Summary
Aim
Sleep disorders are common in Alzheimer's disease (AD) and assumed to directly influence cognitive function and disease progression. This study evaluated sleep characteristics in a rat model of AD that was induced by intracerebroventricular streptozotocin (STZ) administration and assessed the possible underlying mechanisms.
Methods
Cognition ability was assessed in the Morris water maze in rats. Sleep parameters were analyzed by electroencephalographic and electromyographic recordings. Neuronal activity in brain areas that regulate sleep‐wake states was evaluated by double‐staining immunohistochemistry. High‐performance liquid chromatography with electrochemical detection was used to detect neurotransmitter levels.
Results
Fourteen days after the STZ injection, the rats exhibited sleep disorders that were similar to those in AD patients, reflected by a significant increase in wakefulness and decreases in nonrapid eye movement (NREM) sleep and rapid eye movement (REM) sleep. The c‐Fos expression analysis indicated that neuronal activity and the number of neurons in the dorsal raphe nucleus and locus coeruleus decreased in STZ‐injected rats. In the ventrolateral preoptic nucleus (VLPO), the activity of γ‐aminobutyric acid (GABA) neurons was suppressed. In the arousal‐driving parabrachial nucleus (PBN), GABAergic activity was suppressed, whereas glutamatergic activity was promoted. The neurotransmitter analysis revealed a reduction in GABA in the VLPO and PBN and elevation of glutamate in the PBN. A direct injection of the GABAA receptor antagonist bicuculline in the PBN in normal rats induced a similar pattern of sleep disorder as in STZ‐injected rats. A microinjection of GABA in the PBN improved sleep disorders that were induced by STZ.
Conclusion
These results suggest that the reduction in GABAergic inhibition in the PBN and VLPO may be involved in sleep disorders that are induced by STZ. Our novel findings encourage further studies that investigate mechanisms of sleep regulation in sporadic AD.
Keywords: Alzheimer's disease, parabrachial nucleus, sleep disorders, streptozotocin, γ‐aminobutyric acid
1. INTRODUCTION
Sleep disturbances are common in Alzheimer's disease (AD). Recent findings in humans support the concept that sleep disturbances are a consequence of disease progression in AD and might also precede symptom onset and drive disease pathology.1, 2 Improving sleep quality and circadian timing can have immediate positive effects on the quality of life of AD patients and their caregivers, and optimizing these parameters earlier in life might also provide a means of preventing or delaying the development of AD.3 Understanding the mechanisms by which sleep and circadian disturbances influence the progression of AD may reveal therapeutic targets that are engaged in healthy middle‐aged individuals. Sleep and circadian function may thus represent modifiable risk factors for the development of AD that are both diagnostically and therapeutically accessible.2 Therefore, research is needed to elucidate the role of sleep in the genesis of AD in humans and evaluate sleep parameters as therapeutic and diagnostic targets in AD.
Animals are a useful model to explore the mechanisms of sleep disorders in AD. However, existing evidence of the association between sleep disorders and the pathophysiology of AD in animal studies has been restricted to investigations of transgenic animals. Transgenic animals have been produced to overexpress mutations that are associated with familial AD (fAD), such as amyloid precursor protein (APP), presenilin 1 (PS1), and PS2.4 However, the development of sleep disorders in sporadic AD (sAD) is still poorly understood because of a lack of suitable animal models. Epidemiologically, >95% of AD cases are sAD.5 Therefore, further studies are needed to understand the etiopathogenesis of sleep disorders in sAD.
The intracerebroventricular (i.c.v.) administration of streptozotocin (STZ) at a subdiabetogenic dose in rats and mice is regarded as a credible method to trigger sAD and produce behavioral, neurochemical, and histological features that resemble those that are found in sAD in humans.6, 7 It is characterized by cognitive impairment, cholinergic deficits, glial activation, neuronal loss, amyloid angiopathy, and other AD‐like alterations in the brain.8, 9 Thus, i.c.v. STZ administration might be a useful model for studying the mechanisms of AD and drug screening. If such a model causes sleep disorders that are similar to those in sAD patients, then this may be helpful for further understanding the associations between cognitive deficits and sleep disorders in sAD. However, to our knowledge, no studies have reported sleep features that are associated with this model. Therefore, this study evaluated sleep characteristics in rats that were given i.c.v. STZ, and we sought to identify the potential mechanisms that are related to sleep alterations in this model.
2. MATERIALS AND METHODS
2.1. Animals
A total of 200 male Wistar rats (280‐300 g, Grade I, purchased from the Animal Center of Peking University, Beijing, China) were used in this study. The rats were individually housed in plastic cages under an artificial 12‐hour/12‐hour light/dark cycle (lights on 9:00 am to 9:00 pm) in a temperature‐controlled room (23 ± 1°C, 50%‐60% humidity). Zeitgeber time 0 (ZT 0) and ZT 12 corresponded to lights on (9:00 am) and lights off (9:00 pm), respectively. Food and water were available ad libitum. All experiments were conducted in accordance with European Community guidelines on the use of experimental animals and approved by the Peking University Committee on Animal Care and Use.
2.2. Electroencephalography/electromyography and cannula surgery
Under xylazine (6 mg/kg, i.p.) and ketamine (80 mg/kg, i.p.) anesthesia, the rats were implanted with electrodes for the polysomnographic recording of electroencephalogram (EEG) and electromyogram (EMG) and guide cannulas (26‐gauge; Plastics One, Roanoke, VA, USA) for drug administration into the lateral ventricle or/and parabrachial nucleus (PBN). To evaluate the effects of brain tissue damage that is caused by cannulation surgery on memory and sleep‐wake stages, a control group of rats was used that did not undergo surgery for cannula implantation. After positioning in a stereotaxic instrument, guide cannula was implanted for the injection of STZ or vehicle using the following stereotaxic coordinates: anterior/posterior, −1.0 mm; medial/lateral, −1.5 mm; dorsal/ventral, −3.5 mm.10 In the parabrachial nucleus (PBN) microinjection experiments, additional double‐guide cannula (26‐gauge; Plastics One) was implanted with the tip 1 mm above the PBN: anterior/posterior, −9.3 mm; medial/lateral, ±2.3 mm; dorsal/ventral, −5.8 mm.10 Subsequently, the cannulas and EEG electrodes were fixed to the skull with dental cement, anchored by three stainless‐steel screws. Two stainless‐steel wire electrodes were placed in the neck muscles for EMG recordings. During the phase of recovery from surgery, the animals were kept on a heating pad where they were monitored for irregularities in respiration, postoperative pain, and the ability to return to sternal recumbence. After surgery, the rats were injected with penicillin for 3 days and allowed to recover for 7 days prior to the experiments.
2.3. Intracerebroventricular streptozotocin injection
After 7 days recovery from the surgery, STZ (Sigma‐Aldrich, St. Louis, MO, USA) was administered i.c.v. twice at an interval of 48 hours to produce memory impairments in rats.11 Each rat received i.c.v. injection of 3.0 mg/kg STZ in 4 μL artificial cerebrospinal fluid (aCSF; 147 mmol/L NaCl, 2.9 mmol/L KCl, 1.6 mmol/L MgCl2, 1.7 mmol/L CaCl2, and 2.2 mmol/L dextrose). STZ was slowly injected in the ventricle using a Hamilton microsyringe that was connected to the 33‐gauge injection cannula (Plastics One) over a 5‐minutes period. The needle was left in place for an additional 3 minutes to allow for diffusion. The vehicle group received 4 μL of aCSF in the lateral ventricles. The STZ injection was repeated 48 hours after the first injection. Subsequently, the body condition and dehydration of the rats were monitored daily after the injection.
2.4. Quantification of arousal states
Sleep‐wake stages were analyzed by EEG and EMG recordings for 24 hours beginning at 9:00 am on day 14 after the STZ injection. Before sleep recording, the rats were connected to the recording apparatus for at least 1 day for habituation. For the electrophysiological recordings, all rats were placed in an electrically shielded box in a noise‐attenuated environment with a lightweight shielded cable that was plugged into the connector on the rat's head and attached to a counterbalanced swivel. The signals were routed to an electroencephalograph (model MP 150; BIOPAC Systems, Goleta, CA, USA). Recordings were performed at 9:00 am and lasted for 24 hours. The behaviors of the animals were monitored by video surveillance with EEG/EMG recordings. The EEG signals were amplified, filtered (0.5–60 Hz), digitized at a sampling rate of 128 Hz, and recorded using AcqKnowledge software (BIOPAC Systems). The EEG/EMG recordings were subjected to manual scoring using SleepSign 2.0 software (BIOPAC Systems) based on the following criteria: wakefulness (low‐amplitude EEG activity and high‐voltage EMG activity), rapid eye movement (REM) sleep (fast Fourier transform [FFT] theta percentage of EEG ≥60%, desynchronized EEG, the absence of tonic EMG, and occasional body twitches while maintaining a recumbent sleep posture), slow‐wave sleep (SWS; FFT delta percentage of EEG ≥ 70%, large‐amplitude, synchronous EEG with sleep spindles present, greatly diminished tonic EMG, eyes closed, and recumbent posture), and light sleep (LS; FFT delta percentage of EEG <70%, high‐amplitude slow or spindle EEG activity, and low‐amplitude EMG activity). Non‐REM (NREM) sleep time was equal to SWS time + LS time. The total sleep time was equal to NREM sleep time + REM sleep time. REM sleep latency was measured as time from sleep onset to the beginning of the first REM sleep episode.
2.5. Morris water maze
The Morris water maze (MWM) test began at 9:00 am, immediately after sleep monitoring, from day 15 after the STZ injection (n = 8‐10). The apparatus consisted of a 185‐cm‐diameter cylindrical tank filled with water (26 ± 1°C), which was opaque by black nontoxic paint, and a 15 cm circular platform in one of the four identical quadrants. Cues were placed on each wall of the quadrant, including a colorful (1 m tall) plastic toy wagon that jutted from the wall, a checker board (1 m× 1 m), a blue fitness ball (75 cm diameter), and a cross of black stripes (each stripe = 1.5 m × 30 cm).
During the spatial reference memory task, the platform was hidden 1‐2 cm below the surface of the water, and its location remained constant during all the training sessions (hidden days 1‐5, HD1‐5). The rat was placed near the edge of the pool with its head directed toward the wall of the pool (in one of the starting locations randomly) in each session. All the rats swam until they located and climbed onto the platform, and the latency to find the platform was recorded. If the rat did not find the platform within 120 seconds, then it was manually guided to the platform and allowed to remain on the platform for 15 seconds. In this case, a maximum latency of 120 seconds was recorded. Two parameters were measured: latency to reach the critical annulus (the virtual contour of the removed platform) and swimming speed. One day after the reference memory acquisition phase, a probe test was conducted with the platform removed. During this test, the animals could swim freely in the pool for 120 seconds. The percentage of time spent in the central quadrant in the probe test was measured.
Subsequently, visible platform trials were conducted to evaluate the visual function of rats. Rats were trained in four daily sessions to locate the cued platform (visible days 1‐3, VD 1‐3), each session consisting of four 120‐seconds trials with a 15‐minutes intertrial interval. The platform location was changed for each cued platform trial.
2.6. Tissue immunofluorescence and image analysis
The rats were decapitated at 12:00 pm on day 14 after the STZ or vehicle injection (n = 6‐8). Under chloral hydrate (300 mg/kg, i.p.) anesthesia, the rats were transcardially perfused with cold phosphate‐buffered saline (PBS) followed by 4% paraformaldehyde in PBS (pH 7.4). The perfusion, cryoprotection, and frozen brain sections were prepared as described previously.12, 13 Coronal cryostat sections (20 μm) were cut in the coronal plane using a freezing microtome (Leica CM1850; Leica Microsystems UK, Milton Keynes, UK) based on a rat brain atlas, which encompassed the perifornical area (Pef, bregma −2.8 to −3.4 mm), tuberomammillary nucleus (TMN, bregma −3.8 to −4.3 mm), dorsal raphe nucleus (DRN, bregma −7.6 to −8.3 mm), locus coeruleus (LC, bregma −9.7 to −10.2 mm), parabrachial nucleus (PBN, bregma −8.8 to −9.8 mm), and ventrolateral preoptic nucleus (VLPO, bregma −0.3 to −0.8 mm).
Double immunofluorescence (neurotransmitter synthesizing enzymes in relevant sleep‐wake related nuclei and c‐Fos protein) was performed as described previously.14 Briefly, the sections were washed in PBS (3 × 5 minutes) and then incubated in cold acetone for 30 minutes, followed by washing in PBS (3 × 5 minutes). Antigen retrieval was conducted in citrate buffer (pH 6.0) via microwave. After the sections were returned to room temperature naturally, they were immersed in PBS that contained 5% nonspecific donkey serum and 0.3% Triton X‐100 for 30 minutes. The sections were then incubated in the appropriate primary antibodies for specific neurotransmitter markers and c‐Fos diluted in PBS that contained 1.5% nonspecific donkey serum and 0.3% Triton X‐100 for 12‐48 hours at 4°C. The sections were washed in PBS and incubated for 120 minutes at room temperature with the appropriate secondary antibodies. The following primary antibodies were used: mouse antibody for glutamic acid decarboxylase (GAD, 1:1000; MAB‐5406, Millipore, Burlington, MA, USA), mouse antibody for vesicular glutamate transporter 2 (v‐GLUT2, 1:200; ab79157, Abcam, Cambridge, UK), rabbit antibody for tyrosine hydroxylase (TH, 1:2000; sc‐14007, Santa‐Cruz, Dallas, TX, USA), sheep antibody for tryptophan hydroxylase (TrpOH, 1:2000, 9260‐2505, Bio‐Rad, Hercules, CA, USA), goat antibody for Orexin (1:100; sc‐8070, Santa‐Cruz), rabbit antibody for adenosine deaminase (ADA, 1:800; AB176, Millipore), rabbit antibody for c‐Fos (1:200; sc‐52, Santa‐Cruz), and goat antibody for c‐Fos (1:400; ab87655, Abcam). The following secondary antibodies were used: Green Donkey Anti‐Mouse IgG (1:500; E032211, EarthOx, Millbrae, CA, USA), Green Donkey Anti‐Goat IgG (1:500; A‐11055, Thermo, Waltham, MA, USA), Green Donkey Anti‐Rabbit IgG (1:500; A24221, IFkine, Wuhan, Hubei, China), Green Donkey Anti‐Sheep IgG (1:500; ab150177, Abcam), Red Donkey Anti‐Rabbit IgG (1:500; A24421, IFkine), and Red Donkey Anti‐Goat IgG (1:500; A‐11058, Thermo).
The sections were examined under an Olympus IX71 fluorescent microscope (Olympus, Tokyo, Japan) or Leica TCS SP8 confocal microscope (Leica Microsystems, Wetzlar, Germany). The brightness and contrast of the captured images were adjusted using Photoshop CS 6.0 software (Adobe, San Jose, CA, USA). Nucleus‐specific neurotransmitter markers were labeled green. c‐Fos was labeled red. DAPI was labeled blue. Neurons with clear visible nucleus were identified by colabelling with specific neurotransmitter markers and DAPI. The density of nucleus‐specific neurotransmitter marker + cells was calculated. The density of c‐Fos+ and nucleus‐specific neurotransmitter marker + cells were calculated. At least three serial sections of each area were used to count the immunoreactive nuclei bilaterally. The data from each group are expressed as mean ± SEM.
2.7. Estimation of GABA and glutamate
The neurotransmitter analysis was conducted as described by Cao with slight modifications14. The rats were decapitated at 12:00 pm on day 14 after the STZ or vehicle injection (n = 6‐8). After the rats were decapitated, the brains were quickly removed to a prechilled brain matrix with a 1.0 mm coronal slice thickness (RWD Life Technology, Shenzhen, Guangzhou, China; catalog no. 68709). Coronal slices that contained VLPO (0 and 1 mm posterior to bregma) or PBN (8 and 10 mm posterior to bregma) tissue were obtained with the brain matrix. The VLPO was located beside the optic chiasm (OX) at the edge of the coronal section. The PBN was located between the superior cerebellar peduncle (SCP) and ventral spinocerebellar tract (VSC). Based on these anatomical characteristics, tissue that surrounded the VLPO and PBN was punched using a hypodermic needle 12 gauge) guided by the Paxinos and Watson10 rat brain atlas. All the tissues were stored separately in prechilled microcentrifuge tubes at −80°C until assayed. The PBN and VLPO were dissected to extract with 0.2 mol/L perchloric acid by ultrasonic homogenation. The levels of GABA and glutamate were determined by reverse‐phase high‐performance liquid chromatography (HPLC) combined with precolumn derivatization with O‐phthalaldehyde and electrochemical detection under the conditions of the mobile phase (0.1 mol/L Na2HPO4, 0.13 mmol/L Na2EDTA, and 28% CH3OH [pH 6.00 with H3PO4]) and ECD detection (analytical cell: 5011A, E1 = −10 mV, E2 = +600 mV; guard cell: 5020, EGC = +650 mV). GABA and glutamate standards were purchased from Sigma‐Aldrich.
2.8. Microinjection in the parabrachial nucleus (PBN)
A Hamilton syringe that was connected to the 33 gauge injection cannula (Plastics One) was used for the microinjections. Drug or vehicle in a volume of 0.2 μL was delivered through the injection cannula that extended 1 mm beyond the guide cannula over a 2‐minutes period. Drug or vehicle might be diffused to the lateral parabrachial nucleus (dorsal, external, and internal parts), medial parabrachial nucleus and superior cerebellar peduncle, and ventral spinocerebellar tract. To allow the drug to diffuse from the tip completely, the injection cannula was kept in place for an additional 3 minutes. A group of rats received microinjection of the GABA receptor antagonist bicuculline (50 or 100 pmol/side, dissolved in aCSF) or vehicle in the PBN (n = 8‐11). Another group of rats received microinjection of the GABA (5 or 10 μmol/side, dissolved in aCSF) or vehicle in the PBN on day 14 after the STZ injection (n = 8‐11). EEG and EMG recordings were performed for 24 hours, from 9:00 am, immediately after bicuculline or GABA intra‐PBN application.
2.9. Histological verification of microinjection sites
At the end of the experiments, 20 μm thick sections were to histologically verify the cannula placements under light microscopy. According to Paxinos and Watson10 rat brain atlas, only the data from rats whose injection sites were within the limits of the lateral ventricle and/or PBN are presented in this study. Under deep anesthesia with chloral hydrate 300 mg/kg, i.p.), the rats were transcardially perfused with cold PBS, followed by 4% paraformaldehyde in PBS pH 7.4). The brains were immediately removed, blocked, and frozen. The brainstem block was coronally sectioned from caudal to rostral using a cryostat Leica Microsystems, Nussloch, Germany). Serial sections (20 μm thick) were slide mounted, dried, fixed in paraformaldehyde, and stained with hematoxylin‐eosin or cresyl violet. Stained tissue sections were digitized using a Super Nikon Cool scan 4000 ED Film Scanner (Nikon, Melville, NY, USA). The microinjection sites were identified and assigned stereotaxic coordinates by comparison with a rat brain atlas. The photomicrograph of representative cannula placement for i.c.v. injection or PBN microinjection is displayed in Figure 2A or Figure 5A.
2.10. Statistical analysis
The data were analyzed using SPSS 20.0 software (Chicago, IL, USA) and are expressed as mean ± SEM. The escape latency in the MWM during the training period was analyzed using two‐way repeated‐measures analysis of variance (ANOVA). Significant group differences were analyzed using Tukey's multiple comparison post hoc test when appropriate. The data from the probe day of the MWM, sleep‐wake stage analysis, neurotransmitter detection, and immunohistochemistry were analyzed using one‐way ANOVA followed by the Student‐Newman‐Keuls post hoc test. Values of P < 0.05 were considered statistically significant.
3. RESULTS
3.1. Streptozotocin‐injected rats exhibited learning and memory deficits
Similar to previous studies,15, 16 the post hoc tests indicated no significant difference in navigation path length on the first day among groups during the acquisition phase of the test. On day 15 after the STZ injection, the rats exhibited deficits in spatial learning, reflected by a longer latency to find the hidden platform compared with the vehicle groups (significant main effect of treatment, P < 0.01, Figure 1A). During the visible platform trials, all of the rats performed equally well (P > 0.05, Figure 1A). No significant difference in swim speed was observed among the three groups (data not shown), suggesting that the learning impairment was not attributable to motor or visual deficits. During the subsequent probe trial, STZ‐injected rats spent less time in the target quadrant than the vehicle group (significant main effect of treatment, P < 0.01, Figure 1B), indicating that the rats in our model exhibited impairments in memory retention. No significant differences in learning and memory were observed between the control and vehicle groups.
Figure 1.
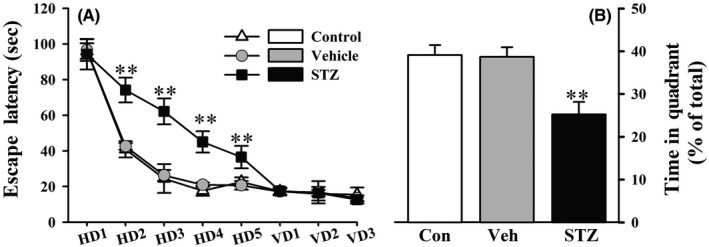
Rats on day 15 after the STZ injection exhibited poor performance in the learning and memory trials. In the MWM, STZ‐injected rats exhibited a longer escape latency (A) to reach the hidden platform during acquisition training. The escape latencies to find the platform during 5 d of hidden platform trials (HD 1‐5) and visible platform trials (VD 1‐3) were analyzed using repeated‐measures ANOVA (group × day), followed by Tukey's multiple comparison post hoc test. The time spent in target quadrant during the probe trial (B) was compared using one‐way ANOVA followed by Tukey's multiple comparison post hoc test. The data are expressed as mean ± SEM (n = 8‐10 per group). **P < 0.01, vs vehicle group
3.2. Streptozotocin‐injected rats exhibited disruption of the sleep‐wake cycle
Consistent with alterations of sleep architecture in AD patients, the analysis of EEG‐based vigilance staging on day 14 after the STZ injection indicated that the rats spent more time awake and less time in NREM sleep and REM sleep, especially SWS, during the 24‐hours day compared with vehicle‐treated rats (P < 0.05 or <0.01, Figure 2B). The bouts of wakefulness significantly increased, and the duration of wakefulness significantly decreased in the STZ group (P < 0.01, Figure 2C,D). The sleep episode analysis revealed that the STZ injection significantly increased the percentage of LS relative to total sleep (LS%) and LS bouts and decreased LS duration compared with the vehicle group (P < 0.01, Figure 2B‐D). The STZ injection significantly suppressed SWS, reflected by a decrease in the percentage of SWS relative to total sleep (SWS%), SWS bouts, and SWS duration (P < 0.01, Figure 2 B‐D). The STZ injection significantly reduced REM sleep bouts (P < 0.05, Figure 2C). The injection of STZ significantly prolonged sleep latency (P < 0.01) and REM sleep latency (P < 0.01, Figure 2E,F).
Figure 2.
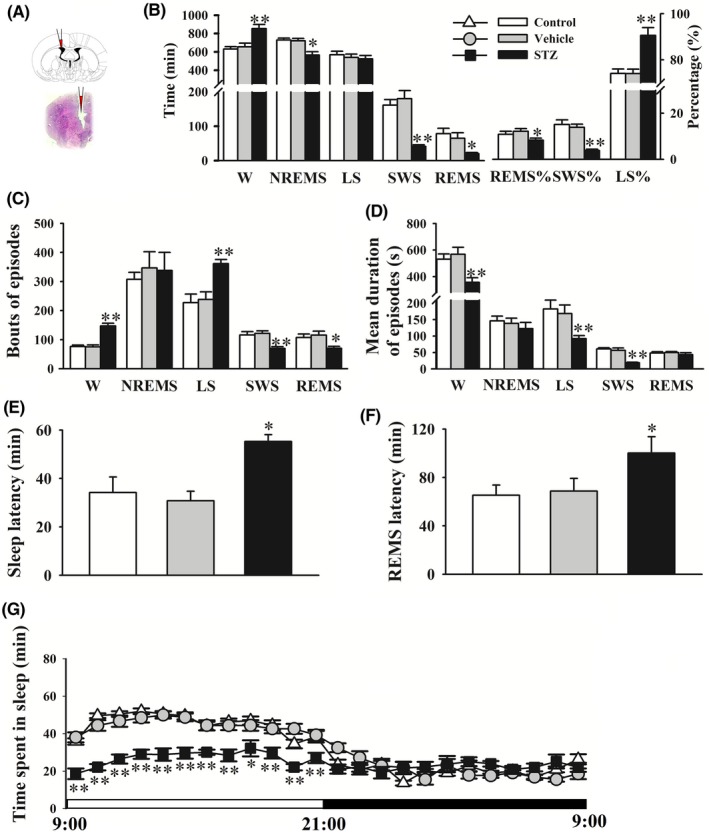
STZ‐injected rats exhibited an increase in wakefulness and decrease in sleep. Sleep parameters included hourly time courses of wakefulness. A, The photomicrograph of representative cannula placement for i.c.v. injection. B, Time spent in wakefulness (W), nonrapid eye movement sleep (NREMS), light sleep (LS), slow‐wave sleep (SWS), and rapid eye movement sleep (REMS), percentage of REMS/LS/SWS relative to total sleep (REMS%, SWS%, LS%). C, D, Bouts and mean duration of W, NREMS, LS, SWS, and REMS episodes. E, F, Sleep latency and REM sleep latency. G, Time spent in sleep per 1 h. The data are expressed as mean ± SEM (n = 8‐10 per group). *P < 0.05 and **P < 0.01 vs vehicle (Student‐Newman‐Keuls test)
A previous study showed that disruption of the sleep‐wake cycle that is induced by STZ was dramatic during the light period.16 The increase in light‐phase wakefulness among STZ‐injected rats emerged at the expense of reductions of REM sleep and NREM sleep. However, the data that were obtained during the dark period revealed no significant differences in wakefulness, NREM sleep, and REM sleep between vehicle and STZ groups.16 In this study, the distribution of total sleep was further assessed in 1‐hour bins. The time‐course of hourly sleep‐wake stage analysis showed that the STZ injection significantly decreased sleep time compared with the vehicle group in every 1‐hour period during the light phase from 9:00 am to 9:00 pm. However, hourly sleep time was unaffected by the STZ during the dark phase, which is consistent with the previous study.16 No significant differences in sleep parameters were observed between the control and vehicle groups.
3.3. Decreases in REM and NREM sleep in STZ‐injected rats were associated with the dysregulation of neuronal activity in sleep/wake regulating brainstem regions
Neuronal activity was evaluated in several regions that regulated sleep‐wake states to study the possible mechanism of the wake‐promoting effect that was observed in STZ‐injected rats. The density of TH+ (noradrenergic) neurons in the LC and TrpOH+ (serotonergic) neurons in the DRN significantly decreased in STZ‐injected rats (P < 0.01, Figure 3A,B,E,F). We also found that STZ‐injected rats expressed fewer dual c‐Fos+ and TH+ neurons in the LC and dual c‐Fos+ and TrpOH+ neurons in the DRN (P < 0.01, Figure 3A,B,E,F). Interestingly, no significant difference was found in the density of orexin + neurons in the Pef or ADA+ (histaminergic) neurons in the TMN. The c‐Fos+ cell density in orexinergic neurons and histaminergic neurons was unaffected by the STZ injection (Figure 3C,D,G,H). These results indicate that the alterations of sleep parameters in STZ‐injected rats might not be associated with changes in neuronal activity in the Pef and TMN.
Figure 3.
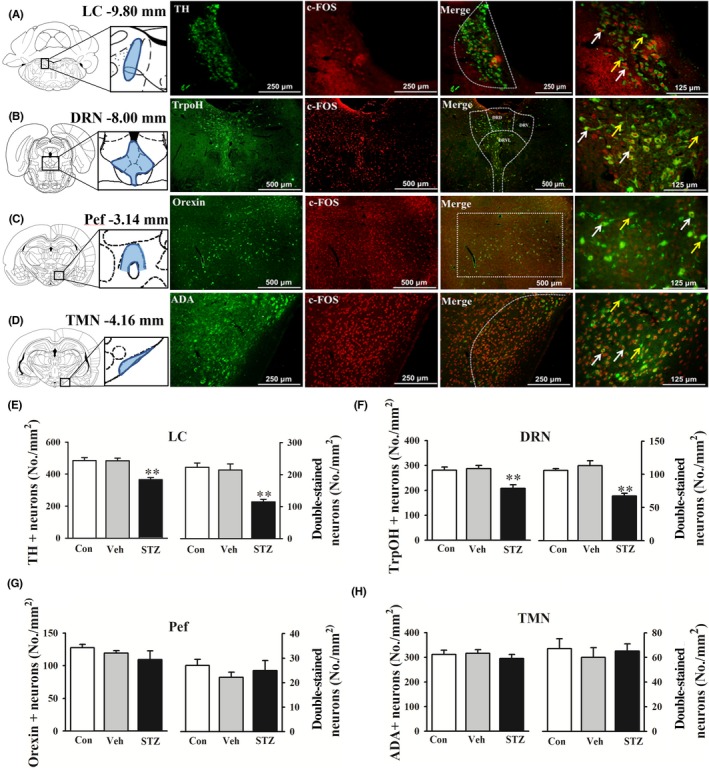
STZ i.c.v. injection affected neuronal activity in sleep‐wake regulating nucleus. The nucleus‐specific neurotransmitter markers were labeled green. c‐Fos was labeled red. A‐D, Double‐stained neurotransmitter markers and c‐Fos in the locus coeruleus (LC), dorsal raphe nucleus (DRN), perifornical area (Pef), and tuberomammillary nucleus (TMN). Tyrosine hydroxylase (TH) was labeled as noradrenergic neurons marker. Tryptophan hydroxylase (TrpOH) was labeled as serotonergic neurons marker. Adenosine deaminase (ADA) was labeled as histaminergic neurons maker. Yellow arrows indicate c‐Fos‐ and nucleus‐specific neurotransmitter marker + neurons. White arrows indicate dual c‐Fos+ and nucleus‐specific neurotransmitter marker + neurons. E‐H, The density of specific neurons and double‐stained cells was calculated. The data are expressed as mean ± SEM (n = 6‐8 per group). **P < 0.01, vs vehicle group (Student‐Newman‐Keuls test)
GABAergic neurons in the VLPO are sleep‐promoting neurons. The density of GAD+ (GABAergic) neurons decreased in STZ‐injected rats (P < 0.05). The STZ injection decreased c‐Fos expression of GABAergic neurons in the VLPO (P < 0.01, Figure 4A,D). Based on the effects of glutamatergic and GABAergic neurons in the PBN on sleep‐wake regulation,17 we evaluated neuronal activity in the PBN to explore the mechanisms of changes in states of behavioral arousal in STZ‐injected rats. Consistent with previous reports,18 glutamatergic neurons were distributed extensively in the whole PBN, and GABAergic neurons were distributed in the caudal part of the PBN. The density of v‐GLUT2+ neurons and density of GAD+ neurons in the caudal part of the PBN (−9.3 mm from bregma) were unaffected by the STZ injection. However, consistent with the hyperarousal that was observed in STZ‐injected rats, c‐Fos expression increased in glutamatergic neurons and decreased in GABAergic neurons in the caudal part of the PBN (P < 0.01, Figure 4B,C,E,F).
Figure 4.
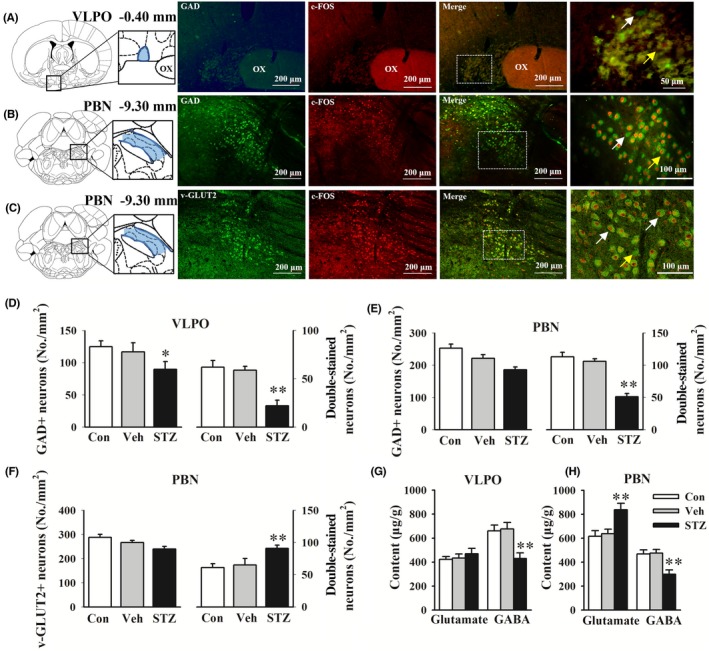
STZ i.c.v. injection affected neuronal activity in sleep‐wake regulating nucleus. The nucleus‐specific neurotransmitter markers were labeled green. c‐Fos was labeled red. A‐C, Double‐stained neurotransmitter markers and c‐Fos in the ventrolateral preoptic area (VLPO) and parabrachial nucleus (PBN). Glutamic acid decarboxylase (GAD) was labeled as GABAergic neurons marker. Vesicular glutamate 2 transporter (v‐GLUT2) was labeled as glutamatergic neurons marker. Yellow arrows indicate c‐Fos− and nucleus‐specific neurotransmitter marker + neurons. White arrows indicate dual c‐Fos+ and nucleus‐specific neurotransmitter marker + neurons. D‐F, The density of specific neurons and double‐stained cells was calculated. G, H, Glutamate and GABA levels in the VLPO and PBN were analyzed by HPLC. The data are expressed as mean ± SEM (n = 6–8 per group). *P < 0.05, **P < 0.01, vs vehicle group (Student‐Newman‐Keuls test). ox, optic chiasm
These data indicate that alterations of sleep parameters in STZ‐injected rats might be related to the suppression of GABAergic inhibition in the PBN, which subsequently resulted in activation of the glutamatergic system. We next evaluated alterations of neurotransmitters in related brain regions to verify the possible aforementioned mechanism. Compared with the vehicle group, STZ‐injected rats exhibited significant decreases in GABA levels in the VLPO and PBN, with an elevation of glutamate levels in the PBN (Figure 4G,H). No significant differences in neuronal activity or neurotransmitter levels were observed between the control and vehicle groups.
3.4. Intra‐PBN bicuculline‐induced sleep phenotype paralleled sleep disturbances in STZ‐injected rats
We next examined whether dysregulation of the GABAergic system in the PBN is related to sleep disturbances in rats. An additional group of rats was surgically implanted with cannulas and received a microinjection of the GABA receptor antagonist bicuculline (50 or 100 pmol/side) in the PBN. No significant differences in sleep parameters were observed between the control and vehicle groups. Low‐dose intra‐PBN bicuculline application (50 pmol/side) decreased the sleep tendency during the 24‐hours day. The sleep‐wake analysis indicated that the rats in the bicuculline (100 pmol/side) group spent more time awake and less time in NREM sleep and REM sleep especially SWS, during the 24‐hours day compared with vehicle‐treated rats (P < 0.05 or <0.01, Figure 5B). The intra‐PBN application of bicuculline (100 pmol/side) significantly increased the percentage of light sleep relative to total sleep (LS%) and decreased the percentage of SWS relative to total sleep (SWS%, P < 0.05, Figure 5C). The administration of bicuculline (100 pmol/side) significantly prolonged sleep latency and REM sleep latency (P < 0.05, Figure 5D,E). The microinjection of bicuculline (100 pmol/side) in the PBN significantly decreased sleep time during the light period (P < 0.01) but not during the dark period relative to the vehicle (Figure 5F and G). The time‐course of hourly sleep time indicated that the intra‐PBN microinjection of bicuculline decreased sleep time beginning in the first 1‐hour period to the fourth 1‐hour period (Figure 5H). These data indicate that the intra‐PBN bicuculline‐induced sleep phenotype paralleled the sleep disturbances that were observed in STZ‐injected rats.
Figure 5.
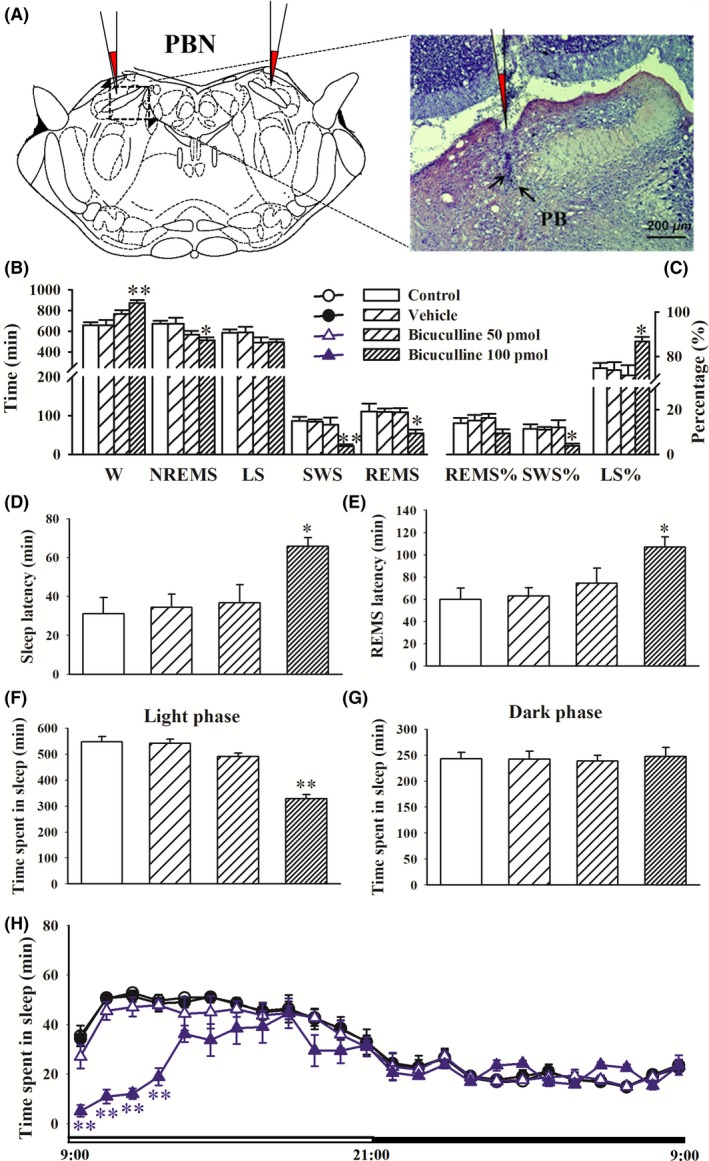
Microinjection of bicuculline in the PBN promoted wakefulness and decreased sleep. Sleep recording was performed 24 h immediately after vehicle or bicuculline (50 or 100 pmol/side) intra‐PBN application. A, Schematic representation of the cannula implantation sites 1 mm above the PBN and bright‐field photomicrograph of a coronal brain section showing the injector tip (black arrowhead). B‐E, Time spent in wakefulness (W), rapid eye movement sleep (REMS), nonrapid eye movement sleep (NREMS), and slow‐wave sleep (SWS) per 1 h. F, G, Sleep latency and REM sleep latency. H, Time spent in sleep per 1 h. The data are expressed as mean ± SEM (n = 8‐11 per group). *P < 0.05, **P < 0.01, vs vehicle group (Student‐Newman‐Keuls test)
3.5. Microinjection of GABA in the PBN attenuated the reduction in STZ‐induced sleep
To examine whether the sleep disruptions that were observed in STZ‐injected rats might be related to deficits of GABA in the PBN, we microinjected GABA (5 or 10 μmol/side) in the PBN. Sleep‐wake stages in normal rats were unaffected by GABA treatment in the PBN. Compared with vehicle‐treated rats, STZ‐injected rats exhibited a significant increase in wake time on day 14 but decreases in NREM sleep, SWS, and REM sleep time (P < 0.05 or <0.01). The intra‐PBN microinjection of GABA (10 μmol/side) significantly reversed STZ‐induced sleep deficits, reflected by a decrease in wake time and increases in NREM sleep and SWS time (P < 0.05, Figure 6A). The intra‐PBN microinjection of GABA (10 μmol/side) significantly shortened sleep latency (P < 0.05) but not REM sleep latency in STZ‐injected rats (Figure 6B). The intra‐PBN microinjection of GABA (10 μmol/side) significantly reversed the STZ‐induced disturbance of sleep structure, reflected by normalization of the percentage of SWS and light sleep relative to total sleep (P < 0.05, Figure 6C). The reversing effects of intra‐PBN GABA treatment on STZ‐induced sleep‐wake disruptions were significant during the light period but not during the dark period (Figure 6D‐F). Further analysis of the sleep‐wake data in rats that received an intra‐PBN microinjection of GABA indicated that the increase in hourly sleep duration was increased by GABA (10 μmol/side) administration beginning in the first 1‐hour period to the fourth 1‐hour period (Figure 6F).
Figure 6.
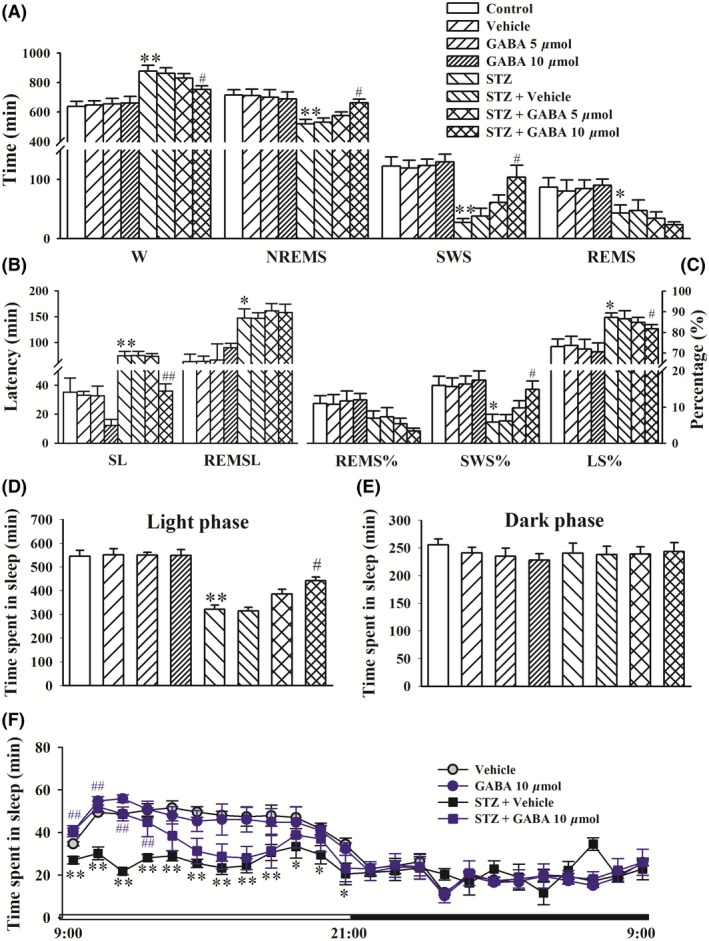
Microinjection of GABA in the PBN ameliorated sleep disturbances induced by STZ. GABA (5 or 10 μmol/side) or vehicle was microinjected in the PBN on day 14 after the STZ injection. Sleep recordings were performed for 24 h immediately after GABA intra‐PBN application. A, Time spent in wakefulness (W), nonrapid eye movement sleep (NREMS), light sleep (LS), slow‐wave sleep (SWS), and rapid eye movement sleep (REMS). B, Sleep latency and REM sleep latency. C, Percentage of REMS/LS/SWS relative to total sleep (REMS%, SWS%, LS%). D, Time spent in sleep per 1 h. The data are expressed as mean ± SEM (n = 8‐11 per group). *P < 0.05, **P < 0.01, vs vehicle group; # P < 0.05, ## P < 0.01, vs STZ + Veh group (Student‐Newman‐Keuls test)
4. DISCUSSION
Clinically, learning and memory deficits are early symptoms and hallmarks of AD.19, 20 Experimental observations indicate that pathological and behavioral changes in the rat model of AD that is induced by an i.c.v. injection of STZ recapitulate the symptoms and pathogenesis of sAD patients.21 In the present study, memory performance in STZ‐injected rats was evaluated using behavioral assays of learning and memory to confirm that our rats were a valid model of sAD. We found that STZ‐injected rats spent more time awake and less time in NREM and REM sleep, accompanied by a longer sleep latency and REM sleep latency, and this pattern of sleep disturbances was consistent with nocturnal insomnia in sAD patients. During the dark phase, STZ‐injected rats spent more time asleep at the expense of wakefulness. The link between AD and sleep disorders is bidirectional. The most common sleep problem in AD is an exaggerated tendency toward phase advance, characterized by frequent daytime napping, difficulties falling asleep at night, nocturnal sleep fragmentation, and early morning awakening.2, 22 This pattern may be similar to STZ‐injected rats.
The DRN and LC are part of the general arousal system in the brain. The immunohistochemistry data indicated that either the density of c‐Fos‐expressing serotonergic neurons in the DRN or density of c‐Fos‐expressing noradrenergic neurons in the LC decreased in STZ‐injected rats, whereas no alterations of orexinergic neurons in the Pef or histaminergic neurons in the TMN were observed. The activity of GABAergic neurons in the VLPO also decreased in STZ‐injected rats. These data suggest that these generally known arousal or sleep‐promoting systems might be dysfunctional in STZ‐injected rats, and neither system is directly associated with STZ‐induced sleep disorders. Alzheimer's disease is the most common neurodegenerative disease, characterized by neuronal loss and the deposition of β‐amyloid and neurofibrillary tangles in the hippocampus and cortex, resulting in cognitive impairment. The present results also indicated neuronal loss in the LC and DRN in STZ‐injected rats. Experimental observations suggest that noradrenergic neurons in the LC and serotonergic neurons in the DRN promote wakefulness and inhibit NREM sleep and REM sleep.13, 23, 24 From this standpoint, the suppressive effects of STZ on serotonergic and noradrenergic neurons may be expected to promote sleep. However, the present study showed that STZ significantly decreased sleep time.
The PBN is located in the pons and provides extensive, largely glutamatergic, ascending projections to the cerebral cortex and descending projections to medullary regions. These projections enable the PBN to exert powerful control over a wide range of neurobiological functions, including thermoregulation, respiration, pain, fear, and feeding.25, 26 The PBN also plays an indispensable role in generating and maintaining wakefulness.27 Animal data indicate a role for inhibitory GABAergic neurons in the VLPO and PBN in the initiation and maintenance of sleep, and glutamatergic neurons in the PBN play an important role in waking behavior.28, 29 Qiu et al found that PBN glutamatergic neurons receive GABAergic inhibitory inputs from the VLPO to facilitate the consolidation of NREM sleep, whereas a group of GABAergic neurons in the PBN are active during NREM sleep and involved in controlling the NREM sleep state.30 The genetically driven disruption of glutamate transmission by PBN neurons results in a large reduction in total wake time and a slowing of waking EEG.27 The present study found that c‐Fos expression decreased in GABAergic neurons in the arousal‐driving PBN in STZ‐injected rats but increased in glutamatergic neurons. HPLC revealed that GABA levels significantly decreased in both the VLPO and PBN, but glutamate levels in the PBN increased in STZ‐injected rats. These results suggest that lower activity of the GABAergic system and activation of the glutamatergic system in the PBN might be related to the promotion of wakefulness and reductions of NREM sleep, SWS, and REM sleep in STZ‐injected rats. Additionally, STZ‐injected rats exhibited lower c‐Fos expression in GABAergic neurons and lower levels of GABA in the VLPO. The lower activity of the GABAergic system in the VLPO may lead to the disinhibition of glutamatergic neurons in the PBN, thus resulting in the promotion of wakefulness in STZ‐injected rats. Inactivation of the GABAergic system by microinjections of bicuculline in the PBN in normal rats resulted in a disordered sleep pattern that was similar to STZ‐injected rats. These results indicate that suppression of the GABAergic system in the PBN might be involved in the development of sleep disorders. Furthermore, a direct injection of GABA in the PBN may improve disturbances in NREM sleep and SWS in STZ‐injected rats without influencing REM sleep. Altogether, the present results suggest that inactivation of the GABAergic system in the PBN may be associated with the development of sleep disorders in STZ‐injected rats. However, activation of the GABAergic system in the PBN did not reverse the decrease in REM sleep, suggesting that the mechanisms of STZ‐induced sleep disorders may not exclusively involve the GABAergic system in the PBN.
Projections from the PBN that are relayed by the basal forebrain to the cerebral cortex may play a critical role in behavioral or electrocortical arousal.28 In STZ‐injected rats, the preoptic area, hypothalamus, lateral hypothalamus, and thalamus may also mediate the PBN's control of wakefulness because these forebrain structures are also densely innervated by the PBN and provide direct innervations of the cortex.17 However, the neurocircuitry through which PBN GABAergic neurons drive arousal in STZ‐injected rats remains unclear, and the role of these respective PBN afferent pathways in arousal needs further investigation.
Alzheimer's disease patients often exhibit disruptions of circadian rhythm and mistimed sleep, which can cause great morbidity and is a major cause of institutionalization.22 Although these sleep and circadian abnormalities were once mistaken as consequences of the disease process, accumulating evidence suggests that circadian disturbances likely occur early in the disease process and may contribute to the pathogenesis of AD.31 The association between disruptions of sleep and circadian rhythm and the progression of AD presents an opportunity to target preclinical symptoms of AD to prevent the further progression of neurodegeneration.32, 33 Potential pharmacological treatments may decrease the prevalence or mitigate the severity of AD. However, because of the lack of suitable models for pharmacological evaluation, the pathogenic mechanisms of sleep disorders in AD are still unclear. Our results suggest that STZ‐injected rats may be a useful animal model that simulates the features of sleep and circadian rhythm disorders in AD patients.
In conclusion, the present results indicate that the reduction in GABAergic inhibition in the PBN plays an important role in sleep disorders that are induced by i.c.v. STZ administration. Our study provides novel insights into the pathological mechanisms of sleep disorders in sAD, which may contribute to screening potential therapeutic agents to ameliorate sleep disorders in sAD patients. We could not establish a direct link between impairments in the GABAergic system in the PBN and sleep disorders, and the neurocircuitry through which PBN GABAergic neurons drive arousal in STZ‐injected rats remains unclear. Nonetheless, our novel findings may encourage future studies that test the hypothesis that alterations of GABAergic function in the PBN, in addition to the generally known circuitry that regulates sleep‐wake states, may account for disruptions of sleep and circadian rhythm in sAD patients.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
This study was funded by grants from the National Natural Science Foundation of China (No. 81573407, 81302746, and 81202511).
Cui S‐Y, Song J‐Z, Cui X‐Y, et al. Intracerebroventricular streptozotocin‐induced Alzheimer's disease‐like sleep disorders in rats: Role of the GABAergic system in the parabrachial complex. CNS Neurosci Ther. 2018;24:1241–1252. 10.1111/cns.13032
The first two authors contributed equally to this work.
Contributor Information
Su‐Ying Cui, Email: csy@bjmu.edu.cn.
Yong‐He Zhang, Email: zhyh@hsc.pku.edu.cn.
REFERENCES
- 1. Busche MA, Kekus M, Forstl H. Connections between sleep and Alzheimer's disease: insomnia, amnesia and amyloid. Nervenarzt. 2017;88:215‐221. [DOI] [PubMed] [Google Scholar]
- 2. Urrestarazu E, Iriarte J. Clinical management of sleep disturbances in Alzheimer's disease: current and emerging strategies. Nat Sci Sleep. 2016;8:21‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mander BA, Winer JR, Jagust WJ, Walker MP. Sleep: a novel mechanistic pathway, biomarker, and treatment target in the pathology of Alzheimer's disease? Trends Neurosci. 2016;39(8):552‐566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guzmán EA, Bouter Y, Richard BC, et al. Abundance of Aβ 5‐x like immunoreactivity in transgenic 5XFAD, APP/PS1KI and 3xTG mice, sporadic and familial Alzheimer's disease. Mol Neurodegener. 2014;9(1):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lista S, O'Bryant SE, Blennow K, et al. Biomarkers in sporadic and familial Alzheimer's disease. J Alzheimers Dis. 2015;47(2):291‐317. [DOI] [PubMed] [Google Scholar]
- 6. Lee Y, Kim YH, Park SJ, et al. Insulin/IGF signaling‐related gene expression in the brain of a sporadic Alzheimer's disease monkey model induced by intracerebroventricular injection of streptozotocin. J Alzheimers Dis. 2014;38(2):251‐267. [DOI] [PubMed] [Google Scholar]
- 7. Pawe G. Intracerebroventricular streptozotocin injections as a model of Alzheimer's disease: in search of a relevant mechanism. Mol Neurobiol. 2016;53(3):1741‐1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mehla J, Pahuja M, Gupta YK. Streptozotocin‐induced sporadic Alzheimer's disease: selection of appropriate dose. J Alzheimers Dis. 2013;33(1):17‐21. [DOI] [PubMed] [Google Scholar]
- 9. Yeo HG, Lee Y, Jeon CY, et al. Characterization of cerebral damage in a monkey model of Alzheimer's disease induced by intracerebroventricular injection of streptozotocin. J Alzheimers Dis. 2015;46(4):989‐1005. [DOI] [PubMed] [Google Scholar]
- 10. Paxinos GA, Watson C. The Rat Brain in Stereotaxic Coordinates, 4th edn San Diego, CA: Academic Press; 1998. [Google Scholar]
- 11. Xiong H, Zheng C, Wang J et al. The neuroprotection of liraglutide on Alzheimer‐like learning and memory impairment by modulating the hyperphosphorylation of Tau and neurofilament proteins and insulin signaling pathways in mice. J Alzheimers Dis. 2013;37(3):623‐635. [DOI] [PubMed] [Google Scholar]
- 12. Yu B, Cui S‐Y, Zhang X‐Q, et al. Mechanisms underlying footshock and psychological stress‐induced abrupt awakening from posttraumatic “Nightmares”. Int J Neuropsychopharmacol. 2016;19(4):pyv113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cui SY, Li SJ, Cui XY, et al. Ca(2+) in the dorsal raphe nucleus promotes wakefulness via endogenous sleep‐wake regulating pathway in the rats. Mol Brain. 2016;9(1):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cao Q, Jiang Y, Cui SY, et al. Tenuifolin, a saponin derived from Radix Polygalae, exhibits sleep‐enhancing effects in mice. Phytomedicine. 2016;23(14):1797‐1805. [DOI] [PubMed] [Google Scholar]
- 15. Paweł G. Intracerebroventricular streptozotocin Injections as a model of Alzheimer's Disease: in search of a relevant mechanism. Mol Neurobiol. 2016;53(3):1741‐1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Song JZ, Cui SY, Cui XY, et al. Dysfunction of GABAergic neurons in the parafacial zone mediates sleep disturbances in a streptozotocin‐induced rat model of sporadic Alzheimer's disease. Metab Brain Dis. 2018;33(1):127‐137. [DOI] [PubMed] [Google Scholar]
- 17. Saper CB, Fuller PM. Wake‐sleep circuitry: an overview. Curr Opin Neurobiol. 2017;44:186‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Geerling JC, Yokota S, Rukhadze I, Roe D, Chamberlin NL. Kölliker‐fuse GABAergic and glutamatergic neurons project to distinct targets. J Comp Neurol. 2017;525(8):1844‐1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhu S, Wang J, Zhang Y, et al. The role of neuroinflammation and amyloid in cognitive impairment in an APP/PS1 transgenic mouse model of Alzheimer's disease. CNS Neurosci Ther. 2017;23(4):310‐320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu Y, Xu YF, Zhang L, et al. Effective expression of Drebrin in hippocampus improves cognitive function and alleviates lesions of Alzheimer's disease in APP (swe)/PS1 (ΔE9) mice. CNS Neurosci Ther. 2017;23(7):590‐604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lestercoll N, Rivera EJ, Soscia SJ, Doiron K, Wands JR, de la Monte SM. Intracerebral streptozotocin model of type 3 diabetes: relevance to sporadic Alzheimer's disease. J Alzheimers Dis. 2006;9(1):13‐33. [DOI] [PubMed] [Google Scholar]
- 22. Musiek ES, Xiong DD, Holtzman DM. Sleep, circadian rhythms, and the pathogenesis of Alzheimer disease. Exp Mol Med. 2015;47(3):e148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE. Sleep state switching. Neuron. 2010;68(6):1023‐1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ogier M, Bricca G, Bader M, Bezin L. Locus coeruleus dysfunction in transgenic rats with low brain angiotensinogen. CNS Neurosci Ther. 2016;22(3):230‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Saper CB, Loewy AD. Commentary on: efferent connections of the parabrachial nucleus in the rat. C.B. Saper and A.D. Loewy, Brain Research 197:291–317, 1980. Brain Res. 2016;1645:15‐17. [DOI] [PubMed] [Google Scholar]
- 26. Saper CB, Loewy AD. Efferent connections of the parabrachial nucleus in the rat. Brain Res. 1980;197(2):291‐317. [DOI] [PubMed] [Google Scholar]
- 27. Fuller PM, Sherman D, Pedersen NP, Saper CB, Lu J. Reassessment of the structural basis of the ascending arousal system. J Comp Neurol. 2011;519(5):933‐956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Scammell TE, Arrigoni E, Lipton JO. Neural circuitry of wakefulness and sleep. Neuron. 2017;93(4):747‐765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Torterolo P, Sampogna S, Chase MH. A restricted parabrachial pontine region is active during non‐rapid eye movement sleep. Neuroscience. 2011;190:184‐193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Qiu MH, Chen MC, Fuller PM, Lu J. Stimulation of the pontine parabrachial nucleus promotes wakefulness via extra‐thalamic forebrain circuit nodes. Curr Biol. 2016;26(17):2301‐2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hahn EA, Wang HX, Andel R, Fratiglioni L. A change in sleep pattern may predict Alzheimer disease. Am J Geriatr Psychiatry. 2014;22(11):1262‐1271. [DOI] [PubMed] [Google Scholar]
- 32. Kabeshita Y, Adachi H, Matsushita M, et al. Sleep disturbances are key symptoms of very early stage Alzheimer disease with behavioral and psychological symptoms: a Japan multi‐center cross‐sectional study (J‐BIRD). Int J Geriatr Psychiatry. 2017;32(2):222‐230. [DOI] [PubMed] [Google Scholar]
- 33. Lim MM, Gerstner JR, Holtzman DM. The sleep–wake cycle and Alzheimer's disease: what do we know? Neurodegener Dis Manag. 2014;4(5):351‐362. [DOI] [PMC free article] [PubMed] [Google Scholar]


