Summary
Aims
The treatment of schizophrenia with antipsychotics is still unsatisfactory. Therefore, the search for new treatments and prevention is crucial, and animal models are fundamental tools for this objective. Preclinical and clinical data evidence the antipsychotic profile of sodium nitroprusside (SNP), a nitric oxide (NO) donor. We aimed to investigate SNP in treating and/or preventing the schizophrenia‐related behaviors presented by the spontaneously hypertensive rats (SHR) strain.
Methods
Wistar rats (WR) and SHRs were submitted to two schemes of treatment: (i) a single injection of SNP or vehicle in adulthood; (ii) a long‐term early treatment from 30 to 60 postnatal day with SNP or vehicle. The following behaviors were evaluated 24 hours after the acute treatment or 30 days after the long‐term treatment: locomotion, social interaction, and contextual fear conditioning.
Results
Spontaneously hypertensive rats presented hyperlocomotion, decreased social interaction, and impaired contextual fear conditioning. Single injection of SNP decreased social interaction in both strains and induced a deficit in contextual fear conditioning in WR. Oppositely, early treatment with SNP prevented the behavioral abnormalities in adult SHRs without promoting any effects in WR.
Conclusion
Our preclinical data point to SNP as a preventive and safe strategy with a broad range of effectiveness to the positive, negative, and cognitive symptoms of schizophrenia.
Keywords: animal models, prevention, schizophrenia, SHR strain, sodium nitroprusside
1. INTRODUCTION
Schizophrenia is a debilitating disease that affects about 1% of the world population.1 The symptomatology is characterized by positive and negative symptoms as well as cognitive deficits.2 The onset of the disorder is marked by the first psychotic episode at late adolescence/early adulthood and is preceded by a prodromal phase characterized by attenuated negative and cognitive symptoms.3 Although the development of antipsychotic medications that block dopaminergic type 2 receptors represented an advance in pharmacotherapy of schizophrenia, it still poses important limitations. They ameliorate mainly positive symptoms and are associated with severe motor and/or metabolic side effects and high rates of treatment nonresponse.4, 5 In this sense, the search for new treatments and, perhaps more important, for preventive strategies is crucial. Individuals with prodromal signs or recent functional decline associated with genetic risk are considered at ultra‐high risk for developing schizophrenia.6 In accordance, efforts to develop preventive and safe strategies for ultra‐high‐risk individuals have been made.7
Pharmacological, biochemical, and genetic data support the role of the nitrergic system in the pathophysiology of schizophrenia.8, 9, 10, 11 The blockade of glutamatergic NMDA receptors, which leads to a decrease in nitric oxide (NO) production and consequently to GMPc levels, has been extensively linked to schizophrenia. In parallel, while a diminished level of NO metabolites has been found in the serum and cerebrospinal fluid in schizophrenic patients, antipsychotics increase serum levels of NO. In addition, postmortem studies have shown alterations in the nitric oxide synthase activity in different brain regions, and polymorphisms of the neuronal nitric oxide synthase have been suggested as a risk factor for schizophrenia. Strikingly, recent evidence points to the antipsychotic effect of sodium nitroprusside (SNP), a nitric oxide donor. In a double‐blind clinical trial, acutely relapsed schizophrenia patients present a long‐lasting improvement of positive, negative, depressive, and anxiety symptoms after a single infusion of SNP.12 In addition, cognitive deficits in these patients were also ameliorated by SNP.13 Moreover, these antipsychotic effects of SNP were described in pharmacological animal models in which the schizophrenia‐related behaviors are induced by dopaminergic agonists or glutamatergic NMDA antagonists—for review, see.11 Nevertheless, the preventive long‐term effect of an early repeated treatment with SNP has not yet been evaluated.
In contrast to the pharmacological schizophrenia animal models, the spontaneously hypertensive rats (SHR) strain presents spontaneous schizophrenia‐related behavioral abnormalities: increase in locomotion (modeling positive symptoms), decreased social interaction (mimicking negative symptoms), and deficits in contextual fear conditioning (an emotional memory task). These behavioral alterations are specifically improved by antipsychotics and aggravated by proschizophrenia manipulations.14, 15, 16, 17 Additionally, the course of these behavioral abnormalities is in accordance with what is seen in the clinic: hyperlocomotion is presented only in adult SHRs while deficits in social interaction and in fear conditioning can be seen in 30‐ and 45‐day‐old SHRs, respectively (S.T.Niigaki, F.F.Peres, D.A.Gouvea, R.Levin, V.Almeida, N.D.Silva, M.C.Diana, M.A.Suiama, V.C.Abilio submitted). Interestingly, cardiovascular comorbidities, including hypertension, are presented in schizophrenia patients.18 Some functional and neurochemical alterations seen in the SHR strain underlie schizophrenia physiopathology and may lead to neurovascular changes and, putatively, be related to the behavioral abnormalities presented by this strain: an increase in brain and vascular oxidative stress,18, 19 an increase in neuroinflammation,18, 20 and a hyperpermeability of the blood‐brain and blood‐cerebrospinal fluid barrier.18, 21, 22 Considering specifically the nitrergic system, a decrease in brain and endothelial levels of NO and/or NOS activity have been reported in the SHR strain.23, 24 In addition, we have reported in the SHR strain a decrease in the gene expression of glutamate AMPA‐R (Gria1) and NMDA‐R (Grin1) in the nucleus accumbens as well as in Gad2 (glutamate decarboxylase 2), Chrnb4 (cholinergic receptor, nicotinic, beta 4), Slc5a7 (choline transporter), and Qrfpr (pyroglutamylated RFamide peptide receptor) in the prefrontal cortex. We suggested that these alterations might be related to the behavioral abnormalities presented by the SHR strain.25, 26 Accordingly, this animal model has been used to investigate potential new treatments as well as early preventive strategies.27, 28, 29, 30, 31, 32 Therefore, the aim of the present work was to investigate the potential antipsychotic effect of SNP in treating and/or preventing the behavioral abnormalities presented by the SHR strain.
2. METHODS
2.1. Animals
Adult (4‐month‐old) and young (1‐month‐old) male Wistar rats (WRs) and SHRs from our own colony were used. SHR strain was developed by selecting WRs with hypertensive phenotype and brother‐sister mating.33 Previous experiments show that SHRs from our colony develop hypertension around 60 days of age (mean and standard error for 60‐day‐old WRs and SHRs, respectively, 155.58 ± 3.30 and 179.67 ± 3.10 mm Hg) remaining hypertensive in adulthood (mean and standard error for 90‐day‐old WRs and SHRs, respectively, 155.38 ± 3.46 and 184.08 ± 4.33 mm Hg).
Animals were maintained in groups of five in Plexiglas cages (41 × 34 × 16.5 cm) under controlled environmental conditions (22‐23°C, light/dark cycle: lights on 6:30 am‐6:30 pm) with food and water available ad libitum. This study was approved by the Ethics Committee of Federal University of São Paulo (N 526654). Procedures followed the guidelines of the Committee on Care and Use of Laboratory Animal Resources, National Research Council, USA.
2.2. Drugs and experimental design
Sodium nitroprusside (NITROP—Hypofarma, Brazil) was diluted in vehicle (0.9% NaCl saline solution). SNP and its vehicle were administered intraperitoneally in a volume of 1.0 mL/kg.
In experiment 1, adult (4‐month‐old) WR and SHR (n = 8‐10/group) received a single injection of VEH or SNP (0.5, 2.5 or 5.0 mg/kg). Twenty‐four hours later, rats were submitted to the assessment of locomotor activity and social interaction, followed by the training session of contextual fear conditioning. On the next day, they were submitted to the testing session of contextual fear conditioning.
In experiment 2, WRs and SHRs (n = 8‐10/group) were treated with VEH or SNP (0.5, 1.0 or 2.5 mg/kg) from 30 to 60 postnatal day (PND). Body weight was assessed every 4‐5 days during treatment. Schizophrenia‐related behaviors were assessed in adulthood (PND 90‐100), with social interaction and locomotor activity being evaluated prior to the contextual fear conditioning task (Figure 1). A decrease in the doses used in this experiment was performed based on a possible effect in general activity with the highest dose used in experiment 1.
Figure 1.
Experimental design used in experiment 2 to evaluate the preventive effects of sodium nitroprusside (SNP) on the spontaneously hypertensive rat (SHR) strain
The route of administration, volume, and doses were chosen based on previous work showing beneficial effects of SNP in animal models of schizophrenia.34, 35, 36, 37 The interval between acute administration of SNP and the behavioral evaluations (24 hours—experiment 1) was based on previous clinical and preclinical studies evidencing the beneficial effect of a single injection of SNP 4 weeks later in patients and up to 1 week later in rats.12, 38
2.3. Schizophrenia‐related behavioral analyses
All behavioral assessments were recorded and analyzed by trained observers—previously submitted to evaluation of concordance—that were blind to rats’ experimental condition.
2.3.1. Social interaction and locomotor activity
Social interaction and locomotor activity were evaluated simultaneously as described before.14 Pairs of unfamiliar rats from the same experimental condition were placed in an open‐field arena (97 cm in diameter and 32.5 cm high, with an open top and a floor divided into 19 similar quadrants). Social behavior and locomotor activity were quantified live during 10 minutes for each rat. Locomotor activity was assessed by the number of floor squares entered. Social interaction was calculated by the sum of time spent sniffing, following or in passive social interaction (when animals lie next to each other). In addition, the percentage of central floor squares entered was calculated to be used as an indicator of anxiety—the higher the percentage, the lower the anxiety levels.
2.3.2. Contextual fear conditioning
The contextual fear conditioning task consisted on two consecutive days, as described previously.15 On the first day (training session), the animals were placed in a dark chamber with a grid floor (22 × 22 × 22 cm). After 140 seconds, 0.4 mA foot shocks lasting 5 seconds were applied every 30 seconds. Twenty‐four hours later each animal was placed in the same dark chamber, without receiving foot shocks. Animal's behavior was recorded by a video camera, and the assessment was conducted over a screen. Freezing (complete immobility of the animal with the absence of vibrissae movements and sniffing) duration was scored during 5 minutes.
2.4. Statistical analysis
Levene's and Shapiro‐Wilks tests were used to determine whether the data were parametric. When nonparametric (social interaction in experiment 2), data were logarithmically transformed to allow a parametric analysis.
Data were analyzed by two‐way ANOVA (strain × treatment) followed by Duncan's test or by three‐way ANOVA with repeated measures (strain × treatment × time) for the body weight in experiment 2. The P < 0.05 was used as a criterion for statistical significance. Statistical analyzes were conducted on the software SPSS 20.
3. RESULTS
3.1. Experiment 1: effects of single injection of SNP in adult rats
3.1.1. Locomotor activity
Two‐way ANOVA detected a significant effect of strain [F (1,57) = 6.221; P = 0.016]. SHRs displayed increased locomotor activity, and treatment with SNP did not modify this behavior (Figure 2A).
Figure 2.
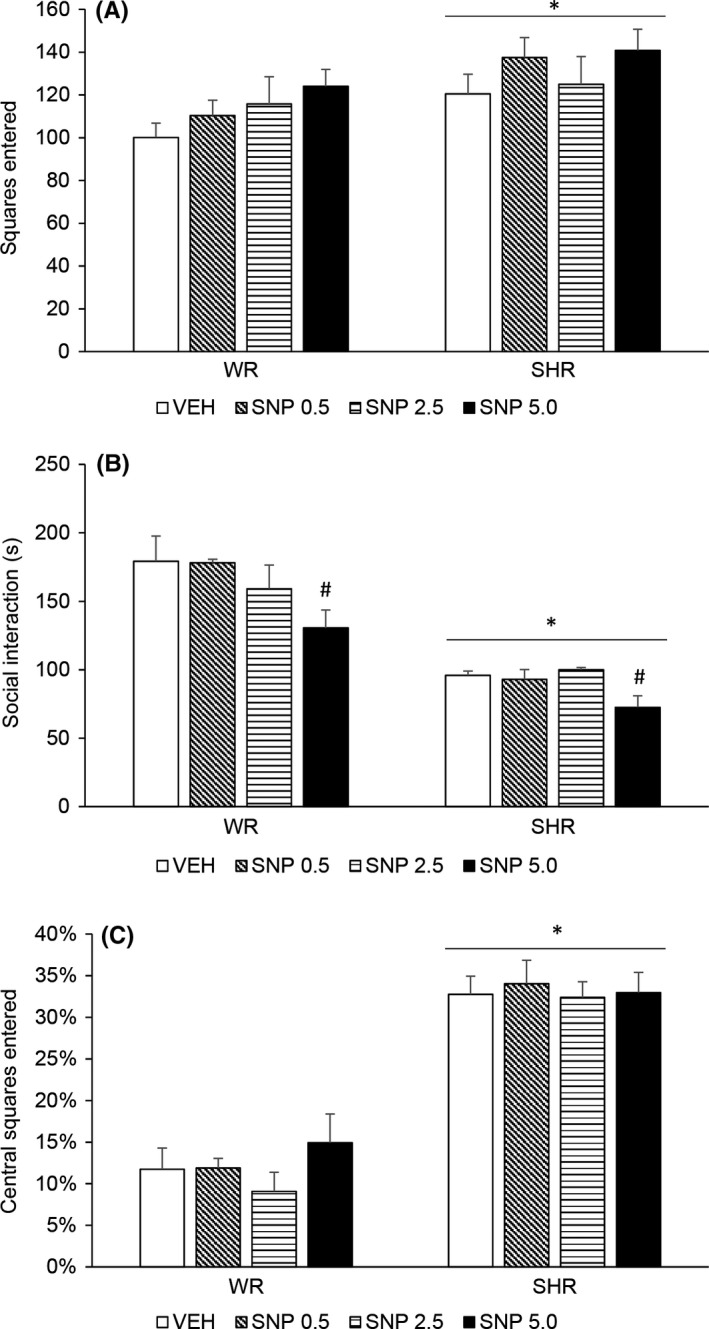
Locomotor activity (A), social interaction duration (B), and percentage of floor squares entered (C) of adult (4‐month‐old) WRs and SHRs (n = 8‐10/group) treated with a single injection of vehicle (VEH) or sodium nitroprusside (SNP—0.5, 2.5, or 5.0 mg/kg) and submitted to behavioral assessments 24 hour later. Data reported as mean ± SE. Two‐way ANOVA followed by Duncan's test. *P < 0.05 compared to WRs of same treatment. # P < 0.05 compared to VEH‐treated animals of the same strain
3.1.2. Social interaction
Two‐way ANOVA showed a significant effect of strain [F (1,57) = 75.946; P < 0.001] and treatment [F (3,57) = 3.556; P = 0.020]. SHRs displayed diminished social interaction, and post hoc analysis revealed that treatment with SNP 5.0 mg/kg decreased social interaction in both strains (Figure 2B).
3.1.3. Percentage of central floor squares entered
Two‐way ANOVA detected a significant effect of strain [F (1,68) = 155.856; P < 0.001]. SHRs displayed increased percentage of entrance in central floor squares, which indicates lower anxiety levels. Treatment with SNP did not modify this percentage in any strain (Figure 2C).
3.1.4. Contextual fear conditioning
Two‐way ANOVA showed a significant effect of strain [F (1,72) = 56.848; P < 0.001] and an interaction between treatment and strain [F (3,72) = 4.495; P = 0.006]. SHRs displayed decreased freezing behavior. Post hoc analysis revealed that treatment with SNP 0.5 and 5.0 mg/kg decreased freezing response in WRs (Figure 3).
Figure 3.
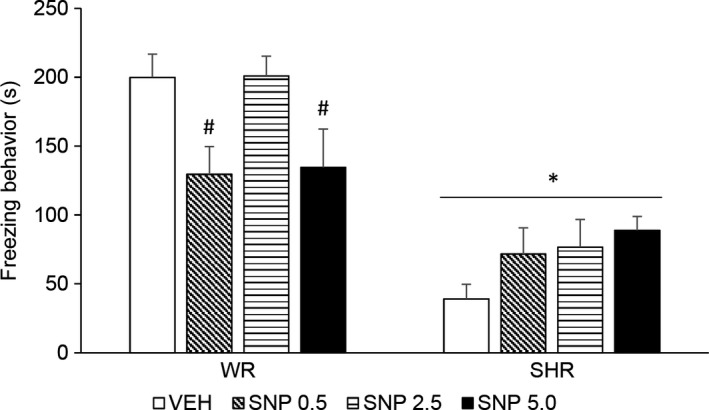
Freezing response during contextual fear conditioning test (s) of adult (4‐month‐old) WRs and SHRs (n = 8‐10/group) treated with a single injection of vehicle (VEH) or sodium nitroprusside (SNP—0.5, 2.5, or 5.0 mg/kg) and submitted to the training and testing sessions of contextual fear conditioning 24 and 48 hour later, respectively. Data reported as mean ± SE. Two‐way ANOVA followed by Duncan's test. * P < 0.05 compared to WRs of same treatment. # P < 0.05 compared to VEH‐treated animals of the same strain
3.2. Experiment 2: effects of prolonged treatment with SNP during periadolescence
3.2.1. Body weight gain
Repeated measures three‐way ANOVA detected a significant effect of time [F (8,576) = 2968.104; P < 0.001] and strain [F (1,72) = 177.285; P < 0.001]. Body weight of both WRs and SHRs increased over time, and SHRs displayed lower body weight. An interaction between strain and time [F (8,576) = 28.021; P < 0.001] suggests that the weight gain rate of SHRs is different from those of Wistar rats. Treatment with SNP did not modify animals’ body weight gain (Table 1).
Table 1.
Body weight gain evaluated every 5‐7 days from the beginning of treatment of WR and SHRs treated with SNP from 30 to 60 postnatal days
| Group | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| WR | |||||||||
| VEI | 104.6 ± 4.92 | 131.1 ± 6.21 | 149.9 ± 6.58 | 165.0 ± 7.14 | 182.4 ± 7.93 | 192.8 ± 8.71 | 204.8 ± 8.29 | 266.3 ± 7.07 | 292.1 ± 8.03 |
| SNP 0.5 | 106.9 ± 5.33 | 134.4 ± 6.70 | 152.6 ± 6.29 | 169.3 ± 6.50 | 186.9 ± 7.29 | 196.4 ± 6.34 | 209.0 ± 8.09 | 278.7 ± 8.76 | 304.6 ± 9.14 |
| SNP 1 | 103.2 ± 3.56 | 129.9 ± 3.77 | 148.7 ± 4.04 | 161.2 ± 3.95 | 181.7 ± 4.42 | 191.9 ± 4.85 | 205.0 ± 6.48 | 276.5 ± 8.02 | 303.2 ± 9.79 |
| SNP 2.5 | 100.2 ± 4.61 | 125.7 ± 5.67 | 144.9 ± 5.89 | 157.3 ± 5.48 | 175.7 ± 6.65 | 185.3 ± 6.94 | 198.0 ± 7.90 | 265.7 ± 7.24 | 294.1 ± 8.51 |
| SHR* | |||||||||
| VEI | 74.7 ± 2.48 | 87.3 ± 2.66 | 97.0 ± 2.39 | 111.8 ± 2.13 | 125.6 ± 2.28 | 138.0 ± 2.60 | 153.0 ± 3.04 | 207.0 ± 3.87 | 226.2 ± 4.39 |
| SNP 0.5 | 75.0 ± 2.52 | 88.4 ± 3.25 | 99.3 ± 3.57 | 115.0 ± 3.86 | 130.4 ± 4.96 | 143.6 ± 5.31 | 161.9 ± 5.58 | 214.5 ± 6.63 | 236.5 ± 7.00 |
| SNP 1 | 79.4 ± 3.62 | 90.9 ± 3.78 | 101.9 ± 4.61 | 119.1 ± 5.76 | 134.7 ± 7.08 | 149.4 ± 7.38 | 165.4 ± 8.04 | 218.8 ± 8.79 | 238.0 ± 8.86 |
| SNP 2.5 | 75.4 ± 4.10 | 85.9 ± 3.27 | 99.0 ± 4.16 | 112.2 ± 3.83 | 126.4 ± 4.50 | 137.0 ± 4.88 | 151.4 ± 5.53 | 200.8 ± 6.50 | 222.9 ± 6.16 |
SHR, spontaneously hypertensive rats; SNP, sodium nitroprusside; WR, Wistar rats; *p<0.05 compared to WRs of same treatment.
3.2.2. Locomotor activity
Two‐way ANOVA detected a significant effect of strain [F (1,65) = 21.251; P < 0.001] and treatment [F (3,65) = 4.112; P = 0.010]. SHRs displayed increased locomotor activity. Although there was no interaction effect, post hoc analysis revealed that only SHRs treated with SNP 0.5 or 2.5 mg/kg displayed decreased locomotor activity when compared to VEH‐treated SHRs (without differing from WRs). Treatment with SNP 0.5 or 2.5 mg/kg attenuated the hyperlocomotion in SHRs (Figure 4A).
Figure 4.
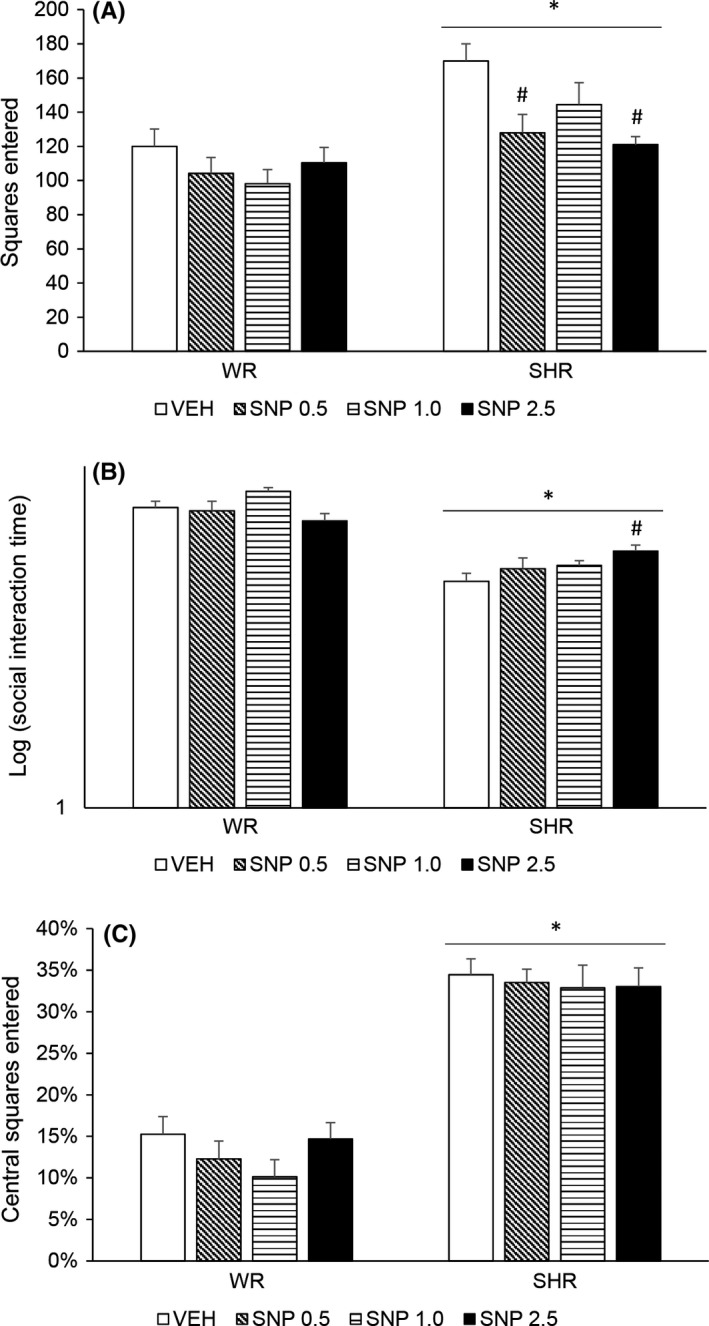
Locomotor activity (A), social interaction duration (B), and percentage of floor squares entered (C) of adult WRs and SHRs (n = 8‐10/group) treated with vehicle (VEH) or sodium nitroprusside (SNP—0.5, 1.0, or 2.5 mg/kg) during periadolescence (30‐60 postnatal day). Data reported as mean ± SE. Two‐way ANOVA followed by Duncan's test. *P < 0.05 compared to WRs of same treatment. # P < 0.05 compared to VEH‐treated animals of the same strain
3.2.3. Social interaction
Two‐way ANOVA showed a significant effect of strain [F (1,63) = 128.823; P < 0.001] and an interaction between strain and treatment [F (3,63) = 3.888; P = 0.013]. Post hoc analysis revealed that SHRs displayed diminished social interaction that was attenuated by the highest dose of SNP (2.5 mg/kg) (Figure 4B).
3.2.4. Percentage of central floor squares entered
Two‐way ANOVA detected a significant effect of strain [F (1,66) = 178.074; P < 0.001]. SHRs displayed increased percentage of entrance in central floor squares, which indicates lower anxiety levels. Treatment with SNP did not modify this percentage in any strain (Figure 4C).
3.2.5. Contextual fear conditioning
Two‐way ANOVA showed a significant effect of strain [F (1,72) = 19.324; P < 0.001], treatment [F (1,72) = 3.498; P = 0.020] and an interaction between strain and treatment [F (1,72) = 5.075; P = 0.003]. SHRs displayed decreased freezing behavior and treatment with SNP, in all doses, increased this behavior (Figure 5).
Figure 5.
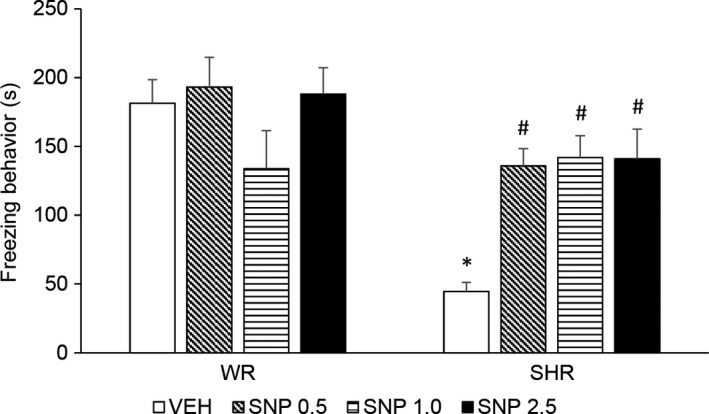
Freezing response during contextual fear conditioning test (s) of adult WRs and SHRs (n = 8‐10/group) treated with vehicle (VEH) or sodium nitroprusside (SNP—0.5, 1.0, or 2.5 mg/kg) during periadolescence (30‐60 postnatal day). Data reported as mean ± SE. Two‐way ANOVA followed by Duncan's test. *P < 0.05 compared to WRs of same treatment. # P < 0.05 compared to VEH‐treated animals of the same strain
4. DISCUSSION
The search for new treatments and preventive strategies for schizophrenia is paramount. In relation to advances in its treatment, SNP emerges as a potential target. SNP's antipsychotic profile was first suggested by preclinical studies—for review, see.11 In animal models using behavioral alterations induced by NMDA antagonists, SNP was able to attenuate hyperlocomotion,34, 37, 38 short‐ and long‐term object recognition memory34, 36 and social interaction deficits.36 In addition, the deficit in prepulse inhibition of startle induced by a dopamine agonist is also improved by SNP.35 These promising preclinical data were followed by a randomized double‐blind, placebo‐controlled trial in which twenty schizophrenia inpatients in the first 5 years of the disease presented an improvement of positive, negative, anxiety, and depression symptoms within hours after a single infusion of SNP. These strikingly effects persisted for 4 weeks.12 The cognitive deficits of these patients were also ameliorated when evaluated up to 8 hours after the infusion.13
In contrast to the above‐mentioned data, SNP was not able to improve hyperlocomotion, deficits in social interaction and in contextual fear conditioning presented by the SHR strain when administered in the adulthood. Although schizophrenia‐related behaviors acutely induced in pharmacological animal models are well‐stablished, the SHR strain presents the advantage of developing schizophrenia‐related behavioral abnormalities spontaneously.14, 15, 16, 17 Hence, the absence of effect of SNP presented herein might be related to a broader and more chronic mechanism underlying the behavioral abnormalities spontaneously displayed in this animal model when compared to the acute specific changes in the dopamine and NMDA neurotransmission induced by those pharmacological models. In this respect, an antipsychotic effect of SNP on the psychotic symptoms and on spatial working memory deficits in schizophrenia patients was not observed.39 The authors argue that the beneficial effect of SNP previously seen12, 13 may occur in patients with a shorter history of illness and/or with a more acute exacerbation of symptoms. This same rationale could be related to the antipsychotic effect of SNP seen in the acute pharmacological‐induced animal models of schizophrenia but not observed in the spontaneous, more chronic, behavioral deficits presented by the SHR strain. From another standpoint, it might be argued that the absence of effect of SNP could be due to the interval of 24 hours between its administration and the behavioral evaluations. Nevertheless, previous studies have demonstrated the effects of SNP on improving the long‐term memory deficit induced by a NMDA antagonist 48 hours after its administration,34 and a decrease in hyperlocomotion induced by a NMDA antagonist 1 week later the administration of SNP.38
Still considering the acute effect of SNP, it should be noticed that a decrease in social interaction was observed in both strains after treatment with the highest dose. This decrease in social interaction could be related to a decrease in general activity. Nevertheless, no changes in locomotion were observed. In parallel, an inverted U‐shaped curve was observed for freezing time presented by WRs (the lowest and the highest doses decreasing it). A possible anxiolytic effect of SNP might be involved in this effect, as nitric oxide donors diminish anxiety36, 40 and anxiolytics might decrease fear conditioning.15, 41 Contrary to this assumption, an anxiety parameter observed in the open‐field—percentage of central floor squares entered—was not modified by any dose of SNP. In any way, this effect was seen only in WRs, indicating that these two strains respond qualitatively different to SNP. In the same way, we have previously described that the known effect of a benzodiazepine in impairing the contextual fear conditioning is not seen in SHR rats.15 Interestingly, we have shown that the antipsychotics haloperidol and clozapine impair contextual fear conditioning in WRs, in opposition to their beneficial effect on the contextual fear conditioning deficit presented by SHRs.15
In relation to a potential preventive effect, the long‐term early treatment with SNP inhibited the manifestation of the schizophrenia‐related behavioral abnormalities presented by adult SHRs. Hyperlocomotion was significantly prevented by the lowest and the highest dose of SNP (with a trend at the intermediate dose); social interaction deficit (seen already in 30‐day‐old SHRs) was reversed by the highest dose; and contextual fear conditioning deficit (already presented at 45‐day‐old SHRs) was abolished by all the doses tested. Importantly, these effects were observed only in the SHR strain, indicating the specificity of SNP to schizophrenia‐related behavioral abnormalities presented by this strain. As far as we know, this is the first evidence of the potential of an early treatment with SNP in preventing or reversing the development of positive‐, negative‐, and cognitive‐related behaviors in animal model of schizophrenia.
Considering the 15% conversion rate to psychosis of ultra‐high‐risk patients as well as the risks of a peripubertal treatment,7, 42 it is crucial to evaluate possible harmful outcomes in healthy subjects. Noteworthy, the early treatment with SNP did not induce any behavioral changes in control animals. In addition, body weight gain of both strains was not modified by the SNP treatment. One might argue that the SHRs behavioral abnormalities in some extent could be due to the body weight difference between WRs and SHRs. For elucidating this possibility, we performed an ANCOVA analysis using weight as a covariable. The inclusion of the weight on the statistical model did not modify the results, that is, SHRs’ displayed all the behavioral abnormalities and SNP prevented all of them even when controlling the results for the body weight differences.
The SHR strain has been used to evaluate pharmacological and nonpharmacological preventive strategies. Although antipsychotics (S.T.Niigaki, F.F.Peres, D.A.Gouvea, R.Levin, V.Almeida, N.D.Silva, M.C.Diana, M.A.Suiama, V.C.Abilio submitted), cannabidiol (F.F.Peres, M.C.Diana, R.Levin, M.A.Suiama, V.Almeida, A.M.Vendramini, A.W.Zuardi, J.E.C.Hallak, J.Á.Crippa, V.C.Abilio submitted), and environmental enrichment32 have proven to present preventive effects, early treatment with SNP was the only one to prevent positive‐, negative‐, and cognitive‐related deficits at the same time without inducing any behavioral changes in control animals. Taken as a whole, the present data point to a broad spectrum of prevention of SNP with a safe profile.
The antipsychotic mechanism of SNP has been related to a direct action on NMDA receptor function as well as to its ability to counteract the decrease in nitric oxide and in cGMP‐mediated cascade due to the NMDA hypofunction associated with the pathophysiology of schizophrenia.11, 43 In accordance, a decrease in metabolites of nitric oxide has been described in the plasma and cerebrospinal fluid of schizophrenia patients.44, 45The decreased serum level of nitric oxide is attenuated by a 6‐week treatment with the antipsychotic risperidone being associated with an improvement in psychotic symptoms.45 Also, nitric oxide and its metabolites are inversely correlated to the severity of negative symptoms.46, 47 In parallel, a modulation by nitric oxide donors of the dopaminergic system, extensively associated with the pathophysiology of schizophrenia,48 has been postulated.9, 49 Dopamine, D1 and D2, receptors modulate NO production and nitric oxide synthase activity in striatal neurons.9 In addition, it has been speculated that dopamine transporters are inhibited by NO, which could lead to an increase in dopamine levels in the prefrontal cortex and, through a modulatory control over mesolimbic areas, to a normalization of dopamine overactivity in the striatum.49
Considering the long‐term preventive effect of SNP, it should also be taken into account that a disruption in nitric oxide signaling has been associated with long‐term behavioral and neuroanatomical abnormalities that might be associated with schizophrenia—see.50, 51 The maturation of neurons and synaptogenesis are modulated by NO. Improper migration of NADPH‐d, a nitric oxide synthase, neurons might lead to a disturbance in the nitrergic transmission interfering with the neurodevelopmental process culminating with the manifestation of schizophrenia later in life.51 In this sense, neonatal nitric oxide synthase inhibition leads to an increase in dopamine agonists‐ and stress‐induced hyperlocomotion as well as deficits in social interaction, prepulse inhibition of startle, latent inhibition, and short‐term recognition memory.50, 51, 52, 53 In this respect, the hypertension of the SHR strain has been associated, among other factors, to a decrease of nitric oxide availability due to an increase in peripheral free radicals.54 The same decrease in nitric oxide availability due to an increase in oxidative stress might occur in the central nervous system. Although speculative, the long‐term treatment with SNP during periadolescence—which would parallel the age of ultra‐high‐risk individuals—might counteract a possible disruption of nitric oxide signaling in the SHR strain leading to a protective effect against the development of the schizophrenia‐related behaviors seen in adulthood or during the periadolescence, corresponding to a prodromal phase. The protective mechanisms associated with the long‐term beneficial effect of SNP are currently under investigation in our group. In this respect, alterations in the nitrergic system, increased brain oxidative stress, and neuroinflammation have been described in the SHR strain19, 20, 23, 24 and might underlie the beneficial effect of SNP presented here.
In conclusion, the present study indicates the SNP as a safe preventive strategy with a broad range of effectiveness. Translated to the clinical context, these preclinical data point to a potential of SNP in attenuating the emergence of positive, negative, and cognitive symptoms of ultra‐high‐risk individuals that would convert to schizophrenia. Importantly, preventive treatment of those who would not convert appears to be safe. The preventive effect and safety of SNP in other animal models of schizophrenia is currently under investigation by our group and would strengthen these findings.
CONFLICT OF INTEREST
JECH and JAC have received travel support from and are medical advisors of BSPG‐Pharm. RB has received research grants from AstraZeneca, Janssen Cilag, Novartis, Roche.
ACKNOWLEDGMENTS
VCA, RAB, ATL, JECH, and JASC are recipients of Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil) productivity fellowships. Research was supported in part by grants from (i) Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP); (ii) Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq); (iii) Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES); (iv) Fundação de Apoio ao Ensino, Pesquisa e Assistência do Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo (FAEPA, Brazil); (v) Center for Interdisciplinary Research on Applied Neurosciences (NAPNA), University of São Paulo, São Paulo, Brazil (NAPNA); and (vi) National Institute for Translational Medicine (INCT‐TM; CNPq/FAPESP, Brazil). The authors would like to thank Maria Vieira Seles for the capable assistance.
Diana MC, Peres FF, Justi V, et al. Sodium nitroprusside is effective in preventing and/or reversing the development of schizophrenia‐related behaviors in an animal model: The SHR strain. CNS Neurosci Ther. 2018;24:624–632. 10.1111/cns.12852
REFERENCES
- 1. Nicholl D, Akhras KS, Diels J, Schadrack J. Burden of schizophrenia in recently diagnosed patients: healthcare utilisation and cost perspective. Curr Med Res Opin. 2010;26:943‐955. [DOI] [PubMed] [Google Scholar]
- 2. Thaker GK, Carpenter WT Jr. Advances in schizophrenia. Nat Med. 2001;7:667. [DOI] [PubMed] [Google Scholar]
- 3. Larson MK, Walker EF, Compton MT. Early signs, diagnosis and therapeutics of the prodromal phase of schizophrenia and related psychotic disorders. Expert Rev Neurother. 2010;10:1347‐1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Briles JJ, Rosenberg DR, Brooks BA, Roberts MW, Diwadkar VA. Review of the safety of second‐generation antipsychotics: are they really “atypically” safe for youth and adults? Prim Care Companion CNS Disord. 2012;14:PCC.11r01298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hasan A, Falkai P, Wobrock T, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 1: update 2012 on the acute treatment of schizophrenia and the management of treatment resistance. World J Biol Psychiatry. 2012;13:318‐378. [DOI] [PubMed] [Google Scholar]
- 6. Gee DG, Cannon TD. Prediction of conversion to psychosis: review and future directions. Rev Bras Psiquiatr. 2011;33(Suppl 2):s129‐s142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Insel TR. Rethinking schizophrenia. Nature. 2010;468:187‐193. [DOI] [PubMed] [Google Scholar]
- 8. Bernstein HG, Keilhoff G, Steiner J, Dobrowolny H, Bogerts B. Nitric oxide and schizophrenia: present knowledge and emerging concepts of therapy. CNS Neurol Disord Drug Targets. 2011;10:792‐807. [DOI] [PubMed] [Google Scholar]
- 9. Pitsikas N. The role of nitric oxide donors in schizophrenia: basic studies and clinical applications. Eur J Pharmacol. 2015;766:106‐113. [DOI] [PubMed] [Google Scholar]
- 10. Nasyrova RF, Ivashchenko DV, Ivanov MV, Neznanov NG. Role of nitric oxide and related molecules in schizophrenia pathogenesis: biochemical, genetic and clinical aspects. Front Physiol. 2015;6:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Crippa JA, Hallak JE, Abilio VC, de Lacerda AL, Zuardi AW. Cannabidiol and sodium nitroprusside: two novel neuromodulatory pharmacological interventions to treat and prevent psychosis. CNS Neurol Disord Drug Targets. 2015;14:970‐978. [DOI] [PubMed] [Google Scholar]
- 12. Hallak JE, Maia‐de‐Oliveira JP, Abrao J, et al. Rapid improvement of acute schizophrenia symptoms after intravenous sodium nitroprusside: a randomized, double‐blind, placebo‐controlled trial. JAMA Psychiatry. 2013;70:668‐676. [DOI] [PubMed] [Google Scholar]
- 13. Maia‐de‐Oliveira JP, Abrao J, Evora PR, et al. The effects of sodium nitroprusside treatment on cognitive deficits in schizophrenia: a pilot study. J Clin Psychopharmacol. 2015;35:83‐85. [DOI] [PubMed] [Google Scholar]
- 14. Calzavara MB, Levin R, Medrano WA, et al. Effects of antipsychotics and amphetamine on social behaviors in spontaneously hypertensive rats. Behav Brain Res. 2011;225:15‐22. [DOI] [PubMed] [Google Scholar]
- 15. Calzavara MB, Medrano WA, Levin R, et al. Neuroleptic drugs revert the contextual fear conditioning deficit presented by spontaneously hypertensive rats: a potential animal model of emotional context processing in schizophrenia? Schizophr Bull. 2009;35:748‐759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Calzavara MB, Medrano WA, Levin R, Libanio TC, de Alencar Ribeiro R, Abilio VC. The contextual fear conditioning deficit presented by spontaneously hypertensive rats (SHR) is not improved by mood stabilizers. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1607‐1611. [DOI] [PubMed] [Google Scholar]
- 17. Levin R, Calzavara MB, Santos CM, Medrano WA, Niigaki ST, Abilio VC. Spontaneously Hypertensive Rats (SHR) present deficits in prepulse inhibition of startle specifically reverted by clozapine. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1748‐1752. [DOI] [PubMed] [Google Scholar]
- 18. Najjar S, Pahlajani S, De Sanctis V, Stern JNH, Najjar A, Chong D. Neurovascular unit dysfunction and blood‐brain barrier hyperpermeability contribute to schizophrenia neurobiology: a theoretical integration of clinical and experimental evidence. Front Psychiatry. 2017;8:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Newaz MA, Yousefipour Z, Nawal NN. Modulation of nitric oxide synthase activity in brain, liver, and blood vessels of spontaneously hypertensive rats by ascorbic acid: protection from free radical injury. Clin Exp Hypertens. 2005;27:497‐508. [DOI] [PubMed] [Google Scholar]
- 20. Haspula D, Clark M. Regulation of neuroinflammatory cytokines by Angiotensin and Cannabinoid systems in SHR astrocytes. FASEB J. 2017;31(1 Supplement):lb554. [Google Scholar]
- 21. Mueller SM. The blood‐brain barrier in young spontaneously hypertensive rats. Acta Neurol Scand. 1982;65:623‐628. [DOI] [PubMed] [Google Scholar]
- 22. Kucuk M, Kaya M, Kalayci R, et al. Effects of losartan on the blood‐brain barrier permeability in long‐term nitric oxide blockade‐induced hypertensive rats. Life Sci. 2002;71:937‐946. [DOI] [PubMed] [Google Scholar]
- 23. Sarkar O, Li Y, Anand‐Srivastava MB. Nitric oxide attenuates overexpression of Gialpha proteins in vascular smooth muscle cells from SHR: role of ROS and ROS‐mediated signaling. PLoS ONE. 2017;12:e0179301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Qadri F, Arens T, Schwarz EC, Hauser W, Dendorfer A, Dominiak P. Brain nitric oxide synthase activity in spontaneously hypertensive rats during the development of hypertension. J Hypertens. 2003;21:1687‐1694. [DOI] [PubMed] [Google Scholar]
- 25. Santoro ML, Santos CM, Ota VK, et al. Expression profile of neurotransmitter receptor and regulatory genes in the prefrontal cortex of spontaneously hypertensive rats: relevance to neuropsychiatric disorders. Psychiatry Res. 2014;219:674‐679. [DOI] [PubMed] [Google Scholar]
- 26. Diana MC, Santoro ML, Xavier G, et al. Low expression of Gria1 and Grin1 glutamate receptors in the nucleus accumbens of Spontaneously Hypertensive Rats (SHR). Psychiatry Res. 2015;229:690‐694. [DOI] [PubMed] [Google Scholar]
- 27. Almeida V, Levin R, Peres FF, et al. Cannabidiol exhibits anxiolytic but not antipsychotic property evaluated in the social interaction test. Prog Neuropsychopharmacol Biol Psychiatry. 2013;41:30‐35. [DOI] [PubMed] [Google Scholar]
- 28. Almeida V, Peres FF, Levin R, et al. Effects of cannabinoid and vanilloid drugs on positive and negative‐like symptoms on an animal model of schizophrenia: the SHR strain. Schizophr Res. 2014;153:150‐159. [DOI] [PubMed] [Google Scholar]
- 29. Levin R, Almeida V, Peres FF, et al. Antipsychotic profile of cannabidiol and rimonabant in an animal model of emotional context processing in schizophrenia. Curr Pharm Des. 2012;18:4960‐4965. [DOI] [PubMed] [Google Scholar]
- 30. Levin R, Peres FF, Almeida V, et al. Effects of cannabinoid drugs on the deficit of prepulse inhibition of startle in an animal model of schizophrenia: the SHR strain. Front Pharmacol. 2014;5:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Peres FF, Levin R, Almeida V, et al. Cannabidiol, among other cannabinoid drugs, modulates prepulse inhibition of startle in the SHR animal model: implications for schizophrenia pharmacotherapy. Front Pharmacol. 2016;7:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Santos CM, Peres FF, Diana MC, et al. Peripubertal exposure to environmental enrichment prevents schizophrenia‐like behaviors in the SHR strain animal model. Schizophr Res. 2016;176:552‐559. [DOI] [PubMed] [Google Scholar]
- 33. Okamoto K, Aoki K. Development of a strain of spontaneously hypertensive rats. Jpn Circ J. 1963;27:282‐293. [DOI] [PubMed] [Google Scholar]
- 34. Kandratavicius L, Balista PA, Wolf DC, et al. Effects of nitric oxide‐related compounds in the acute ketamine animal model of schizophrenia. BMC Neurosci. 2015;16:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Issy AC, Pedrazzi JF, Yoneyama BH, Del‐Bel EA. Critical role of nitric oxide in the modulation of prepulse inhibition in Swiss mice. Psychopharmacology. 2014;231:663‐672. [DOI] [PubMed] [Google Scholar]
- 36. Trevlopoulou A, Touzlatzi N, Pitsikas N. The nitric oxide donor sodium nitroprusside attenuates recognition memory deficits and social withdrawal produced by the NMDA receptor antagonist ketamine and induces anxiolytic‐like behaviour in rats. Psychopharmacology. 2016;233:1045‐1054. [DOI] [PubMed] [Google Scholar]
- 37. Bujas‐Bobanovic M, Bird DC, Robertson HA, Dursun SM. Blockade of phencyclidine‐induced effects by a nitric oxide donor. Br J Pharmacol. 2000;130:1005‐1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Maia‐de‐Oliveira JP, Lobao‐Soares B, Ramalho T, et al. Nitroprusside single‐dose prevents the psychosis‐like behavior induced by ketamine in rats for up to one week. Schizophr Res. 2015;162:211‐215. [DOI] [PubMed] [Google Scholar]
- 39. Stone JM, Morrison PD, Koychev I, et al. The effect of sodium nitroprusside on psychotic symptoms and spatial working memory in patients with schizophrenia: a randomized, double‐blind, placebo‐controlled trial. Psychol Med. 2016;46:3443‐3450. [DOI] [PubMed] [Google Scholar]
- 40. Masood A, Huang Y, Hajjhussein H, et al. Anxiolytic effects of phosphodiesterase‐2 inhibitors associated with increased cGMP signaling. J Pharmacol Exp Ther. 2009;331:690‐699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Smith KS, Engin E, Meloni EG, Rudolph U. Benzodiazepine‐induced anxiolysis and reduction of conditioned fear are mediated by distinct GABAA receptor subtypes in mice. Neuropharmacology. 2012;63:250‐258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hartmann JA, Yuen HP, McGorry PD, et al. Declining transition rates to psychotic disorder in “ultra‐high risk” clients: investigation of a dilution effect. Schizophr Res. 2016;170:130‐136. [DOI] [PubMed] [Google Scholar]
- 43. Oliveira JP, Lobao B, Machado‐de‐Sousa JP, Baker GB, Dursun S, Hallak JE. Targeting the NMDA receptor‐nitric oxide‐cyclic GMP pathway to develop non‐dopaminergic antipsychotic medications for schizophrenia. Rev Bras Psiquiatr. 2011;33:223‐224. [DOI] [PubMed] [Google Scholar]
- 44. Ramirez J, Garnica R, Boll MC, Montes S, Rios C. Low concentration of nitrite and nitrate in the cerebrospinal fluid from schizophrenic patients: a pilot study. Schizophr Res. 2004;68:357‐361. [DOI] [PubMed] [Google Scholar]
- 45. Lee BH, Kim YK. Reduced plasma nitric oxide metabolites before and after antipsychotic treatment in patients with schizophrenia compared to controls. Schizophr Res. 2008;104:36‐43. [DOI] [PubMed] [Google Scholar]
- 46. Suzuki E, Nakaki T, Nakamura M, Miyaoka H. Plasma nitrate levels in deficit versus non‐deficit forms of schizophrenia. J Psychiatry Neurosci. 2003;28:288‐292. [PMC free article] [PubMed] [Google Scholar]
- 47. Nakano Y, Yoshimura R, Nakano H, et al. Association between plasma nitric oxide metabolites levels and negative symptoms of schizophrenia: a pilot study. Hum Psychopharmacol. 2010;25:139‐144. [DOI] [PubMed] [Google Scholar]
- 48. Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III–the final common pathway. Schizophr Bull. 2009;35:549‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Maia‐de‐Oliveira JP, Lobao‐Soares B, Baker GB, Dursun SM, Hallak JE. Sodium nitroprusside, a nitric oxide donor for novel treatment of schizophrenia, may also modulate dopaminergic systems. Schizophr Res. 2014;159:558‐559. [DOI] [PubMed] [Google Scholar]
- 50. Dec AM, Kohlhaas KL, Nelson CL, et al. Impact of neonatal NOS‐1 inhibitor exposure on neurobehavioural measures and prefrontal‐temporolimbic integration in the rat nucleus accumbens. Int J Neuropsychopharmacol. 2014;17:275‐287. [DOI] [PubMed] [Google Scholar]
- 51. Black MD, Selk DE, Hitchcock JM, Wettstein JG, Sorensen SM. On the effect of neonatal nitric oxide synthase inhibition in rats: a potential neurodevelopmental model of schizophrenia. Neuropharmacology. 1999;38:1299‐1306. [DOI] [PubMed] [Google Scholar]
- 52. Black MD, Varty GB, Arad M, et al. Procognitive and antipsychotic efficacy of glycine transport 1 inhibitors (GlyT1) in acute and neurodevelopmental models of schizophrenia: latent inhibition studies in the rat. Psychopharmacology. 2009;202:385‐396. [DOI] [PubMed] [Google Scholar]
- 53. Morales‐Medina JC, Mejorada A, Romero‐Curiel A, et al. Neonatal administration of N‐omega‐nitro‐L‐arginine induces permanent decrease in NO levels and hyperresponsiveness to locomotor activity by D‐amphetamine in postpubertal rats. Neuropharmacology. 2008;55:1313‐1320. [DOI] [PubMed] [Google Scholar]
- 54. Torok J. Participation of nitric oxide in different models of experimental hypertension. Physiol Res. 2008;57:813‐825. [DOI] [PubMed] [Google Scholar]


