Abstract
Acute pancreatitis can present as sudden, expected death and, therefore, fall under the jurisdiction of the medical examiner/coroner (ME/C). Although its etiologies are varied, alcohol abuse, trauma, and drugs are important to consider in the forensic setting. It is therefore important for the forensic pathologist to have an understanding of these and other etiologies, to have a functional knowledge of the pancreatic anatomy and physiology, and to be able to diagnose acute pancreatitis and distinguish it from postmortem artifact. This review will highlight the forensic aspects of acute pancreatitis, with particular focus on acute hemorrhagic pancreatitis. This will include an overview of the developmental anatomy and normal physiology of the pancreas, the various causes of pancreatitis that may result in deaths coming to the attention of the ME/C, the underlying pathophysiology of the disease, the postmortem diagnosis of acute pancreatitis, and ancillary studies that support the diagnosis. Acad Forensic Pathol. 2018 8(2): 239-255
Keywords: Forensic pathology, Pancreatitis, Hemorrhagic pancreatitis, Trauma, Alcohol
Introduction
The pancreas plays critical roles in digestion, glucose metabolism, and the maintenance of homeostasis. Perhaps, this is why the famous Austrian surgeon, Theodor Billroth, is credited with stating, “God put the pancreas in the back because he did not want surgeons messing with it” (1). In the United States, approximately 210 000 patients are admitted to a hospital annually for treatment of acute pancreatitis (2, 3). Much less commonly, acute pancreatitis will present as sudden, expected death and will be investigated by the medical examiner/coroner (ME/C). In this setting, it is relatively rare, accounting for less than 1% of deaths (4 –7). This review will highlight the forensic aspects of sudden death from acute pancreatitis, with particular focus on acute hemorrhagic pancreatitis. Specifically, it will include pancreatic anatomy and physiology, common etiologies of acute pancreatitis, and the postmortem diagnosis of acute pancreatitis.
Discussion
Anatomy/Physiology
In order to best understand the pathophysiology of acute pancreatitis, it is important to a have a functional understanding of the pancreatic anatomy. Embryologically, the pancreas originates from the duodenum as dorsal and ventral pancreatic buds between the fourth and fifth weeks of gestation (8). The ventral bud rotates to become the uncinate process and main pancreatic duct (of Wirsung) and is closely related to the bile duct and common bile duct. The dorsal bud becomes the body and tail of the gland and in some instances retains the accessory pancreatic duct (of Santorini), which may have a separate entrance in the duodenum (9). As will be discussed, the relationship between the pancreatic ducts and common bile duct can have an impact on the development of gallstone pancreatitis.
The pancreas is a retroperitoneal organ located at the level of the first and second lumbar vertebrae (10). This is important to keep in mind in the setting of trauma with injury to these structures, and the potential effects on the pancreas. Additionally, there are significant vascular structures in close proximity to the posterior aspect of the head and neck of the gland including the aorta, superior mesenteric artery, splenic artery, and junction of the superior mesenteric and splenic veins (portal vein) (8, 10).
Physiologically, the pancreas consists of both endocrine (islets of Langerhans) and exocrine components (acini). Approximately 10% of the gland is made up of islets that secrete three major hormones: insulin, glucagon, and somatostatin (11). The remaining 90% of the gland consists of the exocrine components that produce digestive enzymes estimated at 1500 – 2500 mL per day (10). These are packaged into zymogens as proenzymes where their release is facilitated through the actions of trypsin (12). The release and actions of these enzymes play important roles in both postmortem autolysis and in the pathophysiology of pancreatitis and the resultant alterations in homeostasis.
Epidemiology
Acute pancreatitis is an inflammatory disease that results in a spectrum of mortality related to the severity of disease. Mild acute pancreatitis, also known as edematous pancreatitis, tends to be self-limiting and has a mortality of less than 1% (13, 14), while severe acute pancreatitis, or hemorrhagic pancreatitis, is associated with mortality rates ranging from 10-30% (15 –18). Typically, deaths resulting from acute pancreatitis would not fall under the jurisdiction of the ME/C; however, acute pancreatitis can be a cause of sudden unexpected death and can be seen in the forensic setting. Although the incidence of fatal acute pancreatitis in the ME/C setting is not known, the reported incidence of acute pancreatitis first diagnosed at autopsy ranges between 30-42% (19 –21). In a forensic autopsy-based study, Tsokos and Braun researched acute pancreatitis deaths at the Institute of Legal Medicine in Hamburg, Germany between 2000 and 2004 (4). During that time, 6178 autopsy examinations were performed and 27 cases of fatal acute pancreatitis were identified, resulting in a 0.44% frequency of the disease. Other forensic and autopsy studies have reported a frequency of fatal acute pancreatitis between 0.2% and 0.8% (5 –7).
Pathophysiology
The majority of cases of acute pancreatitis are caused by biliary disease (gallstone pancreatitis) or alcohol use/abuse. Together, these two etiologies account for approximately 75% of the cases of acute pancreatitis seen clinically in the United States; with 40% attributed to biliary disease and 35% attributed to alcohol use/abuse (22, 23). In gallstone pancreatitis, a stone becomes lodged in the bile duct or sphincter of Oddi. It is proposed that this results in injury to the exocrine acinar cells through increased pancreatic duct pressures (12, 24). Therefore, variations in the bile duct/pancreatic duct anatomy can potentially be a factor in the development or severity of gallstone pancreatitis. In the forensic setting, however, acute pancreatitis is most commonly seen in the context of alcoholism, either alone or superimposed on chronic pancreatitis (4, 25, 26). In part, this is due to the inherent selection bias of alcohol-related deaths frequently falling under ME/C jurisdiction. Alcohol (ethanol) causes pancreatitis through a group of related mechanisms. First, it leads to intracellular accumulation of digestive enzymes and there premature activation and release. It also increases the permeability of pancreatic ductules, which allows enzymes to reach the acinar cells, and it increases the protein content of digestive enzymes and decreases the bicarbonate levels and trypsin inhibitor concentrations, thereby promoting protein plugs that block duct outflow (12, 27, 28).
There are a wide variety of less common causes, as seen in Table 1 (29 –32). Specific to the ME/C, pancreatitis should be considered in a delayed sudden death after abdominal trauma or after injury to the upper lumbar vertebrae. In jurisdictions that use “therapeutic complications” as a manner of death, pancreatitis after endoscopic retrograde cholangiopancreatography (ERCP) and related to medications may fall under ME/C jurisdiction. More rarely, pancreatitis from a toxin (e.g., scorpion or snake) can occur. Another etiology that is more specific to forensic practice is hypothermia, in which hemorrhagic pancreatitis has been described (33). However, in a review of studies on pancreatic changes in hypothermia, the presence of hemorrhagic pancreatitis was not consistently seen and varied from 0-67% (34).
Table 1:
Causes of Acute Pancreatitis
| More Common | Choledocholithiasis |
| Alcoholism | |
| Post endoscopic retrograde cholangiopancreatography | |
| Drugs | |
| Abdominal trauma | |
| Less Common | Malignancy/tumor |
| Hypertriglyceremia | |
| Hyperparathyroidism | |
| Hypercalcemia | |
| Viral infection (e.g., mumps, Coxsackie B, Hantavirus) | |
| Developmental abnormalities (e.g., pancreas divisum, annular pancreas) | |
| Toxins (e.g., scorpion or snake bite) |
Independent of etiology, the common pathway of acute pancreatitis is injury to the acinar cells. Once these cells are injured, a host of changes occurs that result in the effective autodigestion of the parenchyma. These include fusion of lysosomal and zymogen granules with resultant activation of trypsinogen to trypsin, activation of the zymogen cascade, and initiation of the inflammatory cell response (12). As the inflammatory response grows, it can become systemic and affect the cardiovascular, respiratory, and renal systems (35, 36). This can result in systemic inflammatory response syndrome (SIRS), shock, diffuse intravascular coagulopathy (DIC), and electrolyte abnormalities (30).
Autopsy Pathology
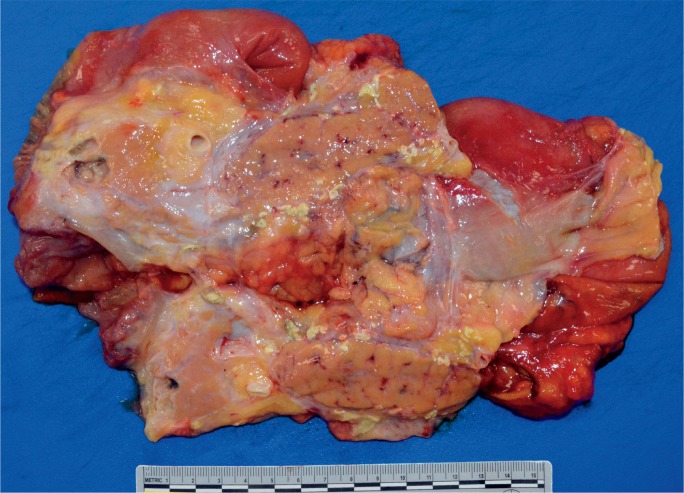
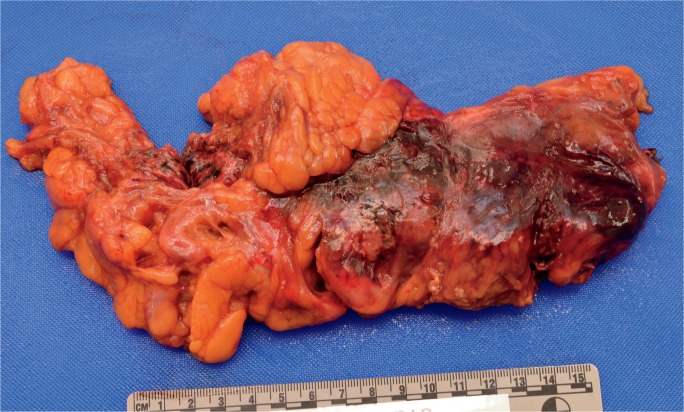
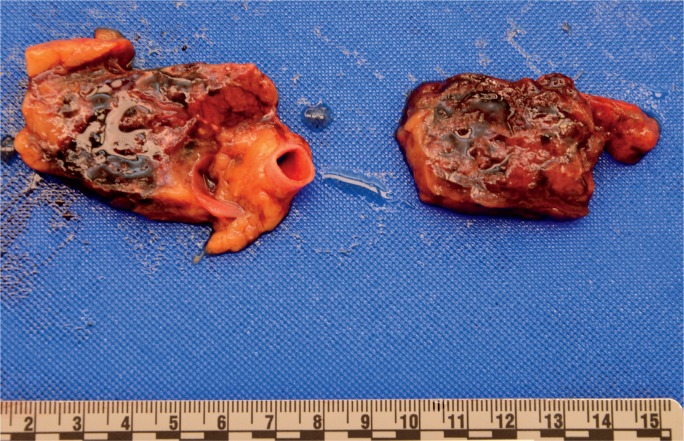
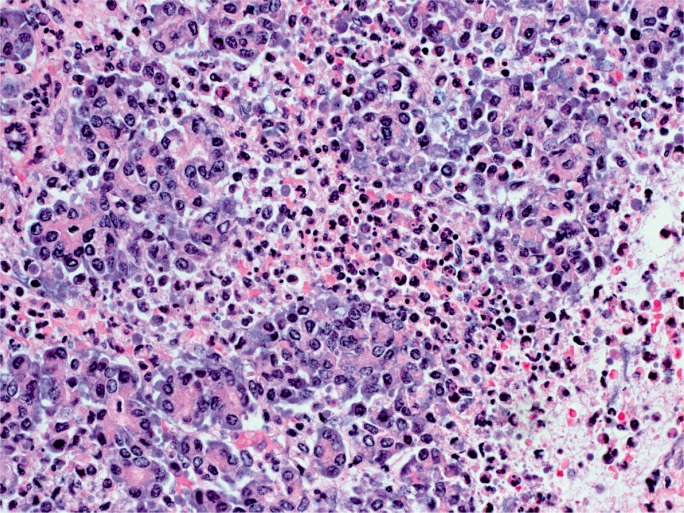
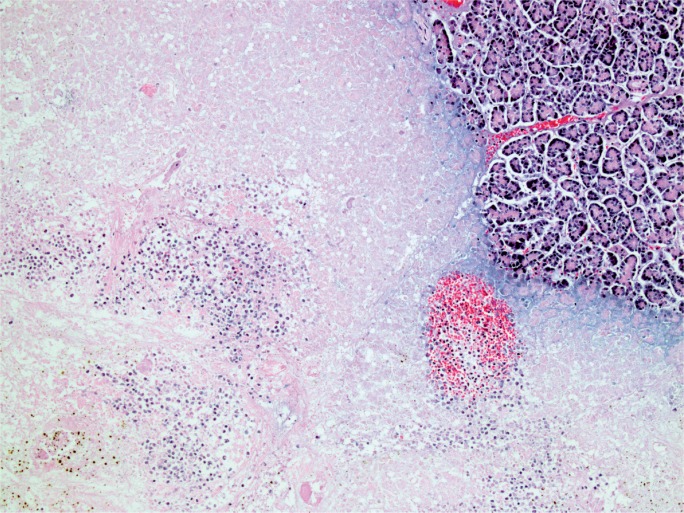
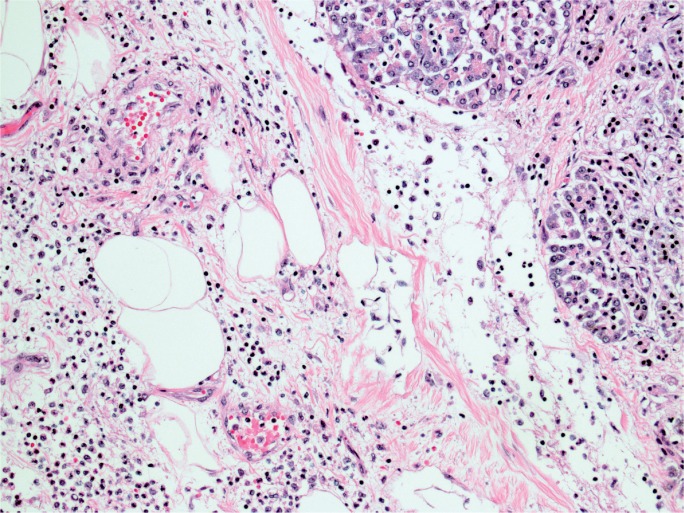
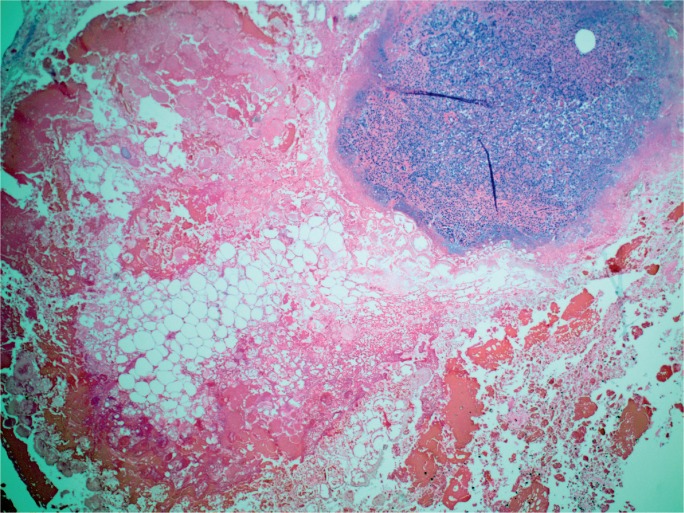
The gross appearance of acute pancreatitis can vary greatly from mild hyperemia of portions of the gland to frank hemorrhagic necrosis that extends to the adjacent tissue and beyond. Some examples are shown in Images 1 to 6. As is the case with many aspects of forensic pathology, it is important for the autopsy pathologist to differentiate genuine disease from postmortem artifact. This is especially true in the pancreas, where the native enzymes and proteins produce rapid autolytic changes. As the cells break down in the postmortem state, digestive enzymes/chemicals that are typically contained in cells and ducts act on the parenchyma to produce degeneration and chemical necrosis. This process is not unlike the pathophysiology of acute pancreatitis where, despite the etiology, the acinar cells become injured and release their contents into the surrounding tissue, which results in additional injury. Given these similarities, it should not be surprising that postmortem autolysis and antemortem pancreatitis might appear similar in the gross state. The only way to definitively confirm the presence of pancreatitis is to examine the tissue microscopically and identify the acute inflammatory cell infiltrate necessary to make such a diagnosis (Images 7 and 8). The acute inflammation is often best seen in the interstitial tissue, which is somewhat spared from the chemical breakdown (Image 9). Other features reported to be helpful in distinguishing pancreatitis from autolysis are fat necrosis and calcium deposition (37); however, fat necrosis without inflammation is frequently seen in postmortem autolysis. It is also important to consider the overall state of decomposition of the body, the positioning of the body, and the distribution of change in the pancreas, as more advanced decomposition, prone positioning, and diffuse interstitial hyperemia/hemorrhage, are more likely to be associated with postmortem autolysis.
Image 1:
Gross appearance of acute pancreatitis with fat necrosis in fileted pancreas.
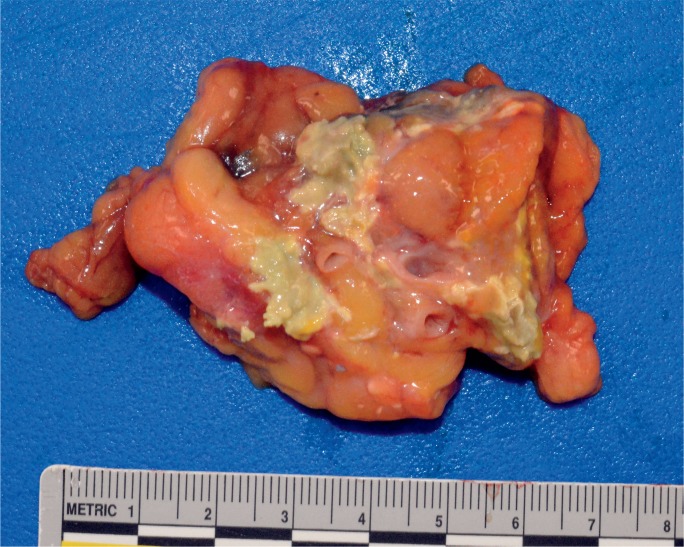
Image 2:
Gross appearance of pancreas with fat necrosis (chalky, yellow discoloration).
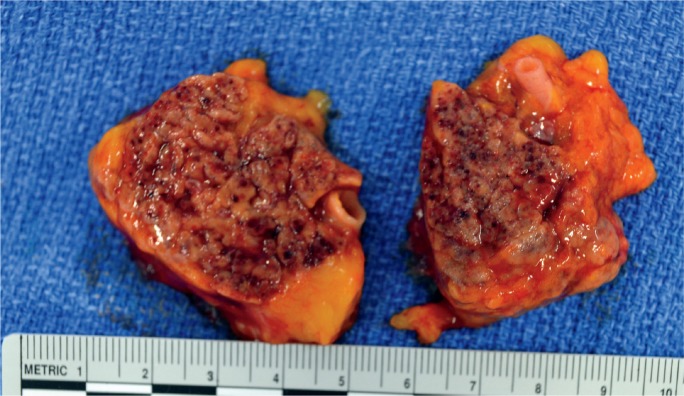
Image 3:
Gross appearance of acute hemorrhagic pancreatitis (mild).
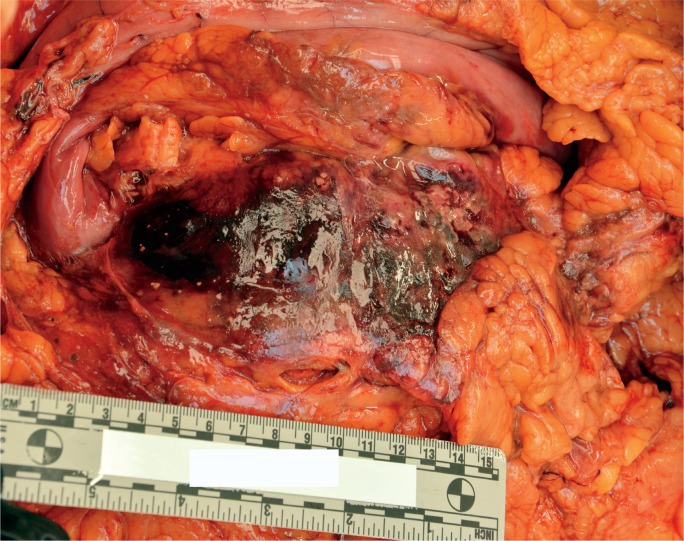
Image 4:
In situ appearance of acute hemorrhagic pancreatitis with retroperitoneal hemorrhage. (Image courtesy of Washoe County Regional Medical Examiner’s Office).
Image 5:
Gross appearance of acute hemorrhagic pancreatitis after evisceration of tissue block. (Image courtesy of Washoe County Regional Medical Examiner’s Office).
Image 6:
Gross appearance of acute hemorrhagic pancreatitis in sectioned pancreas. (Image courtesy of Washoe County Regional Medical Examiner’s Office).
Image 7:
Acute inflammation of pancreatic acinar cells (H&E, x400).
Image 8:
Pancreatic necrosis and inflammation adjacent to normal pancreatic tissue (H&E, x200).
Image 9:
Pancreas with interstitial edema and acute inflammation (H&E, x200).
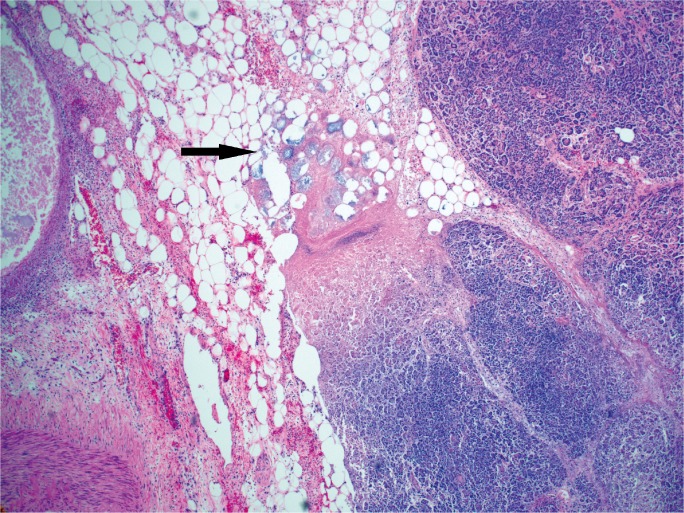
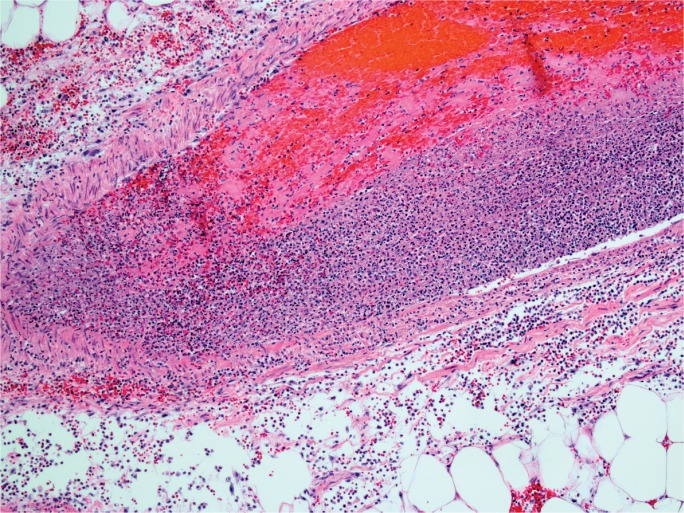
Although acute pancreatitis without hemorrhage is capable of causing death, hemorrhagic pancreatitis is more commonly reported in autopsy-based studies involving sudden death (4, 6, 7, 25). It is likely that the combination of DIC and local auto-digestion result in the hemorrhage seen in pancreatitis (Images 10 to 12). In an experimental study, Bakarev proposed that pancreatic fat necrosis and hemorrhagic necrosis were separate morphological and functional entities (38). The study showed that common bile duct ligation alone produces findings of fat necrosis, while common bile duct ligation in addition to injection of phospholipid A2 produces hemorrhagic necrosis, and with injection of trypsin, produces massive hemorrhage. If hemorrhagic pancreatitis results in significant retroperitoneal bleeding, it is possible that the overlying posterior peritoneal lining can be disrupted and intra-peritoneal hemorrhage (hemoperitoneum) can occur. This is most commonly reported in association with rupture of pancreatic pseudocysts (39, 40), but there are also case reports describing acute intra-pertioneal hemorrhage in the setting of acute hemorrhagic pancreatitis (41 –44) and a single report of this in the forensic literature (45). In this report, five cases of acute hemorrhagic pancreatitis were described, all of which had associated hemorrhage in the peritoneal cavity and destruction of the elastic tissue of the vessels. From a pathophysiologic perspective, this can occur if there is hemorrhage in the retroperitoneal tissue that produces sufficient pressure to disrupt the posterior peritoneal lining, similar to intra-peritoneal hemorrhage from a ruptured abdominal aortic aneurysm. In the forensic setting, it is important to consider this as an alternative to trauma when there is pancreatic hemorrhage with or without retroperitoneal or intra-peritoneal hemorrhage.
Image 10:
Interstitial hemorrhage in acute hemorrhagic pancreatitis (H&E, x40).
Image 11:
Hemorrhage and fat necrosis (arrow) in acute hemorrhagic pancreatitis (H&E, x40).
Image 12:
Acute inflammation of vessel with thrombosis in acute hemorrhagic pancreatitis (H&E, x100). (Image courtesy of Washoe County Regional Medical Examiner’s Office).
Ancillary Testing
Clinically, acute pancreatitis is broadly classified as either mild or severe. Based on the Atlanta classification, severe acute pancreatitis is marked by either evidence of organ failure (systolic blood pressure below 90 mmHg, arterial partial pressure of oxygen 60 mmHg or lower, serum creatinine level of 2 mg/dL or higher, or gastro-intestinal bleeding greater than 500 mL in 24 hours), local complications (necrosis, abscess, or pseudocyst), Ranson score of 3 or higher, or acute physiologic assessment and chronic health evaluation (APACHE) score of 8 or higher (35). In addition to these criteria, there are a number of scoring systems used clinically to guide therapy and predict outcomes in acute pancreatitis (Table 2) (32, 35, 46). Most of these involve clinical assessment and laboratory data that are not able to be measured in the postmortem state. Despite this, there are ancillary tests that can be performed after death that may help to confirm the postmortem diagnosis of acute pancreatitis. Specifically, vitreous testing can reveal elevated glucose concentrations (greater than 200 μg/dL) as well as increased vitreous urea nitrogen (VUN) and creatinine concentrations, indicative of hyperglycemia and renal failure, respectively. In addition, testing of postmortem serum amylase and lipase may also be useful, although results must be interpreted with caution depending on the postmortem interval and lack of specificity of low level elevations (47). Imaging studies such as computed tomography (CT), magnetic resonance imaging (MRI), and ultrasound (US) are frequently used to assess pancreatitis in the clinical setting (32). With the increasing use of CT scans in the postmortem setting, this will become a useful adjunct to making the diagnosis of acute pancreatitis. However, as newer modalities such as postmortem CT angiography become more common, the importance of artifacts relative to the pancreas must be considered (48).
Table 2:
Clinical Scoring Systems Used in Acute Pancreatitis
| Ranson’s Criteria | |
| At admission/diagnosis | Age > 55 |
| White blood cell count > 16 000 per mm3 | |
| Blood glucose > 200 mg/dL | |
| Serum lactate dehydrogenase (LDH) > 350 IU/L | |
| Serum aspartate aminotransferase (AST) > 250 IU/L | |
| During initial 24 hours | Hematocrit decrease > 10% |
| Blood urea nitrogen (BUN) increase > 5 mg/dL | |
| Serum calcium < 8 mg/dL | |
| Base deficit > 4 mmol/L | |
| PaO2 < 60 mmHg | |
| Bedside Index of Severity in Acute Pancreatitis (BISAP) | |
| Blood urea nitrogen (BUN) > 25 mg/dL | |
| Abnormal mental status with Glasgow coma scale < 15 | |
| Evidence of systemic inflammatory response syndrome (SIRS) | |
| Age > 60 | |
| Pleural effusion | |
Conclusion
Although infrequent in the forensic setting, acute pancreatitis should be considered in sudden, unexpected deaths, particularly in those related to alcohol abuse and in delayed deaths after abdominal trauma. In this setting, hemorrhagic pancreatitis is more common, and can be associated with frank intra-peritoneal hemorrhage. The gross findings of acute pancreatitis may overlap with those of postmortem autolysis, and therefore microscopic evidence of acute inflammation must be present to confirm the diagnosis. Ancillary studies, including vitreous testing and postmortem serum amylase and lipase testing, may be performed to support the diagnosis and explain the underlying pathophysiology. Where available, postmortem CT imaging may also serve as a useful adjuvant.
Biography
Robert Stoppacher MD, Onondaga County Medical Examiner’s Office
Roles: Project conception and/or design, data acquisition, analysis and/or interpretation, manuscript creation and/or revision, approved final version for publication, accountable for all aspects of the work.
Footnotes
Ethical Approval: As per Journal Policies, ethical approval was not required for this manuscript
Statement of Human and Animal Rights: This article does not contain any studies conducted with animals or on living human subjects
Statement of Informed Consent: No identifiable personal data were presented in this manuscript
Disclosures & Declaration of Conflicts of Interest: The author, reviewers, editors, and publication staff do not report any relevant conflicts of interest
Financial Fisclosure: The author has indicated that he does not have financial relationships to disclose that are relevant to this manuscript
References
- 1). Schein M. Aphorisms & quotations for the surgeon. Shrewsbury (UK): TFM Publishing; 2004. 276 p. [Google Scholar]
- 2). Russo MW, Wei JT, Thiny MT, et al. Digestive and liver diseases statistics, 2004. Gastroenterology. 2004. May; 126(5):1448–53. PMID: 15131804 10.1053/j.gastro.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 3). Swaropp VS, Chari ST, Clain JE. Severe acute pancreatitis. JAMA. 2004. June 16; 291(23):2865–8. PMID: 15199038 10.1001/jama.291.23.2865. [DOI] [PubMed] [Google Scholar]
- 4). Tsokos M, Braun C. Acute pancreatitis presenting as sudden, unexpected death: an autopsy-based study of 27 cases. Am J Forensic Med Pathol. 2007. September;28(3):267–70. PMID: 17721182 10.1097/paf.0b013e3181425615. [DOI] [PubMed] [Google Scholar]
- 5). DiMaio VJ, DiMaio DJ. Natural death as viewed by the medical examiner: a review of 1000 consecutive autopsies of individuals dying of natural disease. J Forensic Sci. 1991. January; 36(1):17–24. PMID: 2007867 10.1520/JFS13000J. [DOI] [PubMed] [Google Scholar]
- 6). Shetty BS, Boloor A, Menezes RG, et al. Postmortem diagnosis of acute haemorrhagic pancreatitis. J Forensic Leg Med. 2010. August; 17(6):316–20. PMID: 20650420 10.1016/j.jflm.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 7). Renner IG, Savage WT, 3rd, Pantoja JL, Renner VJ. Death due to acute pancreatitis. A retrospective analysis of 405 autopsy cases. Dig Dis Sci. 1985. October; 30(10):1005–18. PMID: 3896700 10.1007/bf01308298. [DOI] [PubMed] [Google Scholar]
- 8). Klimstra DS. Pancreas In: Sternberg SS. Histology for pathologists. 2nd ed Philadelphia: Lippencott-Raven; 1997. 1216 p. [Google Scholar]
- 9). Sadler TW. Langman’s Medical Embryology. 6th ed Baltimore: Williams and Wilkins, 1990. 411 p. [Google Scholar]
- 10). Fischer JE, Bower RH, Bell RH. Liver, biliary, and pancreatic function. In: Davis JH. Clinical Surgery: Vol. 1 St. Louis: Mosby, 1987. [Google Scholar]
- 11). Guyton AC. Textbook of medical physiology. 8th ed Philadelphia: W.B. Saunders; 19911056 p. [Google Scholar]
- 12). Medscape [Internet]. New York: Medscape, LLC; c1994–2018. Acute pancreatitis; [cited 2018 Feb 13]. Available from: https://emedicine.medscape.com/article/181364. [Google Scholar]
- 13). Russo MW, Wei JT, Thiny MT, et al. Digestive and liver disease statistics. 2004. Gastroenterology. 2004. May; 126(5):1448–53. PMID: 15131804 10.1053/j.gastro.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 14). Triester SL, Kowdley KV. Prognostic factors in acute pancreatitis. J Clin Gastroenterol. 2002. February; 34(2):167–76. PMID: 11782614. 10.1097/00004836-200202000-00014. [DOI] [PubMed] [Google Scholar]
- 15). Devenis C, Johnson CD, Bassi C, et al. Diagnosis, objective assessment of severity, and management of acute pancreatitis. Santorini Consensus Conference. Int J Pancreatol. 1999. June; 25(3):195–210. PMID: 10453421. [DOI] [PubMed] [Google Scholar]
- 16). Mayerle J, Hlouschek V, Lerch MM. Current management of acute pancreatitis. Nat Clin Pract Gastroenterol Hepatol. 2005. October; 2(10):473–83. PMID: 16224479 10.1038/ncpgasthep0293. [DOI] [PubMed] [Google Scholar]
- 17). Carnovale A, Rabitti PG, Manes G, et al. Mortality in acute pancreatitis; is it an early or a late event? JOP. 2005. September 10; 6(5):438–44. PMID: 16186665. [PubMed] [Google Scholar]
- 18). Cavallini G, Frulloni L, Bassi C, et al. Prospective multicenter survey on acute pancreatitis in Italy (ProInf-AISP): results on 1005 patients. Dig Liver Dis. 2004. March; 36(3):205–11. PMID: 15046191 10.1016/j.dld.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 19). Corfield AP, Cooper MJ, Williamson RC. Acute pancreatitis: a lethal disease of increasing incidence. Gut. 1985. July;26(7):724–9. PMID: 4018637 10.1136/gut.26.7.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Lankisch PG, Schirren CA, Kunze E. Undetected fatal acute pancreatitis: why is the disease so frequently overlooked? Am J Gastroenterol. 1991. March; 86(3):322–6. PMID: 1705388. [PubMed] [Google Scholar]
- 21). Wilson C, Imrie CW. Deaths from acute pancreatitis: why do we miss the diagnosis so frequently? Int J Pancreatol. 1988. May; 3(4): 273–81. PMID: 2455008 10.1007/BF02788456. [DOI] [PubMed] [Google Scholar]
- 22). Whitcomb DC, Yadav D, Adam S, et al. Multicenter approach to recurrent acute and chronic pancreatitis in the United States: the North American Pancreatitis Study 2 (NAPS2). Pancreatology. 2008; 8(4-5):520–31. PMID: 18765957. PMCID: PMC2790781 10.1159/000152001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23). Banks PA. Epidemiology, natural history, and predictors of disease outcome in acute and chronic pancreatitis. Gastrointest Endosc. 2002. December; 56(6 Suppl): S226–30. PMID: 12447272 10.1016/s0016-5107(02)70016-3. [DOI] [PubMed] [Google Scholar]
- 24). Suda K, Miyano T. Bile pancreatitis. Arch Pathol Lab Med. 1985: 109(5); 433–6. PMID: 3838657. [PubMed] [Google Scholar]
- 25). Tumer AR, Dener C. Diagnostic dilemma of sudden deaths due to acute hemorrhagic pancreatitis. J Forensic Sci. 2007. January;52(1):180–2. PMID: 17209933 10.1111/j.1556-4029.2006.00316. [DOI] [PubMed] [Google Scholar]
- 26). Srettabunjong S, Limgitisupasin W. Severe acute hemorrhagic pancreatitis secondary to cholelithiasis as a rare cause of sudden unexpected death in medico-legal case: a case report. Medicine (Baltimore). 2016. August;95(34): e4680 PMID: 27559973. PMCID: PMC5400340 10.1097/MD.0000000000004680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27). Lerch MM Albrecht E, Ruthenburger M, et al. Pathophysiology of alcohol-induced pancreatitis. Pancreas. 2003. November; 27(4):291–6. PMID: 14576489 10.1097/00006676-200311000-00003. [DOI] [PubMed] [Google Scholar]
- 28). Yadav D, Papachristou GI, Whitcomb DC. Alcohol-associated pancreatitis. Gastroenterol Clin North Am. 2007. June; 36(2):219–38, vii PMID: 17533076 10.1016/j.gtc.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 29). Quinlan JD. Acute pancreatitis. Am Fam Physician. 2014. November 1; 90(9):632–9. PMID: 25368923. [PubMed] [Google Scholar]
- 30). Gill JR. Pancreatitis: a forensic perspective. Acad Forensic Pathol. 2016; 6(2):237–48. 10.23907/2016.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31). Kilt TP, Kilt C, Erarsian S. A rare cause of acute pancreatitis: Hantavirus infection. Acta Gastroenterol Belg. 2017. Jan-Mar; 80(1): 59–61. PMID: 29364099. [PubMed] [Google Scholar]
- 32). Carroll JK, Herrick B, Gipson T, Lee SP. Acute pancreatitis: diagnosis, prognosis, and treatment. Am Fam Physician. 2007. May 15; 75(10):1513–20. PMID: 17555143. [PubMed] [Google Scholar]
- 33). DiMaio DJ, Dimaio VJM. Forensic pathology. Boca Raton: CRC Press, 1993. 528 p. [Google Scholar]
- 34). Madea B, Tsokos M, Preuss J. Forensic pathology reviews. Vol 5 Totowa (NJ): Humana Press, c2008. Chapter 1, Deaths due to hypothermia; p. 3–24. [Google Scholar]
- 35). Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis-2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013. January;62(1):102–11. PMID: 23100216 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 36). Steer ML, Garg P, Saluja AK. Pathogenic mechanisms of acute pancreatitis. Curr Opin Gastroenterol. 2012. September; 28(5):507–15. PMID: 22885948. PMCID: PMC3682674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37). Ye GH, Zhang YG, Yu LS, et al. [Acute necrotizing pancreatitis and postmortem autolysis of pancreas]. Fa Yi Xue Za Zhi. 2008. April; 24(2):94–6, 101 PMID: 18605036. [PubMed] [Google Scholar]
- 38). Bakarev MA, Vasilev AV, Protsenko SI. Pancreatic fat necrosis and hemorrhagic necrosis as separate morphologic and functional entities. Bull Exp Biol Med. 2013. April; 154(6):805–9. PMID: 23658929 10.1007/s10517-013-2061-0. [DOI] [PubMed] [Google Scholar]
- 39). Kelly SB, Gauhar T, Pollard R. Massive intraperitoneal hemorrhage from a pancreatic pseudocyst. Am J Gastroenterol. 1999. December; 94(12):3638–41. PMID: 10606335 10.1016/s0002-9270(99)00515-8. [DOI] [PubMed] [Google Scholar]
- 40). Fiati G, Andren-Sandberg A, LaPinta M, et al. Potentially fatal bleeding in acute pancreatitis: pathophysiology, prevention, and treatment. Pancreas. 2003. January; 26(1):8–14. PMID: 12499910 10.1097/00006676-200301000-00002. [DOI] [PubMed] [Google Scholar]
- 41). Querido S, Carvalho I, Moleiro F, Póvoa P. Fatal acute necrohaemorrhagic pancreatitis with massive intraperitoneal and retroperitoneal bleeding: a rare cause of exsanguination. BMJ Case Rep. 2016. January 20; 2016 pii: bcr2015213732 PMID: 26791128. PMCID: PMC4735153 10.1136/bcr-2015-213732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42). Washiro M, Kusashio K, Yasutomi J, et al. A case of acute pancreatitis with spontaneous massive bleeding into peritoneal cavity. Nihon Rinsho Geka Gakkai Zasshi. 2007; 68(12):3083–6. 10.3919/jjsa.68.3083. [DOI] [Google Scholar]
- 43). Puolakkain P, Lempinin M, Schroder T. Fatal pancreatitis: a study of 64 cases. Acta Chir Scand. 1986. May; 152:379–83. PMID: 3739548. [PubMed] [Google Scholar]
- 44). Frey CF. Hemorrhagic pancreatitis. Am J Surg. 1979. May;137(5): 616–23. PMID: 453456 10.1016/0002-9610(79)90034-5. [DOI] [PubMed] [Google Scholar]
- 45). Murty OP, Agarwal A, Krishnan R. Sudden death due to acute pancreatitis: autopsy observations. J Forensic Med Toxicol. 2008; 25(2):47–53. [Google Scholar]
- 46). Chatzicostas C, Roussomoustakaki M, Viachonikolos IG, et al. Comparison of Ranson, APACHE II, and APACHE III scoring systems in acute pancreatitis. Pancreas. 2002. November; 25(4):331–5. PMID:12409825 10.1097/00006676-200211000-00002. [DOI] [PubMed] [Google Scholar]
- 47). Brown TJ, Prahlow J. Postmortem serum amylase and lipase analysis in the diagnosis of acute pancreatitis. Acad Forensic Pathol. 2018. June; 8(2):311–23. 10.23907/2018.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48). Bruguier C, Grabherr S. Atlas of postmortem angiography. Lausanne (Switzerland): Springer International Publishing, 2016. Chapter 19, Radiologic artefacts of postmortem computed tomography angiography; p. 231–50. [Google Scholar]