Abstract
Background
Ideal cardiovascular health (CVH) was proposed by the American Heart Association to promote population health. We aimed to characterize the association between ideal CVH and markers of subclinical cardiovascular disease (CVD).
Hypothesis
We hypothesized that ideal CVH is associated with several markers of subclinical CVD.
Methods
We used data from the Heart Strategies Concentrating on Risk Evaluation (Heart SCORE) study. We assigned 1 for each of the ideal CVH factors met. Endothelial function, expressed as Framingham reactive hyperemia index (fRHI), was measured using the EndoPAT device. Coronary artery calcium (CAC) and carotid intima‐media thickness (CIMT) were quantified using electron beam computed tomography and carotid ultrasonography, respectively.
Results
A total of 1933 participants (mean [SD] age: 59 [7.5] years, 34% male, 44% black) were included. The mean number of ideal CVH factors met was 2.3 ± 1.3, with blacks having significantly lower score compared to whites (2.0 ± 1.2 vs 2.5 ± 1.4, respectively; P < 0.001). Seven hundred and eighty‐nine participants (41%) achieved ≥3 ideal CVH factors. Participants with ≥3 ideal CVH factors (compared to those with <3 factors) had an average of 107 (95% confidence interval [CI]: 50‐165) Agatston units lower CAC, 0.04 (0.01‐0.06) mm lower CIMT, and 0.07 (0.02‐0.12) units higher fRHI, after adjusting for age, sex, race, income, education, and marital status. Participants with ≥3 ideal CVH factors had 50% lower odds (95% CI: 28%‐66%) of having CAC >100 Agatston units.
Conclusion
In a community‐based study with low prevalence of ideal CVH, even achieving three or more ideal CVH factors were associated with lower burden of subclinical CVD, indicating the utility of this construct for disease prevention.
Keywords: coronary artery calcium, endothelial function, ideal cardiovascular health, risk factor, subclinical atherosclerosis
1. BACKGROUND
Cardiovascular disease (CVD) remains the leading cause of morbidity and mortality in the United States.1 In 2010, the American Heart Association (AHA) Strategic Planning Task Force and Statistics Committee established the 2020 Strategic Impact Goals, which were designed to improve cardiovascular health (CVH) of all Americans by 20% and reduce deaths from CVD by 20%.2 To achieve these goals, the AHA‐defined ideal CVH as achieving four lifestyle factors (nonsmoking, body mass index [BMI)] <25 kg/m2, physical activity at goal, and optimal diet consistent with guidelines), and three biometric factors (untreated total cholesterol <200 mg/dL, untreated fasting blood glucose <100 mg/dL, and untreated blood pressure < 120/80 mm Hg). Consequently, there has been interest in exploring ideal CVH metrics and their associations with markers of clinical and subclinical CVD.
Prior studies have reported that subclinical CVD manifested by microvascular endothelial dysfunction3, 4, 5, 6, 7 or coronary artery calcium8, 9, 10, 11, 12, 13 is associated with atherosclerotic plaque burden and future CVD events. On the other hand, Several epidemiological studies have shown an association between favorable ideal CVH profiles and decreased levels of subclinical CVD as measured by coronary artery calcification14, 15, 16 and carotid intima‐media thickness (CIMT).17 However, to the best of our knowledge, the relationship between ideal CVH and microvascular endothelial function has not been studied.
There are also limited studies examining the impact of race on the association between ideal CVH and subclinical CVD.18 We have previously shown a low prevalence of ideal CVH in a community‐based population.19 In that study, black participants had 82% lower odds of having ≥5 factors of ideal CVH after adjusting for age, sex, and income levels. Others and we also have previously reported that black individuals, compared to whites, have worse arterial endothelial dysfunction20, 21, 22 and CVD events,23 but paradoxically lower prevalence of coronary calcium.24, 25 Given these findings, we aimed to characterize the association between ideal CVH and markers of subclinical CVD in a population with approximately equal representation of black and white participants.
2. METHODS
2.1. Study design
We used data from the Heart Strategies Concentrating on Risk Evaluation (HeartSCORE) study. HeartSCORE began in 2003 as a community‐based cohort study of 2000 participants in Allegheny County, PA. Initial aims of the study were to improve risk stratification, identify racial disparities, and evaluate mechanisms for population differences in CVD. The methods of HeartSCORE have been described previously.20 Participants were 45 to 75 years old at entry. Individuals with a comorbid condition that was expected to limit life expectancy to <5 years and individuals unable to undergo annual follow‐up visits were excluded. The study was approved by the Institutional Review Board at the University of Pittsburgh. All subjects provided written informed consent.
2.2. Data collection
Demographic information and medical histories were collected at the baseline visit. Age, race, sex, education level, and annual income were obtained by self‐report. Participants completed a detailed questionnaire about their marital/cohabiting status, maximum education level achieved, annual income, and ability to pay for basic needs. Education was categorized as “some college or higher”, or “less than college.” Low educational level was defined as those who did not complete a high school diploma. Individual annual income was reported as “<$10 K,” “$10K to <$20K,” “$20K to <$40K,” “$40K to <$80K”, and “≥$80 K.” Low income was defined as individuals with an annual income of less than $20 K or those reporting difficulty paying for their basic needs. Single living status was defined as individuals who are not married or not cohabitating with a partner. Participants also completed questionnaire about medications they were taking.
2.2.1. AHA cardiovascular health variables
In accordance with AHA definitions, ideal CVH was defined as the simultaneous presence of four ideal health behaviors (nonsmoking, BMI <25 kg/m2, physical activity at goal level, and diet consistent with current recommendations) and three ideal health factors (untreated total cholesterol <200 mg/dL, untreated BP <120/80 mm Hg, and untreated fasting glucose <100 mg/dL) in the absence of clinical CVD. For each participant, attainment of each component of the ideal CVH metrics was determined as described previously.19 A value of 1 was assigned when ideal health status was achieved and 0 when ideal health status was not achieved. For smoking, ideal behavior was defined as never smoker, or quit smoking for 12 months. Physical activity was evaluated using the lipid research clinic questionnaire,26 which includes questions about type and frequency of physical activity at work and during leisure time and permits classification of individuals as very active, moderately active, and inactive. The questionnaire provided approximations of ideal vs non‐ideal physical activity. The PrimeScreen questionnaire27 was used to evaluate average daily consumption of fruits and vegetables. A cutoff value of 3 servings/day of fruits and vegetables has been shown to correlate closely with 5 servings/day when derived from more extensive food frequency questionnaires.27 The questionnaire was used to classify individuals as having an ideal (≥3 servings/day) or non‐ideal (<3 servings/day) consumption of fruits and vegetables. It was not possible to quantify fish, fiber‐rich whole grains, sodium, and sugar‐sweetened beverages consumption as recommended by AHA. Experienced research nurses measured blood pressure using a manual sphygmomanometer and an appropriately sized cuff twice after 5 minutes of rest in a seated position. The average of the two readings was used to classify BP status.
2.3. Serum markers
Blood samples were drawn at the baseline visit. Total cholesterol was measured in fasting venous blood using standard laboratory techniques at the University of Pittsburgh Medical Center Clinical Laboratory. Measurement of high‐sensitivity C‐reactive protein was performed using an immunoturbidimetric assay on the Roche P Modular system (Roche Diagnostics, Indianapolis, Indiana), using reagents and calibrators from DiaSorin (Stillwater, Minnesota). Serum interleukin‐6 (IL‐6) was measured using commercially available enzyme‐linked immunosorbent assay kits (R&D Systems, Minneapolis, Minnesota). Fasting blood glucose was measured using the glucose oxidase method.
2.4. Coronary artery calcium measurement
Electron beam computed tomography image acquisition was obtained with Imatron C150 scanner (GE Imatron Inc., South San Francisco, California) using 3‐mm intervals to span the heart during a single inspiratory breath‐hold. Calcium scores were calculated by the Agatston method using a densitometric program.28 An experienced reader blinded to subject identities interpreted scans.
2.5. Carotid artery imaging
Carotid artery imaging was carried out using a GE VIVID7 (General Electric Corp. Milwaukee, Wisconsin) ultrasound imaging system and a 7 MHz linear array vascular ultrasound probe. The ultrasound beam was adjusted to obtain longitudinal scans of the carotid arteries to visualize two parallel echogenic lines corresponding to the blood‐intima and media‐adventitia interfaces on the posterior wall. IMT was averaged over 70 to 100 individual measurements taken along a 1‐cm segment of the common carotid artery beginning 0.5 cm from the carotid bifurcation along the far wall of the distal common carotid artery. CIMT was taken as the maximum measurement of either left or right side.
2.5.1. Microvascular endothelial function measurement
Endothelial function was measured using an EndoPAT2000 device (Itamar Medical, Caesarea, Israel) adapted from the protocol used by the Framingham Heart Study as previously reported.20, 23, 29 In brief, digital pulse amplitude signals are measured using the pulse amplitude tonometry (PAT) device placed on the tip of each index finger, one serving as control and the other being the test finger. Arterial flow is interrupted to the test finger by applying occlusive arm pressure using blood pressure cuff, after baseline PAT signal is recorded on both fingers for 5 minutes. After 5 minutes of occlusion, cuff‐pressure is abruptly deflated and PAT signal is measured on both fingers for the subsequent 5 minutes. The data are recorded electronically and analyzed using a computerized, automated algorithm. Pulse amplitude response to hyperemia is calculated from the test (hyperemic) fingertip as the ratio of the post‐occlusion pulse amplitude to the baseline‐pulse amplitude. The result is divided by the corresponding ratio in the control finger to give the PAT ratio (also known as reactive hyperemia index [RHI]). The Framingham reactive hyperemia index (fRHI) is calculated as the natural log‐transformation of the RHI.20, 23, 29
2.5.2. Statistical analyses
Participant characteristics were summarized by number of ideal CVH variables. To ensure the presence of sufficient numbers within each group, we categorized participants into those with three or more ideal CVH variables vs those with less than three ideal CVH variables. Continuous variables are shown as means± SDs and compared across categories of CVH variables using t test. Discrete variables are presented as numbers and percentages, and compared using χ2 tests. Variables with skewed distribution were log‐transformed to achieve approximately normal distributions. The main analyses were performed after dichotomizing ideal CVH at a cut‐off of three factors. We also performed secondary analyses by taking ideal CVH as continuous variable. We analyzed the association between ideal CVH categories and CAC, CIMT, and fRHI as continuous variables using linear regression model. We also estimated the odds of having significant CAC (Agatston score > 100 units) and CIMT (CIMT >1 mm) using logistic regression models. Potential heterogeneity in the association between ideal CVH and markers of subclinical CVD by race was assessed by stratified analyses, as well as by fitting interaction term between race and ideal CVH. Sensitivity analyses using log‐transformed CAC values yielded similar results. All data analyses were performed using Stata software (Stata Corp., version 13, Texas), with two‐sided P values <0.05 considered to be statistically significant.
3. RESULTS
Data were available on up to 1933 participants who self‐identified as black or white and had available information on ideal CVH variables and either endothelial function, coronary artery calcium (CAC) or CIMT measurement. The mean (SD) age of participants was 59 (7.5) years, with 66% of participants being females and 44% black. The distribution of ideal CVH factors across the population is shown in Figure 1. The mean number of ideal CVH factors met (assigning 0 for each factor not met, 1 for factor met, maximum possible 7) was 2.3 ± 1.3, with blacks having a significantly lower number of ideal CVH factors compared to whites (2.0 ± 1.2 vs 2.5 ± 1.4 respectively (P < 0.001). The baseline characteristics of study participants, overall and grouped by number of ideal CVH variables (<3 Ideal CVH factors vs ≥3 ideal CVH factors) are shown in Table 1. A majority of the participants had at least some college education, while 29% reported low income, and 43% reported single living status. A total of 1144 (59%) participants had <3 Ideal CVH factors and 789 (41%) had ≥3 ideal CVH factors. Black participants were significantly less likely to achieve ≥3 ideal CVH factors compared to white participants (odds ratio [OR] 0.53; 95% confidence interval [CI]: 0.44, 0.63). The race‐difference in odds of achieving ≥3 ideal CVH factors was more marked for black females (OR: 0.29; 95% CI: 0.21, 0.41) than for black males (OR 0.61; 95% CI: 0.39, 0.95; P‐value for interaction = 0.008).
Figure 1.
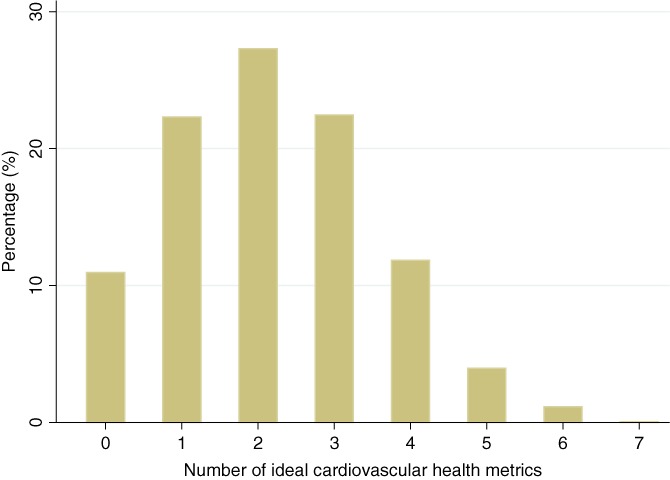
Bar graph showing distribution of ideal cardiovascular health factors
Table 1.
Baseline table by ideal cardiovascular health metricsa
| Factor | N available | Mean (SD) or N (%) | ||
|---|---|---|---|---|
| Overall | <3 ideal CVH factors | ≥3 ideal CVH factors | ||
| Age (years) | 1933 | 59.0 (7.5) | 59.4 (7.4) | 58.5 (7.6) |
| Male | 1933 | 664 (34.4%) | 403 (35.2%) | 261 (33.1%) |
| Black | 1933 | 854 (44.2%) | 578 (50.5%) | 276 (35.0%) |
| Ideal smoking | 1913 | 906 (47.4) | 377 (33.5%) | 529 (67.2%) |
| Ideal BMI | 1911 | 370 (19.4) | 66 (5.9%) | 304 (38.8%) |
| Ideal blood pressure | 1929 | 292 (15.1) | 63 (5.5%) | 229 (29.1%) |
| Ideal glucose | 1893 | 1194 (63.1) | 531 (47.8%) | 663 (84.9%) |
| Ideal cholesterol | 1925 | 483 (25.1) | 171 (15.1%) | 312 (39.5%) |
| Ideal diet | 1743 | 674 (38.7) | 216 (21.4%) | 458 (62.2%) |
| Ideal physical activity | 1906 | 454 (23.8) | 114 (10.2%) | 340 (43.4%) |
| Low income | 1933 | 556 (28.8%) | 370 (32.3%) | 186 (23.6%) |
| Low education | 1933 | 44 (2.3%) | 35 (3.1%) | 9 (1.1%) |
| Single living | 1933 | 823 (42.6%) | 531 (46.4%) | 292 (37.0%) |
| SBP (mm Hg) | 1931 | 136.8 (19.7) | 141.0 (18.8) | 130.7 (19.5) |
| DBP (mm Hg) | 1930 | 80.9 (10.3) | 82.8 (9.8) | 78.2 (10.4) |
| Diabetes | 1923 | 199 (10.3%) | 176 (15.5%) | 23 (2.9%) |
| BMI (kg/m2) | 1912 | 30.2 (6.3) | 31.9 (6.2) | 27.7 (5.6) |
| TC (mg/dL) | 1921 | 213.1 (42.8) | 215.8 (43.7) | 209.1 (41.4) |
| HDL‐C (mg/dL) | 1921 | 57.6 (15.0) | 56.3 (14.4) | 59.5 (15.7) |
| TG (mg/dL) | 1919 | 123.3 (76.0) | 132.9 (78.7) | 109.4 (69.7) |
| Log‐CRP (mg/L) | 1814 | 0.4 (1.2) | 0.6 (1.2) | 0.0 (1.2) |
| Log‐IL6 9 pg/mL) | 1785 | 0.5 (0.7) | 0.7 (0.7) | 0.3 (0.8) |
| fRHI (units) | 1404 | 0.7 (0.5) | 0.7 (0.4) | 0.8 (0.5) |
| CAC (Agaston) | 774 | 160 (396) | 188 (464) | 104 (190)) |
| CIMT (mm) | 782 | 0.82 (0.19) | 0.84 (0.19) | 0.80 (0.20) |
Abbreviations: BMI, body mass index; BP, blood pressure; CAC, coronary artery calcium; CRP, C‐reactive protein; CVH, cardiovascular health; DBP, diastolic blood pressure; fRHI, Framingham reactive hyperemia index; HDL‐C, high‐density lipoprotein cholesterol; IL‐6, interleukin 6; CIMT, carotid intima‐media thickness; SBP, systolic blood pressure; TC, total cholesterol; TG, triglycerides.
P‐value <0.001 for all factor comparisons between <3 and ≥ 3 ideal CVH factors.
The prevalence and mean levels of CVD risk factors (eg, diabetes, blood pressure, and cholesterol) and inflammatory markers (eg, log‐CRP and log‐IL6) were higher among participants with <3 ideal CVH factors compared to those with ≥3 factors. Mean (SD) fRHI, CAC score, and CIMT were 0.7 (±0.5), 160 (±400), and 0.8 (±0.2), respectively. On the whole, those with ≥3 ideal CVH factors had higher fRHI and lower CAC and CIMT as compared to those with <3 ideal CVH factors. (Table 1).
As shown in Table 2, after adjustment for possible confounding variables (age, sex, race, low income, low education, and single living status), having ≥3 ideal CVH factors was associated with a 107 Agatston unit lower CAC (95% CI 50, 165) and 0.04 mm lower CIMT (95% CI 0.01, 0.06). After adjustment for age and sex, fRHI was 0.09 units ([95% CI 0.05, 0.15]) higher in participants with ≥3 ideal CVH factors. The difference in fRHI was slightly attenuated but remained significant after additional adjustment for race, low income, low education, and single living status (0.07 [95% CI 0.02‐0.12]). Individuals with ≥3 ideal CVH factors had lower odds of having CAC >100 Agatston units compared to those with <3 ideal CVH factors; adjusted OR: 0.50 (0.34, 0.72). But the association was not statistically significant for risk of CIMT >1 mm; OR: 0.84 (0.59, 1.18), respectively (Table 3).
Table 2.
Association of ideal cardiovascular health metric (≥3 factors vs <3 factors) with subclinical atherosclerosis (continuous)
| Outcome | Adjustment | N | ≥3 vs < 3 ideal CVH factors: Beta (95% CI) | P‐value |
|---|---|---|---|---|
| CAC | Unadjusted | 774 | −84 (−143, −25) | 0.01 |
| Model 1 | 774 | −102 (−159, −44) | <0.001 | |
| Model 2 | 774 | −108 (−165, −50) | <0.001 | |
| Model 3 | 774 | −107 (−165, −50) | <0.001 | |
| IMT | Unadjusted | 782 | −0.05 (−0.08, −0.02) | <0.001 |
| Model 1 | 782 | −0.04 (−0.07, −0.02) | <0.001 | |
| Model 2 | 782 | −0.04 (−0.06, −0.01) | <0.001 | |
| Model 3 | 782 | −0.04 (−0.06, −0.01) | <0.001 | |
| fRHI | Unadjusted | 1404 | 0.10 (0.05,0.15) | <0.001 |
| Model 1 | 1404 | 0.09 (0.05,0.14) | <0.001 | |
| Model 2 | 1404 | 0.07 (0.02,0.12) | <0.001 | |
| Model 3 | 1404 | 0.07 (0.02,0.12) | 0.01 |
Abbreviations: CAC, coronary artery calcium; CVH, cardiovascular health; fRHI, Framingham reactive hyperemia index; IMT, intima‐media thickness.
Model 1: age and sex, Mode 2: age, sex, and race, and Model 3: age, sex, race, low income, low education, and single living status.
Table 3.
Association of ideal cardiovascular health metric (≥3 factors vs <3 factors) with subclinical atherosclerosis (categorical)
| Outcome | Adjustment | N | ≥3 vs < 3 ideal CVH factors: OR (95%CI) | P‐value |
|---|---|---|---|---|
| CAC > 100 Agatston | Unadjusted | 774 | 0.68 (0.48,0.94) | 0.02 |
| Model 1 | 774 | 0.55 (0.38,0.79) | <0.001 | |
| Model 2 | 774 | 0.50 (0.35,0.72) | <0.001 | |
| Model 3 | 774 | 0.50 (0.34,0.72) | <0.001 | |
| IMT > 1 mm | Unadjusted | 782 | 0.77 (0.55,1.06) | 0.11 |
| Model 1 | 782 | 0.79 (0.56,1.10) | 0.17 | |
| Model 2 | 782 | 0.84 (0.60,1.19) | 0.33 | |
| Model 3 | 782 | 0.84 (0.59,1.18) | 0.32 |
Abbreviations: CAC, coronary artery calcium; CVH, cardiovascular health; IMT, intima‐media thickness.
Model 1: age and sex, Mode 2: age, sex and race, and Model 3: age, sex, race, low income, low education and single living status.
Assessment of the association between ideal CVH and subclinical markers of CVD in subgroups of blacks and whites participants did not indicate the presence of heterogeneity by race (Table 4). Whites with ≥3 ideal CVH factors had a 131 Agatston unit lower CAC (95% CI 58, 204), 0.03 mm lower CIMT (95% CI 0.00, 0.07), and 0.09 unit increased fRHI (95% CI 0.03, 0.15) compared to whites with <3 ideal CVH factors in adjusted analyses. In comparison, blacks with ≥3 ideal CVH factors had 0.05 mm lower CIMT (95% CI 0.00, 0.09) after adjustment. There was a trend toward lower CAC and improved fRHI in blacks with ≥3 ideal CVH factors but results did not reach statistical significance (Table 4). There were similar results when analyzing the odds of significant CAC or CIMT by race (Table S1, Supporting Information). Similarly, we found comparable results when using ideal CVH as a continuous variable instead of dichotomizing at a cut‐off point of three factors (Tables S2A,B). Sensitivity analyses of the association of ideal CVH with CAC on log‐transformed scale yielded comparable results (Tables S3A,B).
Table 4.
Association of ideal cardiovascular health metric (≥3 factors vs <3 factors) with subclinical atherosclerosis (continuous) by race
| Outcome | Adjustment | Whites | Blacks | Interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Association | z‐value | P‐value | N | Association | z‐value | P‐value | z‐value | P‐value | ||
| CAC | Model 1 | 412 | −130 (−203, −57) | −3.5 | <0.001 | 362 | −71 (−164, 21) | −1.5 | 0.13 | 0.97 | 0.33 |
| Model 2 | 412 | −131 (−204, −58) | −3.53 | <0.001 | 362 | −79 (−172,14) | −1.66 | 0.1 | 0.93 | 0.35 | |
| IMT | Model 1 | 504 | −0.03 (−0.06, −0.00) | −2 | 0.05 | 278 | −0.05 (−0.09, −0.01) | −2.2 | 0.03 | −0.48 | 0.63 |
| Model 2 | 504 | −0.03 (−0.07, −0.00) | −2.13 | 0.03 | 278 | −0.05 (−0.09, −0.00) | −2.18 | 0.03 | −0.39 | 0.7 | |
| fRHI | Model 1 | 825 | 0.09 (0.03,0.16) | 2.96 | <0.001 | 579 | 0.04 (−0.04,0.11) | 1.01 | 0.31 | −1.11 | 0.27 |
| Model 2 | 825 | 0.09 (0.03,0.15) | 2.79 | 0.01 | 579 | 0.04 (−0.04,0.11) | 0.94 | 0.35 | −1.02 | 0.31 | |
Abbreviations: CAC, coronary artery calcium; fRHI, Framingham reactive hyperemia index; IMT, intima‐media thickness.
Model 1: adjusted for age and sex and Model 2: Adjusted for age, sex, income, education, marital status.
4. DISCUSSION
In this community‐based HeartSCORE cohort comprised of black and white participants with a low‐prevalence of ideal CVH, we found that achieving a higher level of ideal CVH was associated with decreased burden of subclinical CVD and improved endothelial function. Compared with individuals with <3 ideal CVH factors, those with ≥3 had overall lower levels of CAC, lower CIMT, and improved endothelial function, after taking age, sex, race, income, education, and marital status into account. Individuals with ≥3 ideal CVH factors had about 50% lower odds of having significant CAC.
Our findings are consistent with other studies, which have similarly found increased CVH to be associated with the decreased burden of subclinical CVD.30, 31 Despite significant racial differences in prevalence of ideal CVH factors, endothelial function, and markers of subclinical CVD (demonstrated by us and prior reports),23, 25 we did not find strong evidence for difference in the association between ideal CVH and these outcomes between blacks and whites, consistent with other reports.18, 30 This study contributes to the developing body of evidence supporting AHAs Life's Simple 7 paradigm of focusing on promotion of overall CVH over a sole focus on single risk factors CVD prevention.
Polonsky et al.30 reported that high and moderate levels of CVH were associated with lower risk of CVD after adjustment for CAC, CIMT, and LV mass. Similarly, Xanthakis et al.31 found that after adjustment for biomarkers and subclinical CVD, ideal CVH remained independently and significantly associated with lower risk for CVD events. These findings suggest the potential presence of other pathological mechanisms that may mediate the association of ideal CVH with CVD risk. One such mechanism unique to our study was assessment of microvascular endothelial dysfunction using fRHI. We showed that higher ideal CVH is associated with better endothelial function, suggesting an additional mechanism by which increasing the number of ideal CVH factors could promote cardiovascular well‐being. Moreover, Osibogun et al., using data from the Multi‐Ethnic Study of Atherosclerosis have shown that ideal CVH is correlated with self‐rated health, which has been shown to be an independent predictor of morbidity and mortality.32 These findings highlight the relevance of ideal CVH metrics to good vascular health.
We found a higher prevalence of ideal CVH in whites as compared to blacks similar to other studies.18, 30 There was a trend toward a stronger association between ideal CVH and CAC or fRHI among whites as compared to blacks, but these differences were not statistically significant. Differences in CIMT, on the other hand, were similar between blacks and whites. It is not clear if the trend toward stronger differences for CAC and fRHI seen among whites is real and if the lack of statistical significance in our study is because of limitation in power. Given the known difference in CAC and fRHI between blacks and whites,20, 21, 22, 23, 24, 25 as well as racial differences in ideal CVH variables and CVD outcomes,18, 31 these findings would merit further evaluation in larger studies.
The limitations of our study merit some consideration. We selected a cut‐off of three factors for ideal CVH to allow for sufficient number of participants within the comparison groups, as there were very few participants with 6 or 7 ideal CVH factors. Xanthakis et al.31 found that for each 1 point higher score of ideal CVH, the odds of having any subclinical disease measure were 23% lower. We found similar results in secondary analyses using ideal CVH as continuous variable. Furthermore, sensitivity analyses using different cut‐off points for categorizing ideal CVH yielded similar results (data available from authors upon request). Second, all seven ideal CVH factors were weighted equally when calculating ideal CVH score, although it is likely that not all the factors have the same magnitude of effect on CVD. Nonetheless, this approach is in line with AHA recommendations and prior reports. Third, we classified individuals as having ideal vs nonideal status for each ideal CVH factor, which is somewhat different to previous studies which have also included intermediate status of each individual ideal CVH factor. Polonsky et al.30 found that adults who had primarily intermediate levels of health factors and behaviors (ie, prehypertension, impaired glucose tolerance, borderline hypercholesterolemia, or medically controlled levels of these risk factors) still experienced a lower burden of subclinical disease and fewer cardiovascular events as compared to those with primarily poor levels. These findings indicate that our dichotomization of ideal CVH (merging intermediate and poor ideal CVH together) probably have attenuated the findings we observed. Finally, our focus was primarily on studying the relationship between ideal CVH and subclinical CVD including microvascular endothelial function, and so we have not looked at clinical CVD outcomes in these analyses. Prior studies have evaluated the association of ideal CVH and clinical endpoints.16, 18, 31, 33, 34, 35
In conclusion, in a community‐based study of black and white participants with low prevalence of ideal CVH, even achieving 3 or more ideal CVH factors was associated with lower burden of subclinical atherosclerosis, indicating the utility of this construct for disease prevention.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to report.
Supporting information
Table S1 Association of ideal CVH metric with subclinical atherosclerosis (categorical) by race
Table S2A. Association of ideal CVH metric (continuous) with subclinical atherosclerosis (continuous)
Table S2B. Association of ideal CVH metric (continuous) with subclinical atherosclerosis (categorical)
Table S3A. Association of ideal CVH metric (≥3 factors vs <3 factors) with log‐transformed calcium score
Table S3B. Association of ideal CVH metric (≥3 factors vs <3 factors) with log‐transformed calcium score by race.
ACKNOWLEDGMENT
The present study was funded by the Pennsylvania Department of Health (ME‐02‐384) and the National Institutes of Health (R01HL089292).
Shpilsky D, Bambs C, Kip K, et al. Association between ideal cardiovascular health and markers of subclinical cardiovascular disease. Clin Cardiol. 2018;41:1593–1599. 10.1002/clc.23096
Funding information National Institute of Health, Grant/Award Number: R01HL08929; National Institutes of Health, Grant/Award Number: R01HL089292; Pennsylvania Department of Health, Grant/Award Number: ME‐02‐384
REFERENCES
- 1. Writing Group Members , Lloyd‐Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics, 2010 update: a report from the American Heart Association. Circulation. 2010;121:e46‐e215. [DOI] [PubMed] [Google Scholar]
- 2. Lloyd‐Jones DM, Hong Y, Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American heart Association's strategic impact goal through 2020 and beyond. Circulation. 2010;121:586‐613. [DOI] [PubMed] [Google Scholar]
- 3. Matsuzawa Y, Sugiyama S, Sumida H, et al. Peripheral endothelial function and cardiovascular evens in high‐risk patients. J Am Heart Assoc. 2013;10:1‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Widlansky ME, Gocke N, Keaney JF Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;7:1149‐1160. [DOI] [PubMed] [Google Scholar]
- 5. Anderson TJ, Charbonneau F, Title LM, et al. Microvascular function predicts cardiovascular events in primary prevention: long‐term results from the firefighters and their endothelium (FATE) study. Circulation. 2011;123:163‐169. [DOI] [PubMed] [Google Scholar]
- 6. Rubinshtein R, Kuvin JT, Soffler M, et al. Assessment of endothelial function by non‐invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur Heart J. 2010;31:1142‐1148. [DOI] [PubMed] [Google Scholar]
- 7. Matsuzawa Y, Sugiyama S, Sugamura K, et al. Digital assessment of endothelial function and ischemic heart disease in women. J Am Coll Cardiol. 2010;55:1688‐1696. [DOI] [PubMed] [Google Scholar]
- 8. Sangiorgi G, Rumberger JA, Severson A, et al. Arterial calcification and not lumen stenosis is highly correlated with atherosclerotic plaque burden in humans: a histologic study of 723 coronary artery segments using nondecalcifying methodology. J Am Coll Cardiol. 1998;31:126‐133. [DOI] [PubMed] [Google Scholar]
- 9. Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336‐1345. [DOI] [PubMed] [Google Scholar]
- 10. Rumberger JA, Simons DB, Fitzpatrick LA, Sheedy PF, Schwartz RS. Coronary artery calcium area by electron‐beam computed tomography and coronary atherosclerotic plaque area: a histopathologic correlative study. Circulation. 1995;92:2157‐2162. [DOI] [PubMed] [Google Scholar]
- 11. Greenland P, LaBree L, Azen SP, Doherty TM, Detrano RC. Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals. JAMA. 2004;291:210‐215. [DOI] [PubMed] [Google Scholar]
- 12. Arad Y, Spadaro LA, Goodman K, Newstein D, Guerci AD. Prediction of coronary events with electron beam computed tomography. J Am Coll Cardiol. 2000;36:1253‐1260. [DOI] [PubMed] [Google Scholar]
- 13. Kondos GT, Hoff JA, Sevrukov A, et al. Electron‐beam tomography coronary artery calcium and cardiac events. Circulation. 2003;107:2571‐2576. [DOI] [PubMed] [Google Scholar]
- 14. Saleem Y, Defina LF, Radford NB, et al. Association of a favorable cardiovascular health profile with the presence of coronary artery calcification. Circ Cardiovasc Imaging. 2015;8:15‐20. [DOI] [PubMed] [Google Scholar]
- 15. Silverman MG, Blaha MJ, Krumholz HM, et al. Impact of coronary artery calcium on coronary heart disease events in individuals at the extremes of traditional risk factor burden: the multi‐ethnic study of atherosclerosis. Eur Heart J. 2014;35:2232‐2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ahmed HM, Blaha MJ, Nasir K, et al. Low‐risk lifestyle, coronary calcium, cardiovascular events, and mortality: results from MESA. Am J Epidemiol. 2013;178:12‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kulshreshtha A, Goyal A, Veledar E, et al. Association between ideal cardiovascular health and carotid intima‐media thickness: a twin study. J Am Heart Assoc. 2013;2:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dong C, Rundek T, Wright CB, Anwar Z, Elkind MSV, Sacco RL. Ideal cardiovascular health predicts lower risk of myocardial infraction, stroke, and vascular death across whites, blacks, and Hispanics: the northern Manhattan study. Circulation. 2012;125:2975‐2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bambs C, Kip KE, Dinga A, Mulukutla SR, Aiyer AN, Reis SE. Low prevalence of “ideal cardiovascular health” in a community‐based population. Circulation. 2011;123:850‐857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mulukutla SR, Venkitachalam L, Bambs C, et al. Black race is associated with digital artery endothelial dysfunction: results from the heart SCORE study. Eur Heart J. 2010;31:2808‐2815. [DOI] [PubMed] [Google Scholar]
- 21. American Heart Association . Heart Disease and Stroke Statistics. Dalla, TX: American Heart Association; 2006. [Google Scholar]
- 22. Mensah GA, Morkad AH, Ford ES, et al. State of disparities in cardiovascular health in the United States. Circulation. 2005;111:1233‐1241. [DOI] [PubMed] [Google Scholar]
- 23. Erqou S, Kip KE, Mulukutla SR, Aiyer AN, Reis SE. Endothelial dysfunction and racial disparities in mortality and adverse cardiovascular disease outcomes. Clin Cardiol. 2016;39:338‐344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bild DE, Detrano R, Peterson D, et al. Ethnic difference in coronary calcification: the multi‐ethnic study of atherosclerosis (MESA). Circulation. 2005;111:1313‐1320. [DOI] [PubMed] [Google Scholar]
- 25. Erqou S, Kip KE, Mulukutla SR, Aiyer AN, Reis SE. Racial differences in the burden of coronary artery calcium and carotid intima media thickness between blacks and whites. Neth Heart J. 2015;23:44‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ainsworth BE, Jacobs DR Jr, Leon AS. Validity and reliability of self‐reported physical activity status: the lipid research clinics questionnaire. Med Sci Sports Exerc. 1993;25:92‐98. [DOI] [PubMed] [Google Scholar]
- 27. Rifas‐Shiman SL, Willett WC, Lobb R, Kotch J, Dart C, Gillman MW. PrimeScreen, a brief dietary screening tool: reproducibility and comparability with both a longer food frequency questionnaire and biomarkers. Public Health Nutr. 2001;4:249‐254. [DOI] [PubMed] [Google Scholar]
- 28. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827‐832. [DOI] [PubMed] [Google Scholar]
- 29. Hamburg NM, Keyes MJ, Larson MG, et al. Cross‐sectional relations of digital vascular function to cardiovascular risk factors in the Framingham heart study. Circulation. 2008;117:2467‐2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Polonsky TS, Ning H, Daviglus ML, et al. Association of cardiovascular health with subclinical disease and incident events: the multi‐ethnic study of atherosclerosis. J Am Heart Assoc. 2017;6:e004894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xanthakis V, Enserro DM, Murabito JM, et al. Ideal cardiovascular health: associations with biomarkers and subclinical disease and impact on incidence of cardiovascular disease in the Framingham offspring study. Circulation. 2014;130:1676‐1683. [DOI] [PubMed] [Google Scholar]
- 32. Osibogun O, Ogunmoroti O, Spatz ES, et al. Is self‐rated health associated with ideal cardiovascular health? The multi‐ethnic study of atherosclerosis. Clinical Cardiol. 2018; 41 (9): 1154‐1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Folsom AR, Yatsuya H, Nettleton JA, et al. Community prevalence of ideal cardiovascular health, by the AHA definition, and relation to cardiovascular disease incidence. J Am Coll Cardiol. 2011;57:1690‐1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ford ES, Greenlund KJ, Hong Y. Ideal cardiovascular health and mortality from all causes and diseases of the circulatory system among adults in the United States. Circulation. 2012;125:987‐995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Guo L, Zhang S. Association between ideal cardiovascular health metrics and risk of cardiovascular events or mortality: a meta‐analysis of prospective studies. Clin Cardiol. 2017;40:1339‐1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Association of ideal CVH metric with subclinical atherosclerosis (categorical) by race
Table S2A. Association of ideal CVH metric (continuous) with subclinical atherosclerosis (continuous)
Table S2B. Association of ideal CVH metric (continuous) with subclinical atherosclerosis (categorical)
Table S3A. Association of ideal CVH metric (≥3 factors vs <3 factors) with log‐transformed calcium score
Table S3B. Association of ideal CVH metric (≥3 factors vs <3 factors) with log‐transformed calcium score by race.


