Abstract
Background
Left ventricular hypertrophy (LVH) is an independent predictor of new‐onset atrial fibrillation. Whether LVH can predict the recurrence of arrhythmia after radiofrequency catheter ablation (RFCA) in patients with paroxysmal atrial fibrillation (PAF) remains unclear.
Hypothesis
PAF patients with baseline‐electrocardiographic LVH has a higher recurrence rate after RFCA procedure compared with those without LVH.
Methods
A total of 436 patients with PAF undergoing first RFCA were consecutively enrolled and clustered into 2 groups based on electrocardiogram (ECG) findings: non‐ECG LVH (218 patients) and ECG LVH (218 patients). LVH was characterized by the Romhilt‐Estes point score system; the score ≥5points were defined as LVH.
Results
At 42 months' (interquartile range, 18.0–60.0 months) follow‐up after RFCA, 151 (69.3%) patients in the non‐ECG LVH group and 108 (49.5%) patients in the ECG LVH group maintained sinus rhythm without using antiarrhythmic drugs (P < 0.001). Patients with ECG LVH tended to experience a much higher prevalence of stroke and recurrence of atrial arrhythmia episodes compared with those without ECG LVH (log‐rank P < 0.001). Multivariate analysis found the presence of ECG LVH and left atrial diameter to be independent risk factors for recurrence after adjusting for confounding factors.
Conclusions
The presence of ECG LVH was a strong and independent predictor of recurrence in patients with PAF following RFCA.
Keywords: Atrial Fibrillation, Electrocardiogram, Left Ventricular Hypertrophy, Radiofrequency Catheter Ablation
1. INTRODUCTION
Left ventricular hypertrophy (LVH) diagnosed by electrocardiography (ECG) can predict higher risk for developing new‐onset atrial fibrillation (AF) in patients with cardiovascular (CV) diseases.1 Further, the presence of LVH is found to be independent predictor of adverse CV outcomes in AF patients,2 and AF patients with LVH show a higher recurrence rate of AF when they received pharmacological rhythm‐control therapy.3 Radiofrequency catheter ablation (RFCA) is now widely used as a preferential therapy instead of pharmacological therapy for patients with AF, but little is known about the impact of LVH on the outcome of RFCA treatment among AF patients. Considering the low success rate (ranging from 50% to 90%), the complexity of the technique, the enormous expense involved, and a small but definite risk of serious complications associated with RFCA, it is unclear whether patients who exhibit LVH and AF will benefit from RFCA as a preferential therapeutic strategy.
ECG diagnosis of LVH has advantages in speed and simplicity of diagnosis. Of the widely used ECG LVH criteria, the Romhilt‐Estes score system was calibrated by cardiac magnetic resonance examination in a prior study.4 Our study aimed to investigate the relationship of LVH and atrial arrhythmia occurrence in paroxysmal AF (PAF) patients who are undergoing RFCA to set a new and convenient predictor for the clinical outcome for these patients.
2. METHODS
2.1. Study sample
From January 2008 to May 2015, 218 PAF patients with ECG LVH were consecutively enrolled in this study. The ECG LVH was defined using the Romhilt‐Estes point score system (≥5 points; see Supporting Information, Table S1, in the online version of this article).5 The exclusion criteria were patients with the any of the following: previous RFCA, congenital heart disease, rheumatic heart disease, significant valvular heart disease (moderate or severe), cardiomyopathy (acquired or inherited, including hypertrophy cardiomyopathy), bundle‐branch block, pacemaker‐implantation surgery, and impaired systolic function including left ventricular ejection fraction (LVEF) <50% on transthoracic echocardiography (TTE) and atrial thrombus on transesophageal echocardiography (TEE). As an age‐ and sex‐controlled group, 218 PAF patients without ECG LVH were chosen during the same period. All patients' clinical data were obtained and physical examination and laboratory examination including ECG, chest X‐ray, TTE, and TEE were undertaken before RFCA. The study complied with the Declaration of Helsinki and was approved by the institutional review board of Beijing Anzhen hospital.
2.2. Electrocardiography
The standard 12‐lead ECG examination (MAC 800; GE Healthcare, Wauwatosa, WI) during sinus rhythm before RFCA were obtained in all patients. In the present study, PAF patients who had Romhilt‐Estes scores ≥5 points were enrolled in the ECG LVH group. Two investigators (S.‐N.L. and L.W.) independently assessed the ECG LVH on all ECGs. There was a very high degree of reproducibility of ECG measurement, as reflected by a correlation coefficient of 0.95.
2.3. Transthoracic echocardiography and transesophageal echocardiography
TTE (IE33, S5–1; Philips Inc., Amsterdam, The Netherlands) was performed in all patients. Left atrial anterior–posterior diameter (LAD), LVEF, interventricular septum (IVS), left ventricular posterior wall (LVPW), and left ventricular end‐diastolic dimension were measured by M‐mode echocardiography on parasternal long‐axis images in the LV end‐diastolic phase. TEE was performed in all patients to ensure no thrombus in patients' atrium or left atrium appendage in 24 hours before RFCA.
2.4. Radiofrequency catheter ablation
Our single‐catheter technique for AF ablation has been previously described6 and all patients signed the informed consent. All patients stopped using antiarrhythmic drugs (AADs) ≥5 half‐lives before RFCA, but amiodarone was stopped ≥3 months before RFCA. After transseptal puncture under sedation, a bolus of intravenous heparin (100 U/kg) was administered. During the procedure, an activated clotting time of >300 seconds was maintained. Then, pulmonary vein (PV) venography was performed. A 3.5‐mm open‐irrigation ablation catheter (NaviStar ThermoCool; Biosense Webster, Diamond Bar, CA) was advanced into the LA for mapping and ablation with the 3D electroanatomical mapping system (Carto XP or Carto 3; Biosense Webster). PV electrical isolation was achieved by continuous circumferential PV ablation. For PAF patients combined with typical right atrial flutter, cavotricuspid isthmus was ablated. If AF or other types of sustained atrial tachyarrhythmia persisted after initial ablation, direct‐current cardioversion was performed to restore sinus rhythm. Then residual gaps of the ablation lines (including mitral isthmus ablation, left atrial roof line ablation, and superior vena cava isolation) were mapped and ablated. The endpoint of the procedure was PV isolation with complete block of all ablated lines. The ablation catheter was exchanged with a circular mapping catheter that was utilized to verify the isolation of PVs. Conduction block across the lines was validated by pacing maneuvers. Detailed ablation data are available in Supporting Information, Table S2, in the online version of this article.
2.5. Postablation care and long‐term follow‐up
All patients received one kind of AAD (propafenone, sotalol, or amiodarone) treatment, and it was discontinued if no atrial tachycardia was recorded 3 months post‐RFCA. An anticoagulation drug (warfarin, dabigatran, or rivaroxaban) was also used for ≥3 months and continued based on the patients' risk for thromboembolism determined using CHADS2 or CHA2DS2−VASc score.7 A 12‐lead ECG were performed once a week, and 24‐hour Holter monitoring and clinical assessments were performed at 1, 2, 3, 6, and 12 months after the initial ablation and every 6 months thereafter. The patients were asked to record all episodes of any symptoms suggestive of arrhythmia, such as palpitations, dizziness, or shortness of breath, and report them to us as soon as possible. In such cases, an immediate ECG and 24‐hour Holter monitoring during a symptomatic period were suggested in the nearest clinic. Recurrence was defined as an atrial arrhythmia episode including AF, atrial flutter, and other atrial tachycardia lasting >30 seconds without using AADs after the first 3‐month blanking period.8
2.6. Statistical analysis
Continuous variables were presented as mean ±SD and assessed for normality using the Kolmogorov–Smirnov test. Continuous variables were compared using 1‐way analysis of variance (ANOVA) if normally distributed or the nonparametric Mann–Whitney test if not normally distributed. Categorical variables were expressed as percentages (%) and analyzed using the Pearson χ2 test. A Cox regression model was used to identify independent variables predicting the recurrence. Variables that were statistically significant (P < 0.1) in the univariate regression model were selected into a multivariate regression model. A Kaplan–Meier curve and a log‐rank test were used to calculate the arrhythmia recurrence‐free survival. A 2‐sided value of P < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS software, version 20.0 (IBM Corp., Armonk, NY).
3. RESULTS
3.1. Baseline characteristics of patients
Patients were divided into 2 groups: the control group and the ECG LVH group. No statistically significant differences were observed in age, sex distribution, body mass index, coronary artery disease, diabetes mellitus, hyperlipidemia, LVEF, and left ventricular end‐diastolic dimension between the 2 groups. Patients in the ECG LVH group had higher prevalence of hypertension and stroke, higher mean arterial pressure and CHA2DS2‐VASc scores, larger left atrial diameter, and larger left ventricular wall thickness including thicker IVS and LVPW compared with those in the control group (Table 1).
Table 1.
Baseline characteristics of patients
| No‐ECG LVH, n = 218 | ECG LVH, n = 218 | P Value | |
|---|---|---|---|
| Age, y | 60.18 ±9.84 | 61.58 ±10.79 | 0.159 |
| Male sex | 141 (64.7) | 144 (66.1) | 0.763 |
| BMI, kg/m2 | 25.89 ±3.25 | 25.50 ±3.14 | 0.202 |
| HTN | 114 (52.3) | 141 (64.7) | 0.009 |
| CAD | 24 (11) | 28 (12.8) | 0.556 |
| DM | 33 (15.1) | 35 (16.1) | 0.792 |
| Hyperlipidemia | 30 (13.8) | 29 (13.3) | 0.889 |
| Stroke/TIA | 13 (6) | 27 (12.4) | 0.020 |
| MAP, mm Hg | 94.93 ± 10.95 | 99.19 ± 98.95 | <0.001 |
| LAD, mm | 37.13 ± 5.01 | 38.75 ± 5.71 | 0.002 |
| LVEF, % | 65.42 ± 6.21 | 64.67 ± 6.38 | 0.224 |
| IVS, mm | 9.57 ± 1.30 | 10.16 ±1.70 | 0.001 |
| LVPW, mm | 9.25 ± 1.14 | 9.74 ±1.25 | <0.001 |
| LVEDD, mm | 47.43 ± 4.557 | 48.28 ± 4.56 | 0.056 |
| CHA2DS2‐VASc score | 1.51 ± 1.08 | 1.84 ±1.27 | 0.003 |
Abbreviations: BMI, body mass index; CAD, coronary artery disease; CHA2DS2‐VASc, congestive heart failure, HTN, age ≥ 75 y, DM, stroke/TIA, vascular disease, age 65–74 y, sex category (female); DM, diabetes mellitus; ECG, electrocardiography; HTN, hypertension; IVS, interventricular septum; LAD, left atrial diameter; LVEDD, left ventricular end‐diastolic dimension; LVEF, left ventricular ejection fraction; LVH, left ventricular hypertrophy; LVPW, left ventricular posterior wall; MAP, mean arterial pressure; SD, standard deviation; TIA, transient ischemic attack. Data are presented as n (%) or mean ±SD.
3.2. Clinical outcomes after RFCA
After a median follow‐up period of 42.0 months (interquartile range, 18.0–60.0 months), 259 patients stayed in sinus rhythm, 161 patients developed atrial tachycardia recurrence, 16 patients were lost to follow‐up, and 8 patients died of noncardiac causes (4 died in stable sinus rhythm, and 4 died in AF) during the follow‐up period. Almost all patients did as the recommendations given by doctors when discharged except the patients lost to follow‐up (n = 16). The follow‐up data are shown in Table 2. Patients who developed atrial tachycardia recurrence were recommended to use AADs, undergo cardioversion, or receive a repeat RFCA. For more detailed follow‐up data, see Supporting Information, Table S3, in the online version of this article.
Table 2.
Long‐term follow‐up results
| No LVH, n = 218 | ECG LVH, n = 218 | P Value | |
|---|---|---|---|
| Sinus rhythm | 151 (69.3)a | 108 (49.5.0)a | <0.001 |
| Recurrence | 62 (28.4) | 99 (45.4) | — |
| Lost to follow‐up | 5 (2.3) | 11 (5.0) | — |
Abbreviations: AF, atrial fibrillation; ECG, electrocardiography; LVH, left ventricular hypertrophy. Data are presented as n (%).
Four patients died in stable sinus rhythm in the control group, whereas 4 died in AF rhythm in the ECG LVH group.
3.3. Risk factors for atrial tachycardia recurrence
Using univariate analysis, atrial tachycardia recurrence was associated with age, CHA2DS2‐VASc score, ECG LVH, and LAD (all P < 0.05; Table 3). However, only the ECG LVH and LAD presented as independent predictors of recurrence in multivariate analysis, with odds ratios of 1.940 (95% confidence interval: 1.365–2.757) and 1.044 (95% confidence interval: 1.013–1.076). The Kaplan–Meier curve showed the difference in long‐term recurrence after ablation in the 2 groups (log‐rank test P < 0.001; Figure 1).
Table 3.
Univariate and multivariate logistic regression analyses to assess determinants of recurrence
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| All Patients | OR | 95% CI | P Value | OR | 95% CI | P Value |
| Age, per 10 y | 1.020 | 1.004–1.037 | 0.013 | 1.013 | 0.992–1.034 | 0.218 |
| Male sex | 0.777 | 0.557–1.084 | 0.137 | |||
| HTN | 1.132 | 0.814–1.573 | 0.462 | |||
| CAD | 1.078 | 0.658–1.767 | 0.765 | |||
| DM | 1.002 | 0.643–1.564 | 0.991 | |||
| Hyperlipidemia | 1.085 | 0.669–1.760 | 0.742 | |||
| Stroke | 1.251 | 0.754–2.075 | 0.386 | |||
| MAP, per 10 mm Hg | 1.011 | 0.995–1.028 | 0.177 | |||
| BMI, kg/m2 | 0.996 | 0.946–1.048 | 0.870 | |||
| CHA2DS2‐VASc score | 1.177 | 1.030–1.345 | 0.017 | 1.045 | 0.877–1.244 | 0.623 |
| ECG LVH | 2.078 | 1.479–2.920 | <0.001 | 1.940 | 1.365–2.757 | <0.001 |
| Echo parameters | ||||||
| LAD | 1.056 | 1.025–1.189 | <0.001 | 1.044 | 1.013–1.076 | 0.005 |
| LVEF | 0.603 | 0.981–1.033 | 0.603 | |||
| IVS | 1.083 | 0.975–1.204 | 0.139 | |||
| LVPW | 1.069 | 0.935–1.223 | 0.328 | |||
| LVEDD | 1.009 | 0.974–1.045 | 0.636 | |||
Abbreviations: BMI, body mass index; CAD, coronary artery disease; CHA2DS2‐VASc, congestive heart failure, HTN, age ≥ 75 y, DM, stroke/TIA, vascular disease, age 65–74 y, sex category (female); CI, confidence interval; DM, diabetes mellitus; ECG, electrocardiography; Echo, echocardiographic; HTN, hypertension; IVS, interventricular septum; LAD, left atrial dimension; LVEDD, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; LVH, left ventricular hypertrophy; LVPW, left ventricular posterior wall; MAP, mean arterial pressure; OR, odds ratio; TIA, transient ischemic attack. Variables that were statistically significant (P < 0.1) in the univariate regression model were selected into a multivariate regression model.
Figure 1.
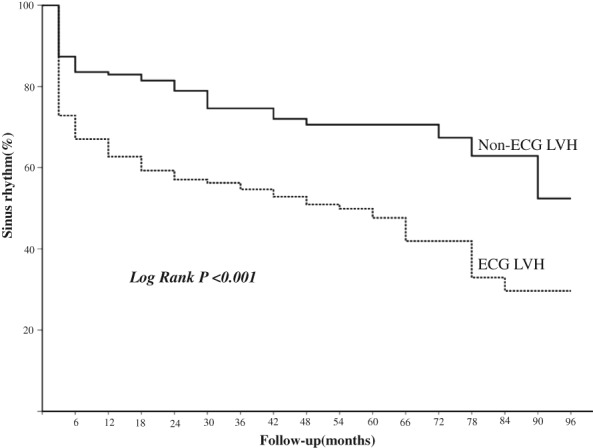
Kaplan–Meier survival analysis for long‐term sinus‐rhythm maintenance after a single RFCA. PAF patients with ECG LVH have higher risk of recurrence (dotted line) compared with those without ECG LVH (solid line). Abbreviations: ECG, electrocardiography; LVH, left ventricular hypertrophy; PAF, paroxysmal atrial fibrillation; RFCA, radiofrequency catheter ablation
4. DISCUSSION
The main finding of the present study is that ECG LVH is a strong and independent predictor of arrhythmia recurrence among PAF patients who received RFCA. The cardiac remodeling of PAF patients having LVH is more severe than patients without LVH. These findings imply that LVH is a powerful and valuable ECG biomarker to identify PAF patients who are at high risk of recurrence after RFCA. Data presented show that patients diagnosed with LVH have significantly larger LAD, IVS, and LVPW than do patients without LVH, indicating a more severe cardiac remodeling in the former group.
The relationship between LVH and AF has been discussed in many prior studies. ECG LVH is an independent predictor of new‐onset AF independently of CMR‐LVH.1 Furthermore Verdecchia et al. demonstrated LVH prediction of risk of stroke, CV death, all‐cause death, and myocardial infarction in anticoagulated patients with nonvalvular AF.9 With the post hoc analysis of the Atrial Fibrillation Follow‐up Investigation of Rhythm Management (AFFIRM) trial, Shah et al… demonstrated that concentric LVH was predictive of AF recurrence in patients who received a pharmacologic rhythm‐control strategy (odds ratio: 1.49, 95% confidence interval: 1.10–2.01, P = 0.01).3 Badheka et al even found that AF patients with LVH experienced a higher mortality when they chose rhythm‐control therapy and concluded that strict rate‐control therapy may be associated with better outcomes in these patients.10 However, our study is the first to evaluate the association between LVH and the long‐term outcome in PAF patients who received RFCA as rhythm‐control therapy. We did not observe the mortality difference between the 2 groups, and after RFCA procedures, PAF patients with ECG LVH could also safely maintain stable sinus rhythm (see Supporting Information, Table S2, in the online version of this article). But the stroke incidence was truly higher in PAF patients with ECG LVH than in patients without ECG LVH (6% vs 12.4%; P = 0.02). We can easily understand that patients with ECG LVH could experience higher blood pressure, higher prevalence of HTN, and more severe cardiac remodeling, but further studies are needed to clarify the association between stroke and ECG LVH.
Several mechanisms are involved in the pathophysiology between LVH and the occurrence of AF. Patients with LVH often have abnormal LV relaxation and impaired filling. As afterload becomes greater, the atrium and PV dilate to compensate, which is a main cause of the occurrence of AF in patients with LVH. In the present study, PAF patients with LVH have a larger LAD, indicating that they experienced a much more severe atrial structural remodeling. Akkaya et al found that AF patients with LVH had a higher degree of left atrial fibrosis, which was considered an important substrate of AF.11 Medi et al found 3 characteristics of atrial electrical remodeling in AF patients with LVH: global conduction slowing, regional conduction delay, and increased AF inducibility, which might partially explain the high correlation between LVH and AF.12
We also independently estimated the LVH by echocardiography examination and found the well consistency between echocardiography examination and ECG examination in diagnosis of LVH with a kappa value of 0.757(0.61–0.80 is substantial agreement, 0.81–0.99 is almost perfect agreement) (see Supporting Information, Table S4, in the online version of this article). Whereas both univariate and multivariate logistic regression analysis revealed ECG LVH to be risk factor for predicting the arrhythmia recurrence after RFCA, the association between the left ventricular wall thickness and the arrhythmia recurrence is not statistically significant. It may imply that ECG‐diagnosed LVH might be a surrogate for other electrical or structural features associated with the recurrence of AF beyond the simple anatomical LVH. Further studies are needed to clarify the underlying association.
4.1. Clinical perspectives
Taking into account the increasing widespread use of RFCA for AF and the close relationship between LVH and AF, the present findings have important clinical implications for managing AF patients undergoing RFCA. This study provides evidence that patients with ECG LVH have a high risk of AF recurrence after RFCA. Previous studies showed that LVH might diminish or disappear with the regression of hypertrophy after antihypertensive therapy, and the regression of LVH was associated with a lower incidence of AF and CV morbidity and mortality in hypertensive patients.13 Therefore, we may improve the outcome of RFCA in PAF patients having LVH through much stricter blood pressure control or other therapeutic measures to reverse LVH. However, further studies will be required to determine whether the therapy aimed specifically at regressing LVH before RFCA or adopting more aggressive ablation strategies will truly reduce the arrhythmia recurrence in these PAF patients.
4.2. Study limitations
This was a retrospective study that included a relatively small number of patients for such heterogeneous collective, a number that may be too low to draw a definite conclusion. The Romhilt‐Estes score system was validated in patients with aortic valve disease, which may not necessarily apply to patients with AF undergoing RFCA. Also, the follow‐up was based on clinical visits, 12‐lead ECG, and 24‐hour ECG Holter; lack of longer monitoring (ECG Holter of ≥4 to 7 days) may result in underestimation of AF recurrences.
5. CONCLUSION
PAF patients with baseline ECG LVH have higher arrhythmia recurrence after undergoing RFCA. These patients may need more aggressive strategies to improve prognosis before receiving RFCA as a rhythm‐control therapy.
Author contributions
The study was designed by Song‐Nan Li, Lu Wang, Jian‐Zeng Dong, and Chang‐Sheng Ma. Data collection was done by Song‐Nan Li, Lu Wang, Ri‐Bo Tang, Rong‐Hui Yu, De‐Yong Long, Cai‐Hua Sang, and Chen‐Xi Jiang. Data were analyzed by Song‐Nan Li, Lu Wang, and Xin Du. The manuscript was prepared by Song‐Nan Li, Lu Wang, Nian Liu, and Rong Bai. Critical revision to the manuscript was done by Jian‐Zeng Dong, Rong Bai, Nian Liu, and Chang‐Sheng Ma. Song‐Nan Li and Lu Wang contributed equally to this work.
Conflicts of interest
The authors declare no potential conflicts of interest.
REFERENCES
- 1. Chrispin J, Jain A, Soliman EZ, et al. Association of electrocardiographic and imaging surrogates of left ventricular hypertrophy with incident atrial fibrillation: MESA (Multi‐Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2014;63:2007–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wachtell K, Okin PM, Olsen MH, et al. Regression of electrocardiographic left ventricular hypertrophy during antihypertensive therapy and reduction in sudden cardiac death: the LIFE Study. Circulation. 2007;116:700–705. [DOI] [PubMed] [Google Scholar]
- 3. Shah N, Badheka AO, Grover PM, et al. Influence of left ventricular remodeling on atrial fibrillation recurrence and cardiovascular hospitalizations in patients undergoing rhythm‐control therapy. Int J Cardiol. 2014;174:288–292. [DOI] [PubMed] [Google Scholar]
- 4. Buchner S, Debl K, Haimerl J, et al. Electrocardiographic diagnosis of left ventricular hypertrophy in aortic valve disease: evaluation of ECG criteria by cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2009;11:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Levy D, Labib SB, Anderson KM, et al. Determinants of sensitivity and specificity of electrocardiographic criteria for left ventricular hypertrophy. Circulation. 1990;81:815–820. [DOI] [PubMed] [Google Scholar]
- 6. Dong J, Liu X, Long D, et al. Single‐catheter technique for pulmonary vein antrum isolation: is it sufficient to identify and close the residual gaps without a circular mapping catheter? J Cardiovasc Electrophysiol. 2009;20:273–279. [DOI] [PubMed] [Google Scholar]
- 7. Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 8. Calkins H, Kuck KH, Cappato R, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation. Recommendations for patient selection, procedural techniques, patient management and follow‐up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Heart Rhythm. 2012;9:632.e621–696.e621.22386883 [Google Scholar]
- 9. Verdecchia P, Reboldi G, Di Pasquale G, et al; RE‐LY Study Investigators . Prognostic usefulness of left ventricular hypertrophy by electrocardiography in patients with atrial fibrillation (from the Randomized Evaluation of Long‐Term Anticoagulant Therapy Study). Am J Cardiol. 2014;113:669–675. [DOI] [PubMed] [Google Scholar]
- 10. Badheka AO, Shah N, Grover PM, et al. Outcomes in atrial fibrillation patients with and without left ventricular hypertrophy when treated with a lenient rate‐control or rhythm‐control strategy. Am J Cardiol. 2014;113:1159–1165. [DOI] [PubMed] [Google Scholar]
- 11. Akkaya M, Higuchi K, Koopmann M, et al. Relationship between left atrial tissue structural remodeling detected using late gadolinium enhancement MRI and left ventricular hypertrophy in patients with atrial fibrillation. Europace. 2013;15:1725–1732. [DOI] [PubMed] [Google Scholar]
- 12. Medi C, Kalman JM, Spence SJ, et al. Atrial electrical and structural changes associated with longstanding hypertension in humans: implications for the substrate for atrial fibrillation. J Cardiovasc Electrophysiol. 2011;22:1317–1324. [DOI] [PubMed] [Google Scholar]
- 13. Okin PM, Wachtell K, Devereux RB, et al. Regression of electrocardiographic left ventricular hypertrophy and decreased incidence of new‐onset atrial fibrillation in patients with hypertension. JAMA. 2006;296:1242–1248. [DOI] [PubMed] [Google Scholar]
Supporting information
Supplementary Table S1: Romhilt‐Estes score system
Supplementary Table S2: Detailed ablation data of all patients
Supplementary Table S3: More followed‐up data:
Supplementary Table S4: Consistency analysis of ECG and ECHO examination in diagnosis of LVH
ACKNOWLEDGMENTS
The authors thank Professor Louisa Dunmall (Queen Mary University of London) for reviewing and revising our manuscript language.
Li S‐N, Wang L, Dong J‐Z, et al. Electrocardiographic left ventricular hypertrophy predicts recurrence of atrial arrhythmias after catheter ablation of paroxysmal atrial fibrillation. Clin Cardiol. 2018;41:797–802. 10.1002/clc.22957
Funding information This work was supported by the National Science Foundation Council of China (No. 81370291), the Capital Health Research and Development of Special (No. 2014‐1‐2061)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1: Romhilt‐Estes score system
Supplementary Table S2: Detailed ablation data of all patients
Supplementary Table S3: More followed‐up data:
Supplementary Table S4: Consistency analysis of ECG and ECHO examination in diagnosis of LVH


