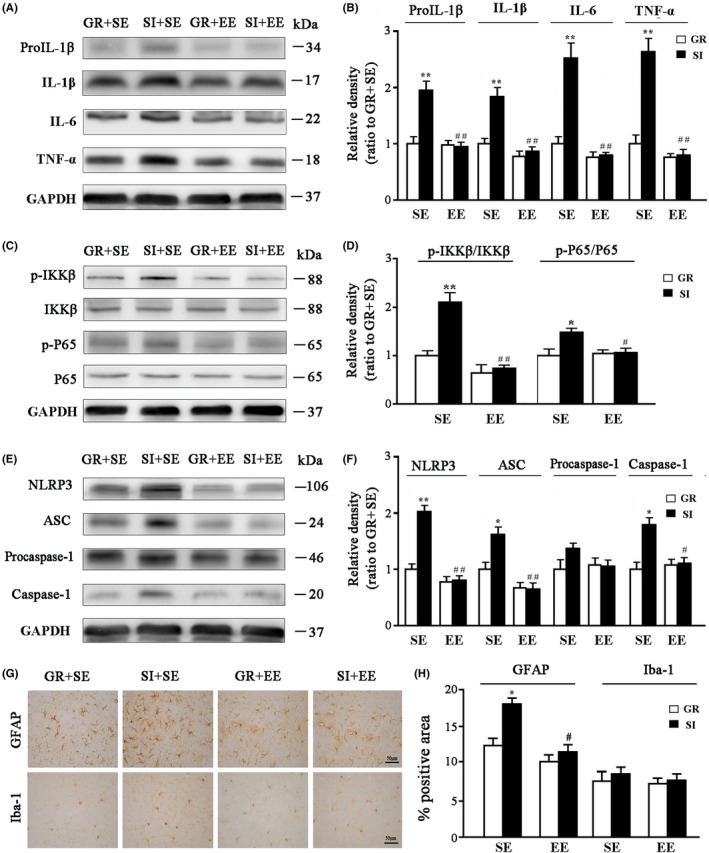
Analyses of proinflammatory factor expression, activation of NLRP3 signaling pathway, and reactive gliosis in the hippocampus. (A,B) Representative bands of Western blot and densitometry analysis of pro‐IL‐1β, IL‐1β, IL‐6, and TNF‐α protein levels. (C‐F) Representative immunoblot of proteins involved in the first (C,D) and second (E,F) signaling pathways of NLRP3 inflammasome activation. (G,H) Immunohistochemical staining and semiquantitative analysis for GFAP and Iba‐1 expression in the hippocampus. Data represent mean ± SEM from 4 mice per group. The statistical analysis was performed by ANOVA with post hoc Student‐Newman‐Keuls test. *P < 0.05, GR vs SI; #P < 0.05, ##P < 0.01, EE vs SE
Inflammasomes are multiprotein complexes that induce inflammation in response to cellular infection or stress.32 Several types of inflammasomes have been identified involving different sensors/receptors in the NOD‐like receptor (NLR) family that initiates the assembly of the complex, among which the NLR protein 3 (NLRP3) inflammasome is well documented in mediating inflammatory molecular expressions in a variety of neurological diseases including AD.33, 34, 35 NLRP3 inflammasome signaling pathways include NF‐κB activation and inflammasome formation.36 Thus, we examined levels of p‐IKKβ, IKKβ, p‐P65, and P65, several key markers involved in the NF‐κB pathway, in the hippocampus among different groups of APP/PS1 mice. p‐IKKβ/IKKβ and p‐P65/P65 ratios were increased by SI (F 1,12 = 17.43, P < 0.001; F 1,12 = 5.847, P = 0.02, respectively), whereas decreased by EE (F 1,12 = 35.215, P < 0.001; F 1,12 = 5.84, P = 0.02, respectively), with significant interaction effects (F 1,12 = 12.177, P = 0.001; F 1,12 = 5.805, P = 0.023, respectively; Figure 4C,D). The enriched physical environment inhibited NF‐κB signaling pathway activation, as revealed by decreased p‐IKKβ/IKKβ and p‐P65/P65 ratios in SI+EE mice, compared with those in SI+SE mice (P = 0.003; P = 0.0269, respectively). We also investigated NLRP3 inflammasome signaling pathway, including NLRP3, apoptosis‐associated speck‐like protein containing card (ASC), procaspase‐1, and caspase‐1 expression levels in the hippocampus. SI increased the above parameters in the hippocampus of APP/PS1 mice (NLRP3: F 1,12 = 32.98, P < 0.001; ASC: F 1,12 = 6.811, P = 0.012; caspase‐1: F 1,12 = 12.111, P = 0.001, respectively), but EE decreased (NLRP3: F 1,12 = 63.906, P < 0.001; ASC: F 1,12 = 32.244, P < 0.001; caspase‐1: F 1,12 = 7.904, P = 0.009, respectively), and significant interaction effects were also observed (NLRP3: F 1,12 = 5.805, P = 0.023; ASC: F 1,12 = 7.347, P = 0.009; caspase‐1: F 1,12 = 11.61, P = 0.001, respectively). Compared to SI+SE mice, SI+EE mice showed low levels of NLRP3 (P = 0.0012), ASC (P = 0.0058) and caspase‐1 (P = 0.0195; Figure 4E,F).
Astrocytes and microglia are strongly affected in individuals with social stress‐induced psychotic disorders and in animal models of chronic psychosocial stress.37 Activated glial cells are main contributors to NF‐κB‐NLRP3 inflammasome‐mediated inflammatory process.38 Thus, we evaluated whether an enriched physical environment inhibited glial activation of SI mice. EE decreased the percentage of GFAP‐positive astrocytes area in the hippocampus (F 1,12 = 13.143, P = 0.001), while SI increased (F 1,12 = 10.957, P = 0.001), and a statistically significant effect of SI × EE interaction was identified (F 1,12 = 5.59, P = 0.025). SI+SE mice had an increased area percentage of GFAP expression compared with GR+SE controls (P = 0.033), indicating a mild activation of astrocytes. Nevertheless, the enriched physical environment rescued this change (P = 0.0113, SI+EE vs SI+SE) (Figure 4G,H). Activation of microglia was unchanged after SI, and effects of housing, environment, or their interaction were not significant on the percentage of Iba‐1–positive area in the hippocampus (F 1,12 = 0.846, P = 0.385; F 1,12 = 0.566, P = 0.473; F 1,12 = 0.063, P = 0.808).
4. DISCUSSION
Due to the continuing depth of global population aging, the incidence of AD is reaching epidemic levels with tremendous social and financial burdens. Most AD cases are sporadic, and their occurrences are influenced by both genetic and environmental factors.7 Precise analysis of various environmental factors that affect the occurrence of AD is of great significance for alleviating the current severe situation of the disease prevention and control. In the present study, by the use of different rearing environments, we have systematically investigated the interaction between physical and social factors of EE on AD‐like pathophysiological changes in the early stages of APP/PS1 mice.
We demonstrated that 8 weeks of exposure to EE improves spatial learning and memory of young APP/PS1 mice housed in a standard environment. In line with this, previous studies have revealed that enrichment has a beneficial effect on cognitive function of various AD mouse models, such as APPswe transgenic mice,18 APP23 transgenic mice,21 Tg2576 mice,39 APPswe/PS1dE9 mice,17, 40, 41 and PS1/PDAPP mice.19 However, effects of EE on the levels of Aβ in AD transgenic mice are controversial, showing three different results: decreases,19, 39, 41 increases,16, 23 and no changes.18, 40
Apart from different genetic animal models of AD utilized,15 these discrepancies may be due to the different time points at which enrichment is initiated. For example, Verret et al39 reported that EE training before the onset of Aβ‐deposition does not affect Aβ expression, but attenuates Aβ‐deposition in the aged APP23 transgenic mice. Consistently, in the present study, we demonstrated that exposure to EE does not affect Aβ production in the hippocampus of young APP/PS1 mice. There is also no difference in hippocampal apoptosis and neuroinflammation between GR+EE and GR+SE groups. However, synaptic proteins SYP and PSD‐95 are increased in the hippocampus of GR+EE mice relative to those of GR+SE mice. Similarly, previous studies on AD transgenic mice 16, 40 or wild‐type mice 13, 42 also showed an improving effect of EE on hippocampal expression of genes/proteins related to synaptic plasticity. Together, these results suggest that the spatial cognition reserved by EE is associated with increased experience‐dependent synaptogenesis in young APP/PS1 mice prior to the amyloidosis onset.
Social withdraw aggravates the onset and development of various neuropsychological disorders including AD.43 SI has been shown to induce brain oxidative and nitrosative stress 44, 45 and impair or inhibit immune response.46, 47 Notably, isolated housing increases Aβ levels and γ‐secretase activity in the hippocampus of adult APP/PS1 mice.48, 49 Our previous studies reported that 17‐month‐old male APP/PS1 mice received SI for 3 months showed severe Aβ plaque accumulation in the hippocampus, with increased γ‐secretase and decreased neprilysin expression.24 In the present study, 1‐month‐old APP/PS1 mice were subjected to SI conditions for 8 weeks and did not show Aβ accumulation and changes in the protein expressions of three APP hydrolases and hydrolysates. However, SI exposure exacerbates spatial cognitive dysfunction, synapse protein loss, and neuroinflammation in the two different stages. This clue high indicates that social stress‐related inflammation may play vital roles in the onset stage of AD.
Indeed, levels of inflammatory mediators, such as C‐reactive protein, complement factors, chemokines, and inflammatory cytokines, are elevated long before the clinical symptoms of AD.50, 51 Statin use can significantly reduce the incidence of AD, which is mainly associated with its anti‐inflammatory effects.52 Simvastatin improves cognitive impairment and attenuates synaptic loss in AD mouse models, and these beneficial effects are related to its anti‐inflammation and independent of Aβ load.53 In addition, accumulative evidence suggests that inflammatory response caused by social stress is the principal mechanism of secondary pathological changes, such as neuronal apoptosis and synaptic loss.54, 55, 56 Together, the present study provided new evidence for SI as a deteriorative factor in the onset of AD.
As mentioned above, the current literature mainly focuses on effects of single EE or SI during AD progression.57 In the present study, we organically combine these two factors together and determine how enriched physical environment influences pathology of socially isolated APP/PS1 mice. We demonstrated that enriched physical environment counteracts hippocampus‐related spatial learning and memory caused by SI before the amyloidosis onset in young adult APP/PS1 mice. These beneficial effects are mainly associated with reduced inflammation, cell apoptosis, and synaptic loss. Our results suggest that improving living environment such as larger living spaces, furnishings, reading, intelligence games, and training may help to delay the process of mild cognitive impairment or early AD patients who live alone. This finding will help establish nonpharmacological interventions via providing enrich physical environment to slow or even prevent the occurrence of AD even under SI situation.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
This work was supported by grants from the National Natural Science Foundation of China (81671070 and 81271210).
Cao M, Hu P‐P, Zhang Y‐L, et al. Enriched physical environment reverses spatial cognitive impairment of socially isolated APPswe/PS1dE9 transgenic mice before amyloidosis onset. CNS Neurosci Ther. 2018;24:202–211. 10.1111/cns.12790
REFERENCES
- 1. Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med. 2010;362:329‐344. [DOI] [PubMed] [Google Scholar]
- 2. Caselli RJ, Beach TG, Knopman DS, et al. Alzheimer disease: scientific breakthroughs and translational challenges. Mayo Clin Proc. 2017;92:978‐994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Canter RG, Penney J, Tsai LH. The road to restoring neural circuits for the treatment of Alzheimer's disease. Nature. 2016;539:187‐196. [DOI] [PubMed] [Google Scholar]
- 4. Cass SP. Alzheimer's Disease and exercise: a literature review. Curr Sports Med Rep. 2017;16:19‐22. [DOI] [PubMed] [Google Scholar]
- 5. Cacioppo JT, Hawkley LC. Social isolation and health, with an emphasis on underlying mechanisms. Perspect Biol Med. 2003;46:S39‐S52. [PubMed] [Google Scholar]
- 6. Di Marco LY, Marzo A, Muñoz‐Ruiz M, et al. Modifiable lifestyle factors in dementia: a systematic review of longitudinal observational cohort studies. J Alzheimers Dis. 2014;42:119‐135. [DOI] [PubMed] [Google Scholar]
- 7. Friedler B, Crapser J, McCullough L. One is the deadliest number: the detrimental effects of social isolation on cerebrovascular diseases and cognition. Acta Neuropathol. 2015;129:493‐509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. El Haj M, Jardri R, Larøi F, et al. Hallucinations, loneliness, and social isolation in Alzheimer's disease. Cogn Neuropsychiatry. 2016;21:1‐13. [DOI] [PubMed] [Google Scholar]
- 9. Wilson RS, Krueger KR, Arnold SE, et al. Loneliness and risk of Alzheimer disease. Arch Gen Psychiatry. 2007;64:234‐240. [DOI] [PubMed] [Google Scholar]
- 10. Dooley J, Bailey C, McCabe R. Communication in healthcare interactions in dementia: a systematic review of observational studies. Int Psychogeriatr. 2015;27:1277‐1300. [DOI] [PubMed] [Google Scholar]
- 11. Klimova B, Maresova P, Valis M, et al. Alzheimer's disease and language impairments: social intervention and medical treatment. Clin Interv Aging. 2015;10:1401‐1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gordon MF, Lenderking WR, Duhig A, et al. Development of a patient‐reported outcome instrument to assess complex activities of daily living and interpersonal functioning in persons with mild cognitive impairment: the qualitative research phase. Alzheimers Dement. 2016;12:75‐84. [DOI] [PubMed] [Google Scholar]
- 13. Frick KM, Fernandez SM. Enrichment enhances spatial memory and increases synaptophysin levels in aged female mice. Neurobiol Aging. 2003;24:615‐626. [DOI] [PubMed] [Google Scholar]
- 14. Bennett JC, McRae PA, Levy LJ, et al. Long‐term continuous, but not daily, environmental enrichment reduces spatial memory decline in aged male mice. Neurobiol Learn Mem. 2006;85:139‐152. [DOI] [PubMed] [Google Scholar]
- 15. Nithianantharajah J, Hannan AJ. Enriched environments, experience ‐dependent plasticity and disorders of the nervous system. Nat Rev Neurosci. 2006;7:697‐709. [DOI] [PubMed] [Google Scholar]
- 16. Jankowsky JL, Xu G, Fromholt D, et al. Environmental enrichment exacerbates amyloid plaque formation in a transgenic mouse model of Alzheimer disease. J Neuropathol Exp Neurol. 2003;62:1220‐1227. [DOI] [PubMed] [Google Scholar]
- 17. Jankowsky JL, Melnikova T, Fadale DJ, et al. Environmental enrichment mitigates cognitive deficits in a mouse model of Alzheimer's disease. J Neurosci. 2005;25:5217‐5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Arendash GW, Garcia MF, Costa DA, et al. Environmental enrichment improves cognition in aged Alzheimer's transgenic mice despite stable amyloid‐β deposition. NeuroReport. 2004;15:1751‐1754. [DOI] [PubMed] [Google Scholar]
- 19. Costa DA, Cracchiolo JR, Bachstetter AD, et al. Enrichment improves cognition in AD mice by amyloid‐related and unrelated mechanisms. Neurobiol Aging. 2007;28:831‐844. [DOI] [PubMed] [Google Scholar]
- 20. Herring A, Lewejohann L, Panzer A‐L, et al. Preventive and therapeutic types of environmental enrichment counteract beta amyloid pathology by different molecular mechanisms. Neurobiol Dis. 2011;42:530‐538. [DOI] [PubMed] [Google Scholar]
- 21. Polito L, Chierchia A, Tunesi M, et al. Environmental enrichment lessens cognitive decline in APP23 mice without affecting brain sirtuin expression. J Alzheimers Dis. 2014;42:851‐864. [DOI] [PubMed] [Google Scholar]
- 22. Fischer A. Environmental enrichment as a method to improve cognitive function. What can we learn from animal models? NeuroImage. 2016;131:42‐47. [DOI] [PubMed] [Google Scholar]
- 23. Jankowsky JL, Slunt HH, Gonzales V, et al. APP processing and amyloid deposition in mice haplo‐insufficient for presenilin 1. Neurobiol Aging. 2004;25:885‐892. [DOI] [PubMed] [Google Scholar]
- 24. Huang H, Nie S, Cao M, et al. Characterization of AD‐like phenotype in aged APPSwe/PS1dE9 mice. Age (Dordr). 2016;38:303‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xu Z, Xiao N, Chen Y, et al. Deletion of aquaporin‐4 in APP/PS1 mice exacerbates brain Aβ accumulation and memory deficits. Mol Neurodegener. 2015;10:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pereda‐Pérez I, Popović N, Otalora BB, et al. Long‐term social isolation in the adulthood results in CA1 shrinkage and cognitive impairment. Neurobiol Learn Mem. 2013;106:31‐39. [DOI] [PubMed] [Google Scholar]
- 27. Xu ZQ, Huang H, Chen YL, et al. Different expression patterns of amyloid‐β protein precursor secretases in human and mouse hippocampal neurons: a potential contribution to species differences in neuronal susceptibility to amyloid‐β pathogenesis. J Alzheimers Dis. 2016;51:179‐195. [DOI] [PubMed] [Google Scholar]
- 28. Krolow R, Noschang C, Arcego DM, et al. Isolation stress exposure and consumption of palatable diet during the prepubertal period leads to cellular changes in the hippocampus. Neurochem Res. 2013;38:262‐272. [DOI] [PubMed] [Google Scholar]
- 29. Chu J, Lauretti E, Praticò D. Caspase‐3‐dependent cleavage of Akt modulates tau phosphorylation via GSK3β kinase: implications for Alzheimer's disease. Mol Psychiatry. 2017;22:1002‐1008. [DOI] [PubMed] [Google Scholar]
- 30. Garcia‐Alloza M, Robbins EM, Zhang‐Nunes SX, et al. Characterization of amyloid deposition in the APPswe/PS1dE9 mouse model of Alzheimer disease. Neurobiol Dis. 2006;24:516‐524. [DOI] [PubMed] [Google Scholar]
- 31. Glass CK, Saijo K, Winner B, et al. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918‐934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821‐832. [DOI] [PubMed] [Google Scholar]
- 33. Heneka MT, Kummer MP, Stutz A, et al. NLRP3 is activated in Alzheimer's disease and contributes to pathology in APP/PS1 mice. Nature. 2013;493:674‐678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shao BZ, Xu ZQ, Han BZ, et al. NLRP3 inflammasome and its inhibitors: a review. Front Pharmacol. 2015;6:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Freeman LC, Ting JP. The pathogenic role of the inflammasome in neurodegenerative diseases. J Neurochem. 2016;1:29‐38. [DOI] [PubMed] [Google Scholar]
- 36. Mitchell S, Vargas J, Hoffmann A. Signaling via the NFκB system. Wiley Interdiscip Rev Syst Biol Med. 2016;8:227‐241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bellucci A, Westwood AJ, Ingram E, et al. Induction of inflammatory mediators and microglial activation in mice transgenic for mutant human P301S tau protein. Am J Pathol. 2004;165:1643‐1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Beauquis J, Pavía P, Pomilio C, et al. Environmental enrichment prevents astroglial pathological changes in the hippocampus of APP transgenic mice, model of Alzheimer's disease. Exp Neurol. 2013;239:28‐37. [DOI] [PubMed] [Google Scholar]
- 39. Verret L, Krezymon A, Halley H, et al. Transient enriched housing before amyloidosis onset sustains cognitive improvement in Tg2576 mice. Neurobiol Aging. 2013;34:211‐225. [DOI] [PubMed] [Google Scholar]
- 40. Stuart KE, King AE, Fernandez‐Martos CM, et al. Mid‐life environmental enrichment increases synaptic density in CA1 in a mouse model of Aβ‐associated pathology and positively influences synaptic and cognitive health in healthy ageing. J Comp Neurol. 2017;525:1797‐1810. [DOI] [PubMed] [Google Scholar]
- 41. Lazarov O, Robinson J, Tang YP, et al. Environmental enrichment reduces Aβ levels and amyloid deposition in transgenic mice. Cell. 2005;120:701‐713. [DOI] [PubMed] [Google Scholar]
- 42. Nithianantharajah J, Levis H, Murphy M. Environmental enrichment results in cortical and subcortical changes in levels of synaptophysin and PSD‐95 proteins. Neurobiol Learn Mem. 2004;81:200‐210. [DOI] [PubMed] [Google Scholar]
- 43. Dong H, Goico B, Martin M, et al. Modulation of hippocampal cell proliferation, memory, and amyloid plaque deposition in APPsw (Tg2576) mutant mice by isolation stress. Neuroscience. 2004;127:601‐609. [DOI] [PubMed] [Google Scholar]
- 44. Fleshner M, Frank M, Maier SF. Danger signals and inflammasomes: stress‐evoked sterile inflammation in mood disorders. Neuropsychopharmacology. 2017;42:36‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Filipović D, Todorović N, Bernardi RE, et al. Oxidative and nitrosative stress pathways in the brain of socially isolated adult male rats demonstrating depressive‐ and anxiety‐like symptoms. Brain Struct Funct. 2017;222:1‐20. [DOI] [PubMed] [Google Scholar]
- 46. Cruces J, Venero C, Pereda‐Pérez I, et al. A higher anxiety state in old rats after social isolation is associated to an impairment of the immune response. J Neuroimmunol. 2014;277:18‐25. [DOI] [PubMed] [Google Scholar]
- 47. Powell ND, Sloan EK, Bailey MT, et al. Social stress up‐regulates inflammatory gene expression in the leukocyte transcriptome via β‐adrenergic induction of myelopoiesis. Proc Natl Acad Sci USA. 2013;110:16574‐16579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hsiao YH, Chen PS, Chen SH, et al. The involvement of Cdk5 activator p35 in social isolation‐triggered onset of early Alzheimer's disease‐related cognitive deficit in the transgenic mice. Neuropsychopharmacology. 2011;36:1848‐1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hsiao YH, Kuo JR, Chen SH, et al. Amelioration of social isolation‐triggered onset of early Alzheimer's disease‐related cognitive defict by N‐acetylcysteine in a transgenic mouse model. Neurobiol Dis. 2012;45:1111‐1120. [DOI] [PubMed] [Google Scholar]
- 50. Strandberg TE, Tilvis RS. C‐reactive protein, cardiovascular risk factors, and mortality in a prospective study in the elderly. Arterioscler Thromb Vasc Biol. 2000;20:1057‐1060. [DOI] [PubMed] [Google Scholar]
- 51. Swardfager W, Lanctot K, Rothenburg L, et al. A meta‐analysis of cytokines in Alzheimer's disease. Biol Psychol. 2010;68:930‐941. [DOI] [PubMed] [Google Scholar]
- 52. Geifman N, Brinton RD, Kennedy RE, et al. Evidence for benefit of statins to modify cognitive decline and risk in Alzheimer's disease. Alzheimers Res Ther. 2017;9:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Li L, Cao D, Kim H, et al. Simvastatin enhances learning and memory independent of amyloid load in mice. Ann Neurol. 2006;60:729‐739. [DOI] [PubMed] [Google Scholar]
- 54. Voloboueva LA, Giffard RG. Inflammation, mitochondria, and the inhibition of adult neurogenesis. J Neurosci Res. 2011;89:1989‐1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ramesh G, Benge S, Pahar B, et al. A possible role for inflammation in mediating apoptosis of oligodendrocytes as induced by the Lyme disease spirochete Borrelia burgdorferi. J Neuroinflmmation. 2012;9:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hong S, Beja‐Glasser VF, Nfonoyim BM, et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science. 2016;352:712‐716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jeong YH, Kim JM, Yoo J, et al. Environmental enrichment compensates for the effects of stress on disease progression in Tg2576 mice, an Alzheimer's disease model. J Neurochem. 2011;119:1282‐1293. [DOI] [PubMed] [Google Scholar]


