Abstract
Background
Trastuzumab (TZ) therapy requires careful monitoring of left ventricular (LV) ejection fraction (LVEF) because it can be potentially cardiotoxic. However, LVEF is an imperfect parameter and there is a need to find other variables to predict cardiac dysfunction early. Left atrium (LA) enlargement has proven to be a powerful predictor of adverse outcomes in several disease entities.
Hypothesis
Baseline LA volume enlargement might predict TZ‐related LV dysfunction.
Methods
HER2‐positive breast cancer patients receiving TZ and undergoing transthoracic echocardiography at baseline and at follow‐up every 3 months were retrospectively recruited. One‐hundred sixty‐two patients formed the study population.
Results
Baseline LAVI was dilated in 14 patients (8.6%). Mean follow‐up was 14 ± 4 months. Cardiotoxicity occurred in 24 patients (14.8%). LAVI was an independent predictor of TZ‐induced LV dysfunction in a clinical model, after adjustment for age and hypertension (odds ratio per 5‐mL/m2 LAVI increase: 1.34, 95% confidence interval: 1.03‐1.82, P = 0.03); and in a hemodynamic model, including ventricular sizes and systolic blood pressure level (odds ratio per 5‐mL/m2 LAVI increase: 1.34, 95% confidence interval: 1.01‐1.81, P = 0.04). The predicted probability of developing cardiotoxicity increased progressively, in parallel with LAVI values.
Conclusions
Baseline LA dilatation emerges as a condition associated with the development of cardiotoxicity in HER2‐positive breast cancer patients treated with TZ.
Keywords: Cardiotoxicity, Left Atrial Volume, Left Ventricular Dysfunction, Trastuzumab
1. INTRODUCTION
Breast cancer (BC) is the most common malignancy in women worldwide.1 Approximately 20% to 25% of all BC patients show an overexpression of human epidermal growth factor receptor‐2 (HER2), which is associated with more aggressive tumor proliferation and increased mortality.2 Trastuzumab (TZ) is a human monoclonal antibody specifically directed to the extracellular portion of the HER2 membrane protein. Its introduction in BC therapy in both adjuvant and metastatic situations has dramatically improved the prognosis of women with HER2‐positive (HER2+) BC.
Because of its potential cardiotoxicity, TZ requires careful monitoring of left ventricular (LV) function,3 which is commonly done through measurement of LV ejection fraction (LVEF). However, LVEF fails to detect early subtle changes, and, when reduced, it reflects a marker of advanced myocyte damage accompanied by a poor prognosis.4, 5 A few possible parameters of early cardiac dysfunction have been proposed,6, 7, 8 but so far no clear predictor has been defined. Left atrium (LA) enlargement has proven to be a powerful predictor of adverse outcomes in several disease entities.9, 10, 11 However, no data are available correlating TZ‐related LV dysfunction to baseline left atrial volume index (LAVI). Therefore, the aim of this single‐center observational study was to investigate the relationship between baseline LAVI and cardiotoxicity development, and to test the hypothesis that baseline LA volume enlargement could predict TZ‐related LV dysfunction.
2. METHODS
We retrospectively reviewed patients with histologically confirmed diagnosis of BC at an early stage or locally advanced (stages I–IIIC) featuring overexpression of HER2 and treated with TZ in an adjuvant or neoadjuvant setting between 2006 and 2014 at the Oncology Department of our institute. Patients treated with an anthracycline‐based regimen also were included. The exclusion criteria were metastatic cancer at diagnosis, baseline LVEF <50%, presence of cardiomyopathy, more than mild valve disease, valve prosthesis, pacemaker or defibrillator, previous heart or renal transplant, severe renal insufficiency, and previous episode of heart failure.
Baseline oncological details, comorbidities, cardiovascular risk factors, medications, and oncological treatment regimens (including anthracyclines, if used), were collected by chart review. A comprehensive transthoracic 2D echocardiographic examination was performed in each patient with the patient in the left lateral position. LV volumes and LVEF were measured by the biplane Simpson method at baseline and at follow‐up every 3 months. LA volume was measured at the end of LV systole from the apical 4‐chamber view, using the modified Simpson rule. LAVI was calculated as LA volume divided by the body surface area, and it was considered abnormally increased if it was ≥34 mL/m2, as indicated by the most recent clinical guidelines.12 All the measurements were performed at the moment of the images acquisition by an experienced operator.
Cardiotoxicity was defined as a decrease in LVEF <50% or an absolute decrease of >10 points below the baseline value with or without signs and/or symptoms of heart failure confirmed by a cardiologist through a comprehensive clinical evaluation,3 according to the clinical practice of the Oncology Department of our institute.
2.1. Statistical analysis
Data are reported as mean ± SD or number and percentage when appropriate. Continuous variables were compared by paired or unpaired t test. To assess normality distributions of the measured variables, a Kolmogorov–Smirnov test was used. The χ2 test was used for comparison of categorical variables. Logistic regression was performed to evaluate the relation between LAVI and the development of cardiotoxicity. Different models were tested, selecting variables based on biological plausibility and on their association with cardiotoxicity: a first unadjusted model, including LAVI only; a clinical model including LAVI, age, and history of hypertension (HTN); and a hemodynamic model including LAVI, systolic blood pressure level, LV size, and the presence of mitral regurgitation (MR). Lastly, the predicted probability for each unit of LAVI increase in the hemodynamic model was plotted to better display the type of relationship between the LAVI and cardiotoxicity event. A P value <0.05 was considered statistically significant. SPSS software version 20 (IBM Corp., Armonk, NY) was used for the statistical analyses.
3. RESULTS
A total of 162 patients formed the study cohort, with a mean age of 58 ± 12 years (range, 31–87 years). Oncological characteristics of the study population are reported in Table 1. The majority of the patients had early BC (n = 147) and underwent mastectomy or breast‐conserving surgery (n = 149). Anthracycline‐based therapy protocols included doxorubicin (240 mg/m2) or epirubicin (360 mg/m2), followed by TZ alone or with taxanes, and were administered in 80 patients. Mean follow‐up was 14 ± 4 months. Clinical and echocardiographic parameters of the study population are presented in Table 2. All patients showed normal LV volumes, well‐preserved LVEF function, and an average LAVI of 26 ± 7 mL/m2 before starting treatment. A baseline LA dilatation was detected in 14 patients (8.6%). These patients were older, presented with a history of systemic HTN (64% vs 32%; P = 0.02), were taking prescribed antihypertensive agents (64% vs 21%; P = 0.001), and had presence of trivial or mild MR (71% vs 45%; P = 0.01) more frequently as compared with patients with normal LAVI. No significant differences were found in blood pressure values or presence of dyslipidemia, diabetes mellitus, or family history of cardiac disease.
Table 1.
Oncological characteristics of the study population
| Variable | Value |
|---|---|
| Cancer type | |
| Ductal | 149 |
| Lobular | 13 |
| Grading | |
| 1 | 49 |
| 2 | 98 |
| 3 | 15 |
| Cancer side | |
| Left | 89 |
| Right | 65 |
| Bilateral | 8 |
Table 2.
Clinical and echocardiographical parameters of the study population and comparison between patients with baseline LAVI <34 mL/m2 and ≥34 mL/m2
| Total Population, N = 162 | LAVI <34 mL/m2, n = 148 | LAVI ≥34 mL/m2, n = 14 | P Value | |
|---|---|---|---|---|
| Age at baseline, y | 59 ± 12 | 59 ± 12 | 65 ± 11 | 0.05 |
| BSA, m2 | 1.67 ± 0.14 | 1.67 ± 0.14 | 1.69 ± 0.09 | 0.6 |
| SBP, mm Hg | 125 ± 14 | 124 ± 15 | 130 ± 12 | 0.1 |
| DBP, mm Hg | 75 ± 9 | 75 ± 1 | 75 ± 2 | 0.9 |
| CV risk factors | ||||
| HTN | 57 (35) | 48 (32) | 9 (64) | 0.02 |
| Hypercholesterolemia | 24 (15) | 21 (14) | 3 (21) | 0.4 |
| DM | 6 (4) | 5 (3) | 1 (7) | 0.4 |
| PAD | 8 (5) | 7 (5) | 1 (7) | 0.6 |
| CV treatments | ||||
| ACEI/ARB | 20 (12) | 14 (9) | 6 (42) | 0.0003 |
| β‐Blockers | 25 (15) | 18 (12) | 7 (50) | 0.0002 |
| Diuretics | 18 (11) | 17 (11) | 1 (7) | 0.6 |
| CCB | 9 (5) | 4 (3) | 5 (35) | <0.0001 |
| Anthracyclines | 80 (49) | 77 (52) | 3 (21) | 0.02 |
| Cardiotoxicity | 24 (15) | 17 (11) | 7 (50) | 0.0001 |
| Echocardiographic parameters | ||||
| LVEDV, mL | 89 ± 19 | 87 ± 18 | 101 ± 14 | 0.01 |
| LVEDVi, mL/m2 | 54 ± 12 | 53 ± 12 | 60 ± 8 | 0.04 |
| LVESV, mL | 32 ± 9 | 31 ± 9 | 39 ± 8 | 0.003 |
| LVESVi, mL/m2 | 19 ± 5 | 18 ± 5 | 23 ± 5 | 0.004 |
| LVEF, % | 64 ± 5 | 64 ± 5 | 60 ± 5 | 0.007 |
| LAV, mL | 44 ± 13 | 41 ± 9 | 62 ± 17 | <0.0001 |
| LAVI, mL/m2 | 26 ± 7 | 24 ± 5 | 43 ± 10 | <0.0001 |
| DTE, ms | 205 ± 50 | 203 ± 50 | 248 ± 48 | 0.08 |
| Mitral regurgitation | 82 (51) | 72 (45) | 10 (71) | 0.01 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BSA, body surface area; CCB, calcium channel blocker; CV, cardiovascular; DBP, diastolic blood pressure; DM, diabetes mellitus; DTE, mitral E‐wave deceleration time; HTN, hypertension; LAV, left atrial volume; LAVI, left atrial volume index; LVEDV, left ventricular end‐diastolic volume; LVEDVi, left ventricular end‐diastolic volume index; LVEF, left ventricular ejection fraction; LVESV, left ventricular end‐systolic volume; LVESVi, left ventricular end‐systolic volume index; PAD, peripheral arterial disease; SBP, systolic blood pressure; SD, standard deviation.
Data are presented as n (%) or mean ± SD.
During the follow‐up, cardiotoxicity occurred in 24 patients (14.8%). Mean time to detection of cardiotoxicity was 7 ± 4 months. No difference in the incidence of cardiotoxicity between patients receiving anthracyclines before TZ and patients not receiving such therapy was found (15/80 [19%] vs 9/82 [11%]; P = 0.16). These 2 subgroup of patients were very homogeneous; no significant difference was found in terms of cardiovascular risk factors. Notably, 7/14 (50%) of patients with baseline dilated LAVI subsequently developed TZ‐related cardiotoxicity, with an odds ratio (OR) per 5‐mL/m2 LAVI increase of 1.47 (95% confidence interval [CI]: 1.13‐1.97, P = 0.004). Baseline LAVI was shown to be an independent predictor of TZ‐induced LV dysfunction in a clinical model, after adjustment for age and HTN (OR per 5‐mL/m2 LAVI increase: 1.34, 95% CI: 1.03‐1.82, P = 0.03). In addition, the LAVI role was confirmed in a hemodynamic model including LV end‐diastolic volume and systolic blood pressure level (OR per 5‐mL/m2 LAVI increase: 1.34, 95% CI: 1.01‐1.81, P = 0.04); there were no missing data for this model (Table 3). Forcing MR in the hemodynamic model did not rule out the role of LAVI (OR: 1.34, 95% CI: 1.02‐1.81, P = 0.041). When analyzed as a continuous variable, the predicted probability of cardiotoxicity development grew in parallel to LAVI values, with no plateau effect (Figure 1).
Table 3.
Logistic regression analysis assessing association with cardiotoxicity in a clinical and hemodynamic model
| OR (95% CI) | P Value | |
|---|---|---|
| Clinical model | ||
| Age | 0.64 (0.06‐6.78) | 0.7 |
| HTN | 2.59 (0.95‐7.26) | 0.06 |
| LAVI per 5 mL/m2 | 1.34 (1.03‐1.82) | 0.03 |
| Hemodynamic model | ||
| EDVi per 5 mL/m2 | 1.09 (0.89‐1.31) | 0.3 |
| SAP per 5 mmHg | 1.20 (1.04‐1.40) | 0.01 |
| LAVI per 5 mL/m2 | 1.34 (1.02‐1.80) | 0.03 |
Abbreviations: CI, confidence interval; EDVi, end‐diastolic volume index; HTN, hypertension; LAVI, left atrial volume index; OR, odds ratio; SAP, systolic arterial pressure.
Figure 1.
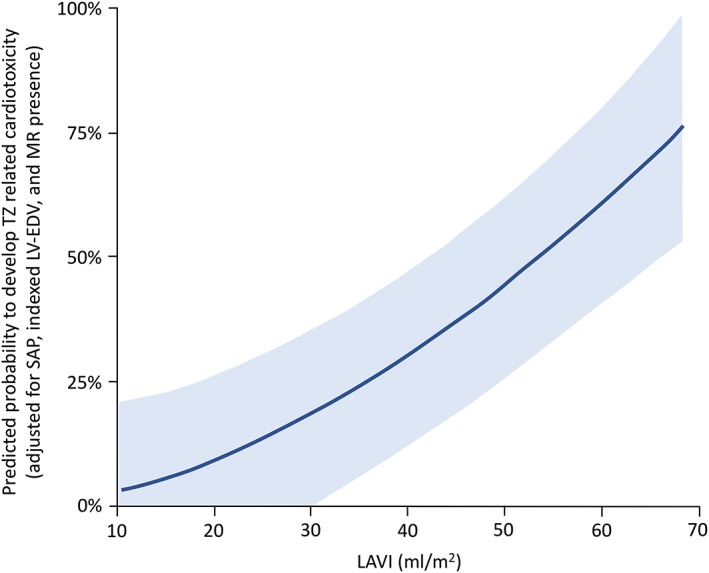
Probability of developing cardiotoxicity in relation to LA volume values at baseline. Abbreviations: LA, left atrial; LAVI, left atrial volume index; LVEDV, left ventricular end‐diastolic volume; MR, mitral regurgitation; SAP, systolic arterial pressure; TZ, trastuzumab
4. DISCUSSION
In clinical practice, the rationale for early recognition of TZ‐induced cardiac damage is related to the availability of effective treatment options and to the acknowledgment that delayed intervention has limited success. Therefore, identification of parameters useful for risk definition of BC patients treated with TZ is now of great interest.
The main findings of the present study are (1) baseline LAVI was independently associated with TZ‐related cardiotoxicity in a group of HER2+ BC patients, and (2) LAVI resulted in characterizing the risk of developing cardiotoxicity in a continuous fashion.
This is the first report linking LAVI and risk of cardiotoxicity in this context. Half of the patients with an abnormal baseline LAVI developed LV dysfunction during follow‐up. Importantly, these patients with dilated baseline LAVI were older and presented more frequently with HTN and prescription antihypertensive agents, all conditions that could cue a subclinical diastolic dysfunction. However, beyond LAVI, none of the conventional parameters of systolic or diastolic impairment (mitral inflow pattern and tissue Doppler parameters) were able to predict cardiotoxicity. The explanation is intuitive if we consider that LA dilatation represents a sensible marker of chronic changes in LV filling pressure, which can be superior and less dynamic than the other classical diastolic parameters. Baseline LAVI was a predictor of cardiotoxicity in both our clinical and hemodynamic models; but more important, when LAVI was analyzed as a continuous variable, the risk of cardiotoxicity grew in parallel with the baseline chamber volume. There is increasing evidence that LA structural and functional abnormalities result from alterations in extracellular matrix and ion channels reflecting pathophysiological changes in renin secretion, levels of angiotensin II, aldosterone, transforming growth factor‐β1, sympathetic stimulation, and markers of systemic inflammation,13 suggesting possible targets for preventive therapy in HER2+ BC patients at risk of developing TZ‐related LV dysfunction and subsequently needing discontinuation of such monoclonal antibody therapy.
HTN is a well‐known predictor of cardiotoxicity development during TZ therapy,14 and it was confirmed to be associated with a higher risk of TZ‐related cardiac dysfunction in our series. The hypothesized mechanisms that may lead to cancer‐promoting effects are the generation of large amounts of reactive oxygen species15 and interference in the myocyte survival pathways.16 Although the number of cardiac adverse events associated with the isolated use of TZ may increase with previous use of anthracycline,17, 18 in the present study there was no difference in prevalence of cardiotoxicity between those patients who had previously undergone anthracycline treatment and those who had not. This can be due to the deferred administration timing of the biological therapy in respect to the anthracyclines. Indeed, TZ cardiotoxicity is increased when given simultaneously with anthracyclines.19 Likewise, to reduce the risk of cardiac damage, no high dosage and/or continuous infusion of the latter therapy was given.
Overall, the increased incidence of cardiotoxicity in the subset of patients with dilated baseline LAVI likely reflects a more adverse cardiac risk profile and reduced cardiac reserve. Therefore, research is needed to stratify patients into different classes of cardiotoxicity risk and to guide the management of such patients. In addition, given the growing number of long‐term BC survivors, patients need to be characterized not only for the risk of the transient cardiac injury during TZ administration, but also for long‐term or permanent cardiac effects. It is known that LA enlargement carries important clinical and prognostic implications. Currently, echocardiography allows the evaluation of BC patients. LVEF is the most used parameter to monitor cardiac injury, and a simple, feasible, and inexpensive echocardiographic 2D parameter such as LAVI could add useful information and help to display for each patient a comprehensive evaluation of his entire cardiovascular risk profile even in terms of adverse outcomes, as previously shown in patients with diabetes.20
4.1. Study limitations
This study presents a number of limitations. First, it has a retrospective nature and data were retrieved from chart review; thus, cardiac biomarkers such as troponin and N‐terminal pro‐brain natriuretic peptide, recently proposed as novel approaches for early identification of patients more prone to develop myocardial dysfunction and cardiac events after chemotherapy,21, 22 were not available for all the patients. Furthermore, we acknowledge that even a mild renal dysfunction may represent a condition at higher risk of TZ‐related cardiotoxicity.23 In the present study we excluded all the patients with overt renal dysfunction; however, data regarding glomerular filtration were not available for all the patients, and mild/subclinical renal impairment could not be tested on the regression model. Second, the study sample is relatively small, but this is balanced by the selection of a uniform population with no previous oncologic and cardiac diseases. Nevertheless, it is possible to believe that the presented data can be useful in daily practice and the benefit may be related also to the absence of interference with standard echocardiographic examination, LAVI being a regularly performed echocardiographic parameter without adding time‐consuming analysis. The assessment of LA function by volumetric or deformation indices (strain rate) could add further information for clinical practice. Prospective longitudinal studies are needed to further investigate these exploratory data.
5. CONCLUSION
Baseline LAVI provides unique information to detect a condition at higher risk of developing cardiotoxicity in HER2+ BC patients treated with TZ. Thus, LAVI should be routinely assessed in such groups of BC patients needing a TZ course of treatment. Furthermore, the LA enlargement can result from alterations that may be addressed by new therapeutic interventions.
Conflicts of interest
The authors declare no potential conflicts of interest.
Bergamini C, Dolci G, Rossi A, et al. Left atrial volume in patients with HER2‐positive breast cancer: One step further to predict trastuzumab‐related cardiotoxicity. Clin Cardiol. 2018;41:349–353. 10.1002/clc.22872
REFERENCES
- 1. Jemal A, Bray F, Center MM, et al. Global cancer statistics [published correction appears in CA Cancer J Clin. 2011;61:134]. CA Cancer J Clin. 2011;61:69–90. [DOI] [PubMed] [Google Scholar]
- 2. Slamon DJ, Leyland‐Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. [DOI] [PubMed] [Google Scholar]
- 3. Zamorano JL, Lancellotti P, Rodriguez Muñoz D, et al; ESC Committee for Practice Guidelines . 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:2768–2801. [DOI] [PubMed] [Google Scholar]
- 4. Ewer MS, Lenihan DJ. Left ventricular ejection fraction and cardiotoxicity: is our ear really to the ground? J Clin Oncol. 2008;26:1201–1203. [DOI] [PubMed] [Google Scholar]
- 5. Eidem BW. Identification of anthracycline cardiotoxicity: left ventricular ejection fraction is not enough. J Am Soc Echocardiogr. 2008;21:1290–1292. [DOI] [PubMed] [Google Scholar]
- 6. Bergamini C, Torelli F, Ghiselli L, et al. Left ventricular end‐diastolic volume as early indicator of trastuzumab‐related cardiotoxicity in HER2+ breast cancer patients: results from a single‐center retrospective study. Minerva Cardioangiol. 2017;65:278–287. [DOI] [PubMed] [Google Scholar]
- 7. Fallah‐Rad N, Walker JR, Wassef A, et al. The utility of cardiac biomarkers, tissue velocity and strain imaging, and cardiac magnetic resonance imaging in predicting early left ventricular dysfunction in patients with human epidermal growth factor receptor II–positive breast cancer treated with adjuvant trastuzumab therapy. J Am Coll Cardiol. 2011;57:2263–2270. [DOI] [PubMed] [Google Scholar]
- 8. Dores H, Abecasis J, Correia MJ, et al. Detection of early sub‐clinical trastuzumab‐induced cardiotoxicity in breast cancer patients [article in English, Portuguese]. Arq Bras Cardiol. 2013;100:328–332. [PubMed] [Google Scholar]
- 9. Lønborg JT, Engstrøm T, Møller JE, et al. Left atrial volume and function in patients following ST elevation myocardial infarction and the association with clinical outcome: a cardiovascular magnetic resonance study. Eur Heart J Cardiovasc Imaging. 2013;14:118–127. [DOI] [PubMed] [Google Scholar]
- 10. Dalsgaard M, Egstrup K, Wachtell K, et al. Left atrial volume as predictor of valve replacement and cardiovascular events in patients with asymptomatic mild to moderate aortic stenosis. Echocardiography. 2013;30:1008–1014. [DOI] [PubMed] [Google Scholar]
- 11. Rossi A, Temporelli PL, Quintana M, et al; MeRGE Heart Failure Collaborators . Independent relationship of left atrial size and mortality in patients with heart failure: an individual patient meta‐analysis of longitudinal data (MeRGE Heart Failure). Eur J Heart Fail. 2009;11:929–936. [DOI] [PubMed] [Google Scholar]
- 12. Lang RM, Badano LP, Mor‐Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1.e14–39.e14. [DOI] [PubMed] [Google Scholar]
- 13. Casaclang‐Verzosa G, Gersh BJ, Tsang TS. Structural and functional remodeling of the left atrium: clinical and therapeutic implications for atrial fibrillation. J Am Coll of Cardiol. 2008;51:1–11. [DOI] [PubMed] [Google Scholar]
- 14. Russo G, Cioffi G, Gori S, et al; ICARO (Italian Cardio‐Oncological Network) . Role of hypertension on new onset congestive heart failure in patients receiving trastuzumab therapy for breast cancer. J Cardiovasc Med (Hagerstown). 2014;15:141–146. [DOI] [PubMed] [Google Scholar]
- 15. Largent JA, Bernstein L, Horn‐Ross PL, et al. Hypertension, antihypertensive medication use, and breast cancer risk in the California Teachers Study cohort. Cancer Causes Control. 2010;21:1615–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Seicean S, Seicean A, Alan N, et al. Cardioprotective effect of β‐adrenoceptor blockade in patients with breast cancer undergoing chemotherapy: follow‐up study of heart failure. Circ Heart Fail. 2013;6:420–426. [DOI] [PubMed] [Google Scholar]
- 17. Seidman A, Hudis C, Pierri MK, et al. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol. 2002;20:1215–1221. [DOI] [PubMed] [Google Scholar]
- 18. Eschenhagen T, Force T, Ewer MS, et al. Cardiovascular side effects of cancer therapies: a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2011;13:1–10. [DOI] [PubMed] [Google Scholar]
- 19. Slamon D, Eiermann W, Robert N, et al. Phase III randomized trial comparing doxorubicin and cyclophosphamide followed by docetaxel (AC > T) with doxorubicin and cyclophosphamide followed by docetaxel and trastuzumab (AC > TH) with docetaxel, carboplatin and trastuzumab (TCH) in HER2neu‐positive early breast cancer patients: BCIRG 006 study. Cancer Res. 2009;69(suppl). doi: 10.1158/0008-5472.SABCS-09-62. [DOI] [Google Scholar]
- 20. Zoppini G, Bonapace S, Bergamini C, et al. Evidence of left atrial remodeling and left ventricular diastolic dysfunction in type 2 diabetes mellitus with preserved systolic function. Nutr Metab Cardiovasc Dis. 2016;26:1026–1032. [DOI] [PubMed] [Google Scholar]
- 21. Goel S, Simes RJ, Beith JM. Exploratory analysis of cardiac biomarkers in women with normal cardiac function receiving trastuzumab for breast cancer. Asia‐Pac J Clin Oncol. 2011;7:276–280. [DOI] [PubMed] [Google Scholar]
- 22. Sawaya H, Sebag IA, Plana JC, et al. Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circ Cardiovasc Imaging. 2012;5:596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Russo G, Cioffi G, Di Lenarda A, et al. Role of renal function on the development of cardiotoxicity associated with trastuzumab‐based adjuvant chemotherapy for early breast cancer. Intern Emerg Med. 2012;7:439–446. [DOI] [PubMed] [Google Scholar]


