Abstract
The liver is subject to a variety of extrinsic and intrinsic insults that manifest with both specific and nonspecific patterns of necrosis. In the autopsy setting, these patterns are often encountered as incidental findings or even causes of death. There are several etiologies of hepatic necrosis, including toxins, drug injuries, viral infections, ischemic injuries, and metabolic disease, all of which possess overlapping gross and histologic presentations. Nonetheless, patterned necrosis in the context of clinical and demographic history allows for the forensic pathologist to develop a differential diagnosis, which may then be pruned into a specific or likely cause. The aim of the following review is to elucidate these patterns in the context of the liver diseases from which they arise with the goal developing a differential diagnosis and ultimate determination of etiology. Acad Forensic Pathol. 2018 8(2): 256-295
Keywords: Forensic pathology, Liver necrosis, Acute liver failure, Hepatitis
Introduction
The liver is subject to a variety of insults that induce acute and chronic changes. Acute hepatitis lasts less than six months but may progress to chronic hepatitis with repeated insults or unresolved injury. In its most severe form, hepatic injury evolves into acute liver failure (ALF) or acute-on-chronic liver failure (ACLF), two conditions with a high mortality rate secondary to a rapid decline in liver function. In all of these conditions, necrosis is a common feature, arising from multiple etiologies that manifest with both specific and nonspecific histologic patterns. The following review will discuss these etiologies and as sociated patterns in the context of the liver diseases from which they arise with the goal of aiding forensic pathologists in developing a differential diagnosis and identifying specific causes of hepatic necrosis encountered at autopsy.
Discussion
Apoptosis and Necrosis
Apoptosis and necrosis are the two central modes of cell death in the liver. Apoptosis is a regulated process that may either be physiologic or pathologic. In the former, apoptosis culls aged cells, functions in embryologic development, and is vital to adulthood remodeling (e.g., follicular atresia following ovulation). Necrosis, on the other hand, is abrupt cell death that is almost always pathologic. In the liver, necrosis is often a response to extrinsic insults such as medication/medication metabolites, toxins, infection, ischemia, and trauma. Though rare, intrinsic autoimmune injury is also a source of hepatic injury (1).
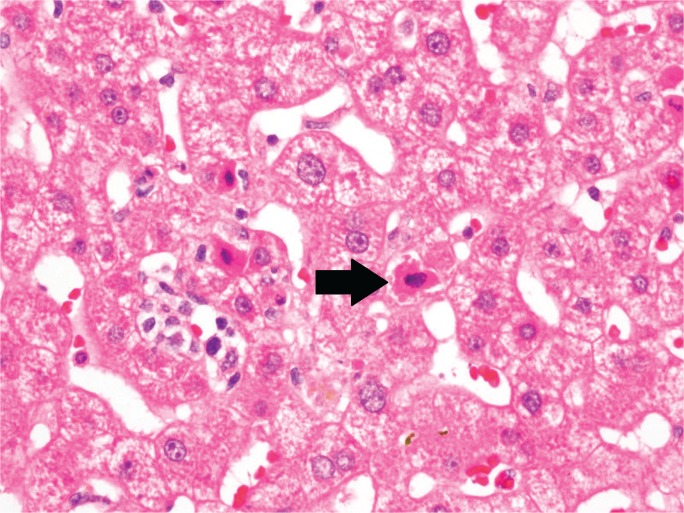
Morphologically, apoptosis displays cellular condensation, nuclear condensation (pyknosis), nuclear fragmentation (karyorrhexis), and cytoplasmic blebbing. Eventually, chromatin and organelles are packaged into vesicles, forming so-called apoptotic, acidophil, or Councilman bodies (Image 1), that are easily phagocytosed by Kupffer cells with little to no inflammatory activation. By contrast, necrosis features cellular swelling with cytoplasmic blebs that lyse the cell, spilling intracellular contents into the surrounding environment, and ultimately eliciting an inflammatory response. This characteristic swelling and consequent karyolysis is referred to as oncotic necrosis (2).
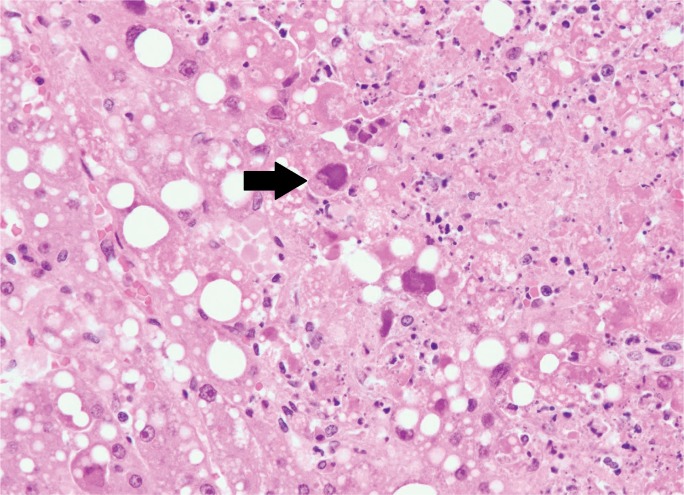
Image 1:
Section of liver showing several hypereosinophilic, apoptotic hepatocytes (arrow) (H&E, x400).
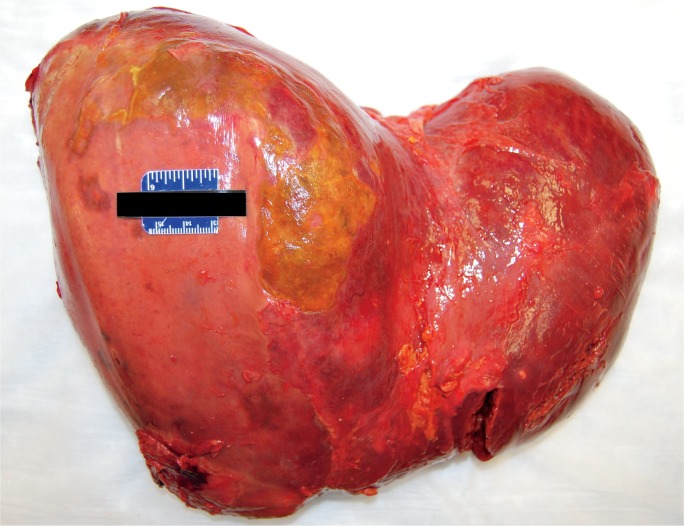
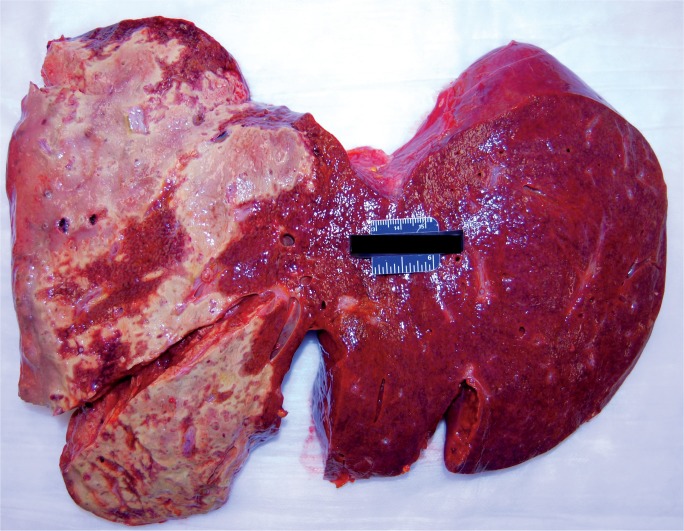
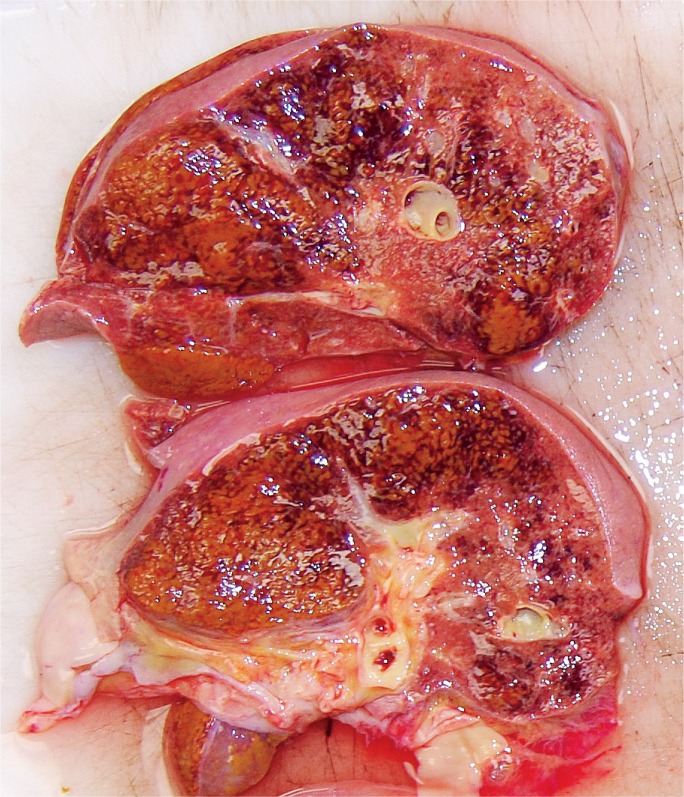
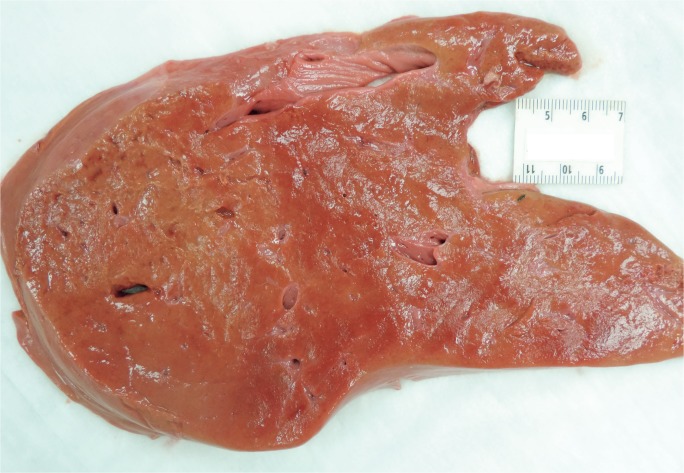
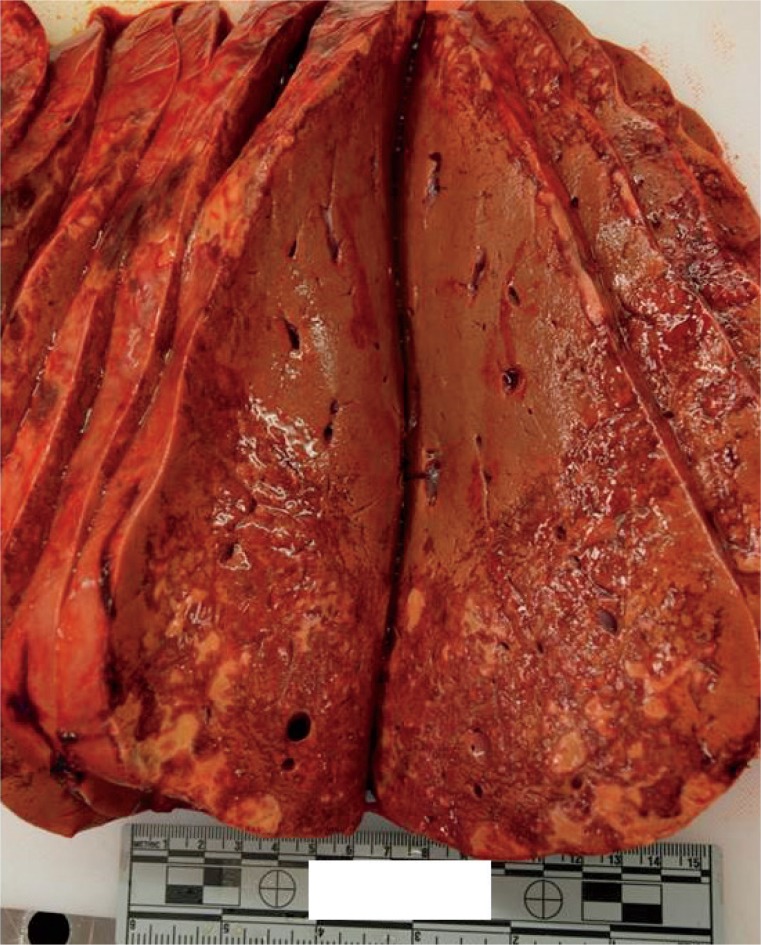
Livers with diffuse necrosis show characteristic gross pathologic findings. Early, the liver may be edematous with foci of necrosis presenting as pale, tan-yellow punctate lesions, while more extensive involvement leads to loss of the liver parenchyma with large patches of necrosis and wrinkling of the capsule (Images 2A and 2B) (3).
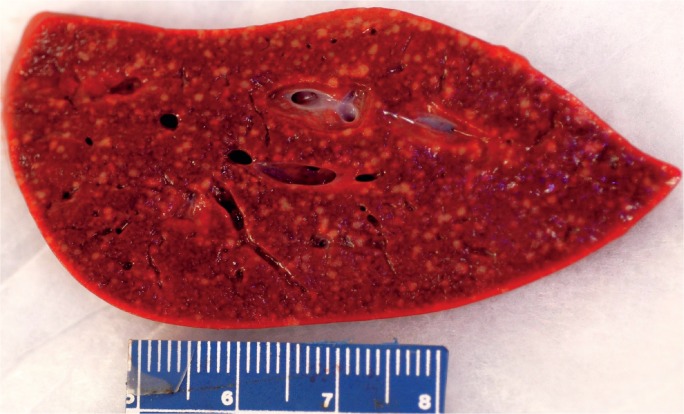
Image 2A:
Anterior surface of a liver with massive hepatic necrosis associated with acute pancreatitis showing wrinkling along with patchy areas of red and yellow-orange discoloration.
Image 2B:
Cross-section of a liver with massive hepatic necrosis associated with acute pancreatitis showing widespread necrosis in the left side of the liver.
Liver Anatomy
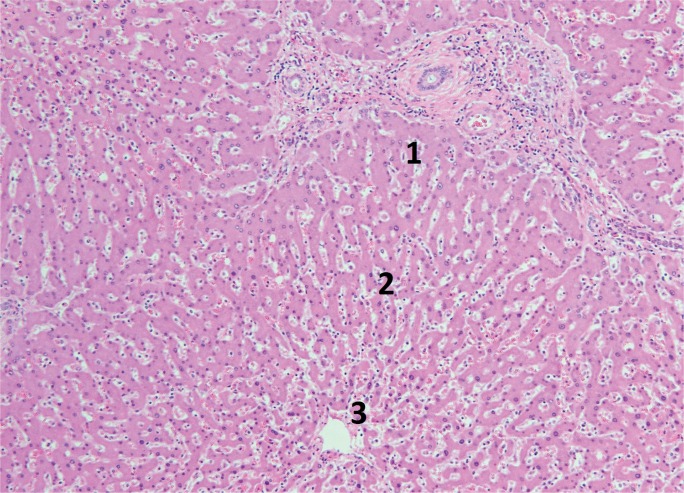
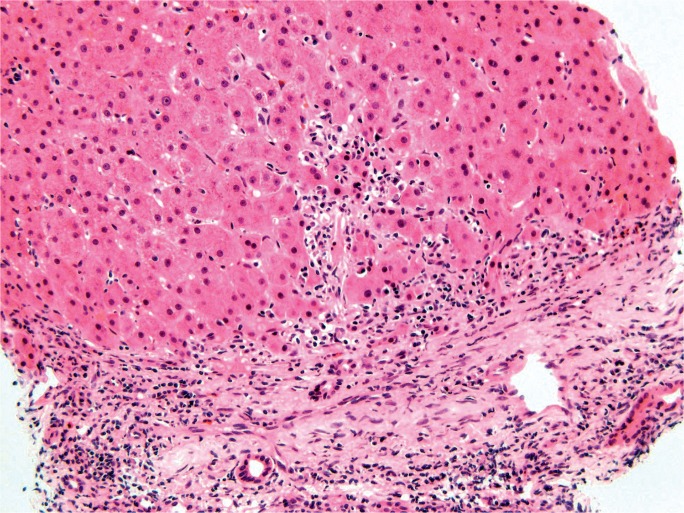
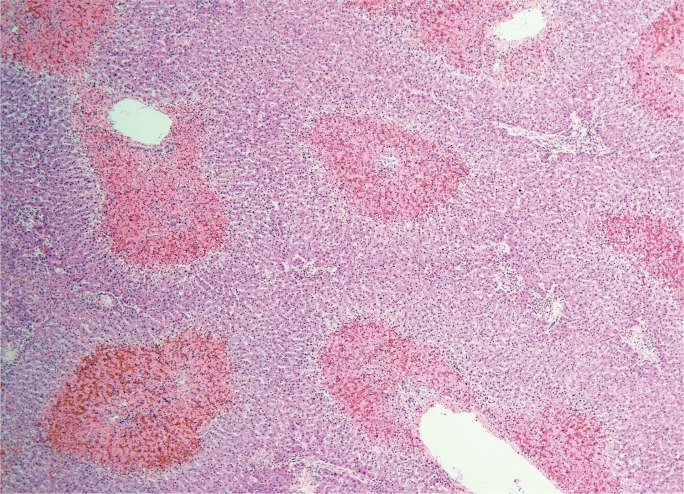
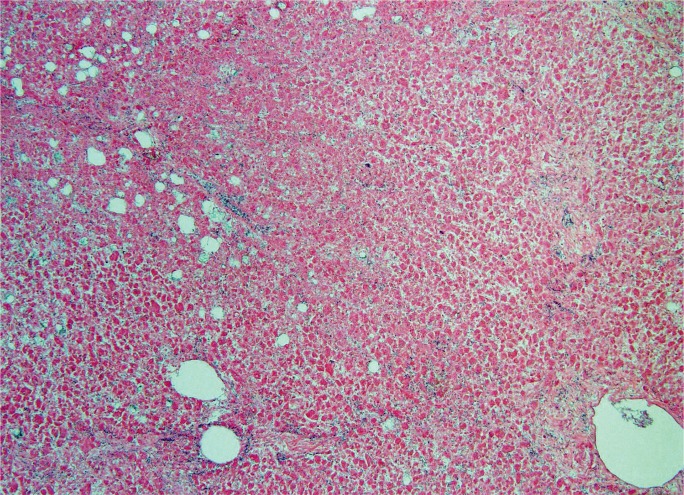
The degree and pattern of necrosis can be useful in determining the etiology and is often described in reference to the anatomy of the hepatic lobule and acinus. The hepatic acinus partitions liver lobules into zones according to their proximity to afferent arterioles. Zone 1 (perilobular) is most proximal to the hepatic arterioles, zone 2 is between the central vein and hepatic arterioles (midlobular), and zone 3 is adjacent to the central vein (centrilobular) (Image 3). Injury following these acinar patterns is referred to as zonal necrosis. Bridging necrosis describes cell death that links vasculature, whether the portal veins (portal-portal), the central veins (central-central), or portal and central veins (central-portal) (Image 4). When cell-death diffusely involves some hepatic lobules, it is referred to as submassive necrosis (Image 5), whereas diffuse involvement of all hepatic lobules is termed massive necrosis (Image 6) (3).
Image 3:
Normal liver lobule showing zones with zone 1 nearest the portal triad and zone 3 nearest the central vein (H&E, x100).
Image 4:
Bridging necrosis in a liver due to the toxic effects of ethanol and oxycodone (H&E, x40).
Image 5:
Submassive necrosis in an individual with hepatic veno-occlusive disease (H&E, x40).
Image 6:
Massive necrosis in an individual fulminant hepatic failure of unknown etiology (H&E, x40).
Acute and Chronic Hepatitis
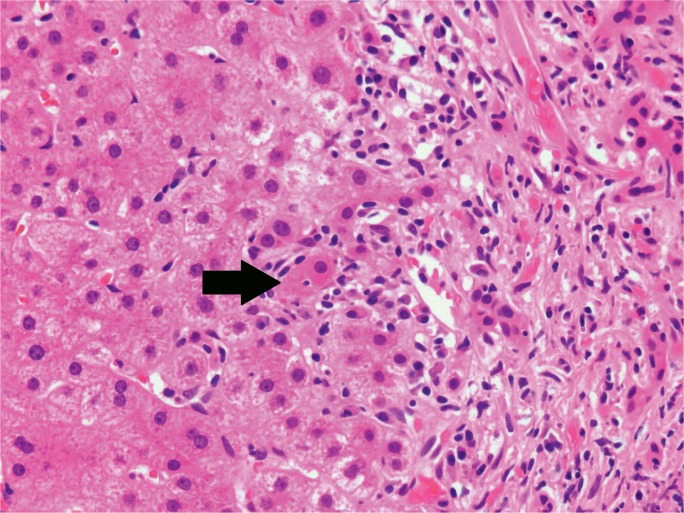
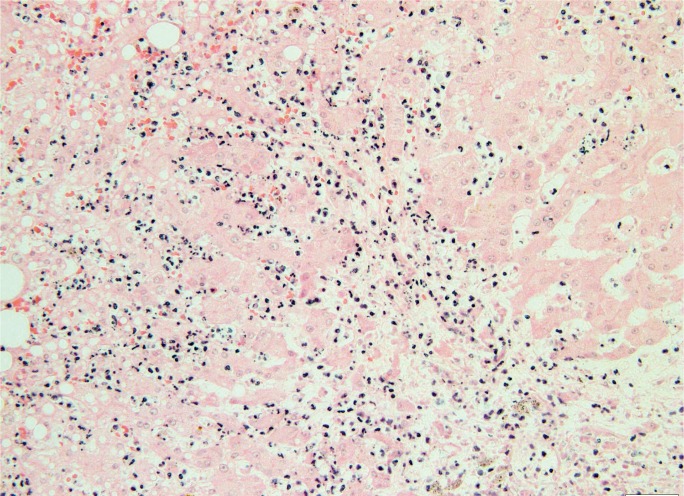
Focal/multi-focal necrosis, sometimes called patchy necrosis, does not follow a zonal pattern, but rather involves random, individual clusters of hepatocytes (Image 7). This pattern is typical of acute hepatitis, falling under the umbrella of “lobular disarray,” which also includes ballooning degeneration of hepatocytes, lobular and sinusoidal inflammation, Kupffer cell hyperplasia, increased apoptotic bodies, and cholestasis. If this process lasts longer than six months, it is deemed chronic, and often presents with progressive fibrosis (3). In some processes, including chronic hepatitis, there is focal necrosis of hepatocytes at the limiting plate between the portal tract connective tissue and start of hepatic parenchyma with an associated lymphocytic infiltrate (Image 8). This process is commonly called piecemeal necrosis, though updates in terminology have renamed it interface hepatitis and troxis necrosis, the latter originating from the Greek term for “nibbling” (4).
Image 7:
Patchy areas of necrosis and hemorrhage in an infant with disseminated herpes simplex virus type 1 (H&E, x40).
Image 8:
Microscopic section showing a lymphocytic portal infiltrate extending into the surrounding parenchyma, also known as piecemeal necrosis, troxis necrosis, or interface hepatitis (H&E, x200).
Acute Liver Failure
Acute and chronic hepatitis may progress to ALF and ACLF. Acute liver failure is characterized by a dramatic decline in hepatic function in a patient without prior liver disease. Three categories of ALF are subdivided based on the time between onset of encephalopathy and first appearance of jaundice: hyperacute (0 -1 week), acute (1-4 weeks), and subacute (4-12 weeks) (5). These subclassifications not only aid in prognosis (hyperacute presentations are favorable to acute and subacute presentations), but are also suggestive of etiology and histopathologic findings.
The incidence of ALF is rare, particularly in the developed world. In the United States, population based studies estimated 5.5 cases per million (6). There are multiple potential etiologies, particularly those involving drug injury and infection, though in many cases the cause is unknown (7). Awareness and interventions, including emergency liver transplant, have greatly improved overall survival to greater than 65% (8, 9), though 45% of adults and 56% of children may recover without the need of liver transplantation (7).
Acute-On-Chronic Liver Failure
Acute liver failure is distinguished from ACLF by the presence of preexisting liver disease and usually involves an acute decompensation of cirrhosis that manifests clinically with acute encephalopathy, ascites, gastrointestinal hemorrhage, or bacterial peritonitis (10). The mortality rate is high in ACLF and correlates with the number of extra-hepatic organ failures. In a large prospective study, the 28-day mortality increased from 32% in patients with two organ failures to 78.6% in patients with three organ failures or more. In this study, ACLF was most common in young patients with active alcoholism and frequently involved extra-hepatic kidney failure. In more than 40% of cases, a precipitating event that initiated acute decompensation was not identified. In those where a precipitating event was identified, infection was the most common source (11). Other precipitants include acute alcoholic hepatitis after binge-drinking, drug injury, hepatic ischemia secondary to hypotension, and acute viral infection (12).
Hepatic Injury and Necrosis
Alcohol
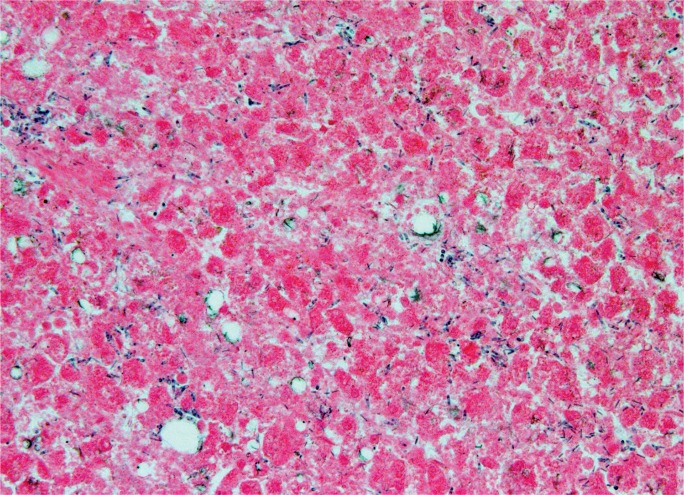
Alcoholic liver disease exists on a spectrum from early steatosis to acute hepatitis, fibrosis, and cirrhosis. Steatosis generally begins in zone 3 and extends toward the portal triads in more severe cases. Lipid deposition begins with small droplets (microvesicular), but with time accumulates into larger droplets (macrovesicular) that distend the hepatocyte membrane and displace the nucleus (13).
Chronic ethanolism induces the cytochrome P450 pathway, leading to an increase in reactive oxygen species that both signals lipid peroxidation and the formation of acetaldehyde protein adducts that directly damage mitochondria. Intrahepatic glutathione, which normally reduces these reactive oxygen species, is consequently depleted, predisposing hepatocytes to oxidative injury. Prolonged oxidative stress and adduct formation incite hepatocellular injury, with derangement of the microtubulin network and accumulation of eosinophilic, intracytoplasmic cytokeratins (Mallory bodies) (Image 9). As membrane stability declines, ballooning degeneration and eventual oncotic necrosis occurs (Image 10). Mallory bodies themselves are immunogenic actors that signal downstream activation of tumor necrosis factor (TNF)-alpha, activating cell-death and neutrophil recruitment (14, 15).
Image 9:
Section from an individual with alcoholic hepatitis showing prominent Mallory bodies (arrow) (H&E, x400).
Image 10:
Marked ballooning degeneration of hepatocytes (H&E, x400).
Chronic alcohol abuse not only produces cytotoxic reactive oxygen species and acetaldehyde adducts, but also leads to bacterial overgrowth in the gastrointestinal tract, increased gut permeability, and the transfer of bacteria to the liver (16, 17). This combination activates innate immunity, particularly Kupffer cells, and promotes the inflammatory response that characterizes alcoholic hepatitis.
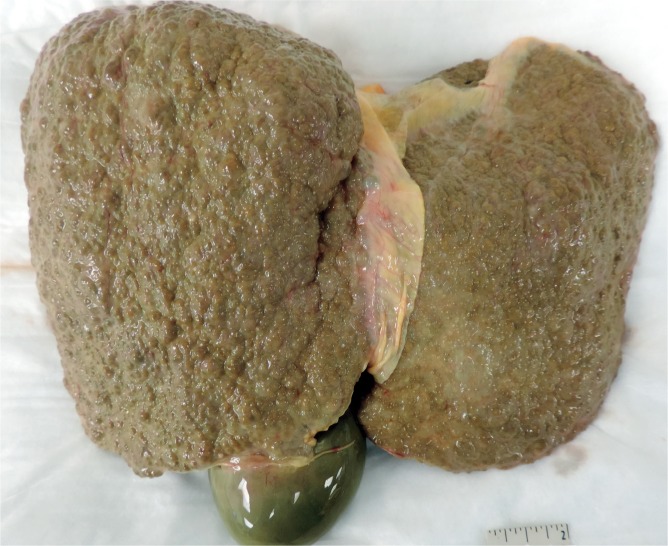
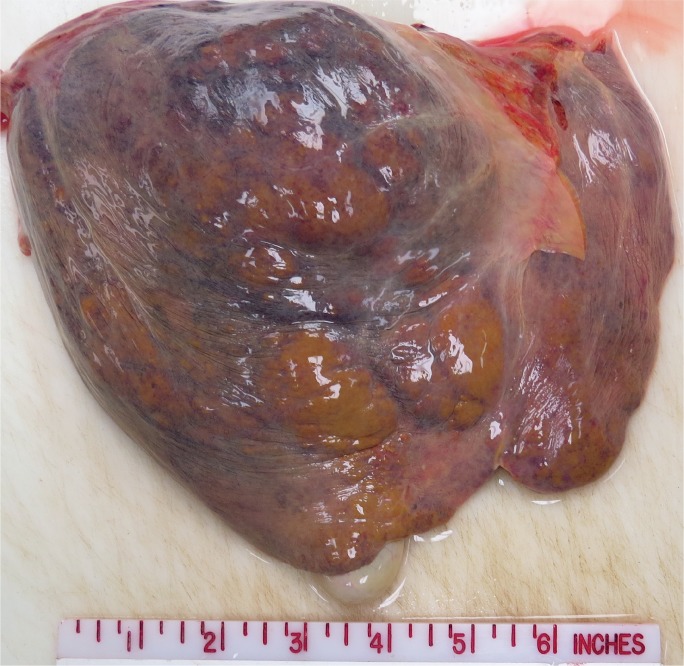
Acute hepatitis is a feared complication of alcoholic liver disease that occurs in 35% to 40% of patients (18). The mortality rate is 6.8% (19), but increases with age, female sex, and concomitant viral hepatitis C infection (20). Acute alcoholic hepatitis involves an acute lobular infiltrate against a background of steatosis. Portal inflammation is typically inconspicuous but may show small collections of lymphocytes. A dense portal infiltrate should raise suspicion of chronic hepatitis, particularly hepatitis B and C infection. Because cytochrome P450 enzymes are focused in centrilobular hepatocytes, necrosis generally begins in zone 3, heralded by ballooning degeneration that devolves into karyolysis. Neutrophils satellite these ballooning hepatocytes and Mallory bodies. In active and ongoing injury, apoptotic bodies are present that have not yet been phagocytosed by Kupffer cells. Additional histologic features include glycogenated hepatocytes, megamitochondria, cholestasis, and portal ductular reactions (21, 22). Grossly, alcoholic livers are enlarged and fatty secondary to steatosis (Image 11), but, with prolonged injury and fibrotic change, shrink and become multi-nodular (i.e., cirrhosis) (Image 12).
Image 11:
Gross photograph of an enlarged, fatty liver in an individual with alcoholic hepatitis.
Image 12:
Gross photograph of a cirrhotic liver.
Hepatic necrosis and inflammation are potent activators of perisinusoidal stellate cells, which initiate fibrogenesis. Fibrosis begins in zone 3, webbing outward in a perisinusoidal pattern that produces the classic “chicken wire” appearance. In later stages, centrilobular fibrosis extends to the portal triads, creating central-portal and portal-portal fibrotic crosslinks. Regenerative parenchyma encircled by fibrous septae creates the micronodular appearance seen in cirrhotic livers (13). Active alcoholism in a patient with otherwise compensated cirrhosis may superimpose an acute hepatitis, leading to rapid decline and ACLF (12). In these cases, the degree of bridging necrosis, along with other histologic features such as apoptosis, Mallory bodies, and eosinophilic degeneration of hepatocytes, has been associated with poor prognosis (23).
Drug Induced Liver Injury
There are two mechanisms of adverse drug reactions that broadly delineate histopathological findings: intrinsic and idiosyncratic. An intrinsic reaction results in predictable dose-dependent injury secondary to the drug itself or a byproduct of the drug. By contrast, idiosyncratic drug reactions are less predictable and likely driven by an immunogenic response to drug metabolites. The timeline between ingestion of a drug and an idiosyncratic reaction is likewise unpredictable, occurring anywhere between a couple of days and up to a year from first administration (24).
Intrinsic Injury
Acetaminophen is the most common cause of both intrinsic hepatotoxicity and ALF in Europe and the United States (9, 25, 26). Acetaminophen is sold as an over-the-counter analgesic or in combination with other drugs, such as anticholinergics and opioids. In a study using data collected from the ALF study group between 1998 and 2012, over half of acetaminophen-related ALF was due to acetaminophen-opioid mixtures. These patients were older (mean age 39 years), with increased comorbidities, and more likely to unintentionally overdose (27). Other population studies have shown that unintentional acetaminophen is responsible for 25% of ALF in pediatric patients (6).
Under recommended doses (3-4 g/day), acetaminophen is generally a safe and effective analgesic. The majority of acetaminophen is conjugated with sulfate and glucuronide and safely excreted in the urine. The remainder is oxidized through the cytochrome P450 pathway, producing the reactive metabolite N-acetyl-p-benzoquinone imine (NAPQI) as a byproduct, which is detoxified by glutathione. Toxic concentrations of acetaminophen saturate sulfate and glucuronide conjugation and thereby shunt the reaction toward the cytochrome P450 pathway, producing excess NAPQI while at the same time depleting glutathione stores. NAPQI is then free to trigger widespread mitochondrial dysfunction and ultimately hepatic necrosis (28).
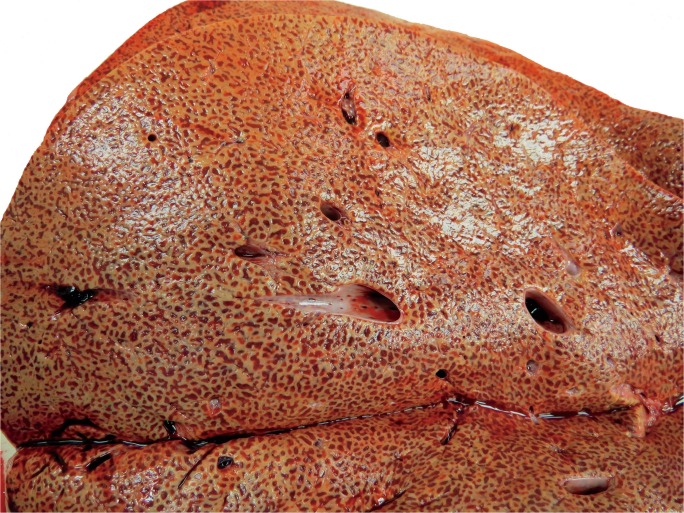
Cytochrome P450 enzymes are located in centrilobular hepatocytes, which explains the characteristic pattern of zone 3 coagulative necrosis seen in acetaminophen toxicity (Images 13 and 14). In contrast to alcoholic hepatitis, there is generally sparse inflammation, though an increase in Kupffer cells/macrophages may be identified secondary to damage-associated molecular patterns (DAMPs) released from necrotic hepatocytes (28, 29). Other agents, such as halothane and carbon tetrachloride, also induce zone 3 necrosis with little inflammation (29).
Image 13:
Gross image demonstrating profound centrilobular necrosis.
Image 14A:
Microscopic section showing prominent centrilobular necrosis (H&E, x40).
Image 14B:
Microscopic section showing prominent centrilobular necrosis (H&E, x100)
Idiosyncratic Injury
Idiosyncratic hepatotoxicity accounts for up to 11% of ALF in adult patients (7). There are a great variety of prescription and non-prescription agents responsible for idiosyncratic reactions. A US prospective study found that nearly half of the drugs implicated in liver injury were antimicrobials (e.g., sulfonamides, ketoconazole), followed by central nervous system agents (e.g., monoamine oxidase inhibitors, phenytoin, valproate). Herbal and dietary supplements accounted for 9% of reactions, most commonly linked to products promoting weight loss and muscle growth (30). Idiosyncratic reactions follow a subacute clinical course and affect older populations, particularly those 60 years and older (7, 31). Typically, the prognosis of idiosyncratic ALF without transplant is poor compared to intrinsic injury (9, 32).
Just as there are a wide variety of agents responsible for idiosyncratic injury, there are a number of associated histologic patterns. None of these patterns are pathognomonic, and all may mimic other liver disease processes, which makes determining a specific etiology difficult. Often, microscopy illustrates the extent of injury rather than the precise etiology. Hepatocellular injury may begin as acute hepatitis with patchy necrosis or interface hepatitis and develop into liver failure once necrosis becomes confluent (i.e., massive/submassive necrosis) (Image 15) (33). In contrast to intrinsic injury, idiosyncratic reactions show marked portal inflammation. Lymphocytes are typically present; however, there may also be eosinophils and granulomas, which suggest a more favorable prognosis (33, 34). Isoniazid, monoamine oxidase inhibitors, anti-convulsants, and antimicrobials typically follow this pattern. Amiodarone heptotoxicity mimics alcoholic hepatitis, presenting as a steatohepatitis with marked Mallory bodies and neutrophil satellitosis (29).
Image 15:
Cross section of a liver with acute liver failure and submassive necrosis due to herbal medications and ethanol.
Injuries that follow a subacute clinical course may feature submassive necrosis, which includes segments of regenerative nodules scattered throughout zones of confluent necrosis (Image 16). Regenerative hyperplasia is caused by hepatic progenitor cell activation once 50% of hepatocytes have been lost (35). These nodules may be confused with cirrhotic nodules and a Masson trichome stain may help distinguish the two — the dense type one collagen of cirrhotic nodules stains a bright blue whereas the early regenerative nodules stain a light, grey-blue due to the mixture of collagen and ground substance (28). Other features associated with hepatic necrosis due to idiopathic drug reactions are hepatocyte rosette formation, lobular disarray, and hemorrhage, which represent severe parenchymal injury (33).
Image 16:
Gross photograph of a liver with acute liver failure with regenerative nodules due to isoniazid.
Viral Infection
Hepatotrophic Viruses
Globally, hepatotrophic viral infections are the most common etiology of hepatitis and ALF, particularly infections involving hepatitis A, E, and B (25). Hepatitis A (HAV) follows fecal-oral transmission, generally arising from contaminated food and water supplies. Improved sanitation and vaccination have dramatically decreased its incidence; however, outbreaks have occurred in low-endemic regions involving intravenous drug users and human immunodeficiency virus (HIV) positive men who have sex with men (36). Globally, the World Health Organization has estimated 1.5 million cases of HAV infection occur each year.
Children under the age of six years generally remain asymptomatic while older children and adults have a 70% risk of developing acute liver dysfunction (37). Although HAV is a common cause of acute hepatitis (38), progression to ALF is rare, accounting for approximately 3-4% of cases in the United States between 1998 and 2007 (7, 9).
Similar to HAV, hepatitis E (HEV) infection occurs through fecal-oral transmission. In 2015, an estimated 44 000 deaths occurred due to HEV, accounting for 3.3% of viral hepatitis mortality (39). In the United States, ALF due to HEV is negligible (7, 9), though there are reports suggesting a small proportion of HEV hepatitis may be misdiagnosed as drug-induced liver injury (40). Hepatitis E is a common cause of both acute hepatitis and ACLF. Though ALF is a feared complication in pregnant women, there is a higher risk of infection in males (38).
While HAV and HEV do not progress to chronic hepatitis, hepatitis B (HBV) produces both acute and chronic hepatic infection. In 2010, approximately 248 million individuals were chronically infected with HBV, mostly focused in sub-Saharan and Western Pacific regions (41). In the United States, HBV infections have significantly decreased due to vaccination efforts beginning in the 1990s. Nonetheless, between 2011 and 2012 there were an estimated 847 000 individuals with chronic infection (particularly involving non-Hispanic Asians) and, in 2015, 21 900 cases of acute hepatitis (42, 43). Hepatitis B is responsible for 7% of cases in the United States (7) and may cause ACLF due to either HBV reactivation or acute HBV hepatitis in a compensated, cirrhotic liver (12). Coinfection with hepatitis D (HDV), an HBV dependent virus, may also potentiate acute hepatitis and possibly ALF or ACLF. Hepatitis C (HCV) is a common source of chronic hepatitis, leading to cirrhosis in 20% of cases (3). Though an uncommon cause of ALF, decompensation secondary to an acute insult may result in ACLF; however, progression is less common than ACLF due to alcoholic cirrhosis (11).
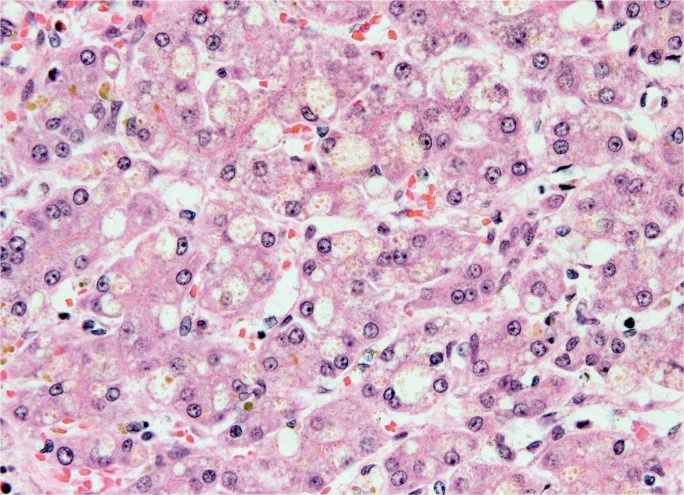
Because HAV, HEV, and HBV share common histologic features, diagnosis often relies on serologic markers. Nonetheless, early viral hepatitis presents with lobular disarray and focal/multifocal necrosis surrounded by a lymphocytic infiltrate (spotty necrosis). In many cases, necrosis does not become diffuse and the parenchymal architecture is not altered. However, in ALF, focal necrosis evolves into submassive and massive necrosis (28). In some studies, HEV is shown to generate more severe confluent necrosis with neutrophil infiltration in the sinusoids (44). Portal inflammation may be more prominent in HAV, with a lymphocytic infiltrate spilling beyond the portal connective tissue and into the hepatic parenchyma. Hepatitis B infected hepatocytes expressing surface antigen contain nuclear, ground-glass inclusions surrounded by a halo (Image 17). Acute HCV infection shows mild portal and lobular inflammation with a lymphocytic infiltrate and negligible interface hepatitis. The degree of ballooning degeneration is also less compared to the other hepatotrophic viruses (45).
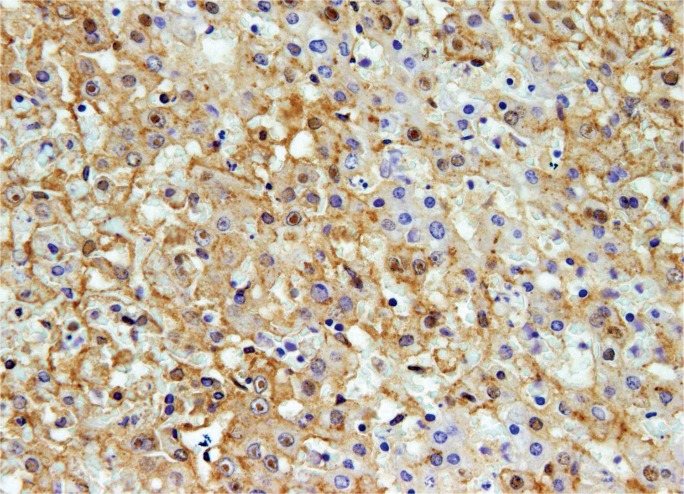
Image 17:
Microscopic section showing a lymphocytic portal infiltrate with adjacent ground glass hepatocytes, characteristic of hepatitis B infection (H&E, x400).
Non-Hepatotrophic Viruses
Though rare, non-hepatotrophic viruses such as herpes virus (HSV), adenovirus, Epstein-Barr virus (EBV), and cytomegalovirus (CMV) generate hepatitis and even ALF, predominantly in newborns, pregnant women, and the immunosuppressed.
In a study examining non-acetaminophen-related pediatric ALF, 25.2% of children 0-6 months of age tested positive for HSV with a mortality rate of 60% within the first 21 days of the study period (46). Herpes simplex virus hepatitis often does not present with classic mucocutaneous lesions, so antemortem suspicion for disseminated infection may be low. As a result, diagnosis is made at autopsy in more than half of cases. Immunosuppression, whether from recent transplantation or coinfection with HIV, and pregnancy in the third trimester are significant risk factors for disseminated herpes; however, up to 24% of affected patients may be immunocompetent (47). Epstein-Barr virus, CMV, and adenovirus affect similar patient populations, though to a lesser degree than HSV (38, 46, 48).
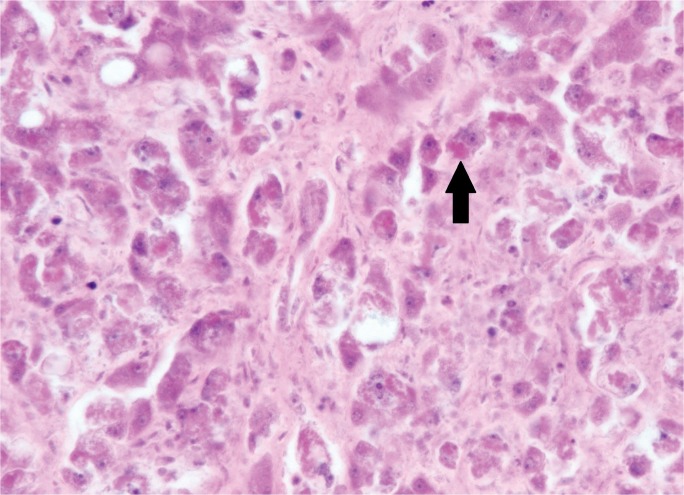
Grossly, non-hepatotrophic hepatitis shows punctate discoloration that correlates histologically with circumscribed areas of patchy necrosis and associated nuclear fragments (“dirty necrosis”). Necrosis may diffusely involve the hepatic parenchyma, particularly in neonates (Image 18). In HSV, the nuclei of infected hepatocytes are enlarged and distorted by eosinophilic, ground-glass nuclear inclusions (Cowdry body inclusions). Cowdry A bodies represent early infection and present with small, central nuclear inclusions separated from the nuclear membrane by a halo (Image 19). Cowdry B inclusions are larger inclusions that eccentrically push nuclear material to the border of the nuclear membrane and are associated with late stage infection (28, 45). Cytomegaolovirus and adenovirus also express cytopathic effect, but their inclusions are often more basophilic and surrounded by a halo (“owl’s eye” inclusions). In addition, basophilic granules are found in the cytoplasm of CMV infected hepatocytes, sometimes surrounded by neutrophilic microabcesses. In adenovirus, inclusions are more often located in hepatocytes peripheral to the areas of necrosis (Image 20) (45, 49). Compared to the other viruses, EBV shows less necrosis and more lymphocytic inflammation that fills the sinusoids and portal tracts (50). As shown, when non-hepatotrophic viral infection is suspected, immunohistochemical stains for a number of viruses are available and useful in confirming the precise viral agent. Though rare in the United States, hepatocelluar injury resulting from dengue virus and the yellow fever virus has been reported, characteristically showing zone 2 necrosis (28).
Image 18A:
In situ gross photograph of neonate who died from unsuspected disseminated herpes infection. Note the pale speckling of the parenchyma.
Image 19B:
Herpes simplex virus type 1 immunohistochemical stain of same specimen (magnification x400).
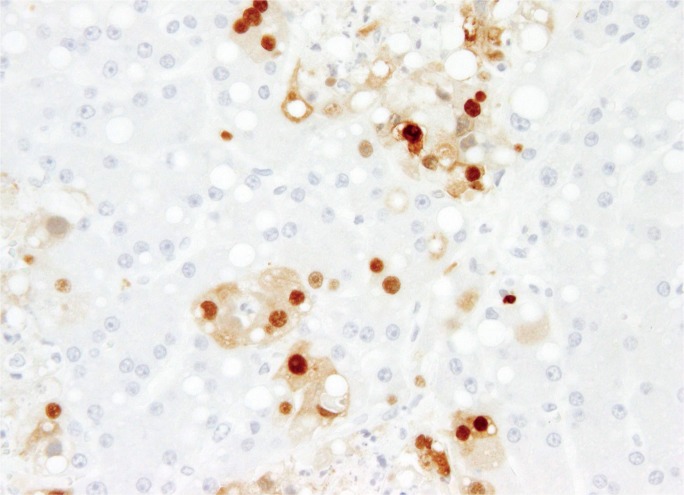
Image 20A:
High power of an adult with disseminated adenovirus infection showing large, irregular, basophilic inclusions (arrow) adjacent to an area of necrosis (H&E, x400).
Image 18B:
Cross-section gross photograph of neonate who died from unsuspected disseminated herpes infection. Note the pale speckling of the parenchyma.
Image 19A:
High power section showing necrosis, hemorrhage, and hepatocytes with Cowdry type A inclusions (circle) (H&E, x400).
Image 20B:
Adenovirus immunostain of same specimen (magnification x400).
Autoimmune
Autoimmune hepatitis (AIH) is a chronic liver disease of unknown etiology. Classically, AIH affects young white females who present with vague symptoms of endocrine dysfunction, hepatitis, elevated IgG, and auto-antibodies (51); however, there has been recent expansion of this phenotype to include both genders, all ages, and multiple ethnic groups (52). Autoimmune hepatitis is most common in northern Europe, accounting for an annual incidence of 1.9 per 100 000 (3), and rare in the United States (53). A combination of genetic predisposition and environmental insult likely triggers AIH. In one study, nearly 10% of AIH were induced by drugs. Within this cohort, nitrofurantoin, commonly used to treat urinary tract infections, and minocycline, commonly used to treat acne, were responsible for over 90% of cases (54).
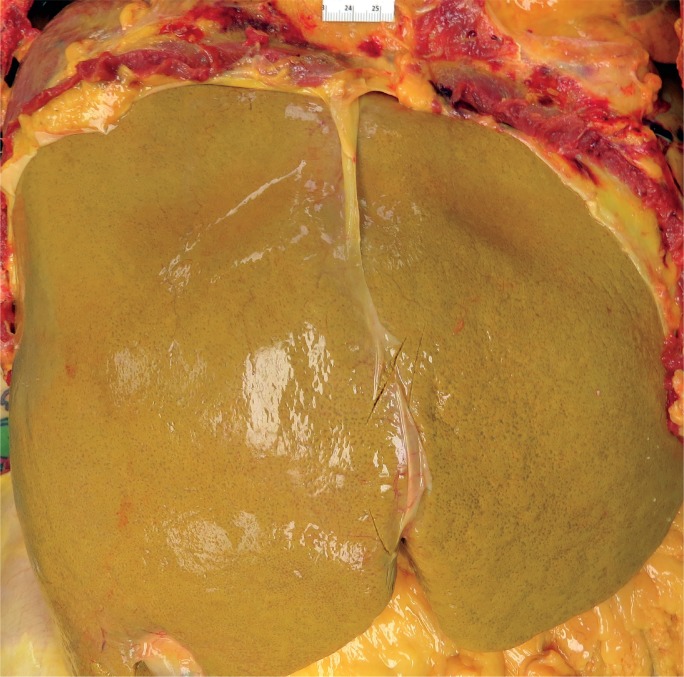
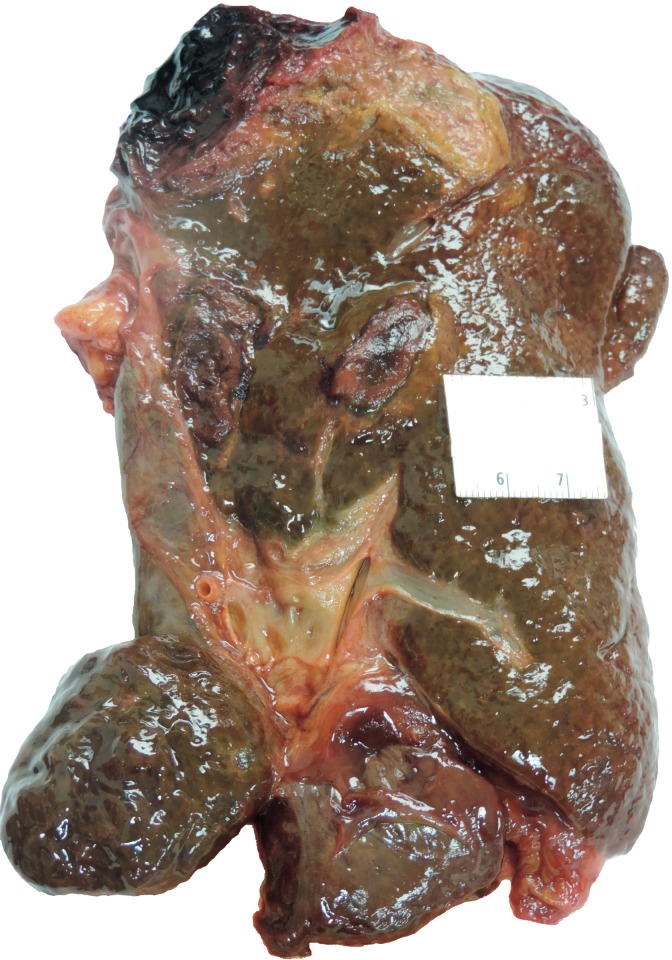
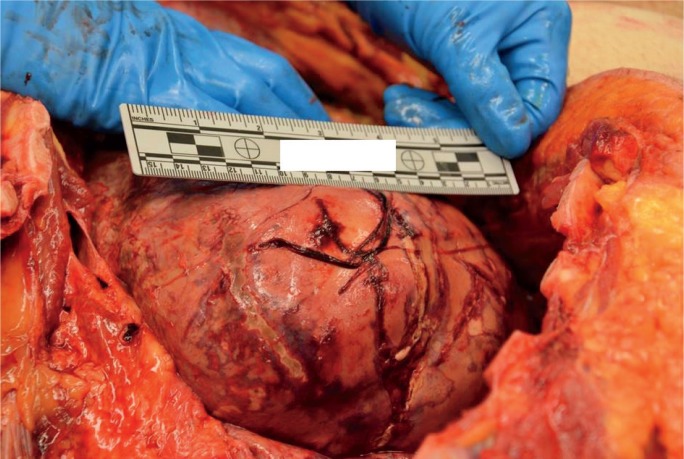
Autoimmune hepatitis may be asymptomatic or range in presentation from acute hepatitis to ALF and ACLF. Acute and fulminant AIH occurs in 25% to 75% (52) and 5% (7) of patients, respectively (Image 21). At presentation, previously asymptomatic patients may already have cirrhosis from chronic, subclinical injury (55, 56). In these instances, acute exacerbation, whether from a de novo flair of autoimmune injury or superimposed viral infection, may lead to ACLF.
Image 21:
Cross-section of a liver with fulminant liver failure due to autoimmune hepatitis. The parenchyma has a yellow-orange discoloration and was markedly softened.
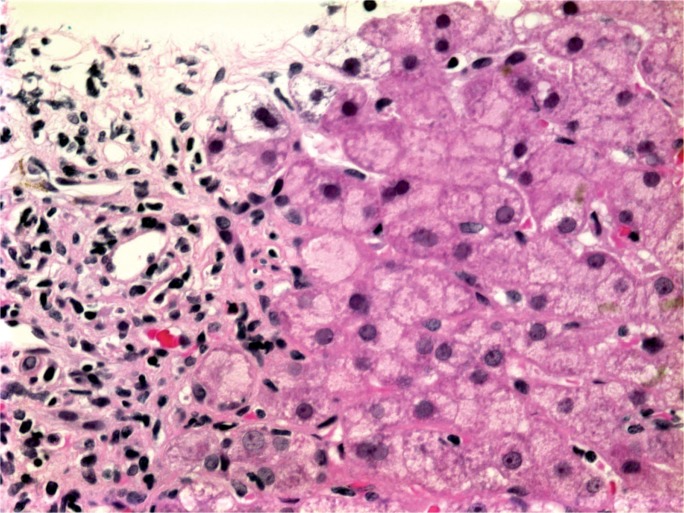
As a chronic hepatitis, AIH presents with interface necrosis (84 to 98% of cases) and varying degrees of background fibrosis. The associated inflammatory infiltrate typically consists of plasma cells, but may also contain lymphocytes and histiocytes (57). Lymphocytes entrapped in the cytoplasm of hepatocytes (emperipolesis) are found in up to 78% of cases (Image 22) (58). Emperipolesis is associated with more severe necroinflammatory disease and may serve a role in the pathogenesis of AIH through the initial induction of hepatocyte apoptosis (59). The hepatic lobules may also be affected by a mononuclear infiltrate with associated lobular disarray. Hepatocyte rosetting, in which regenerative hepatocytes are arranged in a circular formation with a central lumen, is a common feature of AIH. Lobular necrosis ranges in severity, from focal necrosis up to bridging necrosis in 40% of cases (58). Confluent necrosis carries a 1.9-fold risk of progression to cirrhosis (55). Centrilobular necroinflammation has been described in acute and fulminant cases of AIH, making it difficult to distinguish from drug-induced injury and acute viral hepatitis. While not pathognomonic, the combined features of lobular necroinflammation involving zone 3 with a plasmacytic infiltrate, emperipolesis, and hepatocyte rosetting supports the diagnosis of AIH (60).
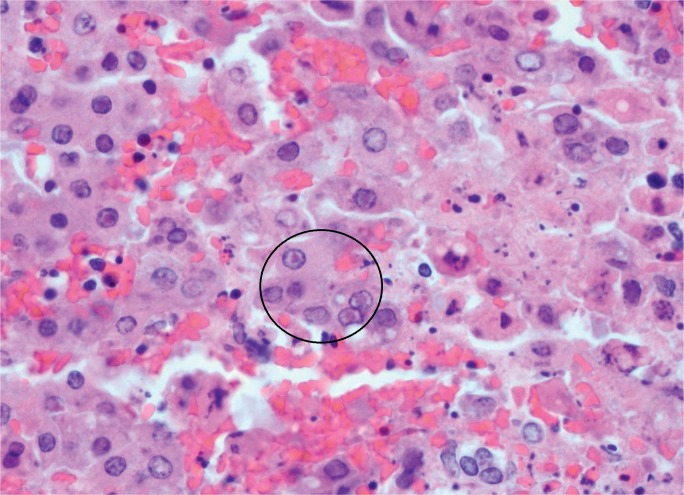
Image 22:
Photomicrograph of a lymphocyte entrapped within a hepatocyte, known as emperipolesis (arrow) (H&E, x200).
Ischemia
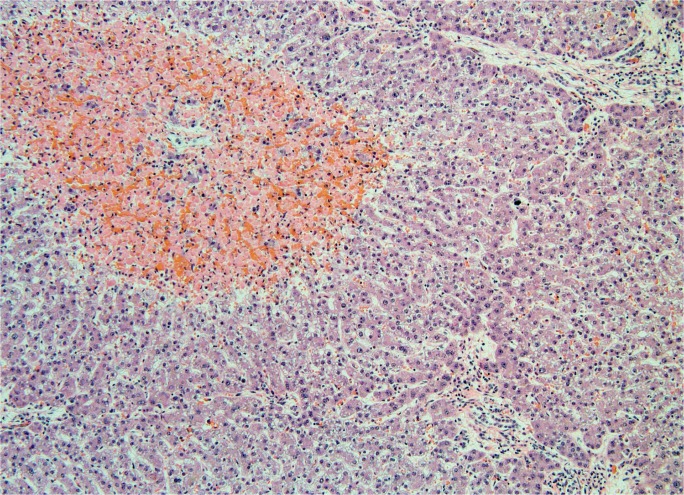
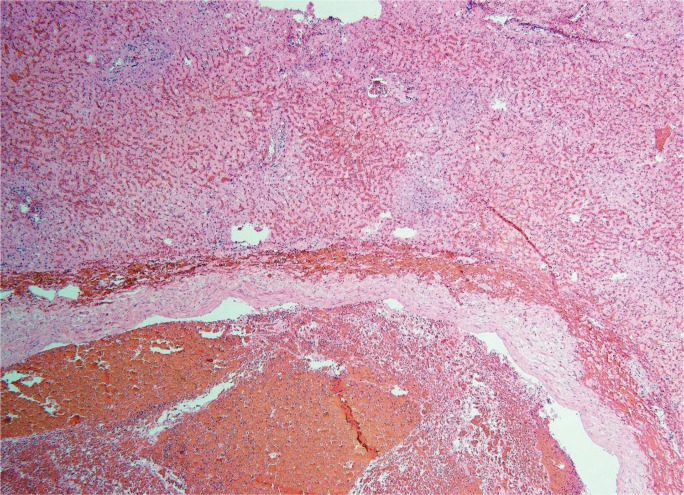
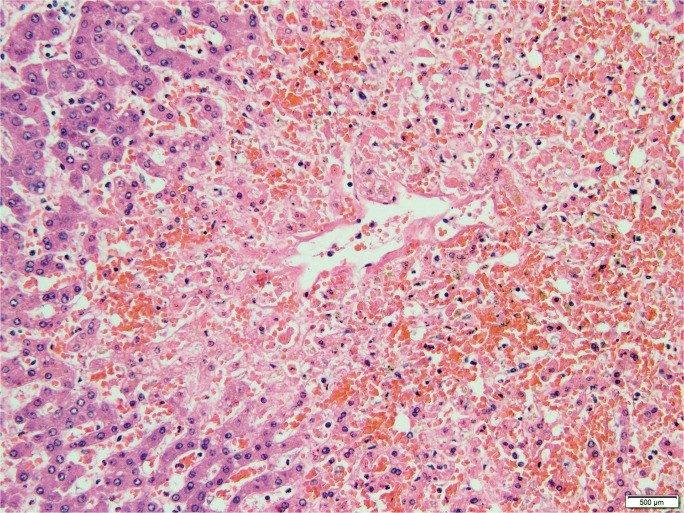
Because circulatory failure is common prior to death, hepatic necrosis associated with cardiac shock is a typical finding at autopsy. Low cardiac output results in increased central venous pressure that back flows into the hepatic vein. Clinically, right-sided heart failure, including causes such as left-sided heart failure, pulmonary hypertension, constrictive pericarditis, and tricuspid regurgitation, leads to passive hepatic congestion, perisinusoidal edema, and ischemic necrosis of hepatocytes (61). Though rare, Budd-Chiari syndrome, in which the hepatic vein becomes thrombosed, produces the same effect (62). Gross findings classically include hepatomegaly with purple discoloration secondary to congestion (Image 23). The cut surface shows a classic nutmeg appearance due to alternating necrosis and hemorrhage. Histologically, there is prominent central vein congestion, sinusoidal edema, and zone 3 necrosis (Image 24). In severe cases, necrosis is diffuse with a central-central bridging pattern. Long standing heart failure may result in fibrotic changes within the central vein wall with fibrosis webbing throughout the sinusoids in a pattern similar to alcoholic liver disease (63).
Image 23:
Gross photograph of a liver with ischemic necrosis. Note the thrombus adjacent to the ruler.
Image 24A:
Microscopic section of ischemic hepatitis showing thrombus within a large blood vessel and associated sinusoidal congestion (H&E, x40).
Image 24B:
Microscopic section of ischemic hepatitis showing early centrilobular necrosis (H&E, x200).
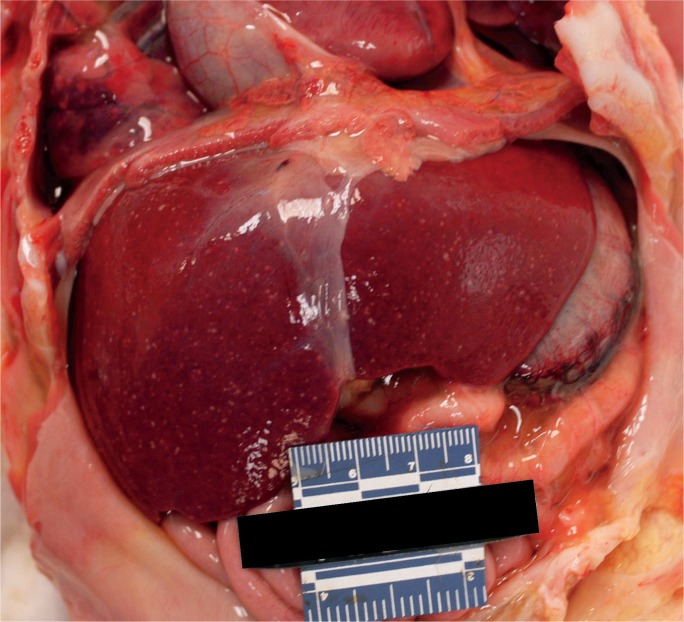
Major hepatic trauma is commonly encountered in the autopsy setting and may present with hepatic necrosis, presumably secondary to ischemia (Image 25). Angioembolization is an initial approach to achieving hemostasis in high-grade liver injuries, though there are many associated complications, including abscess formation, bile leak, and major hepatic necrosis. In a retrospective study of 538 patients with hepatic trauma receiving angioembolization, up 42% of complications were due to major hepatic necrosis, most commonly occurring in patients with penetrating injury. Additionally, gel-foam embolization of either the proper hepatic artery or multiple sites was more likely to potentiate necrosis secondary to diffuse hepatic ischemia (64).
Image 25A:
Gross photograph of traumatic herniation of right hepatic lobe through a diaphragmatic laceration. (Photo courtesy of Washoe County Regional Medical Examiner’s Office).
Image 25B:
Serial sections displaying approximately 50% parenchymal necrosis. (Photo courtesy of Washoe County Regional Medical Examiner’s Office).
Metabolic
Nonalcoholic fatty liver disease (NAFLD) is a common etiology of chronic liver disease that may rarely be a cause of hepatic necrosis and ALF. It is defined by steatosis without alcohol abuse, adverse drug reaction, or viral infection (65). Globally, NAFLD affects 6.3% to 33% (median 20%) of individuals (66). Obesity and type two diabetes mellitus are recognized risk factors. In the United States, Hispanic Americans are at increased risk, followed by Caucasians and African Americans (67). Histologically, steatohepatitis is identical to alcoholic hepatitis, making clinical history paramount.
Wilson disease is an autosomal recessive disorder resulting in impaired copper excretion that accumulates in tissues and organs, particularly the liver. Copper promotes the generation of free radicals that induces a steatohepatitis similar to NAFLD (68). In up to 2% of cases, Wilson disease may progress to ALF (7). In suspected cases, special stains, including rhodanine and orcein, may highlight copper deposits in hepatocytes (68).
Other metabolic causes of hepatic injury include hemochromatosis and galactosemias, which are prominently seen in pediatric populations (7, 48).
Artifact
One finding encountered frequently by the forensic pathologist that may mimic massive hepatic necrosis is autolysis. Both processes may lead to large areas of non-viable hepatocytes that at first glance may be difficult to distinguish. However, autolysis tends to cause a complete “white out” of the hepatic parenchyma with loss of architecture, dropout of the nuclei, and absence of other pathologic features (Image 26). This is in contrast to actual necrosis in which the cells tend to be better preserved and sections may show additional pathologic features (Image 27). Autolysis should be suspected in cases with widespread microscopic changes in the liver, and likely other organs, without a history compelling for liver failure, in individuals with extended postmortem intervals or obesity, and deaths occurring in warm environments or with inadequate refrigeration after death.
Image 26A:
Microscopic section of a liver with marked autolysis showing widespread loss of architecture and tissue breakdown (H&E, x40).
Image 27:
Microscopic section of a liver with massive necrosis showing relative preservation of the hepatocyte nuclei and a brisk acute inflammatory infiltrate (H&E, x400)
Image 26B:
Microscopic section of a liver with marked autolysis showing total dropout of the nuclei, loss of normal hepatocyte struc ture, and scattered growth of microorganisms in the absence of inflammation (H&E, x400).
Conclusion
Due to the number of potential causes, hepatic necrosis is frequently encountered at autopsy and may present with diverse gross and histologic features in individuals of varying demographics. While many of the diseases presented have “classical” pathologic findings, in practice there is significant overlap of gross and microscopic features, making it a challenge to identify a precise etiology. Table 1 provides a summary of some of the more common causes of hepatic necrosis. Ultimately, placing gross and histologic features in the context of clinical and social history is required to develop an appropriate differential diagnosis and cause of death.
Table 1:
Causes of Hepatic Necrosis with Associated Demographics, Pathological Features, and Ancillary Studies Available to Aid in Diagnosis.
| Category | Age | Clinical Presentation | Gross Features | Microscopic Features | Ancillary Studies |
|---|---|---|---|---|---|
| Alcohol | Adulthood | Variable, may result in ALF or ACLF | Enlarged, fatty liver or cirrhosis | Steatosis, lobular inflammation, Mallory bodies | |
| Intrinsic drug (acetaminophen, halothane, carbon tetrachloride) | Varies | ALF | Centrilobular necrosis | Zone 3 necrosis without significant inflammation | Toxicology |
| Idiosyncratic drug (numerous including antimicrobial, herbal, and CNS agents) | Varies; typically adulthood | Subacute liver failure | Varies; possible submassive necrosis and regenerative nodules | Varies | Toxicology |
| Hepatotrophic viruses | Varies, typically adulthood | Varies, including ALF and ACLF | Varies | Portal lymphocytic infiltrate | Serology, PCR, IHC stains |
| Non-hepatottrophic viruses | Varies | ALF | Punctate pale lesions throughout parenchyma | Patchy necrosis, viral inclusions | Serology, PCR, IHC stains |
| Autoimmune | Young adults | Varies, including ALF and ACLF; also endocrine dysfunction, elevated IgG, autoantibodies | Varies | Zone 3 necrosis, plasma cell infiltrate, resetting, and emperipolesis | |
| Ischemia | Varies, typically adulthood | Circulatory collapse, blood loss, CHF | Varies, purple discoloration to passive congestion | Varies, typically Zone 3 necrosis | |
| Metabolic | Childhood | Varies | Varies | Varies | Special stains |
ALF - Acute liver failure
ACLF - Acute-on-chronic liver failure
CNS - Central nervous system PCR - Polymerase chain reaction IHC - Immunohistochemical
CHF - Congestive heart failure
Biography
Daniel C. Butler MD, Medical University of South Carolina - Department of Pathology and Laboratory Medicine
Roles: Project conception and/or design, data acquisition, analysis and/or interpretation, manuscript creation and/or revision, approved final version for publication, accountable for all aspects of the work.
David N. Lewin MD, Medical University of South Carolina - Department of Pathology and Laboratory Medicine
Roles: Project conception and/or design, data acquisition, analysis and/or interpretation, manuscript creation and/or revision, approved final version for publication, accountable for all aspects of the work.
Nicholas I. Batalis MD, Medical University of South Carolina - Department of Pathology and Laboratory Medicine
Roles: Project conception and/or design, data acquisition, analysis and/or interpretation, manuscript creation and/or revision, approved final version for publication, accountable for all aspects of the work.
Footnotes
Ethical Approval: As per Journal Policies, ethical approval was not required for this manuscript
Statement of Human and Animal Rights: This article does not contain any studies conducted with animals or on living human subjects
Statement of Informed Consent: No identifiable personal data were presented in this manuscript
Disclosures & Declaration of Conflicts of Interest: Nicholas I. Batalis is the Associate Editor-in-Chief of Academic Forensic Pathology: The Official Publication of the National Association of Medical Examiners. The authors, reviewers, and publication staff do not report any other relevant conflicts of interest
Financial Disclosure: The authors have indicated that they do not have financial relationships to disclose that are relevant to this manuscript
References
- 1). Guicciardi ME, Gores GJ. Apoptosis: a mechanism of acute and chronic liver injury. Gut. 2005. July; 54(7):1024–33. PMID: 15951554. PMCID: PMC1774601 10.1136/gut.2004.053850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2). Guicciardi ME, Malhi H, Mott JL, Gores GJ. Apoptosis and necrosis in the liver. Compr Physiol. 2013. April; 3(2):977–1010. PMID: 23720337. PMCID: PMC3867948 10.1002/cphy.c120020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3). Theise ND. Robbins basic pathology. 9th ed Philadelphia: Saunders/Elsevier; c2013. Chapter 15, Liver, gallbladder, and biliary tract; p.603–44. [Google Scholar]
- 4). Wang MX, Morgan T, Lungo W, et al. “Piecemeal” necrosis: renamed troxis necrosis. Exp Mol Pathol. 2001. October; 71(2):137–46. PMID: 11599920 10.1006/exmp.2001.2397. [DOI] [PubMed] [Google Scholar]
- 5). O’Grady JG, Schalm SW, Williams R. Acute liver failure: redefining the syndromes. Lancet. 1993. July 31; 342(8866):273–5. PMID: 8101303 10.1016/0140-6736(93)91818-7. [DOI] [PubMed] [Google Scholar]
- 6). Bower WA, Johns M, Margolis HS, et al. Population-based surveillance for acute liver failure. Am J Gastroenterol. 2007. November; 102(11):2459–63. PMID: 17608778 10.1111/j.1572-0241.2007.01388.x. [DOI] [PubMed] [Google Scholar]
- 7). Lee WM, Squires RH, Jr, Nyberg SL, et al. Acute liver failure: summary of a workshop. Hepatology. 2008. April; 47(4):1401–15. PMID: 18318440. PMCID: PMC3381946 10.1002/hep.22177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8). Bernal W, Hyyrylainen A, Gera A, et al. Lessons from look-back in acute liver failure? A single centre experience of 3300 patients. J Hepatol. 2013. July; 59(1):74–80. PMID: 23439263 10.1016/j.jhep.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 9). Ostapowicz G, Fontana RJ, Schiødt FV, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002. December 17; 137(12):947–54. PMID: 12484709 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- 10). Moreau R, Jalan R, Arroyo V. Acute-on-chronic liver failure: recent concepts. J Clin Exp Hepatol. 2015. March; 5(1):81–5. PMID: 25941435. PMCID: PMC4415197 10.1016/j.jceh.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Moreau R, Jalan R, Gines P, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013. June; 144(7):1426–37. PMID: 23474284 10.1053/j.gastro.2013.02.042. [DOI] [PubMed] [Google Scholar]
- 12). Bernal W, Jalan R, Quaglia A, et al. Acute-on-chronic liver failure. Lancet. 2015. October 17; 386(10003):1576–87. PMID: 26423181 10.1016/s0140-6736(15)00309-8. [DOI] [PubMed] [Google Scholar]
- 13). Theise ND. Histopathology of alcoholic liver disease. Clin Liver Dis. 2013. April 24; 2(2):64–7. 10.1002/cld.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Crawford JM. Histologic findings in alcoholic liver disease. Clin Liver Dis. 2012. November; 16(4):699–716. PMID: 23101978 10.1016/j.cld.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 15). Caulin C, Ware CF, Magin TM, Oshima RG. Keratin-dependent, epithelial resistance to tumor necrosis factor-induced apoptosis. J Cell Biol. 2000. April 3; 149(1):17–22. PMID: 10747083. PMCID: PMC2175089. 10.1083/jcb.149.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16). Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011. November; 141(5):1572–85. PMID: 21920463. PMCID: PMC3214974 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17). Yan AW, Fouts DE, Brandl J, et al. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology. 2011. January; 53(1):96–105. PMID: 21254165. PMCID: PMC3059122 10.1002/hep.24018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18). Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med. 2009. June 25; 360(26):2758–69. PMID: 19553649 10.1056/NEJMra0805786. [DOI] [PubMed] [Google Scholar]
- 19). Liangpunsakul S. Clinical characteristics and mortality of hospitalized alcoholic hepatitis patients in the United States. J Clin Gastroenterol. 2011. September; 45(8):714–9. PMID: 21085006. PMCID: 3135756 10.1097/mcg.0b013e3181fdef1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Singal AK, Kuo YF, Anand BS. Hepatitis C virus infection in alcoholic hepatitis: prevalence patterns and impact on in-hospital mortality. Eur J Gastroenterol Hepatol. 2012. October; 24(10):1178–84. PMID: 22735607 10.1097/meg.0b013e328355cce0. [DOI] [PubMed] [Google Scholar]
- 21). Celli R, Zhang X. Pathology of alcoholic liver disease. J Clin Transl Hepatol. 2014. June; 2(2):103–9. PMID: 26357621. PMCID: PMC4521259 10.14218/JCTH.2014.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22). Sakhuja P. Pathology of alcoholic liver disease, can it be differentiated from nonalcoholic steatohepatitis? World J Gastroenterol. 2014. November 28; 20(44):16474–9. PMID: 25469015. PMCID: PMC4248190 10.3748/wjg.v20.i44.16474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23). Rastogi A, Kumar A, Sakhuja P, et al. Liver histology as predictor of outcome in patients with acute-on-chronic liver failure (ACLF). Virchows Arch. 2011. August; 459(2):121–7. PMID: 21744153 10.1007/s00428-011-1115-9. [DOI] [PubMed] [Google Scholar]
- 24). Hussaini SH, Farrington EA. Idiosyncratic drug-induced liver injury: an update on the 2007 overview. Expert Opin Drug Saf. 2014. January; 13(1):67–81. PMID: 24073714 10.1517/14740338.2013.828032. [DOI] [PubMed] [Google Scholar]
- 25). Bernal W, Auzinger G, Dhawan A, Wendon J. Acute liver failure. Lancet. 2010. July 17; 376(9736):190–201. PMID: 20638564 10.1016/s0140-6736(10)60274-7. [DOI] [PubMed] [Google Scholar]
- 26). Larson AM, Polson J, Fontana RJ, et al. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005. December; 42(6):1364–72. PMID: 16317692 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- 27). Serper M, Wolf MS, Parikh NA, et al. Risk factors, clinical presentation, and outcomes in overdose with acetaminophen alone or with combination products: results from the acute liver failure study group. J Clin Gastroenterol. 2016. January; 50(1):85–91. PMID: 26166142. PMCID: PMC5528869 10.1097/mcg.0000000000000378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28). Lefkowitch JH. The pathology of acute liver failure. Adv Anat Pathol. 2016. May; 23(3):144–58. PMID: 27058243 10.1097/pap.0000000000000112. [DOI] [PubMed] [Google Scholar]
- 29). Ramachandran R, Kakar S. Histological patterns in drug-induced liver disease. J Clin Pathol. 2009. June; 62(6):481–92. PMID: 19474352 10.1136/jcp.2008.058248. [DOI] [PubMed] [Google Scholar]
- 30). Chalasani N, Fontana RJ, Bonkovsky HL, et al. Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008. December; 135(6):1924–34, 1934. e1–4. PMID: 18955056. PMCID: PMC3654244 10.1053/j.gastro.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31). Wei G, Bergquist A, Broomé U, et al. Acute liver failure in Sweden: etiology and outcome. J Intern Med. 2007. September; 262(3):393–401. PMID: 17697161 10.1111/j.1365-2796.2007.01818.x. [DOI] [PubMed] [Google Scholar]
- 32). Björnsson E. Drug-induced liver injury: Hy’s rule revisited. Clin Pharmacol Ther. 2006. June; 79(6):521–8. PMID: 16765139 10.1016/j.clpt.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 33). Kleiner DE, Chalasani NP, Lee WM, et al. Hepatic histological findings in suspected drug-induced liver injury: systematic evaluation and clinical associations. Hepatology. 2014. February; 59(2):661–70. PMID: 24037963. PMCID: PMC3946736 10.1002/hep.26709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34). Björnsson E, Kalaitzakis E, Olsson R. The impact of eosinophilia and hepatic necrosis on prognosis in patients with drug-induced liver injury. Aliment Pharmacol Ther. 2007. June 15; 25(12):1411–21. PMID: 17539980 10.1111/j.1365-2036.2007.03330.x. [DOI] [PubMed] [Google Scholar]
- 35). Katoonizadeh A, Nevens F, Verslype C, et al. Liver regeneration in acute severe liver impairment: a clinicopathological correlation study. Liver Int. 2006. December; 26(10):1225–33. PMID: 17105588 10.1111/j.1478-3231.2006.01377.x. [DOI] [PubMed] [Google Scholar]
- 36). Lin KY, Chen GJ, Lee YL, et al. Hepatitis A virus infection and hepatitis A vaccination in human immunodeficiency virus-positive patients: a review. World J Gastroenterol. 2017. May 28; 23(20):3589–3606. PMID: 28611512. PMCID: PMC5449416 10.3748/wjg.v23.i20.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37). Hepatitis A vaccines. Wkly Epidemiol Rec. 2000. February 4; 75(5):38–44. PMID: 10693358. [PubMed] [Google Scholar]
- 38). Gupta E, Ballani N, Kumar M, Sarin SK. Role of non-hepatotropic viruses in acute sporadic viral hepatitis and acute-on-chronic liver failure in adults. Indian J Gastroenterol. 2015. November; 34(6):448–52. PMID: 26589230 10.1007/s12664-015-0613-0. [DOI] [PubMed] [Google Scholar]
- 39). World Health Organization [Internet]. Geneva: World Health Organization; c2018. Hepatitis E; [updated 2017 Jul 7; cited 2018 Feb 22]. Available from: http://www.who.int/mediacentre/factsheets/fs280/en/. [Google Scholar]
- 40). Davern TJ, Chalasani N, Fontana RJ, et al. Acute hepatitis E infection accounts for some cases of suspected drug-induced liver injury. Gastroenterology. 2011. November; 141(5):1665–72. e1–9. PMID: 21855518. PMCID: PMC3654540. 10.1053/j.gastro.2011.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41). Schweitzer A, Horn J, Mikolajczyk RT, et al. Estimations of world-wide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015. October 17; 386(10003):1546–55. PMID: 26231459. 10.1016/s0140-6736(15)61412-x. [DOI] [PubMed] [Google Scholar]
- 42). Roberts H, Kruszon-Moran D, Ly KN, et al. Prevalence of chronic hepatitis B virus (HBV) infection in U.S. households: National Health and Nutrition Examination Survey (NHANES), 1988-2012. Hepatology. 2016. February; 63(2):388–97. PMID: 26251317 10.1002/hep.28109. [DOI] [PubMed] [Google Scholar]
- 43). Viral hepatitis surveillance: United States, 2015. [Internet]. Atlanta: Centers for Disease Control and Prevention; [cited 2018 Feb 22] 73 p. Available from: https://www.cdc.gov/hepatitis/statistics/2015surveillance/pdfs/2015hepsurveillancerpt.pdf. [Google Scholar]
- 44). Peron JM, Danjoux M, Kamar N, et al. Liver histology in patients with sporadic acute hepatitis E: a study of 11 patients from South-West France. Virchows Arch. 2007. April; 450(4):405–10. PMID: 17333266 10.1007/s00428-007-0382-y. [DOI] [PubMed] [Google Scholar]
- 45). Alves VAF. Acute Viral Hepatitis-Beyond A, B and C [Internet]. Evans (GA): United States & Canadian Academy of Pathology; 2017. [cited 2018 Feb 22]. Available from: https://handouts.uscap.org/AN2017/2017_CM10_alves_0101.pdf. [Google Scholar]
- 46). Schwarz KB, Dell Olio D, Lobritto SJ, et al. Analysis of viral testing in nonacetaminophen pediatric acute liver failure. J Pediatr Gastroenterol Nutr. 2014. November; 59(5):616–23. PMID: 25340974. PMCID: PMC4305349 10.1097/mpg.0000000000000512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47). Norvell JP, Blei AT, Jovanovic BD, Levitsky J. Herpes simplex virus hepatitis: an analysis of the published literature and institutional cases. Liver Transpl. 2007. October; 13(10):1428–34. PMID: 17902129 10.1002/lt.21250. [DOI] [PubMed] [Google Scholar]
- 48). Sundaram SS, Alonso EM, Narkewicz MR, et al. Characterization and outcomes of young infants with acute liver failure. J Pediatr. 2011. November; 159(5):813–818.e1. PMID: 21521221. PMCID: 3177978 10.1016/j.jpeds.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49). Ronan BA, Agrwal N, Carey EJ, et al. Fulminant hepatitis due to human adenovirus. Infection. 2014. February; 42(1):105–11. PMID: 23979854 10.1007/s15010-013-0527-7. [DOI] [PubMed] [Google Scholar]
- 50). Mellinger JL, Rossaro L, Naugler WE, et al. Epstein-Barr virus (EBV) related acute liver failure: a case series from the US Acute Liver Failure Study Group. Dig Dis Sci. 2014. July; 59(7):1630–7. PMID: 24464209. PMCID: PMC4250929 10.1007/s10620-014-3029-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51). Manns MP, Lohse AW, Vergani D. Autoimmune hepatitis—update 2015. J Hepatol. 2015. April; 62(1 Suppl): S100–11. PMID: 25920079 10.1016/j.jhep.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 52). Czaja AJ. Diagnosis and management of autoimmune hepatitis: current status and future directions. Gut Liver. 2016. March; 10(2):177–203. PMID: 26934884. PMCID: PMC4780448 10.5009/gnl15352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53). Francque S, Vonghia L, Ramon A, Michielsen P. Epidemiology and treatment of autoimmune hepatitis. Hepat Med. 2012. March 16; 4:1–10. PMID: 24367228. PMCID: PMC3846915 10.2147/hmer.s16321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54). Björnsson E, Talwalkar J, Treeprasertsuk S, et al. Drug-induced autoimmune hepatitis: clinical characteristics and prognosis. Hepatology. 2010. June; 51(6):2040–8. PMID: 20512992 10.1002/hep.23588. [DOI] [PubMed] [Google Scholar]
- 55). Roberts SK, Therneau TM, Czaja AJ. Prognosis of histological cirrhosis in type 1 autoimmune hepatitis. Gastroenterology. 1996. Mar; 110(3):848–57. PMID: 8608895 10.1053/gast.1996.v110.pm8608895. [DOI] [PubMed] [Google Scholar]
- 56). Czaja AJ. Acute and acute severe (fulminant) autoimmune hepatitis. Dig Dis Sci. 2013. April; 58(4):897–914. PMID: 23090425 10.1007/s10620-012-2445-4. [DOI] [PubMed] [Google Scholar]
- 57). Tiniakos DG, Brain JG, Bury YA. Role of histopathology in auto-immune hepatitis. Dig Dis. 2015; 33 Suppl 2:53–64. PMID: 26642062 10.1159/000440747. [DOI] [PubMed] [Google Scholar]
- 58). de Boer YS, van Nieuwkerk CM, Witte BI, et al. Assessment of the histopathological key features in autoimmune hepatitis. Histopathology. 2015. February; 66(3):351–62. PMID: 25257662 10.1111/his.12558. [DOI] [PubMed] [Google Scholar]
- 59). Miao Q, Bian Z, Tang R, et al. Emperipolesis mediated by CD8 T cells is a characteristic histopathologic feature of autoimmune hepatitis. Clin Rev Allergy Immunol. 2015. June; 48(2-3):226–35. PMID: 25051956 10.1007/s12016-014-8432-0. [DOI] [PubMed] [Google Scholar]
- 60). Nguyen Canh H, Harada K, Ouchi H, et al. Acute presentation of autoimmune hepatitis: a multicentre study with detailed histological evaluation in a large cohort of patients. J Clin Pathol. 2017. November; 70(11):961–969. PMID: 28428284 10.1136/jclinpath-2016-204271. [DOI] [PubMed] [Google Scholar]
- 61). Alvarez AM, Mukherjee D. Liver abnormalities in cardiac diseases and heart failure. Int J Angiol. 2011. September; 20(3):135–42. PMID: 22942628. PMCID: PMC3331650 10.1055/s-0031-1284434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62). O’Grady JG. Budd-chiari syndrome and acute liver failure: a complex condition requiring a rapid response. Liver Transpl. 2017. February; 23(2):133–134. PMID: 28006871. PMID: 28006871 10.1002/lt.24695. [DOI] [PubMed] [Google Scholar]
- 63). Lefkowitch JH, Mendez L. Morphologic features of hepatic injury in cardiac disease and shock. J Hepatol. 1986; 2(3):313–27. PMID: 3722787 10.1016/s0168-8278(86)80043-5. [DOI] [PubMed] [Google Scholar]
- 64). Dabbs DN, Stein DM, Scalea TM. Major hepatic necrosis: a common complication after angioembolization for treatment of high- grade liver injuries. J Trauma. 2009. March; 66(3):621–7; discussion 627-9. PMID: 19276729 10.1097/ta.0b013e31819919f2. [DOI] [PubMed] [Google Scholar]
- 65). Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012. June; 142(7):1592–609. PMID: 22656328 10.1053/j.gastro.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 66). Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011. August; 34(3):274–85. PMID: 21623852 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 67). Fazel Y, Koenig AB, Sayiner M, et al. Epidemiology and natural history of non-alcoholic fatty liver disease. Metabolism. 2016. August; 65(8):1017–25. [DOI] [PubMed] [Google Scholar]
- 68). Pronicki M. Wilson disease - liver pathology. Handb Clin Neurol. 2017; 142:71–75. PMID: 28433112 10.1016/b978-0-444-63625-6.00007-0. [DOI] [PubMed] [Google Scholar]