Summary
Aim
This 4‐week open‐label observational study describes the effect of introducing a microtablet dose dispenser and adjusting doses based on objective free‐living motor symptom monitoring in individuals with Parkinson's disease (PD).
Methods
Twenty‐eight outpatients with PD on stable levodopa treatment with dose intervals of ≤4 hour had their daytime doses of levodopa replaced with levodopa/carbidopa microtablets, 5/1.25 mg (LC‐5) delivered from a dose dispenser device with programmable reminders. After 2 weeks, doses were adjusted based on ambulatory accelerometry and clinical monitoring.
Results
Twenty‐four participants completed the study per protocol. The daily levodopa dose was increased by 15% (112 mg, P < 0.001) from period 1 to 2, and the dose interval was reduced by 12% (22 minutes, P = 0.003). The treatment adherence to LC‐5 was high in both periods. The MDS‐UPDRS parts II and III, disease‐specific quality of life (PDQ‐8), wearing‐off symptoms (WOQ‐19), and nonmotor symptoms (NMS Quest) improved after dose titration, but the generic quality‐of‐life measure EQ‐5D‐5L did not. Blinded expert evaluation of accelerometry results demonstrated improvement in 60% of subjects and worsening in 25%.
Conclusions
The introduction of a levodopa microtablet dispenser and accelerometry aided dose adjustments improve PD symptoms and quality of life in the short term.
Keywords: accelerometry, dose titration, microtablets, observational study, Parkinson's disease
1. INTRODUCTION
Levodopa is a mainstay in the symptomatic treatment of Parkinson's disease (PD), but with time, the effect duration of doses shortens and a narrowing therapeutic window can lead to levodopa‐induced dyskinesia in a substantial proportion of patients.1, 2 Levodopa treatment complications are the result of decreasing dopamine storage capacity as well as pre‐ and postsynaptic plasticity in the brain. Eventually, levodopa dose effect duration shortens from over 5 hours to 1‐3 hours at late stages of PD, closely reflecting the pharmacokinetics of levodopa in plasma.3, 4 To optimize levodopa efficacy, it is desirable to gradually adapt the dosing regimen to the changes in pharmacodynamic effect. Validated clinical rating scales focus on effect sizes, whereas effect duration is usually evaluated based on patient recall or diaries. Recall is unfortunately biased by emotional and medication state‐related factors, and diaries can be challenging to keep and interpret.5, 6
A common strategy for adapting levodopa doses to the onset of motor fluctuations is to fractionate the daytime levodopa doses in many smaller doses. This is, however, not without disadvantages as the increased number of doses makes it challenging to adhere to medication.7 The fractioning strategy is mainly based on pharmacokinetic knowledge and clinical experience8 as there are only a few studies on the efficacy of fractionation with liquid levodopa.9, 10, 11, 12, 13 Fine‐tuning of levodopa dosage with traditional tablets is limited to dose adjustments of 25 mg, which does not always provide enough granularity for adequate individualization of treatment.
Levodopa‐carbidopa microtablets, 5/1.25 mg (LC‐5), are delivered from a dose dispenser device (MyFID®, Sensidose AB, Sollentuna, Sweden) in dosage steps of 5 mg and can facilitate the fine‐tuning and individualization of dosing both regarding time and dose.14 This can result in more stable levodopa plasma concentrations than with levodopa/carbidopa/entacapone tablets.15 The dispenser also provides reminders and a diary‐like function that assists patients in keeping track of and managing their treatment. The product has market authorization in 14 European countries and is subsidized for use in advanced PD in Sweden. The pharmacokinetic and pharmacodynamic profile of LC‐5 microtablets (Flexilev®, Sensidose AB, Sollentuna, Sweden) in patients with PD was recently described.16
Objective measurements of PD motor symptoms based on wearable technology have recently become commercially available. The Personal (outside the US Parkinson) Kinetigraph, PKG (Global Kinetics Corporation, Australia), is a wrist‐worn accelerometry–based system that automatically characterizes and quantifies movement in terms of bradykinesia, dyskinesia, and tremor over a period of 6 days in patients' home environment.17, 18 The PKG device has been approved by the US Food and Drug Administration and has acquired a CE marking. The utility of using free‐living motor symptom monitoring for guiding individual treatment has, however, not been studied comprehensively.
The aim of this study was to examine the effect of introducing device‐assisted oral treatment with levodopa microtablets and adjusting the dose regimen based on information from a PKG measurement in addition to clinical information. The study population was recruited based on prescription records rather than reported fluctuations, and the primary outcome measure was changed in MDS‐UPDRS rating over the study period of 4 weeks. The effect of dose titration per se was evaluated with PKG measurements and self‐reported rating scales.
2. METHOD
2.1. Study population
Patients with PD and stable levodopa medication at intervals of ≤4 hour were recruited based on prescription records obtained from the Neurology Department of the Sahlgrenska University Hospital, Sweden (Figure S1).The inclusion criteria were age >18 years and a diagnosis of idiopathic PD according to the UK Parkinson Disease Society Brain Bank Criteria.19 All concomitant PD treatments (including deep brain stimulation) were allowed except for levodopa/carbidopa intestinal gel (LCIG) infusion. Exclusion criteria were as follows: inability to follow instructions or otherwise comply with the protocol; other musculoskeletal or neurological disorders that would confound assessment of motor function; severe visual impairment; current and bothersome hallucinations or previous hospitalization due to psychosis; and more than 1‐hour travel distance by car to the clinic. The study was conducted in accordance with the Declaration of Helsinki. The study protocol was approved by the Regional Ethical Review Board in Gothenburg, Sweden. Written informed consent was obtained from all participants prior to all study‐related procedures.
2.2. Study design
The study was a longitudinal, observational, 4‐week study of the effect of introducing LC‐5 microtablets delivered from a dosing device (MyFID®) and adjusting dosage based on the PKG recordings. There were two observation periods limited to three visits, and the study was conducted between July 2016 and February 2017 at the Sahlgrenska University Hospital in Gothenburg, Sweden. During the first observation period of 2 weeks, patients used the LC‐5 microtablets dose dispenser (MyFID®) for their regular levodopa dosing schedule after translating their original levodopa preparations to LC‐5 microtablets based on conversion factors described previously.16 At the second visit, the dose regimen was adjusted according to PKG results and a confirmatory clinical interview. Patients used the adjusted dose regimen for 2 weeks and were evaluated clinically at a third visit (see study design details in Figure S2).
2.3. Objective assessments
Participants wore the PKG at the most afflicted side for 6 days before the second and the final visit. The PKG data are processed with a proprietary algorithm and presented in 2‐minute bins as a bradykinesia score (BKS) and a dyskinesia score (DKS). Average scores for the 6‐day period are presented as a graph to facilitate the detection of predictable fluctuations in relation to medication times (Figure 1). The report also contains a graphical representation of the occurrence of tremor episodes and episodes of sleep‐like immobility.17 The fluctuation and dyskinesia score (FDS) is a single measure derived from the interquartile range of BKS and DKS during daytime (09:00‐18:00) over the entire measurement period and reflects the degree of motor state variability.18 The summary scores BKS, DKS, FDS, and percent time with tremor (09:00‐18:00) were used to evaluate the effect of dose titration.
Figure 1.
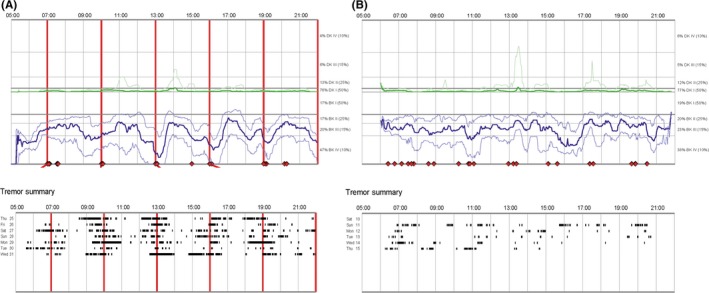
An example of evident wearing‐off phenomena shown in The Parkinson Kinetigraph data logger graph A. The bold blue line represents the median of bradykinesia scores for every 2 min of the day during six days of measurement. The 25th and 75th percentiles of bradykinesia scores are indicated by thin blue lines. The corresponding green line represents dyskinesia scores. The vertical red lines indicate prescribed dose times. The troughs in the bold blue line at medication times correspond to predictable wearing‐off episodes. The black raster pattern represents the timing of tremor episodes. B shows the median of bradykinesia and dyskinesia scores and the timing of tremor episodes in The Parkinson Kinetigraph data logger graph after dose adjustment
The PKG also contains information about fluctuation patterns that are not conveyed by summary scores, and changes in outcome may involve a subset of aspects that vary between patients. Two movement disorder specialists (AJ and DN) with experience in using the PKG for clinical evaluation were therefore asked to evaluate the PKG reports visually. Before evaluation, all information about medication times and medication intake confirmations, as well as dates of measurement, was removed and the reports were presented in a randomized order. The experts were asked to determine whether there was a meaningful difference between the two recordings from the patient and in that case which recording represented a better situation. When the last performed PKG recording was better than the first, the outcome was interpreted as improved.
2.4. Clinical assessments and self‐reported questionnaires
Clinical assessments were carried out by a movement disorder specialist (FB) at baseline and at the final visit. Global PD symptoms were assessed using the Movement Disorder Society Unified Parkinson's Disease Rating Scale (MDS‐UPDRS).20 Self‐ratings were made with the Non‐Motor Symptoms Questionnaire (NMS Quest).21 The 8‐item patient‐rated Parkinson's disease quality‐of‐life scale (PDQ‐8)22 and the EuroQoL 5‐dimension, five response levels together with the associated 0‐ to 100‐point visual analog scale (VAS).23 A utility index was calculated from the EQ‐5D‐5L results (higher indicates a better health state).24 Furthermore, the 19‐item Wearing‐off Questionnaire (WOQ‐19)25 was used to assess the presence of wearing‐off–related motor and nonmotor symptoms. The Montgomery Åsberg Depression Rating Scale self‐reported questionnaire (MADRS‐S)26, 27 was used to evaluate symptoms of depression. All self‐reported questionnaires were filled out on the day before or on the day of the visit. For the purpose of the study, the time period assessed with PDQ‐8 and NMS Quest was restricted to the last week.
2.5. Dose adjustments
The PKG pattern of medication effects on tremor, bradykinesia scores, and dyskinesia scores was used to determine the duration and efficacy of individual doses. This information was used to adjust dose sizes and intervals. The algorithms for dose adjustment were based on basic pharmacokinetic principles: When wearing‐off phenomena could be identified based on bradykinesia scores or tremor, the duration of the preceding dose effects was used to guide the optimal dosing interval. When the morning dose resulted in less reduction in bradykinesia scores or tremor than the following doses, an increase in the morning dose was suggested. When peak dose hyperkinetic episodes were identified, a decrease in the preceding dose size was suggested. When the dose effect appeared suboptimal, a dose increase was suggested. When wearing‐off phenomena were not identified, a moderate increase in dose intervals (up to +30 minutes) was attempted. The information obtained from PKG recordings was confirmed through an informal interview with the subjects before agreeing with the subject on a revised dosing schedule. In this way, the PKG report was used as an educational tool to visualize the effects of medication to the patients, in the way we currently use PKG reports in the clinic as a starting point for discussing dosing schedules with patients.
2.6. Record of medication adherence
The MyFID device records all dispensation of LC‐5 microtablets, doses, and time of dispensing. In addition, it records the dispensing of rescue doses. Total adherence was defined as the total amount (mg) dispensed divided by the total amount prescribed; the days adherence was defined as the percentage of analyzed days on which exactly the prescribed number of doses were dispensed. Adherence to timing was defined as the percentage of doses taken at the prescribed time (±25% of the preceding dose interval). Dose adherence was defined as the total intakes divided by the number of prescribed doses.
2.7. Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics 22 (IBM Corp., Armonk, NY). The primary outcome was the change in MDS‐UPDRS subscale scores at the final visit compared to baseline. Secondary outcomes were the changes in self‐reported questionnaire results from the first to the last visit, including PDQ‐8, EQ‐5D‐5L, NMS Quest, WOQ‐19, and MADRS‐S scores. The tertiary efficacy outcomes were the changes in self‐reported questionnaires between visit one and two and between visit two and three, as well as the changes in objective measure scores derived from PKG recordings before visit 2 and before visit 3.
To analyze differences in outcomes across the 3 visits, repeated‐measures analysis of variance was used for continuous variables and Friedman test for categorical variables. Bonferroni corrections were made for multiple comparisons. Paired t test was used for comparing parametric variables and Wilcoxon signed‐rank test for nonparametric variables. The distribution of PKG outcomes according to clinician evaluations (improved, equal or worsened) was compared to random outcome with a 33.33% likelihood of any of the three possible outcomes using the χ2‐test. Significance was defined as P < 0.05.
3. RESULTS
3.1. Participants
Thirty‐one participants were recruited to a baseline/screening visit. Three participants were excluded due to screening failures. Three participants withdrew during the first observation phase of the study. There was an unexpected death of one participant a few days into period 2 due to cardiac arrest and the autopsy indicated previously undiagnosed cardiac hypertrophy as the underlying cause. A total of 24 participants completed the study in accordance with the protocol (median 68 years, range 58‐82 years; 14 males [58%], Table 1). Data were primarily analyzed using the per‐protocol set. The median duration of diagnosed PD was 9.5 years (range 4‐30). The total levodopa equivalent daily dose (LED) was calculated using the conversion factors proposed by Tomlinson24 with adjustments for the higher bioavailability of dispersible levodopa/benserazide.25 Before introducing LC‐5 microtablets, the mean (SD) for LED was 1100 (556) mg/d of which 730 (330) mg/d was levodopa‐derived. Individual participant demographic data are presented in Table S1.
Table 1.
Demographic characteristics of completing participants
| Baseline characteristic | Participants (n = 24) |
|---|---|
| Gender (n[%]) | |
| Male | 14 (58) |
| Female | 10 (42) |
| Age (years) | |
| Median (range) | 68 (58‐82) |
| Symptom duration (years) | |
| Median (range) | 11 (5‐32) |
| Years since diagnosis (years) | |
| Median (range) | 9.5 (4‐30) |
| Symptom fluctuation duration (years) | |
| Median(range) | 4 (1‐20) |
| Most afflicted side (n[%]) | |
| Left | 11 (46) |
| Right | 12 (50) |
| Bilateral | 1 (4) |
| LED (mg/d) | |
| Mean ± SD | 1100 ± 556 |
| LED derived from levodopa (mg/d) | |
| Mean ± SD | 730 ± 330 |
| Dopamine agonists‐LED (mg/d) | |
| Mean ± SD | 129 ± 96a |
| Weight (kg) | |
| Mean ± SD | 74 ± 15 |
| Concomitant PD treatment (n[%]) | |
| Levodopa | 24 (100) |
| Pramipexole | 11 (46) |
| Entacapone | 4 (17) |
| Ropinirole | 5 (21) |
| Rasagiline | 13 (54) |
| Amantadine | 2 (8) |
| Deep brain stimulation | 3 (13) |
LED, levodopa equivalent daily dose; SD, standard deviation; PD, Parkinson's disease
Mean data are presented for 16 participants that use dopamine agonists.
3.2. Dose titration
After the first PKG measurement, the mean LC‐5 dose was increased by 112 mg (15%, range −60 to 350 mg, t = 5.015, P < 0.001) and the dose intervals were shorted from a mean of 173 to 151 minutes (a decrease by 12%, range of decrease −60 to 44 minutes, t = −3.265, P = 0.003). Furthermore, the morning dose was increased from 136 mg to 153 mg (range of changes −40 to +75 mg, t = 2.808, P = 0.01). There was no significant change in the number of doses per day (t = 0.382, P = 0.706). However, in one patient the number of doses was halved from 29 to 15. With this outlier removed from analysis, dose titration resulted in a change in the median number of daily doses from 5 to 7 (t = −3.943, P = 0.001, Table 2).
Table 2.
Dose regimens in the first and second periods
| First period | Second period | P value | |
|---|---|---|---|
| LED (mg/d) | 1107 ± 556 | 1224 ± 577 | <0.001 |
| Levodopa/carbidopa dose, LC‐5 (mg/d) | 739 ± 332 | 852 ± 374 | <0.001 |
| Number of doses (per day) | 7 ± 5 | 7 ± 2 | 0.71a |
| Dose interval (min) | 173 ± 57 | 151 ± 38 | 0.003 |
| Morning dose (mg) | 136 ± 67 | 153 ± 65 | 0.01 |
| Morning dose interval (min) | 172 ± 70 | 154 ± 42 | 0.053 |
LED, levodopa equivalent daily dose.
Data are mean ± standard deviation; significant level P < 0.05.
After removal of outlier, there was a significant increase in the mean of number of doses from 5.8 to 6.7 (P = 0.001).
3.3. Adherence to medication schedules
The mean total adherence (percent of the prescribed total dose) was 91% (range 36% to 106%) in period 1 and 96% (range 81% to 107%) in period 2. In the first period, 20 of 24 patients had a total adherence >80% and all patients had an adherence >80% in the second period. The days adherence (percent of days with correct number of doses) was 78% (range 0‐100) in period 1 and 73% (range 15‐100) in period 2. Adherence to the timing of doses (percent of doses taken on time) was 88% (range 51‐100) and 91% (range 72‐100) in periods 1 and 2, respectively. Dose adherence (percent of the prescribed total number of intakes) was 97% in period 1 and 96% in period 2. There were no statistically significant differences between period 1 and period 2 regarding adherences. Higher numbers of doses were associated with lower adherence to medication schedules, but despite the higher number of doses in period 2, adherences remained high (Figure 2).
Figure 2.
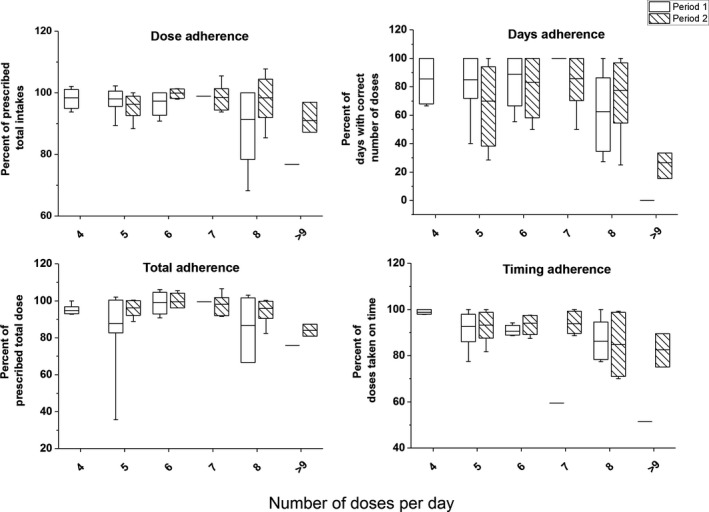
Total, days, timing, and dose adherence by number of prescribed doses per day in periods 1 and 2. The horizontal line in each box represents the mean adherence. The 25th and 75th percentiles of adherence are indicated by the box, and the range is indicated by the whiskers
3.4. Clinical assessments and self‐reported questionnaires
3.4.1. Primary outcome
The introduction of LC‐5 microtablets followed by a PKG‐aided dose titration resulted in improvements in MDS‐UPDRS part II and part III, with 2.7 points (z = −2.65, P = 0.008; effect size r = 0.38) and 4.6 points (z = −2.29, P = 0.022; effect size r = 0.33, Figure 3), respectively. The range of changes in part II was −8 to +7 points and in part III −21 to +12. There were no differences in MDS‐UPDRS part I and part IV. Of the 24 patients, 17 improved in part II and part III, and two did not change while five worsened.
Figure 3.
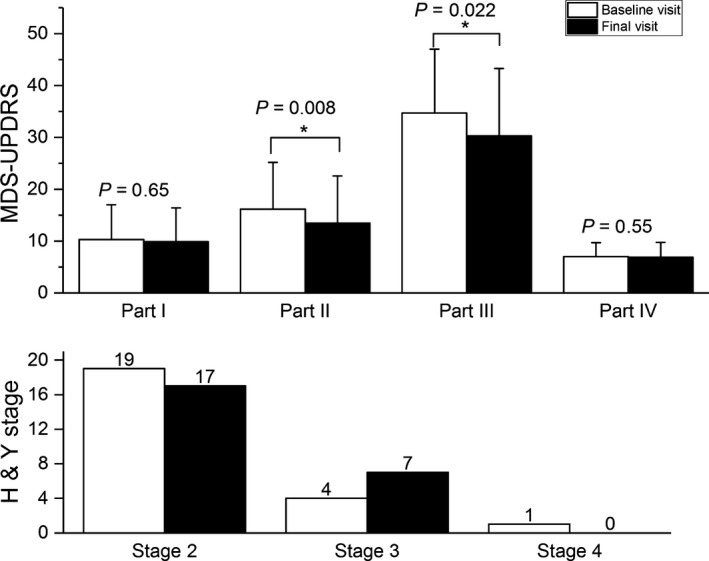
Efficacy of dose titration on clinical outcomes between baseline and final visit. *P < 0.05. H&Y, Hoehn and Yahr stage; MDS‐UPDRS, Movement Disorder Society's version of the Unified Parkinson's Disease Rating Scale
3.4.2. Secondary outcomes
Health‐related quality of life, as measured by PDQ‐8, improved from baseline to final visit (mean diff of summary index score = −6.7, F 2,22 = 7.46, P = 0.003, = 0.40). The number of reported symptoms in the NMS Quest decreased (mean diff = −1.7, χ2 = 7.37, P = 0.025). MADRS‐S scores were better in the final visit compared to baseline visit (mean diff = −3.5, χ2 = 14.91, P = 0.001). WOQ‐19 showed a decrease in the number of reported symptoms from baseline to final visit (χ2 = 8.51, P = 0.014), but no significant change in the number of symptoms improving after the next dose (χ2 = 5.12, P = 0.077). EQ‐5D‐5L scores did not change over the study period (F 2,22 = 1.25, P = 0.31, Table 3).
Table 3.
Self‐reported questionnaires and PKG summary scores
| Baseline visit | Second visit | Final visit | P valuea | Change (baseline to second visit) | P value | Change (second to final visit) | P value | |
|---|---|---|---|---|---|---|---|---|
| Self‐reported questionnaires | ||||||||
| PDQ‐8 (%) | 27.1 ± 16.6 | 24.1 ± 14.9 | 20.4 ± 17.7 | 0.003 | −3.0 ± 7.7 | 0.069 | −3.6 ± 10.1 | 0.091 |
| EQ‐5D‐5L index | 0.67 ± 0.2 | 0.68 ± 0.17 | 0.71 ± 0.18 | 0.31 | 0.02 ± 0.1 | 0.561 | 0.03 ± 0.1 | 0.221 |
| NMS Quest | 10.7 ± 5.3 | 10 ± 5.3 | 9 ± 5.1 | 0.025 | −0.8 ± 3 | 0.238 | −1 ± 2.1 | 0.034 |
| WOQ‐19, Wearing‐off symptoms | 9.4 ± 4.7 | 8.5 ± 4.4 | 7.4 ± 4.7 | 0.014 | −0.8 ± 2.4 | 0.137 | −1.2 ± 1.9 | 0.009b |
| WOQ‐19, Symptoms improving after the next dose | 6.0 ± 3.9 | 6.6 ± 4 | 5.5 ± 4.4 | 0.077 | 0.7 ± 3.2 | 0.277 | −1.1 ± 2.1 | 0.021b |
| MADRS‐S | 10.5 ± 7.8 | 9.6 ± 6.9 | 7 ± 5.9 | 0.001 | −1 ± 5.2 | 0.447 | −2.6 ± 3.1 | 0.001b |
| First period | Second period | Change (First to second period) | P value | |
|---|---|---|---|---|
| PKG summary scores | ||||
| BKS | 25.5 ± 5.6 | 25.0 ± 5.4 | −0.5 ± 3.3 | 0.463 |
| DKS | 3.4 ± 5.1 | 2.8 ± 2.9 | −0.7 ± 4.3 | 0.458 |
| FDS | 9.5 ± 3.2 | 9.6 ± 2.5 | 0.1 ± 2.2 | 0.876 |
| PTT (%) | 3.4 ± 4 | 2.2 ± 1.8 | −1.2 ± 3.3 | 0.085 |
PDQ‐8, 8‐item patient‐rated Parkinson's disease questionnaire; EQ‐5D‐5L, EuroQoL 5‐dimension with five responses levels; NMS Quest, Non‐Motor Symptoms Questionnaire; WOQ‐19, 19‐item Wearing‐off Questionnaire; MADRS‐S, Montgomery Åsberg Depression Rating Scale self‐reported questionnaire. PKG, The Parkinson Kinetigraph data logger; BKS, bradykinesia scores; DKS, dyskinesia scores; FDS, fluctuation and dyskinesia scores; PTT, percent of time with tremor between 09:00 and 18:00.
Data are mean ± standard deviation. Significant level P < 0.05.
From baseline to final visit.
Significant difference from second to final visit. Significant level for P value was adjusted to 0.025 using Bonferroni adjustment for multiple comparisons.
3.5. Objective measurements based on PKG recordings
None of the PKG objective summary scores bradykinesia score (BKS), dyskinesia score (DKS), fluctuation dyskinesia score (FDS), and percent daytime with tremor (PTT) changed significantly following the dose titration (Table 3).
3.5.1. Blinded clinical evaluation of PKG
In a first evaluation round, the experts agreed in their evaluations of 16 of the 24 patients. They were then asked to re‐evaluate 11 cases, including the 8 that they previously did not agree on and 3 randomly selected for each expert to test intrarater repeatability. They were unaware about which 8 patients they had not agreed on. None of the experts changed opinion on the previously agreed cases and they now agreed on 7 of the 8 remaining cases. The last case was agreed upon after an open discussion. The experts found that PKG recordings indicated improvement in 12 patients, no change in three patients, and deterioration in 5 patients after dose adjustment. At least one of the PKG reports from four patients had insufficient data to allow comparison. The outcome distribution in the cases that could be evaluated (n = 20) was significantly different from a random distribution with a 1/3 likelihood for each outcome (χ2 = 6.70, P = 0.035). Only one of the 22 patients who had a technically acceptable PKG recording in the first period was found to be nonfluctuating, two only fluctuated regarding tremor, two displayed poor medication effect in the afternoon, and one was stable regarding bradykinesia but had continuous high dyskinesia scores. The other 16 displayed regular dose‐dependent hypo‐ and/or hyperkinetic motor fluctuations.
3.6. Adverse events
One patient reported vomiting, abdominal pain and nausea before the second visit while being off‐medication, and withdrew from the study. One patient had diarrhea and one patient experienced increased wearing‐off during daytime after introducing LC‐5. Both patients withdrew during the first period. Three serious adverse events occurred during the second period. One patient with a prior history of epilepsy was hospitalized due to a generalized seizure explained by nonadherence to the antiepileptic medication. There was one serious adverse event with migraine that resulted in an overnight hospital stay. One subject suffered a fatal cardiac arrest, and a previously unrecognized cardiac hypertrophy was found at autopsy. None of the serious adverse events were found to be directly related to the change of medication, although the change in medication routines may have contributed to the patient forgetting antiepileptic medication. The adverse events with diarrhea and increased wearing‐off were assessed as probably related to the change of medication.
4. DISCUSSION
Routine management of motor fluctuations in PD is based on the physicians' subjective evaluations and patients' accounts to optimize therapeutic effects. In the present study, patients with PD who were using levodopa at intervals of ≤4 hours significantly improved in MDS‐UPDRS II and III after introducing device‐assisted treatment with LC‐5 microtablets and PKG‐aided dose titration. The dose titration resulted in a 15% increase in daily levodopa intake and a 20‐minute decrease in dose intervals. Improvements were also observed with self‐rating instruments except for the EQ‐5D‐5L. Blinded evaluation of the PKG recordings indicated improved movement patterns in 12 patients (60% of assessable patients). The study suggests that PKG‐aided dose titration with LC‐5 improves symptom control in patients with PD. Although the value of the findings is limited by the open‐label observational study design, the study reflects a feasible approach to optimization of oral levodopa medication in outpatients with PD.
The improvement in MDS‐UPDRS part II and part III was relatively large in comparison with the change of 2.3 to 2.7 points that is considered to be a minimal clinically important difference on the UPDRS motor scores (part III).28 An improvement of 6.7 in PDQ‐8 index scores that was observed at the end of the study is a clinically relevant change.29 The generic quality of life measured with EQ‐5D‐5L showed no improvements, but may be less sensitive for detecting longitudinal changes in PD.30 It is clear from the self‐reported assessments that some improvements occurred during the first period of the study when patients used dosing schemes that were equivalent to their previous medication. The placebo effect of entering a study is an obvious explanation, but it is also likely that using the MyFID dose dispenser may have improved compliance with medication. The adherence recorded by the MyFID device was very high in almost all patients. Unfortunately, we do not know whether adherence improved compared to the patients' ordinary medication schedule from before the study. Previous studies indicate that the medication adherence in PD is mostly lower, at least for timing, even when the daily doses are fewer than the 6‐7 doses per day of the current study.7, 31
The aspect of adherence also has implications for interpreting the finding that summary PKG scores did not improve in period 2 compared to period 1. In our experience, a fair number of patients who use the PKG‐system report that they had less problems than normal because they were helped by the medication reminders from the PKG. During the study, reminders were used on either the MyFID device or on both PKG and MyFID device, thereby minimizing the risk of nonadherence to medication. The summary scores generated from PKG recordings reflect different aspects of movement‐related symptoms, but the recording also contains complex information about symptom variations in relation to the time of day. In this study, that information was used as the primary source for guiding dose titration and although the results were invariably confirmed in clinical interviews, patients on several occasion thought fluctuations were just part of life with PD and did not report them spontaneously or did not see patterns as clearly.
Although no single PKG summary score improved in the study, the blinded clinical evaluation of objective PKG measurements indicated significant improvement in movement profiles following dose titration. PKG is a surrogate measure focused on wrist movements, so improved PKG results are less important than improvements in global rating scales. It is nevertheless fundamental to the validity of using free‐living monitoring that improvements in movement patterns are associated with improvements in other outcome measures.
The daily levodopa dose was increased by roughly 100 mg after dose adjustment. The increase was distributed to periods of the day when the effect of levodopa was insufficient according to the accelerometry report. In this population, wearing‐off was the dominant problem and the PKG dyskinesia summary scores did not change after the dose adjustment. This suggests that the improved outcome was not achieved at a cost of increasing dyskinesia, but by achieving more time with appropriate medication effect. With a dose increase that “fills the gaps” in medication, the risk of inducing treatment‐related adverse effects should be small. However, the follow‐up time of this study was short and long‐term complications may take some time to develop. It would therefore be valuable to perform prospective randomized studies of long‐term effects of using home accelerometry monitoring to guide pharmacological management of PD.
One important aspect of this study is that the population was not recruited based on reported fluctuations, but on a maximum dose interval. The reason was the notion that motor fluctuations may be more common than what is appreciated by patients and physicians and that objective measures could be a way to detect fluctuation patterns that may otherwise escape the clinical assessment. This approach is practical as it uses an easily defined inclusion criterion which could be used for deciding when it is useful to evaluate a patient with ambulatory accelerometry like the PKG. A few patients without pronounced motor fluctuations were included, which may have led to an underestimation of the effect of dose titration. Another aspect is that the study population was very heterogeneous in terms of disease duration, disease severity, and number of daily levodopa doses. The total levodopa equivalent daily doses ranged between 497 and 2478 mg, and there were not any two patients that had the same dosing schedules at the start of the study. This reflects the diversity and differences between individual patients with PD and underlines the need for individualized dosing.
In conclusion, this open‐label study suggests that introducing a levodopa microtablet dispenser and individualized accelerometry‐guided dose adjustments can improve PD symptoms and disease‐related quality of health in the short term.
CONFLICT OF INTEREST
AJ is planning a study that Global Kinetics intends to support with a grant. FB has received honoraria from Global Kinetics Corporation for talks on the clinical use of PKG and has been a member of a European Steering Committee for the same company. JS is an employee of Sensidose AB and hold stock.
Supporting information
ACKNOWLEDGMENTS
We gratefully acknowledge all patients who participated in this study. This study was funded by Vinnova (2014‐03727), the Swedish Foundation for Strategic Research (Grant SBE 13‐0086) and the Swedish Government's Regional (ALF) Agreement on Research (Grant ALFGBG‐429901). Dose dispensers and study drug were provided free of charge by Sensidose AB, Sweden, and the accelerometry reports were provided free of charge by Global Kinetics Corporation, Australia.
Johansson D, Ericsson A, Johansson A, et al. Individualization of levodopa treatment using a microtablet dispenser and ambulatory accelerometry. CNS Neurosci Ther. 2018;24:439–447. 10.1111/cns.12807
REFERENCES
- 1. Gray R, Ives N, Rick C, et al. Long‐term effectiveness of dopamine agonists and monoamine oxidase B inhibitors compared with levodopa as initial treatment for Parkinson's disease (PD MED): a large, open‐label, pragmatic randomised trial. Lancet (London, England) 2014;384:1196‐1205. [DOI] [PubMed] [Google Scholar]
- 2. Ahlskog JE, Muenter MD. Frequency of levodopa‐related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord. 2001;16:448‐458. [DOI] [PubMed] [Google Scholar]
- 3. Lewitt PA. Levodopa for the treatment of Parkinson's disease. N Engl J Med. 2008;359:2468‐2476. [DOI] [PubMed] [Google Scholar]
- 4. Cedarbaum JM. Clinical pharmacokinetics of anti‐parkinsonian drugs. Clin Pharmacokinet. 1987;13:141‐178. [DOI] [PubMed] [Google Scholar]
- 5. Stone AA, Shiffman S, Schwartz JE, Broderick JE, Hufford MR. Patient compliance with paper and electronic diaries. Control Clin Trials. 2003;24:182‐199. [DOI] [PubMed] [Google Scholar]
- 6. Papapetropoulos SS. Patient diaries as a clinical endpoint in Parkinson's disease clinical trials. CNS Neurosci Ther. 2012;18:380‐387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grosset D, Antonini A, Canesi M, et al. Adherence to antiparkinson medication in a multicenter European study. Mov Disord. 2009;24:826‐832. [DOI] [PubMed] [Google Scholar]
- 8. Nyholm D, Stepien V. Levodopa fractionation in Parkinson's disease. J Parkinsons Dis. 2014;4:89‐96. [DOI] [PubMed] [Google Scholar]
- 9. Bennett JP Jr, Turk M, Landow E. Continuous oral administration of L‐dihydroxyphenylalanine (L‐DOPA) solution to patients with advanced Parkinson's disease. Clin Neuropharmacol. 1989;12:285‐292. [DOI] [PubMed] [Google Scholar]
- 10. Kurth MC, Tetrud JW, Irwin I, Lyness WH, Langston JW. Oral levodopa/carbidopa solution versus tablets in Parkinson's patients with severe fluctuations: a pilot study. Neurology. 1993;43:1036‐1039. [DOI] [PubMed] [Google Scholar]
- 11. Metman LV, Hoff J, Mouradian MM, Chase TN. Fluctuations in plasma levodopa and motor responses with liquid and tablet levodopa/carbidopa. Mov Disord. 1994;9:463‐465. [DOI] [PubMed] [Google Scholar]
- 12. Pappert EJ, Goetz CG, Niederman F, Ling ZD, Stebbins GT, Carvey PM. Liquid levodopa/carbidopa produces significant improvement in motor function without dyskinesia exacerbation. Neurology. 1996;47:1493‐1495. [DOI] [PubMed] [Google Scholar]
- 13. Yang HJ, Ehm G, Kim YE, et al. Liquid levodopa‐carbidopa in advanced Parkinson's disease with motor complications. J Neurol Sci. 2017;377:6‐11. [DOI] [PubMed] [Google Scholar]
- 14. Nyholm D, Ehrnebo M, Lewander T, et al. Frequent administration of levodopa/carbidopa microtablets vs levodopa/carbidopa/entacapone in healthy volunteers. Acta Neurol Scand. 2013;127:124‐132. [DOI] [PubMed] [Google Scholar]
- 15. Nyholm D, Lewander T, Gomes‐Trolin C, et al. Pharmacokinetics of levodopa/carbidopa microtablets versus levodopa/benserazide and levodopa/carbidopa in healthy volunteers. Clin Neuropharmacol. 2012;35:111‐117. [DOI] [PubMed] [Google Scholar]
- 16. Senek M, Aquilonius SM, Askmark H, et al. Levodopa/carbidopa microtablets in Parkinson's disease: a study of pharmacokinetics and blinded motor assessment. Eur J Clin Pharmacol. 2017;73:563‐571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Griffiths RI, Kotschet K, Arfon S, et al. Automated assessment of bradykinesia and dyskinesia in Parkinson's disease. J Parkinsons Dis. 2012;2:47‐55. [DOI] [PubMed] [Google Scholar]
- 18. Horne MK, McGregor S, Bergquist F. An objective fluctuation score for Parkinson's disease. PLoS ONE. 2015;10:e0124522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico‐pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181‐184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society‐sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS‐UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23:2129‐2170. [DOI] [PubMed] [Google Scholar]
- 21. Chaudhuri KR, Martinez‐Martin P, Schapira AH, et al. International multicenter pilot study of the first comprehensive self‐completed nonmotor symptoms questionnaire for Parkinson's disease: the NMSQuest study. Mov Disord. 2006;21:916‐923. [DOI] [PubMed] [Google Scholar]
- 22. Jenkinson C, Fitzpatrick R, Peto V, Greenhall R, Hyman N. The PDQ‐8: Development and validation of a short‐form parkinson's disease questionnaire. Psychol Health. 1997;12:805‐814. [Google Scholar]
- 23. Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five‐level version of EQ‐5D (EQ‐5D‐5L). Qual Life Res. 2011;20:1727‐1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wittrup‐Jensen KU, Lauridsen J, Gudex C, Pedersen KM. Generation of a Danish TTO value set for EQ‐5D health states. Scand J Public Health. 2009;37:459‐466. [DOI] [PubMed] [Google Scholar]
- 25. Stacy M, Bowron A, Guttman M, et al. Identification of motor and nonmotor wearing‐off in Parkinson's disease: comparison of a patient questionnaire versus a clinician assessment. Mov Disord. 2005;20:726‐733. [DOI] [PubMed] [Google Scholar]
- 26. Fantino B, Moore N. The self‐reported Montgomery‐Asberg depression rating scale is a useful evaluative tool in major depressive disorder. BMC Psychiatry. 2009;9:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382‐389. [DOI] [PubMed] [Google Scholar]
- 28. Shulman LM, Gruber‐Baldini AL, Anderson KE, Fishman PS, Reich SG, Weiner WJ. The clinically important difference on the unified Parkinson's disease rating scale. Arch Neurology. 2010;67:64‐70. [DOI] [PubMed] [Google Scholar]
- 29. Horvath K, Aschermann Z, Kovacs M, et al. Changes in quality of life in Parkinson's disease: How large must they be to be relevant? Neuroepidemiology. 2017;48:1‐8. [DOI] [PubMed] [Google Scholar]
- 30. Reuther M, Spottke EA, Klotsche J, et al. Assessing health‐related quality of life in patients with Parkinson's disease in a prospective longitudinal study. Parkinsonism Relat Disord. 2007;13:108‐114. [DOI] [PubMed] [Google Scholar]
- 31. Leopold NA, Polansky M, Hurka MR. Drug adherence in Parkinson's disease. Mov Disord. 2004;19:513‐517. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


