Abstract
The Takotsubo cardiomyopathy is often considered autochthonous to the heart, although the primary problem may be not in the heart muscle itself. Instead, similar to several Takotsubo‐like cardiac pathologies seen in acute neurological diseases, it may reflect the capacity of the nervous system to injure the heart. Persuasive evidence exists that shocking emotional stress promotes direct heart injuries. Moreover, clinical and laboratory research shows that cardiac structural damage can occur in the presence of a normal heart, especially in the context of seizures, stroke, and traumatic brain injury or under conditions of psychological stress. The aim of this review is to summarize the clinical implications of these observations, several of which focus on the pivotal role of the insula of Reil in the brain‐heart connection, to unravel the mystery of Takotsubo cardiomyopathy pathogenesis.
Keywords: Insula of Reil, QTc Interval, Takotsubo Cardiomyopathy, Ventricular Repolarization
1. INTRODUCTION
Raconteur's reports of heart disease and sudden death under emotional stress always stimulate the people's imagination and scientific investigation. Fear and grief are deep emotions that play, through the brain system, an important role on the heart. In 1942, Walter Bradford Cannon, pioneer physiologist of human emotions, published an interesting article titled “Voodoo Death,“1 in which he postulated that “voodoo death” is real and may be explained as due to shocking emotional stress by an intense reaction of the sympathetic‐adrenergic system to obvious or repressed terror. This voodoo death is not a phenomenon of a primitive people forgotten by time; as the sudden death or heart disease attributed to disruptive life events or during acute grief for the loss of a great love or a close relation, during mourning, war, or natural catastrophes such as earthquakes, this phenomenon can happen to civilized people too. Up to the present day, it is surprising that the evidence is more impressive than clear in mankind. Takotsubo cardiomyopathy (TC) is probably the most remarkable example of “heartache” and may offer a plausible explanation of how shocking emotional stress promotes direct heart injuries, for this is also referred to as “stress cardiomyopathy” or “broken heart.” The etiology of TC is not yet fully elucidated.
The purpose of this article is to focus on 3 signa to unlock the mystery of the pathogenesis of Takotsubo syndrome: (1) the prolongation of corrected QT (QTc) interval, (2) the trigger role of emotional stress, and (3) the typical pattern of wall motion incongruent with the coronary artery supply region.
2. BACKGROUND
The term Takotsubo cardiomyopathy was coined for the first time by Dr Hikaru Sato2 in 1990, to describe the similarity of a peculiar regional left ventricular (LV) dysfunction to “tako‐tsubo” (the name for an ancient Japanese vase used in octopus fishing). But probably some clinicians already knew it before the actual name was assigned.3 Many alternative names have been used, including stress‐induced cardiomyopathy and broken‐heart syndrome, to express a link with stress. It is often triggered by strong emotions related to accidents, quarrels, unexpected death of a close relation, fear or anxiety, work‐related problems, catastrophic news, or physical stress due to medical or surgical procedures. It predominantly affects women, and 95% of female patients accounted for in the Rhode Island Takotsubo Cardiomyopathy Registry were postmenopausal.4, 5
The TC is usually characterized by reversible regional LV dysfunction with apical ballooning and hyperkinesis of the basal LV segments on echocardiography or ventriculogram. A prolonged QTc interval is detectable in a substantial proportion of patients (47.7%).4 The clinical presentation, with chest pain, dyspnea, electrocardiographic (ECG) ST‐segment changes, and elevated troponin, may mimic myocardial infarction (MI); however, no significant obstructions are detected in coronary vessels. Misdiagnosis represents a considerable clinical problem, and on account of this some patients may incorrectly take unnecessary medications that may trigger eventual side effects.
The TC prognosis was initially thought to be benign, but subsequently large studies and registries have demonstrated that both short‐term mortality and long‐term mortality are higher than previously recognized.6, 7 Templin et al4 reported a 30‐day mortality of 5.9% and a long‐term death rate of 5.6% per patient per year. Moreover, some patients are at risk for recurrence even a few years after the first event. So far, although there has been an exponential increase in the number of articles reporting on the disease around the world, its cause and pathogenesis are still unknown. Several pathogenetic mechanisms have been debated: (1) coronary artery vasospasm, (2) coronary microcirculation dysfunction, (3) obstruction of the LV outflow tract, and (4) increased blood catecholamines. The result of all these hypotheses, however, is quite inconsistent because (1) the apical and mid‐ventricular wall motion does not match with an epicardial coronary artery distribution, (2) the histopathological changes of contraction band necrosis are not congruent with MI, and (3) the plasma catecholamines in the majority of patients with TC show normal or near‐normal values.8
3. DISCUSSION
Since the introduction of the term, anecdotal case series of TC triggered by different types of acute brain diseases and injuries have been described in literature: intracranial hemorrhage, subarachnoid hemorrhage, stroke, epilepsy, Alzheimer disease, head injury, and limbic encephalitis.9, 10, 11, 12, 13, 14 This is not a trivial coincidence, because the patients with TC have a higher prevalence (55.8%) of neurologic or psychiatric disorders.4 There is a strong link between cerebral disease and the heart,15 and the capacity of the brain to injure the heart has been previously investigated, mostly with ECG repolarization abnormalities reported in association with cerebrovascular disease.
The characteristic form known today as the Wellens ECG pattern was observed by serendipity more than half a century ago and consisted essentially of T waves usually negative in anterior precordial leads of considerable amplitude and width and a long QT interval.16 The QTc interval is the ECG manifestation of ventricular depolarization and repolarization, and the autonomic nervous system is an important modulator of ventricular repolarization. The autonomic dysfunction develops in patients with acute brain injury; for this reason, the lengthening of the QTc interval represents one of the most frequent ECG changes in these circumstances. The increased QTc interval frequently observed in patients with TC is not a mere curiosity, but it may be a signum of the brain on the heart as well. Mugnai et al17 observed a QTc >500 ms in 4 patients (17.4%) on admission and in 21 patients (91.3%) during the subacute phase of TC. An admission prolonged QTc interval, however, is a significant predictor of rehospitalization at follow‐up.18 The time course of ECG repolarization changes remains unclear during the clinical phase of this disorder.19 Perazzolo et al20 speculated that the transmural dispersion of repolarization induced by the apicobasal gradient of myocardial edema could play a role in the electrogenesis of the Wellens ECG pattern, as evidenced by the parallel time course of dynamic T‐wave inversion/QT‐interval prolongation and transient LV myocardial edema. The TC is considered, according to some authors, among the causes of acquired long QT syndrome21, 22; therefore, as a prudential practice, it would be better to avoid the occurrence of other risk factors (such as electrolyte imbalance, bradycardia, the use of QT‐prolonging drugs) to prevent the possibility of malignant ventricular proarrhythmia. The QTc interval prolongation in the presence of normal heart de facto may be cerebrogenic, resulting from sympathetic overexcitation by way of cortical activation or damage.
Recent attention has shifted to the insular cortex (IC) as a possible cortical generating site of some of these repolarization changes. The cardiovascular function is regulated by a complex network of cortical, subcortical, and peripheral areas with hierarchical distribution. A key role in this circuitry is played by the IC, a strategic area of the brain that has dense connections with regions in the limbic/paralimbic systems, thalamus, and hypothalamus, as well as in the frontal, temporal, and parietal lobes. The insula of Reil was first named in 1796 by J.C. Reil, prominent German physician, anatomist, physiologist, and professor at the universities of Halle and Berlin in his masterpiece Exercitationum anatomicarum fasciculus primus de structura nervorum (First Volume of Anatomical Practice: On the Structure of Nerves).23 The anatomy and its relationship with cardiovascular system regulation has been investigated extensively by Oppenheimer.24, 25, 26 The right insula seems to exhibit a sympathetic dominance; in fact, the stimulation induces heart rate increase and/or pressor response; on the contrary, the stimulation of the left insula leads to increased parasympathetic tone with heart rate decrease and/or depressor responses. Pressor responses are known to occur with rostral IC stimulation, and depressor effects with caudal insular stimulation. Phasic microstimulation of the rat left insula has resulted in QTc interval prolongation.24 In humans, unlike the rat, the right IC is involved in cardiovascular sympathetic control.25 In this respect, recent studies suggest that IC in the setting of ischemic stroke,27 hepatic encephalopathy,28 and multiple sclerosis29 could play a major role in the pathogenesis of cerebrogenic long QTc interval. The right insular infarction patients were significantly more likely to show a higher incidence of prolonged QTc interval (53% vs 35%).30
Interestingly, TC has also been identified after ischemic stroke, especially that involving the right IC.31, 32 This proposition cannot be regarded as accidental. The IC, which in humans lies beneath the frontoparietal and temporal opercula, has profuse reciprocal connectivity with the limbic system, which is predominantly involved in emotional control.33 Furthermore, the demonstration of a cardiac chronotropic organization within the rat IC indicates how emotional stresses alter cardiac autonomic tone.25 This role of the IC may further explain why an emotional or a physical stressor trigger has been reported in more than two‐thirds of patients with TC.4 It is interesting to note that mental stress has been shown to increase the QT interval and cardiac sympathetic tone34 and that the TC has been described in Japan, where most earthquakes occur. Curiously, the number of patients with TC increased in the 4 weeks after the 2004 Niigata Chuetsu earthquake to 25 cases, compared with only 1 case reported in the 4 weeks before, none in 2003, and 1 in 2002.35 Moreover, the prevalence of depression and anxiety disorders in patients with TC ranges from 21% to 60%,36 and in this regard the IC has been proven to be important in the pathophysiological mechanisms of various neuropsychiatric diseases, such as panic and posttraumatic stress disorders. This remarkable proposition might explain the observed recurrences in patients with TC and persistent adverse psychological factors after the acute episode.
Endomyocardial biopsies in some TC patients demonstrated structural changes associated with a particular histological pattern, such as postmortem cardiac lesions observed in patients with subarachnoid hemorrhage in which the cell dies in a hypercontracted state with early myofibrillar damage and anomalous irregular cross‐band formations.37 These cardiac lesions, termed myocytolysis,38 do not resemble those seen in cardiac ischemia, and there is strong evidence that catecholamines are involved in the etiology. The overexcitation of the sympathetic nervous system mediated through the IC mimics cardiac damage and ECG abnormalities involving repolarization that occur in TC. Prolonged stimulation within the left IC in rats induced QT prolongation associated with myocytolysis; these changes were associated with a significant increase in levels of plasma norepinephrine, which is indicative of a neurally mediated mechanism.20 The above findings suggest that intracranial pathology with insular damage may produce the same myocardial histological changes with prolonged QTc interval seen in TC.39 Using histochemical techniques, similar lesions were identified by forensic pathologists in the hearts of patients who died under conditions of extreme stress (such as a homicidal assault) in the absence of brain trauma.40 Interestingly, on electron microscopic examination, Greenhoot noted that these abnormalities were not centered around the coronary artery, but around the end of intracardiac nerves, implying a neural etiology rather than a cardiac, vascular, or humoral mechanism caused by a blood‐borne route.41, 42 Norberg showed in the heart a nervous network containing norepinephrine.43 Moreover, Kume et al. found increased norepinephrine levels (derived from sympathetic terminal nerves) on direct sampling of coronary sinus blood during angiography in patients with TC.44 This argument supports the hypothesis that the QTc prolongation and cardiac lesions in these patients have a neural signature due to intracardiac release of catecholamines.
Last, the characteristic pattern of ventricular wall‐motion abnormality in TC does not match with a coronary artery system and appears most likely to be correlated with the distribution of myocardial sympathetic nerve terminals,45 providing a further evidence for a neurally mediated mechanism of cardiac injury. We know the precise anatomical distribution of the various branches of the cardiac sympathetic nervous system in the heart thanks to Antonio Scarpa, acclaimed anatomist and neurologist. He was born on May 9, 1752, in Lorenzaga di Motta di Livenza, in the northeastern region of Italy. At the age of 18, he graduated with honors in medicine at the University of Padua and became professor and head of the department of anatomy and surgery at the University of Modena just 2 years later, in 1772. In 1794 he published his greatest work, Tabulae Neurologicae Ad Illustrandam Historiam Anatomicam, Cardiacorum Nervorum, Noni Nervorum Cerebri, Glossopharyngaei, Et Pharyngaei Ex Octavo Cerebri,46 and for the first time in history he showed that the terminal ramifications of the cardiac nerves were directly connected to the muscle fibers of the heart. The efferent neural ramifications7–10,14–17 of cardiac plexuses depicted in the magnificent medical illustration by Scarpa are curiously adjacent to areas of regional LV dysfunction (Figure 1). These observations strengthen the postulate of a neural origin of typical wall motion in TC; in any case, individual differences in the anatomy of cardiac sympathetic innervation might result in the involvement of a variety of LV myocardial segments and explain the anatomical variants (basal and mid‐ventricular).
Figure 1.
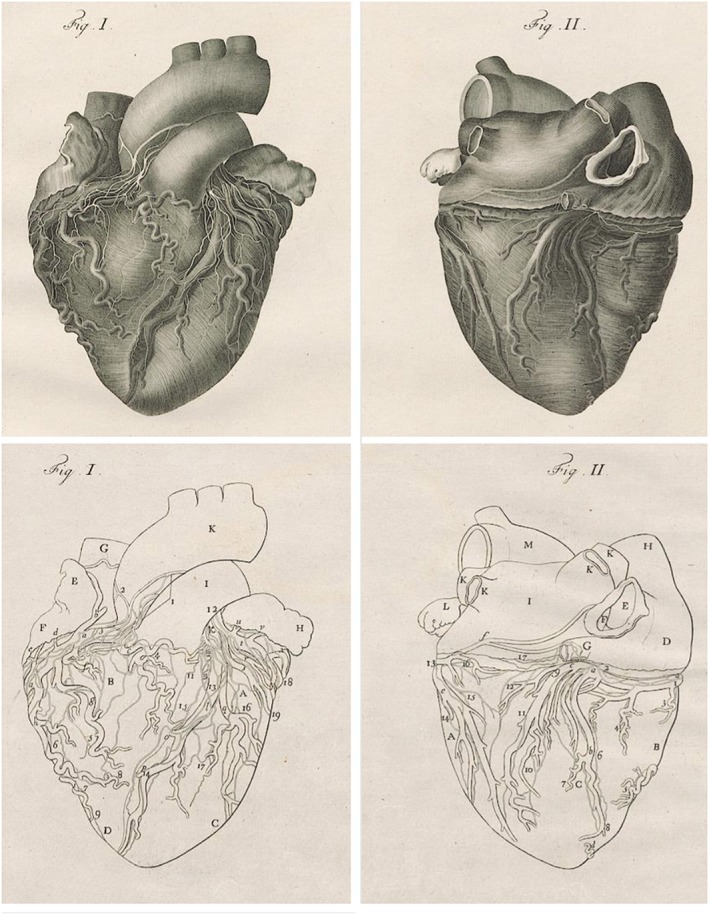
Magnificent copper engravings of cardiac nervi and arteriae coronariae in cordis facies superior (left) and complanata (right) depicted by Faustino Anderloni after Antonio Scarpa's own drawings in Tabulae Neurologicae Ad Illustrandam Historiam Anatomicam (1794).46 Scarpa first accurately illustrated the innervation of the heart, showing in this atlas that the terminal ramifications of the cardiac nerves are directly connected to the muscle fibers of the heart. Letter and number abbreviations name, respectively, coronary vessels and cardiac nerves; further explanations in the original text. University Library Heidelberg, Germany. Image in the public domain from wikisource: http://digi.ub.uni‐heidelberg.de/diglit/scarpa1794
4. CONCLUSION
“We have not power of thinking without signs”, the frequent association with cerebral disease and sudden emotional stress suggests with an abductive reasoning a new hypothesis in TC pathogenesis: the brain, with insula of Reil, beyond the heart. The long QTc interval observed in TC might be cerebrogenic and expresses the brain capacity to injure the heart. The mechanism of transient ventricular myocardial dysfunction could be sympathetically mediated through proximal release of noradrenaline from the terminal nerves of the myocardium. The typical wall‐motion pattern is congruent with cardiac nerves distribution illustrated by Antonio Scarpa. Finally, we have to discontinue using this imprecise term “Takostubo cardiomyopathy,” because the primary problem is not in the heart muscle itself, and rename it “neurocardiac syndrome.” Because every cognition is determined logically by previous cognitions, if the proposed induction and hypothesis are valid, we should reverse the diagnostic and therapeutic paradigm from the heart to the brain, investigating the insula of Reil, so we may significantly contribute to improved patient management and outcomes.
4.1. Conflicts of interest
The authors declare no potential conflicts of interest.
Marafioti V, Turri G, Carbone V, Monaco S. Association of prolonged QTc interval with Takotsubo cardiomyopathy: A neurocardiac syndrome inside the mystery of the insula of Reil. Clin Cardiol. 2018;41:551–555. 10.1002/clc.22910
REFERENCES
- 1. Cannon WB. “Voodoo” death. Am Anthropol. 1942;44:169–181. [Google Scholar]
- 2. Sato H, Tateishi H, Dote K, et al. Tako‐tsubo‐like left ventricular dysfunction due to multivessel coronary spasm In: Kodama K, Haze K, Hori M. (eds). Clinical Aspect of Myocardial Injury: From Ischemia to Heart Failure. Tokyo: Kagakuhyoronsha; 1990:56–64. [Google Scholar]
- 3. Case records of the Massachusetts General Hospital . Weekly clinicopathological exercises. Case 18–1986. A 44‐year‐old woman with substernal pain and pulmonary edema after severe emotional stress. N Engl J Med. 1986;314(19):1240–1247. [DOI] [PubMed] [Google Scholar]
- 4. Templin C, Ghadri JR, Diekmann J, et al. Clinical features and outcomes of Takotsubo (stress) cardiomyopathy. N Engl J Med. 2015;373:929–938. [DOI] [PubMed] [Google Scholar]
- 5. Regnante RA, Zuzek RW, Weinsier SB, et al. Clinical characteristics and four‐year outcomes of patients in the Rhode Island Takotsubo Cardiomyopathy Registry. Am J Cardiol. 2009;103:1015–1019. [DOI] [PubMed] [Google Scholar]
- 6. Lyon AR, Bossone E, Schneider B, et al. Current state of knowledge on Takotsubo syndrome: a position statement from the Task Force on Takotsubo Syndrome of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2016;18:8–27. [DOI] [PubMed] [Google Scholar]
- 7. Pelliccia F, Kaski JC, Crea F, et al. Pathophysiology of Takotsubo syndrome. Circulation. 2017;135:2426–2441. [DOI] [PubMed] [Google Scholar]
- 8. Y‐Hassan S, Henareh L. Plasma catecholamine levels in patients with takotsubo syndrome: implications for the pathogenesis of the disease. Int J Cardiol. 2015;181:35–38. [DOI] [PubMed] [Google Scholar]
- 9. Finsterer J, Wahbi K. CNS disease triggering takotsubo stress cardiomyopathy. Int J Cardiol. 2014;177:322–329. [DOI] [PubMed] [Google Scholar]
- 10. Y‐Hassan S. Takotsubo syndrome triggered by an epileptic seizure may be the cause of abnormal cardiac repolarization seen in patients with epilepsy. Epilepsia. 2011;52:654–655. [DOI] [PubMed] [Google Scholar]
- 11. Mayer SA, Lin J, Homma S, et al. Myocardial injury and left ventricular performance after subarachnoid hemorrhage. Stroke. 1999;30:780–786. [DOI] [PubMed] [Google Scholar]
- 12. Wang TD, Wu CC, Lee YT. Myocardial stunning after cerebral infarction. Int J Cardiol. 1997;58:308–311. [DOI] [PubMed] [Google Scholar]
- 13. Pho KK, Chan MY, Chiba BL. Images in cardiology: reversible left ventricular apical ballooning after head injury. Clin Cardiol. 2005;28:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gelow J, Kruer M, Yadav V, et al. Apical ballooning resulting from limbic encephalitis. Am J Med. 2009;122:583–586. [DOI] [PubMed] [Google Scholar]
- 15. Samuels MA. The brain‐heart connection. Circulation. 2007;116:77–84. [DOI] [PubMed] [Google Scholar]
- 16. Byer E, Ashman R, Toth LA. Electrocardiogram with large, upright T waves and long Q‐T intervals. Am Heart J. 1947;33:796–806. [DOI] [PubMed] [Google Scholar]
- 17. Mugnai G, Vassanelli F, Pasqualin G, et al. Dynamic changes of repolarization abnormalities in takotsubo cardiomyopathy. Acta Cardiol. 2015;70:225–232. [DOI] [PubMed] [Google Scholar]
- 18. Santoro F, Brunetti ND, Tarantino N, et al. Dynamic changes of QTc interval and prognostic significance in takotsubo (stress) cardiomyopathy. Clin Cardiol. 2017;40:1116–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mitsuma W, Kodama M, Ito M, et al. Serial electrocardiographic findings in women with Takotsubo cardiomyopathy. Am J Cardiol. 2007;100:106–109. [DOI] [PubMed] [Google Scholar]
- 20. Perazzolo Marra M, Zorzi A, Corbetti F, et al. Apicobasal gradient of left ventricular myocardial edema underlies transient T‐wave inversion and QT interval prolongation (Wellens' ECG pattern) in Tako‐Tsubo cardiomyopathy. Heart Rhythm. 2013;10:70–77. [DOI] [PubMed] [Google Scholar]
- 21. Ghosh S, Apte P, Maroz N, et al. Takotsubo cardiomyopathy as a potential cause of long QT syndrome and torsades de pointes. Int J Cardiol. 2009;136:225–227. [DOI] [PubMed] [Google Scholar]
- 22. Madias C, Fitzgibbons TP, Alsheikh‐Ali AA, et al. Acquired long QT syndrome from stress cardiomyopathy is associated with ventricular arrhythmias and torsades de pointes. Heart Rhythm. 2011;8:555–561. [DOI] [PubMed] [Google Scholar]
- 23. Reil JC. First Volume of Anatomical Practice: On the Structure of Nerves. Halle, Halae Saxonum, In Officina Curtiana Venalis; 1796. [Google Scholar]
- 24. Oppenheimer SM, Wilson JX, Guiraudon C, et al. Insular cortex stimulation produces lethal cardiac arrhythmias: a mechanism of sudden death? Brain Res. 1991;550:115–121. [DOI] [PubMed] [Google Scholar]
- 25. Oppenheimer SM, Gelb A, Girvin JP, et al. Cardiovascular effects of human insular cortex stimulation. Neurology. 1992;42:1727–1732. [DOI] [PubMed] [Google Scholar]
- 26. Oppenheimer S, Cechetto D. The insular cortex and the regulation of cardiac function. Compr Physiol. 2016;6:1081–1133. [DOI] [PubMed] [Google Scholar]
- 27. Marafioti V, Turri G, Rossi A, et al. Prolonged QTc interval and insula in patients with ischemic stroke: inductive or abductive reasoning? Int J Cardiol. 2014;176:1203–1204. [DOI] [PubMed] [Google Scholar]
- 28. Marafioti V, Benetti V, Montin U, et al. QTc interval prolongation and hepatic encephalopathy in patient candidates for liver transplantation: a valid inference? Int J Cardiol. 2015;188:43–44. [DOI] [PubMed] [Google Scholar]
- 29. Turri G, Calabrese M, Pancheri E, et al. QTc interval in patients with multiple sclerosis: an inference from the insula of Reil? Eur J Neurol. 2017;24:491–496. [DOI] [PubMed] [Google Scholar]
- 30. Sander D, Klingelhöfer J. Changes of circadian blood pressure patterns and cardiovascular parameters indicate lateralization of sympathetic activation following hemispheric brain infarction. J Neurol. 1995;242:313–318. [DOI] [PubMed] [Google Scholar]
- 31. Yoshimura S, Toyoda K, Ohara T, et al. Takotsubo cardiomyopathy in acute ischemic stroke. Ann Neurol. 2008;64:547–554. [DOI] [PubMed] [Google Scholar]
- 32. Jung JM, Kim JG, Kim JB, et al. Takotsubo‐like myocardial dysfunction in ischemic stroke: a hospital‐based registry and systematic literature review. Stroke. 2016;47:2729–2736. [DOI] [PubMed] [Google Scholar]
- 33. Yasui Y, Breder, CD , Saper CB, et al. Autonomic responses and efferent pathways from the insular cortex in the rat. J Comp Neurol. 1991;303:355–374. [DOI] [PubMed] [Google Scholar]
- 34. Negoescu R, Dinca‐Panaitescu S, Filcescu V, et al. Mental stress enhances the sympathetic fraction of QT variability in an RR‐independent way. Integr Physiol Behav Sci. 1997;32:220–227. [DOI] [PubMed] [Google Scholar]
- 35. Watanabe H, Kodama M, Okura Y, et al. Impact of earthquakes on Takotsubo cardiomyopathy. JAMA. 2005;294:305–307. [DOI] [PubMed] [Google Scholar]
- 36. Smeijers L, Szabó BM, Kop WJ, et al. Psychological distress and personality factors in takotsubo cardiomyopathy. Net Heart J. 2016;24:530–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bybee KA, Prasad A. Stress‐related cardiomyopathy syndromes. Circulation. 2008;118:397–409. [DOI] [PubMed] [Google Scholar]
- 38. Baroldi F. Different morphological types of myocardial cell death in man In: Fleckstein A, Rona G, eds. Recent Advances in Studies in Cardiac Structure and Metabolism: Pathophysiology and Morphology of Myocardial Cell Alteration. Vol. 6 Baltimore, MD: University Park Press; 1975. [PubMed] [Google Scholar]
- 39. Oppenheimer S. Cortical control of the heart. Cleve Clin J Med. 2007;74(suppl 1):S27–S29. [DOI] [PubMed] [Google Scholar]
- 40. Cebelin MS, Hirsch CS. Human stress cardiomyopathy: myocardial lesions in victims of homicidal assaults without internal injuries. Hum Pathol. 1980;11:123–132. [DOI] [PubMed] [Google Scholar]
- 41. Greenhoot JH, Reichenbach DD. Cardiac injury and subarachnoid hemorrhage, a clinical, pathological, and physiological correlation. J Neurosurg. 1969;30:521–531. [DOI] [PubMed] [Google Scholar]
- 42. Randall DC, Kaye MP, Randall WC, et al. Response of primate heart to emotional stress before and after cardiac denervation. Am J Physiol. 1976;230:988–995. [DOI] [PubMed] [Google Scholar]
- 43. Norberg KA. Transmitter histochemistry of the sympathetic adrenergic nervous system. Brain Res. 1967;5:125–170. [DOI] [PubMed] [Google Scholar]
- 44. Kume T, Kawamoto T, Okura H, et al. Local release of catecholamines from the hearts of patients with tako‐tsubo‐like left ventricular dysfunction. Circ J. 2008;72:106–108. [DOI] [PubMed] [Google Scholar]
- 45. Randall WC, Priola DV, Ulmer RH. A functional study of distribution of cardiac sympathetic nerves. Am J Physiol. 1963;205:1227–1231. [DOI] [PubMed] [Google Scholar]
- 46. Scarpa A. Tabulae Neurologicae Ad Illustrandam Historiam Anatomicam, Cardiacorum Nervorum, Noni Nervorum Cerebri, Glossopharyngaei, Et Pharyngaei Ex Octavo Cerebri. Ticini: Balthasarem Comini; 1794. [Google Scholar]


