Abstract
The diagnosis of acute pancreatitis, which can occur due to natural and nonnatural causes, is usually made at autopsy based on gross and microscopic examination. However, some pathologists choose to measure serum amylase and lipase levels in postmortem blood samples, which may provide corroborating evidence of acute pancreatitis when evaluated in the context of the autopsy findings. A small series of autopsy cases of deaths related to acute pancreatitis with corresponding postmortem serum amylase and lipase levels and a review of the literature are used to highlight the potential benefits and interpretation issues of postmortem serum amylase and lipase. In autopsies without decomposition, elevated postmortem serum amylase (greater than 1000 U/L) and lipase can provide supportive evidence of acute pancreatitis as a cause of death. However, relying on postmortem serum amylase and lipase alone to diagnose acute pancreatitis is insufficient and unreliable. Rather, one must have the gross and histologic evidence of acute pancreatitis. Acad Forensic Pathol. 2018 8(2): 311-323
Keywords: Forensic pathology, Acute pancreatitis, Postmortem serum amylase and lipase, Autopsy, Gross and histologic examination
Introduction
Pancreatitis leading to death may be on the differential diagnosis for pathologists when performing an autopsy on a decedent with a clinical history that includes chronic alcoholism or known gallstones; however, pancreatitis can be caused by other natural conditions, including hyperlipidemia, as well as nonnatural conditions, including blunt abdominal injury, drugs, and hypothermia (1). The postmortem diagnosis of acute pancreatitis is often relatively straight forward. Various gross findings can suggest a possible diagnosis of acute pancreatitis, including pancreatic hemorrhage and evidence of fat necrosis. It should be highlighted, however, that nonspecific postmortem pancreatic hemorrhage (Image 1), probably related to autolysis, can be seen at autopsy; therefore, gross pancreatic hemorrhage is not sufficient to diagnose pancreatitis. Histologic examination of the pancreas, with identification of acute inflammatory cells, is essential for the diagnosis of acute pancreatitis. Depending on the circumstances of the case, acute pancreatitis may be considered a cause or contributing cause of death.
Image 1:
Nonspecific, postmortem pancreatic “hemorrhage,” related to autolysis, in a 44-year-old male who died from a drug over-dose. There was no inflammation microscopically.
While the diagnosis of acute pancreatitis is largely dependent on gross and microscopic evaluation, some pathologists choose to measure serum amylase and lipase levels in postmortem blood samples. Although results must be evaluated in the context of the gross and histologic findings, elevated levels of serum amylase and lipase can provide adjunct laboratory confirmation of acute pancreatitis. Herein, the authors present a small series of autopsy cases of deaths related to acute pancreatitis wherein postmortem serum amylase and lipase levels are reported.
Methods
In order to highlight the utility and limitations of measuring postmortem serum amylase and lipase, the authors have selected a series of five cases from their files where the cause of death of acute pancreatitis was complemented by postmortem serum amylase and lipase results. Refer to Table 1 for a summary of the five cases.
Table 1:
Case Synopsis.
| Case Number | Decedent Information | Initial Presentation | Medical History | Pancreas Exam | Antemortem Testing | Postmortem Serum Testing | Cause of Death | Time Between LKA/Pronounced Dead to Autopsy |
|---|---|---|---|---|---|---|---|---|
| 1 | 47 yowm | Found dead; two-day history of vomiting | Diabetes mellitus; obesity | Focal hemorrhage;+ fat necrosis; acute inflammation | N/A | Amylase: 1817 U/L Lipase: 5080 U/L |
I – Acute hemorrhagic pancreatitis II – DM; cardiomegaly; obesity |
34 to > 40 hours |
| 2 | 24 yowm | Found unresponsive; recent abdominal pain; drinking large amount of water | Obesity | Multifocal hemorrhage; + fat necrosis; acute inflammation | ED blood glucose: >1200 mg/dL TG: 1715 mg/dL |
Amylase: 1920 U/L Lipase: 6606 U/L |
I – Acute hemorrhagic pancreatitis with acute DM, due to hyper-triglyceridemia II – Morbid obesity |
20 hours |
| 3 | 46 yowm | Found unresponsive after complaining of abdominal pain; recent alcohol binge | Hypertension | Multifocal areas of fat necrosis, with fibrosis & scattered hemorrhage; acute and chronic inflammation | N/A | Amylase: 364 U/L Lipase: 1159 U/L |
I – Acute & chronic pancreatitis II – Hypertensive & atherosclerotic cardiovascular disease |
18 to 20 hours |
| 4 | 57 yowm | Presented to ED after not feeling well for one week; increased thirst | None | Swollen and hemorrhagic; venous thrombosis; necrosis; acute inflammation | ED blood glucose: 1357 mg/dL Amylase: 119 U/L Lipase: 6485 U/L |
Amylase: 268 U/L Lipase: >15 000 U/L |
Acute hemorrhagic pancreatitis, with acute diabetic ketoacidosis, related to hyper-triglyceridemia | 18 hours |
| 5 | 30 yowm | Found dead on couch; recent complaints of feeling ill | Chronic alcoholism | Swollen and hemorrhagic; + fat necrosis; acute inflammation | N/A | Amylase: 2100 U/L Lipase: 9580 U/L |
I – Acute hemorrhagic pancreatitis II – Chronic alcoholism |
37 to 68 hours |
LKA – Last known alive
yowm – Year old white male
N/A – Not applicable
DM – Diabetes mellitus
ED – Emergency department
TG – Triglycerides
Case 1
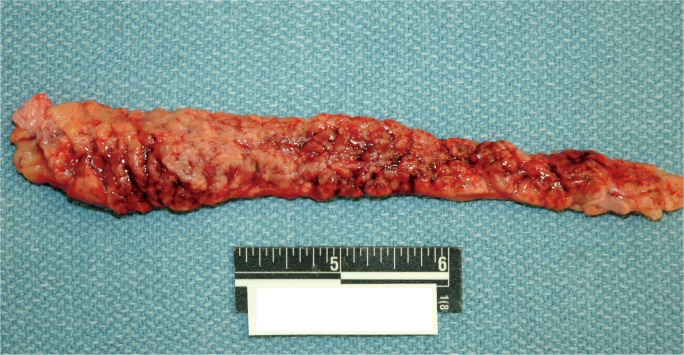
A 47-year-old, obese, diabetic male was found dead at home after a two-day history of vomiting. At autopsy, he was 71 inches tall and weighed 350 pounds (body mass index [BMI] of 48.8 kg/m2). On gross internal exam, his heart weighed 480 g and contained multifocal areas of mild to severe coronary artery atherosclerosis. His pancreas demonstrated marked central hemorrhage (Image 2), and there were multiple foci of yellow-tan chalky discolorations within the pancreas (Image 3) and the surrounding adipose tissue. Microscopic diagnoses consisted of cardiac myocyte hypertrophy and associated interstitial fibrosis, mild hepatic steatosis, mild emphysema, nodular glomerulosclerosis, and acute hemorrhagic pancreatitis with associated fat necrosis. Postmortem urine and blood drug screens were negative. Vitreous electrolytes were unremarkable except for a glucose of 396 mg/dL (acetone negative). Postmortem blood chemistry test results were as follows: serum amylase 1817 U/L (normal 25-105), serum lipase 5080 U/L (normal 16-63), cholesterol 149 mg/dL (normal 120-196), HDL 25 mg/dL (normal 40-85), LDL 72 mg/dL (normal 0-129), chol/HDL ratio 6.0 (normal 1-4), and triglycerides 258 mg/dL (normal 0-150). The cause of death was certified as acute hemorrhagic pancreatitis, with contributing factors of diabetes mellitus, cardiomegaly, and obesity. The manner of death was natural.
Image 2:
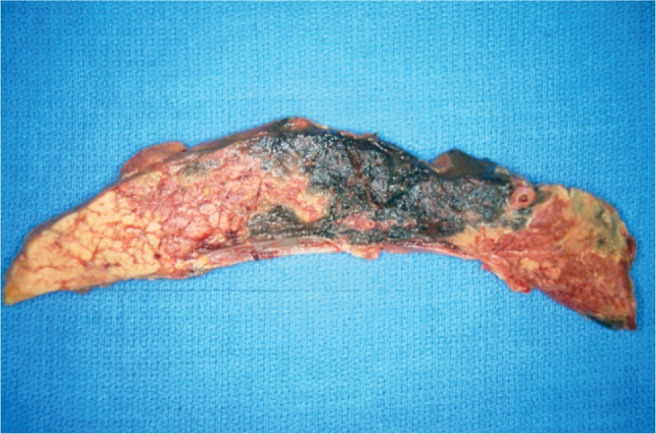
Gross longitudinal section of the pancreas from Case 1. Note the patchy, but prominent areas of hemorrhage. Micro-scopically, there was acute inflammation and hemorrhage.
Image 3:
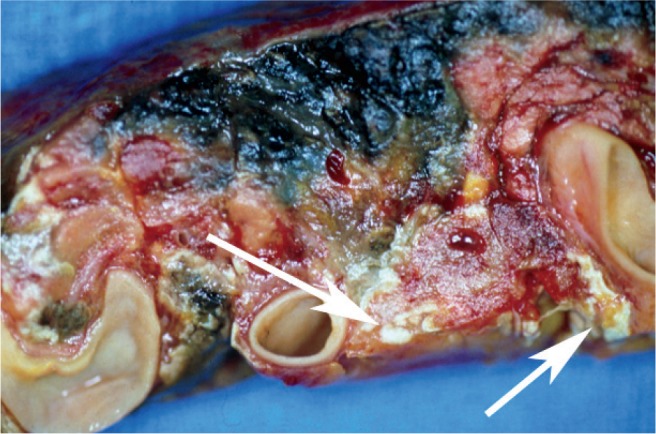
Close-up of a different gross section of the pancreas from Case 1. Note the areas of distinct hemorrhage, as well as the foci of fat necrosis (arrows).
Case 2
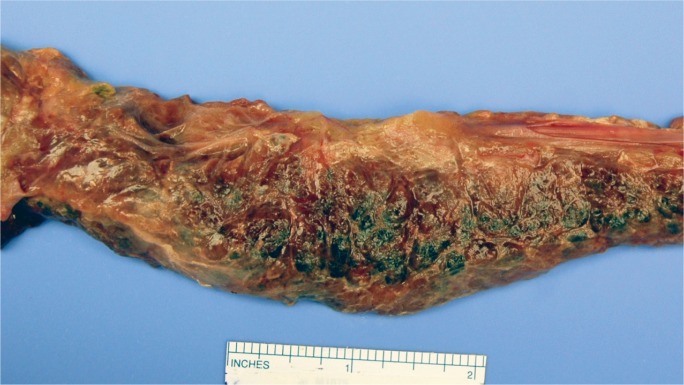
An obese, 24-year-old male was found unresponsive and was emergently transported to a local hospital’s emergency department, where a blood glucose level measured greater than 1200 mg/dL and his blood pH was 6.8. Despite all resuscitative efforts, the patient died. He had recently complained of abdominal pain and had been drinking a large amount of water.
At autopsy, the man weighed 400 pounds and was 75 inches tall (BMI of 50 kg/m2). Significant gross findings included a 560 g heart with mild biventricular dilatation but no associated atherosclerosis, yellow discoloration of the liver, and areas of diffuse hemorrhage within the pancreas (Image 4) with associated white-yellow chalky foci, which extended to include the surrounding adipose tissue. Microscopic findings included cardiac myocyte hypertrophy with associated interstitial fibrosis, chronic bronchial inflammation, hepatic steatosis, and acute hemorrhagic pancreatitis with extensive fat necrosis (Image 5). A urine drug screen was negative. A serum drug screen was positive for acetone (60 mg/dL). Postmortem urinalysis was positive for protein (30 mg/dL), glucose (>1000 mg/dL), and ketones (15 ng/dL). Vitreous electrolytes revealed normal sodium, potassium, and chloride, and the following abnormal concentrations: urea 46 mg/dL; creatinine 1.48 mg/dL, and glucose 700 ng/dL. Postmortem blood chemistry tests included a serum amylase of 1920 U/L and a serum lipase of 6606 U/L. A lipid profile was performed on antemortem hospital blood samples, with the following results: cholesterol 258 mg/dL (normal 135-200), HDL cholesterol 66 mg/dL (normal 40-60), chol/HDL ratio 3.9 (normal 0-6), triglycerides 1715 mg/dL (normal 10-149), and LDL cholesterol unable to calculate due to elevated triglycerides. The cause of death was certified as acute hemorrhagic pancreatitis with acute diabetes mellitus, due to hypertriglyceridemia, with a contributing factor of morbid obesity. The manner of death was natural.
Image 4:
Gross longitudinal section of the pancreas from Case 2. The hemorrhage is more diffuse in this example.
Image 5:
Representative microscopic appearance of the pancreas from Case 2. Note the presence of acute inflammation as well as fat necrosis (H&E, x200).
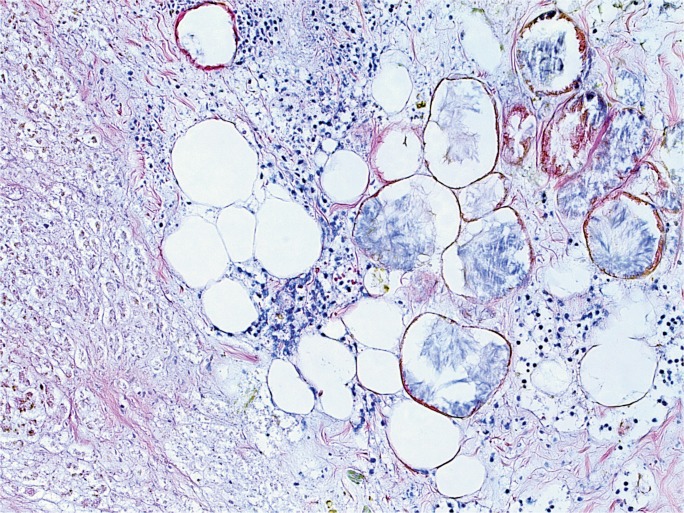
Case 3
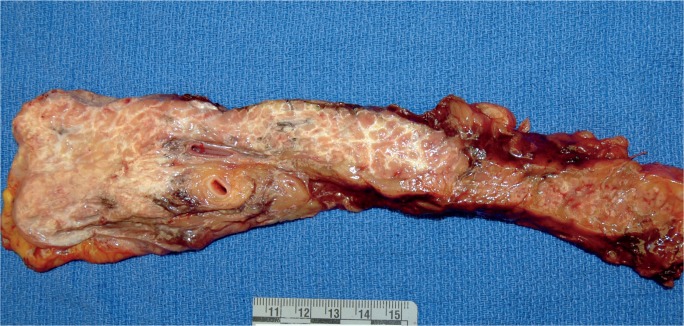
A 46-year-old hypertensive male complained of abdominal pain. His wife went to the store to purchase a laxative, but upon her return, he was unresponsive. All resuscitative efforts failed. He had recently lost his job and had been consuming large amounts of ethanol. In addition, he was taking morphine for his pain.
At autopsy, the 69 inch, 175 pound man (BMI of 25.8 kg/m2) had the following significant gross findings: a 450 g heart, mild to moderate coronary artery atherosclerosis, yellow discoloration of the liver, and a pancreas with numerous areas of white-grey, lacelike fibrotic bands, with scattered surrounding hemorrhage (Image 6). Microscopic examination showed cardiac myocyte hypertrophy with interstitial fibrosis, hepatic steatosis, and patchy acute and chronic pancreatic inflammation with associated fibrosis, hemorrhage, and fat necrosis. Postmortem vitreous electrolytes were normal. A postmortem drug screen was positive for morphine (concentration of 183 ng/mL), but was negative for ethanol. Postmortem blood was also tested for amylase and lipase, with levels of 364 U/L and 1159 U/L, respectively. The cause of death was certified as acute and chronic pancreatitis, with contributing causes of hypertensive and atherosclerotic cardiovascular disease. The manner of death was natural.
Image 6:
Gross longitudinal section of the pancreas from Case 3. In this case, which demonstrated acute and chronic inflammation and fibrosis microscopically, note the widespread, lacelike fibrotic bands, with only focal, patchy areas of grossly-evident hemorrhage.
Case 4
A 57-year-old male had not been feeling well for over a week, with noticeably increased thirst. He presented to a local hospital emergency department, where a blood glucose concentration was 1357 mg/dL. He was admitted with a diagnosis of diabetic ketoacidosis and was placed on insulin therapy. His condition rapidly deteriorated and he experienced cardiorespiratory arrest as he was being prepared for transport to a tertiary care facility. All resuscitative efforts failed and he died approximately 20 hours after initial presentation.
At autopsy, the 76 inch tall man weighed 237 pounds (BMI of 28.8 kg/m2). Significant gross findings included cardiomegaly (540 g), mild to moderate atherosclerosis, and a markedly swollen and hemorrhagic pancreas, with numerous pancreatic and peri-pancreatic veins, including the splenic vein, containing occlusive thrombi. Microscopic examination revealed myocyte hypertrophy with associated interstitial fibrosis, coronary artery atherosclerosis, mild emphysema, moderate steatosis, and acute hemorrhagic pancreatitis with extensive necrosis and venous thrombosis (Image 7). Toxicology testing of the original hospital admission blood was positive for acetone. Antemortem blood sample testing showed the following results: serum lipase 6485 U/L, serum amylase 119 U/L, cholesterol 247 mg/dL, HDL 17 mg/dL, LDL direct 75 mg/dL, non-HDL cholesterol 230 mg/dL, chol/HDL ratio 14.5, triglycerides 964 mg/dL. Postmortem blood sample testing showed the following results: serum lipase >15 000 U/L, serum amylase 268 U/L, cholesterol 248 mg/dL, HDL 12 mg/dL, LDL direct 68 mg/dL, non-HDL cholesterol 236 mg/dL, chol/HDL ratio 20.7, triglycerides 892 mg/dL. The cause of death was certified as acute hemorrhagic pancreatitis with acute diabetic ketoacidosis related to hypertriglyceridemia. The manner of death was natural.
Image 7:
Microscopic section of the pancreas from Case 4, showing an intravascular thrombus, as well as hemorrhage, inflammation, and fat necrosis (H&E, x100).
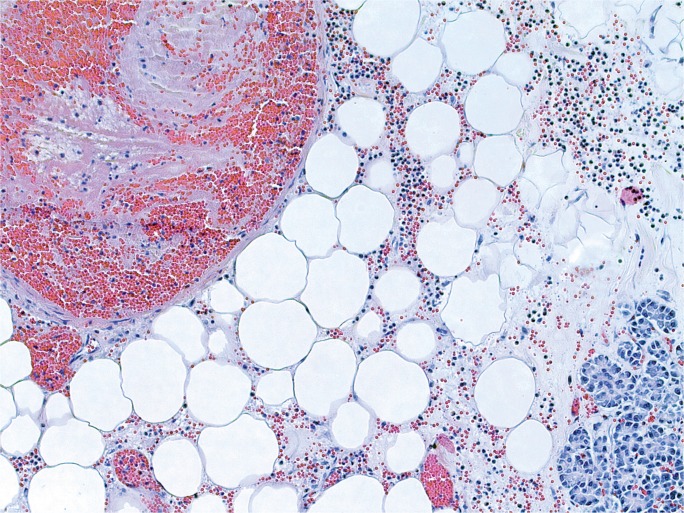
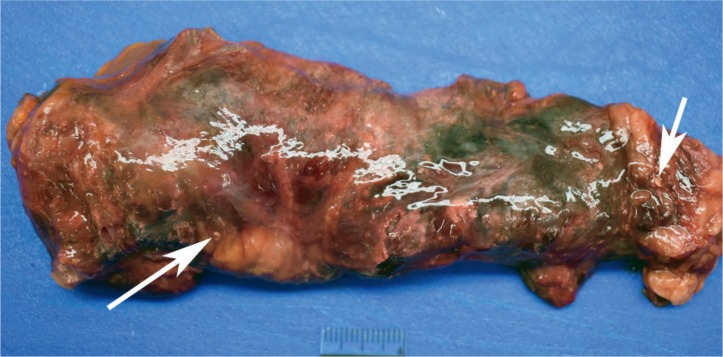
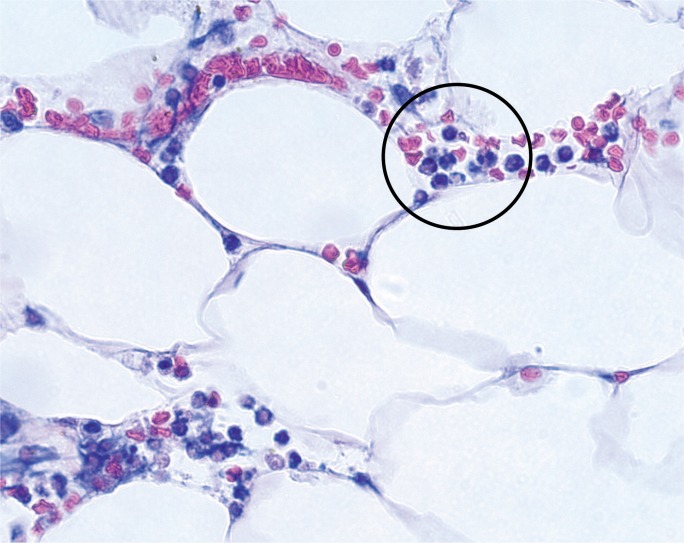
Case 5
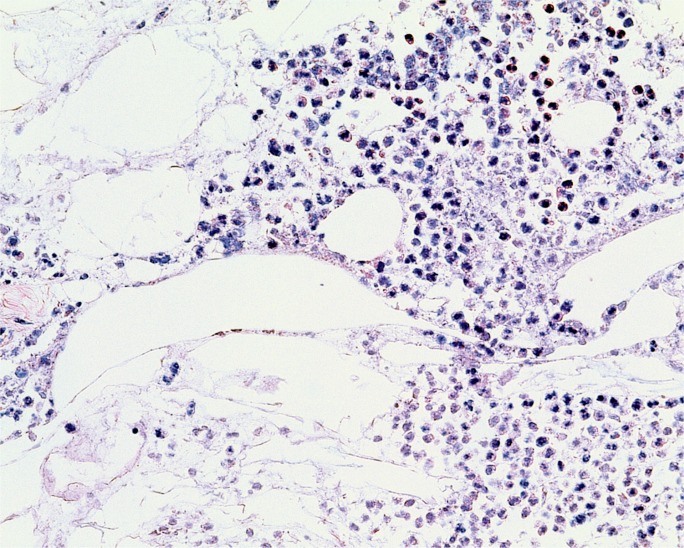
A 30-year-old chronic alcoholic male was found dead on his couch. He had recently complained of feeling ill. At autopsy, the 71 inch, 218 pound man (BMI of 30.4 kg/m2) had a 510 g, mildly-dilated heart, a grossly yellow liver, and a diffusely swollen and hemorrhagic pancreas with areas of white-tan discoloration within the surrounding retroperitoneal adipose tissue (Image 8). Microscopic exam showed cardiac myocyte hypertrophy with interstitial fibrosis, hepatic steatosis with alcoholic hepatitis, and acute pancreatic inflammation with hemorrhage and fat necrosis (Images 9 and 10). Toxicology testing of postmortem blood samples showed therapeutic levels of diphenhydramine. Postmortem serum testing had the following results: amylase 2100 U/L (normal 0-88); lipase 9580 U/L (normal 16-63). Vitreous electrolytes were as follows: sodium 133 mEq/L, potassium 26.7 mEq/L, chloride 90 mEq/L, urea 18 mg/dL, creatinine 1.5 mg/dL, and glucose 125 mg/dL. The cause of death was certified as acute hemorrhagic pancreatitis with a contributing factor of chronic alcoholism. The manner of death was natural.
Image 8:
Gross image of the intact (un-sectioned) pancreas from Case 5. Note the swollen and hemorrhagic appearance, as well as scattered yellow-white foci of fat necrosis, the largest two of which are indicated by arrows.
Image 9:
Microscopic section of the pancreas from Case 5. A majority of the pancreas demonstrated severe autolysis/necrosis, with associated hemorrhage. Focally, there were areas demonstrating somewhat viable appearing inflammatory cells, as seen in this image, where fat necrosis is also evident; however, identification of definite neutrophils was compromised by severe autolysis (H&E, x100).
Image 10:
Microscopic section of the peripancreatic adipose tissue from Case 5, showing rare intact and identifiable neutrophils (encircled). Most of the inflammatory cells (see elsewhere in image) contained nondefined, “smudged” nuclei, consistent with marked autolysis (H&E, x400).
Discussion
Causes of acute pancreatitis include obstructive etiologies, such as a biliary stone; toxins, such as alcohol; drugs; postsurgical complication; genetic predisposition; infections; metabolic abnormalities; autoimmune diseases; pregnancy; and idiopathic reasons (2). In the clinical setting, an elevated serum amylase and/or lipase, in combination with the appropriate clinical presentation and radiographic findings, is strongly suggestive of pancreatitis. While amylase and lipase are typically elevated in this setting, certain etiologies may have serum amylase levels within normal limits due to an interference of the assay, for example by plasma lipids in hypertriglyceridemia (3 –5). Furthermore, an increase in amylase and lipase is not necessarily specific to direct pancreatic pathology. Amylase is secreted by not only the pancreas, but also the salivary glands, small intestine, ovaries, adipose tissue, and skeletal muscle. Also, while an elevation in lipase can indicate acute pancreatitis, it may also represent chronic pancreatitis, acute cholecystitis, or bowel obstruction (6–7). Other diagnoses in which an increase in amylase and lipase has been noted include head injury, abdominal aortic aneurysm, acute liver failure, macroamylasaemia, chronic renal failure, ruptured ectopic pregnancy, toxic epidermal necrolysis, and Stevens-Johnson syndrome (1).
In light of the nonspecificity of the tests, in the clinical setting, serum amylase or lipase greater than three times the upper limit of normal is considered supporting evidence of acute pancreatitis in the correct clinical context (8). Amylase rapidly increases within hours of symptoms and decreases to within normal limits within three to five days. Lipase concentrations also become elevated in the setting of pancreatitis with serum concentrations remaining increased for up to 8 to 14 days after symptoms begin (9 –11). As noted above, though, both amylase and lipase are not specific for the pancreas.
In the postmortem setting, amylase, and to a greater extent, lipase, are not reported as being commonly used by pathologists when considering a diagnosis of pancreatitis. Rather, at autopsy, pathologists rely on gross and microscopic findings to diagnose pancreatitis, including gross hemorrhage and edema, as well as microscopic hemorrhage, parenchymal destruction, acute inflammation, and fat necrosis (12). However, at autopsy, it may be challenging to differentiate pancreatitis from postmortem autolysis (13).
Grossly-identifiable, nonspecific pancreatic hemorrhage is a relatively common finding at autopsy, as seen in Image 1. Presumably, the postmortem extravasation of blood in this setting is related to autolysis involving digestive pancreatic enzymes. Microscopic identification of extravasated blood, without associated inflammation, is the key to recognizing this change as a postmortem artifact. It should also be noted that pancreatic trauma, followed quickly by death, will have a similar histologic appearance (extravasated blood without inflammation).
Coe detailed that postmortem chemistry studies can provide valuable information, including documentation of biochemical changes in cases without significant gross and histologic autopsy findings (14 –16). While some biochemical materials are stable following death, others display both known and unpredictable postmortem changes. Much of the biochemical changes known following death have been studied in the early postmortem period, between death and the start of intravascular hemolysis (14 –16). As is done for routine toxicology testing, values detected from blood collected from femoral and/or subclavian veins are considered the closest representation of antemortem values (14). In the postmortem state, most enzymes, including amylase and lipase, display a rapid and unpredictable variation (14).
A study by Schoning and Strafuss compared canine antemortem versus postmortem serum amylase and lipase up to 48 hours after death (17). Postmortem serum amylase increased no more than 1.5 times the antemortem value and postmortem serum lipase increased up to 14.7 times the antemortem value (17). In humans, a study by Enticknap demonstrated that amylase has been documented to increase after death with values up to three to four times higher (mean of 370 Somogyi units or 684 U/L) on the second day after death (18). Specifically, amylase concentrations demonstrated a biphasic rise after death, increasing rapidly between two and 12 hours after death, followed by a decrease between 13 and 36 hours after death. Amylase then peaked to its highest level between 37 and 48 hours after death, followed be a decrease thereafter (18).
In order to understand the postmortem changes in amylase, a study by Michiue et al. collected postmortem serum amylase concentrations from bilateral cardiac blood specimens in deaths that did not directly involve the pancreas, have a preexisting condition or complication of the pancreas, or have a prolonged death (19). Causes of death in this study included intoxication, hyperthermia, hypothermia, acute brain injury, mechanical asphyxia, drowning, fire fatality, acute ischemic heart disease, and spontaneous cerebral hemorrhage. In most cases, the postmortem serum amylase concentrations in the bilateral cardiac blood specimens were higher than the clinical reference range, likely due to ischemia and hypoxia representing the severity of systemic organ damage (19). When intoxication causes of death were excluded, postmortem serum amylase concentrations were below 1000 U/L in most cases. Therefore, the study concluded that when postmortem serum amylase is greater than 1000 U/L, except in cases where intoxication is the cause of death, this may represent a high likelihood of significant pancreatic pathology, including pancreatitis (19). Similar studies evaluating the utility of lipase were not identified in the literature.
As demonstrated in this case series, all five cases had gross and microscopic evidence of acute pancreatitis. While postmortem serum amylase and lipase are largely supportive of a diagnosis of pancreatitis, relying on postmortem amylase alone, for example in Cases 3 and 4, would have led to missed diagnoses. As discussed previously, for Case 3 in the setting of acute and chronic pancreatitis, amylase decreases to within normal limits three to five days following presentation while lipase remains elevated. For Case 4, an explanation of why amylase is not elevated in the setting of gross and histologic evidence of acute pancreatitis and a greatly elevated lipase is likely due to interference from hypertriglyceridemia, as described previously. However, despite the limitations outlined, postmortem serum amylase and lipase can complement the autopsy gross and histologic findings of pancreatitis (20).
Cases 2 and 4 are examples of cases in which acute pancreatitis developed as a complication of hypertriglyceridemia. In the setting of hyperlipidemic pancreatitis, triglyceride concentrations are often more than 1000 mg/dL (21). Clinically, individuals present with hyperlipidemic pancreatitis in multiple settings, including poorly controlled diabetes mellitus, alcohol use, obesity, or drug use, the most common of which is diabetes mellitus (22, 23). It can be inferred that individuals who present with hyperlipidemic pancreatitis associated with diabetes mellitus can have further destruction of islet cells, leading to a likely final mechanism of death related to acute diabetic ketoacidosis. In both cases, neither individual had a preexisting diagnosis of diabetes mellitus, despite the fact that ketoacidosis was unequivocally present at death. It could not be determined in either case if the diabetes was preexistent or if acute islet cell destruction related to the acute pancreatitis resulted in the acute onset of diabetes and ketoacidosis; however, either possibility seems possible for each case. No obvious renal histologic features of diabetes were identified, and tests were not performed to identify elevated hemoglobin A1C. The presence of either of these two findings would be supportive of preexisting diabetes.
The usefulness of postmortem biochemistry is, in large part, restricted to cases that have autopsy examinations performed within the first 48 hours after death due to increasing postmortem factors that include leakage from cell deterioration and denaturation, degradation, and decomposition of biochemical markers (24). The biochemical profile at the time of death represents multiple factors, including preexisting medical conditions, cause of death, associated complications, survival period, and postmortem changes (24, 25).
In autopsies without decomposition, postmortem serum amylase and lipase can provide supportive evidence of acute pancreatitis as a cause of death, especially when the serum amylase is greater than 1000 U/L without evidence of intoxication (19). However, relying on postmortem serum amylase and lipase alone to diagnose acute pancreatitis is insufficient and unreliable. Rather, one must have the gross and histologic evidence of acute pancreatitis. In cases that demonstrate extensive pancreatic autolysis, increased postmortem serum amylase and lipase can help trigger the pathologist to carefully look at the pancreas or peripancreatic adipose tissue, as demonstrated in Case 5, for any evidence of acute neutrophilic exudate within the autolytic pancreatic parenchyma and/or peripancreatic adipose tissue. An elevated lipase without a corresponding elevation of amylase may be explained by the timing of presentation/death, versus interference by hypertriglyceridemia.
For purposes of establishing the most accurate reflection of antemortem amylase and lipase concentrations, it seems intuitive to collect peripheral blood samples that are furthest from the pancreas, either subclavian or femoral vein, and in cases of trauma-related pancreatitis, at a site most distal from the pancreas and the bulk of the traumatic injuries. Developing a consistent means of sampling multiple sites, including vitreous humor, central and peripheral blood, cerebrospinal fluid, and urine is recommended in order to establish a database of the biochemical profile in individuals at death (24). In order to expand the database on postmortem serum amylase and lipase concentrations, especially when acute pancreatitis is in the differential diagnosis at autopsy, pathologists are encouraged to order postmortem serum amylase and lipase concentrations. Additional postmortem chemistry studies, such as lipid profiles and tests which identify ketoacidosis (vitreous glucose and ketones; blood ketones) may also help to clarify the cause and mechanism of death in cases of pancreatitis.
Conclusion
The diagnosis of acute pancreatitis requires histologic identification of acute pancreatic inflammation. Minor elevations of postmortem serum amylase and lipase are nonspecific, or, in the case of amylase, may be related to the timing of sample collection or artifactually low due to interfering substances. Markedly elevated postmortem serum amylase (greater than 1000 U/L) and lipase concentrations may provide corroborating evidence of acute pancreatitis. This may be especially useful when severe autolysis/necrosis results in difficulties in recognizing acute inflammation histologically. Pathologists should consider the possible causative role of hypertriglyceridemia in cases of acute pancreatitis. Postmortem lipid studies can assist in making such a diagnosis. Pathologists should also consider the possibility of acute diabetic ketoacidosis in persons dying from acute pancreatitis, even in persons without a previous diagnosis of diabetes, as the mechanism of death. Elevated postmortem vitreous glucose and ketones, as well as postmortem blood ketones, can assist in making such a diagnosis. Evidence of renal diabetic changes, or elevated hemoglobin A1C, may provide evidence that diabetes was preexistent.
Authors
Theodore T. Brown MD, Western Michigan University Homer Stryker MD School of Medicine - Pathology
Roles: Project conception and/or design, data acquisition, analysis and/or interpretation, manuscript creation and/or revision, approved final version for publication, accountable for all aspects of the work, principal investigator of the current study.
Joseph A. Prahlow MD, Western Michigan University Homer Stryker MD School of Medicine - Pathology
Roles: Project conception and/or design, data acquisition, analysis and/or interpretation, manuscript creation and/or revision, approved final version for publication, accountable for all aspects of the work, principal investigator of the current study.
Footnotes
Ethical Approval: As per Journal Policies, ethical approval was not required for this manuscript
Statement of Human and Animal Rights: This article does not contain any studies conducted with animals or on living human subjects
Statement of Informed Consent: No identifiable personal data were presented in this manuscript
Disclosures & Declaration Of Conflicts Of Interest: The authors, reviewers, editors, and publication staff do not report any relevant conflicts of interest
Financial Disclosure: The authors have indicated that they do not have financial relationships to disclose that are relevant to this manuscript
References
- 1). Muniraj T, Dang S, Pitchumoni CS. Pancreatitis or not?--Elevated lipase and amylase in ICU patients. J Crit Care. 2015. December; 30(6): 1370–5. PMID 26411523 10.1016/j.jcrc.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 2). Frossard JL, Steer ML, Pastor CM. Acute pancreatitis. Lancet. 2008. January 12; 371(9607):143–52. PMID: 18191686 10.1016/s0140-6736(08)60107-5. [DOI] [PubMed] [Google Scholar]
- 3). Fallat RW, Vester JW, Glueck CJ. Suppression of amylase activity by hypertriglyceridemia. JAMA. 1973. September 10; 225(11):1331–4. PMID: 4740657 10.1001/jama.225.11.1331. [DOI] [PubMed] [Google Scholar]
- 4). Lesser PB, Warshaw AL. Diagnosis of pancreatitis masked by hyperlipemia. Ann Intern Med. 1975. June; 82(6):795–8. PMID: 1138589 10.7326/0003-4819-82-6-795. [DOI] [PubMed] [Google Scholar]
- 5). Warshaw AL, Bellini CA, Lesser PB. Inhibition of serum and urine amylase activity in pancreatitis with hyperlipemia. Ann Surg. 1975. July; 182(1):72–5. PMID: 1147712 10.1097/00000658-197507000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Rompianesi G, Hann A, Komolafe O, et al. Serum amylase and lipase and urinary trypsinogen and amylase for diagnosis of acute pancreatitis. Cochrane Database Syst Rev. 2017. April 21; 4: CD012010 PMID: 28431198 10.1002/14651858.cd012010.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7). Vissers RJ, Abu-Laban RB, McHugh DF. Amylase and lipase in the emergency department evaluation of acute pancreatitis. J Emerg Med. 1999. Nov-Dec; 17(6):1027–37. PMID: 10595892 10.1016/s0736-4679(99)00136-5. [DOI] [PubMed] [Google Scholar]
- 8). Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013. January; 62(1):102–11. PMID: 23100216 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 9). Matull WR, Pereira SP, O’Donohue JW. Biochemical markers of acute pancreatitis. J Clin Pathol. 2006. April; 59(4):340–4. PMID: 16567468. PMCID: PMC1860356 10.1136/jcp.2002.002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). Smotkin J, Tenner S. Laboratory diagnostic tests in acute pancreatitis. J Clin Gastroenterol. 2002. April; 34(4):459–62. PMID: 11907364 10.1097/00004836-200204000-00018. [DOI] [PubMed] [Google Scholar]
- 11). Yadav D, Agarwal N, Pitchumoni CS. A critical evaluation of laboratory tests in acute pancreatitis. Am J Gastroenterol. 2002. June; 97(6):1309–18. PMID: 12094843 10.1016/s0002-9270(02)04122-9. [DOI] [PubMed] [Google Scholar]
- 12). Renner IG, Savage WT, 3rd, Pantoja JL, Renner VJ. Death due to acute pancreatitis. A retrospective analysis of 405 autopsy cases. Dig Dis Sci. 1985. October; 30(10):1005–18. PMID: 3896700 10.1007/bf01308298. [DOI] [PubMed] [Google Scholar]
- 13). Siriwardana RC, Deen KI, Hevawesenthi J. Postmortem sampling of the pancreas for histological examination: what is the optimum cutoff time? JOP. 2010. January 8; 11(1):87–8. PMID: 200655563. [PubMed] [Google Scholar]
- 14). Coe JI. Postmortem chemistry: practical considerations and a review of the literature. J Forensic Sci. 1974. January; 19(1):13–32. PMID: 4853713 10.1520/jfs10066j. [DOI] [PubMed] [Google Scholar]
- 15). Coe JI. Postmortem chemistry update. Emphasis on forensic application. Am J Forensic Med Pathol. 1993. June; 14(2):91–117. PMID: 8328447 10.1097/00000433-199306000-00001. [DOI] [PubMed] [Google Scholar]
- 16). Coe JI. Postmortem chemistry of blood, cerebrospinal fluid, and vitreous humor. Leg Med Annu. 1977; 1976:55–92. PMID: 325316. [PubMed] [Google Scholar]
- 17). Schoning P, Strafuss AC. Postmortem sera and cerebrospinal fluid enzymes. J Forensic Sci. 1980. April; 25(2):344–8. PMID: 6156226 10.1520/jfs12133j. [DOI] [PubMed] [Google Scholar]
- 18). Enticknap JB. Biochemical changes in cadaver sera. J Forensic Med. 1960; 7:135–46. [Google Scholar]
- 19). Michiue T, Ishikawa T, Kawamoto O, et al. Postmortem serum levels of amylase and gamma glutamyl transferase (GGT) as markers of systemic tissue damage in forensic autopsy. Leg Med (Tokyo). 2013. March; 15(2):79–84. PMID: 23165248 10.1016/j.legalmed.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 20). Palmiere C, Lesta Mdel M, Sabatasso S, et al. Usefulness of postmortem biochemistry in forensic pathology: illustrative case reports. Leg Med (Tokyo). 2012. January; 14(1):27–35. PMID: 22177826 10.1016/j.legalmed.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 21). Toskes PP. Hyperlipidemic pancreatitis. Gastroenterol Clin North Am. 1990. December; 19(4):783–91. PMID: 2269517. [PubMed] [Google Scholar]
- 22). Fortson MR, Freedman SN, Webster PD. 3 rd Clinical assessment of hyperlipidemic pancreatitis. Am J Gastroenterol. 1995. December; 90(12): 2134–9. PMID: 8540502. [PubMed] [Google Scholar]
- 23). Yadav D, Pitchumoni CS. Issues in hyperlipidemic pancreatitis. J Clin Gastroenterol. 2003. January; 36(1):54–62. https://doi .org/10.1097/00004836-200301000-00016. [DOI] [PubMed] [Google Scholar]
- 24). Maeda H, Ishikawa T, Michiue T. Forensic biochemistry for functional investigation of death: concept and practical application. Leg Med (Tokyo). 2011. March; 13(2):55–67. PMID: 21269863 10.1016/j.legalmed.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 25). Maeda H, Zhu BL, Ishikawa T, et al. Significance of postmortem biochemistry in determining the cause of death. Leg Med (Tokyo). 2009. April; 11 Suppl 1: S46–9. PMID: 19269240 10.1016/j.legalmed.2009.01.048. [DOI] [PubMed]