Abstract
Statins lower low‐density lipoprotein cholesterol (LDL‐C) and improve clinical outcomes in patients with atherosclerotic cardiovascular disease (CVD). Patients with acute coronary syndromes (ACS) often do not achieve LDL‐C targets despite potent statin treatment, and have a particularly high risk of early recurrent events. Evolocumab, a proprotein convertase subtilisin/kexin type (PCSK9)‐inhibitor resulting in rapid, marked LDL‐C reduction, has been studied in hypercholesterolemic subjects without CVD and stabilized patients with CVD; the feasibility, safety, and efficacy of this treatment initiated in the acute phase of ACS remain unknown. We report the design of evolocumab for early reduction of LDL‐cholesterol levels in patients with ACS (EVOPACS), a phase‐3, multicenter, randomized, double‐blind, placebo‐controlled trial to assess the feasibility, safety, and LDL‐C‐lowering efficacy of evolocumab on top of atorvastatin 40 mg in patients with ACS. The primary endpoint is percent change in LDL‐C from baseline to 8 weeks. Secondary endpoints are adverse events and serious adverse events. Against a background of beneficial cardiovascular effects of statins beyond LDL‐C lowering and in view of preclinical evidence of similar effects of PCSK9 inhibition, the study will also address a variety of exploratory endpoints including the change in C‐reactive protein and other inflammatory biomarkers; platelet reactivity; and occurrence of contrast‐induced acute kidney injury and myocardial injury in patients undergoing cardiac catheterization. An intracoronary imaging sub‐study will investigate the change from baseline in the lipid core burden index in non‐culprit lesions, as assessed by serial near‐infrared spectroscopy. Recruitment began in January 2018 and enrollment of 308 patients is planned.
Keywords: acute coronary syndrome, lipidology, PCSK9 inhibitor
1. INTRODUCTION
Patients with acute coronary syndromes (ACS) have an increased risk of recurrent ischemic events.1 Lowering of low‐density lipoprotein cholesterol (LDL‐C) reduces cardiovascular morbidity and mortality in patients with atherosclerotic cardiovascular disease (CVD).2 In the context of ACS, statins—the accepted standard of care for LDL‐C lowering—result in early, sustained clinical benefit3, 4, 5 and their early initiation in patients presenting with ACS is recommended in current guidelines.1, 6 In patients who cannot achieve sufficient LDL‐C lowering with statins, nonstatin medications are a valuable add‐on option. Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors represent a promising approach for rapid, profound reduction in LDL‐C and incremental reduction of cardiovascular morbidity.7, 8, 9 As statins have a delayed onset of action before target levels are attained and the risk of event recurrence is highest during the first weeks after ACS, PCSK9 inhibitors may be of potential therapeutic interest in this particular setting. Two clinically approved anti‐PCSK9 antibodies, evolocumab and alirocumab, have been evaluated in hypercholesterolemic patients without CVD, stable ischemic heart disease, or stabilized patients with a history of ACS.7, 8 Treatment with PCSK9 inhibitors has not been studied in the very high‐risk, acute (within days) setting of ACS.
In addition to lowering LDL‐C, statins exert vasculoprotective effects including reduction of plasma levels of inflammatory biomarkers,10 stabilization of coronary plaque morphology,11, 12 attenuation of platelet reactivity,13 prevention of myocardial injury,14 and contrast‐induced kidney injury15 in patients undergoing coronary interventions. These favorable effects are of particular relevance in the acute context of ACS, as the residual ischemic risk and high event recurrence are likely related to the enhanced pro‐inflammatory and pro‐thrombotic milieu. The PCSK9 enzyme is expressed in atherosclerotic plaques,16 implicated in vascular inflammation,17 and may affect platelet function and thrombogenicity according to preclinical evidence.18 Whether these mechanistic effects might translate to favorable clinical effects of PCSK9 inhibition beyond the potent LDL‐lowering efficacy remains largely unknown.
Against this background, we designed a randomized, placebo‐controlled trial to assess the feasibility, safety, and LDL‐lowering efficacy of evolocumab administered within 24‐72 hours of symptom onset in patients presenting with ACS. Moreover, the EVOPACS study will assess the impact of evolocumab on a variety of exploratory endpoints including inflammatory biomarkers, platelet reactivity, coronary plaque composition, as well as myocardial and acute kidney injury following coronary interventions. Exploring these mechanistic aspects in the very high‐risk clinical setting of ACS may help identify additional early effects of PCSK9 inhibitors.
2. METHODS
2.1. Study design
EVOPACS is an investigator‐initiated, prospective, randomized, double‐blind, placebo‐controlled, parallel‐group, multi‐center trial (http://www.clinicaltrial.gov NCT03287609). The study evaluates the feasibility, safety and efficacy of evolocumab for very early LDL‐C lowering in ACS patients with LDL‐C levels that are either above guideline‐recommended targets,6 or are not projected to be reduced below these targets under high‐intensity statin therapy. Enrolment of 308 patients is planned at seven centers in Switzerland. The trial began enrollment in January 2018. The protocol was approved by the institutional ethics committees, and written informed consent will be obtained from all study participants.
Patients hospitalized for ACS, according to current definitions1 (non‐ST‐elevation myocardial infarction [NSTEMI] or unstable angina with onset <72 hours; or ST‐elevation myocardial infarction [STEMI] with onset <24 hours) are potentially eligible. Screening is performed in medically stabilized patients as soon as possible upon patient admission, and LDL‐C (fasting or non‐fasting) is measured to determine eligibility. Patients who fulfil all eligibility criteria and provide informed consent are enrolled and randomly assigned to either evolocumab 420 mg subcutaneously (sc) every 4 weeks or matching placebo sc. Clinical visits are scheduled at 4 and 8 weeks. The investigational product (IP) (evolocumab or placebo) is administered at the study site at two time points: at baseline (index ACS event) and after 4 weeks. The study endpoints are assessed at 8 weeks (Figure 1).
Figure 1.
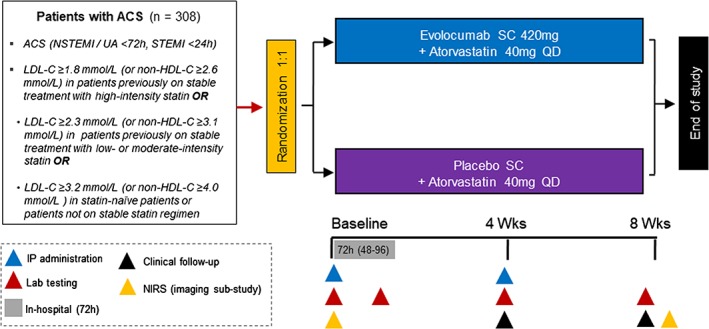
Study design for EVOPACS
Previous studies have shown regression of coronary plaque volume and stabilization of plaque composition following intensive statin treatment.19 In the YELLOW trial, short‐term (7‐week) treatment with high‐intensity statin reduced the lipid content of obstructive coronary lesions, as assessed by means of near‐infrared spectroscopy (NIRS).11 Against this background, an exploratory intracoronary imaging sub‐study will assess changes in the lipid core burden index (LCBI) of obstructive, nonculprit coronary lesions by means of serial NIRS performed at baseline and at 8 weeks. A subset of selected patients who are enrolled in preselected study centers; undergo clinically indicated percutaneous coronary intervention (PCI) of the culprit lesion of the index ACS; and fulfil all additional eligibility criteria will be enrolled in the sub‐study.
2.2. Inclusion and exclusion criteria
Inclusion criteria are summarized in Table 1. Briefly, LDL‐C at screening needs to be (a) above the guideline‐recommended target level of 1.8 mmol/L in patients previously treated with high‐intensity statin (atorvastatin ≥40 mg/day; rosuvastatin ≥20 mg/day; or simvastatin 80 mg/day) for at least 4 weeks prior to enrollment; (b) >2.3 mmol/L in patients previously treated with low/moderate intensity statin; or (c) >3.2 mmol/L in patients previously not treated with statin. The rationale for selecting these cut‐offs is that, for any status of prior statin treatment, LDL‐C is not projected to reach the guideline‐recommended target despite treatment with the study‐specific background statin (atorvastatin 40 mg). These cut‐offs are based on previous evidence of the LDL‐lowering efficacy of atorvastatin 40 mg either as newly‐initiated statin, or in the context of uptitration of a lower‐intensity statin regimen.6 The main exclusion criteria are listed in Table 2.
Table 1.
Inclusion criteria
| Male or female ≥18 years of age |
| Hospitalized for a recent ACS (unstable angina or NSTEMI within <72 hours, STEMI within <24 hours prior to screening) |
LDL‐C levels at screening:
|
| Ability to understand the requirements of the study and to provide informed consent |
Abbreviations: ACS, acute coronary syndromes; HDL‐C, high‐density lipoprotein; LDL‐C, lipoprotein cholesterol; NSTEMI, non‐ST‐elevation myocardial infarction; STEMI, ST‐elevation myocardial infarction.
Table 2.
Main exclusion criteria
| Unstable clinical status (hemodynamic or electrical instability) |
| Uncontrolled cardiac arrhythmia, defined as recurrent and symptomatic ventricular tachycardia or atrial fibrillation or flutter with rapid ventricular response not controlled by medications in the past 3 months prior to screening |
| Severe renal dysfunction, defined by estimated glomerular filtration rate < 30 mL/min/1.73 m2 |
| Active liver disease or hepatic dysfunction, either reported in patient medical record or defined by asparate aminotransferase (AST) or alanine aminotransferase (ALT) levels >3× the upper limit of normal |
| Known allergy to contrast medium, heparin, aspirin, ticagrelor, or prasugrel |
| Reported intolerance to atorvastatin (any dose) or statin intolerance defined by the following criteria: inability to tolerate at least two different statins; intolerance associated with confirmed, intolerable statin‐related adverse effect(s), or significant biomarker abnormalities; symptom or biomarker changes resolution or significant improvement upon dose decrease or discontinuation; and symptoms or biomarker changes not attributable to established predispositions |
| Patients who previously received evolocumab or another PCSK9 inhibitor |
| Patient who received cholesterol ester transfer protein inhibitors within 12 months prior to screening |
| Treatment with systemic steroids or systemic cyclosporine in the past 3 months (eg, intravenous, intramuscular, or per os) |
| Known active infection or major hematologic, metabolic, or endocrine dysfunction in the judgment of the Investigator |
| Current enrolment in another investigational device or drug study |
| Active malignancy requiring treatment |
| Pregnancy. For females of childbearing potential (age < 50 years and last menstruation <12 months prior to screening) who did not undergo tubal ligation, ovariectomy, or hysterectomy, pregnancy will be excluded by a pregnancy test prior to inclusion in the study. |
Abbreviation: PCSK9, proprotein convertase subtilisin/kexin type.
Additional criteria apply for the imaging sub‐study (Tables S1 and S2, Supporting Information), mainly including the presence of a nonculprit de novo coronary lesion with angiographically significant stenosis at baseline (defined by ≥50% angiographic diameter stenosis by visual estimation) that is deemed suitable for treatment in a staged PCI procedure after 8 weeks. Patients with three‐vessel or left‐main coronary artery disease (CAD) or those with a history of coronary artery bypass surgery are excluded from the sub‐study.
2.3. Study objectives and endpoints
The study's primary objective is to assess the effectiveness of evolocumab compared with placebo, administered during the acute phase of ACS, for the reduction of LDL‐C levels at 8 weeks in patients receiving atorvastatin 40 mg. Evaluation of the safety of evolocumab administration in the clinical setting of early ACS is the secondary objective. The primary endpoint is the percent change in LDL‐C from baseline to 8 weeks. The incidence of adverse events (AEs) and serious adverse events (SAEs) from baseline to 8 weeks is the secondary endpoint.
Exploratory endpoints include (a) lipid parameters: change from baseline in total cholesterol, high‐density lipoprotein (HDL)‐C, triglycerides, non‐HDL‐C, apolipoprotein B (ApoB), apolipoprotein A1 (ApoA1), lipoprotein(a), and percent of patients attaining the LDL‐C goal <1.8 mmol/L; (b) inflammatory biomarkers: change in high‐sensitivity C‐reactive protein (hs‐CRP) and other biomarkers of inflammation, percentage of patients attaining the target of hs‐CRP <2 mg/dL or the dual target of LDL‐C (<1.8 mmol/L) and hs‐CRP (<2 mg/dL) at 8 weeks; (c) incidence of contrast‐induced acute kidney injury (CI‐AKI) in patients who undergo diagnostic coronary angiography (with or without PCI); (d) change in high‐sensitivity troponin T from baseline to 72 hours; (e) change in platelet reactivity assessed by impedance aggregometry (Multiplate Analyzer) in whole blood stimulated with the ADP and TRAP tests between baseline and 8 weeks. Moreover, the incidence of centrally adjudicated events [death, cardiovascular death, myocardial infarction (reinfarction), hospitalization for recurrent ACS, hospitalization for heart failure, coronary revascularization, stroke, or transient ischemic attack] will be assessed by an independent Clinical Events Committee blinded to patient treatment allocation.
The objective of the exploratory imaging sub‐study is to evaluate the effect of evolocumab on the change in plaque lipid content as defined by serial NIRS, in nonculprit coronary lesions in patients who present with ACS and receive guideline‐recommended high‐intensity statin therapy (atorvastatin 40 mg). The exploratory endpoints of the sub‐study are change in total LCBI, and change in maximum LCBI in any 4‐mm segment (maxLCBI4mm) from baseline to 8 weeks.
2.4. Treatments and key procedures
Patients enrolled in the study will be treated for the ACS event in accordance with current guidelines.1, 20 Patient management may include either medical treatment alone without invasive management; or coronary angiography if deemed clinically applicable, possibly followed by PCI or coronary artery bypass surgery. Dual antiplatelet therapy (DAPT) consisting of aspirin and a P2Y12 inhibitor will be started as early as possible in all patients. Consistent with current recommendations and in order to reduce the confounding effect of differing antiplatelet treatments on the exploratory endpoint of platelet function, ticagrelor will be the P2Y12 inhibitor of choice, unless contra‐indicated according to clinical judgment. DAPT will be continued at maintenance doses (aspirin ≤100 mg/day, ticagrelor 90 mg twice daily) for a duration that is determined by treating physicians, typically lifelong for aspirin and 12 months for ticagrelor. Guideline‐recommended medical therapy, including beta‐blockers and ACE inhibitors will be administered as appropriate.
After patient enrollment, randomization (based on computer‐generated random numbers and using a central randomization system) is done in a double‐blind fashion with 1:1 allocation. Randomization is stratified according to (a) study center and (b) presence of unchanged statin treatment within ≥4 weeks prior to enrollment. Study patients, investigators, and study site personnel remain blinded to treatment allocation during the entire study duration.
The IP is administered in three consecutive subcutaneous injections at two time‐points (Figure 1). At baseline, the IP is administered as early as possible following randomization. Among patients who undergo clinically indicated coronary angiography (with or without revascularization), IP administration should occur prior to the index coronary angiography whenever possible, but IP administration after coronary angiography is also possible. Blood draws are performed at baseline for measurements of the primary as well as exploratory endpoints (“T0 measurements”). A subsequent blood draw is performed 72 hours later (within an allowed time window of 48‐96 hours) for the measurement of kidney function (assessment of CI‐AKI); high‐sensitivity troponin T (assessment of PCI‐related myocardial injury); and platelet function testing (“T72 measurements”). The second IP administration is performed during the 4‐week visit, and blood draws are performed at the 4‐ and 8‐week visits (Figure 1). Patients are encouraged to complete the planned visits regardless of their adherence to IP administration (intention‐to‐treat [ITT] approach).
All patients will receive background treatment with atorvastatin 40 mg/day throughout the study period. This statin regimen is consistent with current guidelines recommending a high‐intensity statin in patients presenting with ACS.6 Because a proportion of patients are expected to be statin‐naïve at the time of enrollment, and because—unlike previous studies with PCSK9 inhibitors—there is no run‐in period for optimizing statin treatment, we refrained from selecting the highest (80 mg) dose of atorvastatin in order to mitigate the potential of statin‐related adverse effects and increase compliance. If patients were on a different statin regimen prior to enrollment, this is discontinued and replaced by atorvastatin 40 mg. For patients who had been on a more potent statin regimen prior to enrollment (ie, atorvastatin >40 mg or rosuvastatin >20 mg/day), the background therapy throughout the study period will be atorvastatin 80 mg/day. Dose reduction or cessation of atorvastatin is allowed in the event of postulated side effects (eg, muscular pain) or elevation of creatine kinase levels.
For the intracoronary imaging sub‐study, baseline NIRS is performed immediately following successful PCI of the culprit lesion at the time of the index ACS event. Follow‐up NIRS imaging of the same vessel is performed at 8 weeks, prior to attempting a staged PCI in the nonculprit lesion. NIRS recordings will be analyzed by investigators unaware of treatment allocation.
2.5. Statistical analyses
This is a superiority trial powered for the primary endpoint. Assuming an average percentage LDL‐C reduction of 30% in the control arm (placebo sc plus atorvastatin 40 mg) and 44% in the active treatment arm (evolocumab sc plus atorvastatin 40 mg), and adopting a common SD of 36%, a total sample size of 280 patients would provide statistical power of 90% at a significance level of 5% for a two‐sided t test. Anticipating a dropout rate of 10% at 8 weeks, a total of 308 patients should be recruited (154 per arm). The imaging sub‐study is not powered and will aim at enrolling 50 patients.
2.5.1. Analysis of primary endpoints
Efficacy and safety analyses will be performed on the full analysis set, which includes all randomized patients who have received at least one dose of the study IP. The primary analysis will be performed when all randomized subjects either have completed the scheduled study visits or have withdrawn early from the study; at that time point the study will be unblinded. Analysis of the primary endpoint will be conducted with a linear mixed effects model adjusting for the following stratification factors: (a) presence of unchanged statin treatment within ≥4 weeks prior to enrollment as a fixed effect; and (b) study center as a random effect. Analysis will be based on the ITT principle and patients will contribute to the group to which they were randomized. Patients with missing data for the primary end‐point will be excluded from the primary analysis. A key sensitivity analysis for the primary endpoint, using multiple imputation, will be carried out to assign the primary endpoint if follow‐up (week 8) values are missing in more than 5% of patients. The superiority of evolocumab vs placebo will be assessed for all efficacy endpoints, without applying multiplicity adjustments.
2.5.2. Safety analyses
For analyses of safety outcomes, missing data will not be imputed, and AEs will be summarized by treatment group using descriptive statistics. For analyses of clinical endpoints, patient incidence of events will be summarized for each randomized treatment group.
2.5.3. Analyses of exploratory endpoints
A similar approach as for the primary endpoint will be used for the analysis of continuous exploratory outcomes defined as percentage changes. For continuous exploratory outcomes defined as nominal changes, mixed effects (with the study center as a random effect) linear models will be employed with the follow‐up measurement as a response, and with the following explanatory variables: the corresponding baseline measurement; arm (binary variable); and presence of unchanged statin treatment within ≥4 weeks prior to enrollment (binary variable). For binary exploratory endpoints, differences will be assessed using Fisher's exact test.
2.5.4. Covariates and subgroups
The following baseline covariates may be used for subgroup or covariate analyses: unchanged statin treatment within ≥4 weeks prior to enrolment (yes/no); clinical presentation with STEMI vs NSTE‐ACS; age: <65 vs ≥65 years; gender; baseline LDL‐C: <median vs ≥median. Sensitivity analyses will be performed for exploratory outcomes influenced by the performance of coronary angiography (with or without PCI). More specifically, sensitivity analysis for the exploratory endpoint CI‐AKI will be performed in the subgroup of patients who undergo coronary angiography at baseline, and will be stratified in relation to IP administration before vs after coronary angiography. Similarly, sensitivity analysis for the exploratory endpoint change in hs‐troponin T will be performed in the subgroup of patients who undergo PCI at baseline and will be stratified in relation to IP administration before vs after PCI.
The study protocol is a collaborative effort of the Steering Committee (K.C.K., S.W., and F.M.). The Clinical Trials Unit Bern, an academic research organization, will monitor the progress of the trial, have full access to the complete database, and independently generate all analyses. The Steering Committee will be responsible for submitting the results of the study for publication. The authors are solely responsible for drafting all related manuscripts and for their final content. The study is funded by Amgen.
3. RESULTS
On October 25, 2018, a total of 180 patients had been enrolled in the study (mean age 61.1 ± 11.8 years, 17% women). The majority of patients (77%) had been on no statin prior to enrollment, whereas 12% had been on low‐ or moderate‐intensity, and 11% on high‐intensity statin. The index ACS event was NSTE‐ACS in 65% and STEMI in 35% of patients.
4. DISCUSSION
Patients with ACS remain at a high risk of recurrent events, namely MI, stent thrombosis, and repeat revascularization, despite effective contemporary treatments1; the residual ischemic risk may be related to the culprit lesion responsible for the index event, disease progression originating from nonculprit lesions,21 or the increased inflammatory and platelet activation in the acute setting of ACS.22 Lowering LDL‐C levels with statins improves cardiovascular outcomes in patients with established CVD,2 and achievement of very low levels (below guideline‐recommended targets) with more intensive statin therapy or with the addition of nonstatin medications results in an incremental reduction in nonfatal events.2, 7, 8 In the very high‐risk setting of ACS, the clinical benefit conferred by early implementation of intensive statin therapy is observed in the very early phase following the index ACS event—within 4 weeks of treatment in the PROVE‐IT trial3 and within 16 weeks in the MIRACLE trial.4 However, statins have a delayed onset of action before target levels are attained which may be of importance, as the risk of event recurrence is highest during the first weeks after the acute phase of ACS. Moreover, many patients with ACS cannot achieve recommended targets of LDL‐C despite potent statin treatment.23 Thus, there is an unmet need for patients who present with ACS and are not likely to achieve adequate LDL‐C reduction with current oral lipid‐modifying medications. Pharmacologic inhibition of PCSK9 has emerged as an effective treatment for properly selected patients as it results in rapid, profound reductions in LDL‐C levels independently of background lipid‐lowering therapies. Treatment with PCSK9 inhibitors initiated within a median of 3 months or 3.4 years after an ACS has been investigated in two large outcomes studies of alirocumab and evolocumab, respectively7, 8 (Figure 2). The feasibility and usefulness of this treatment in the acute setting of ACS (within hours/days of symptom onset) has not been previously investigated.
Figure 2.
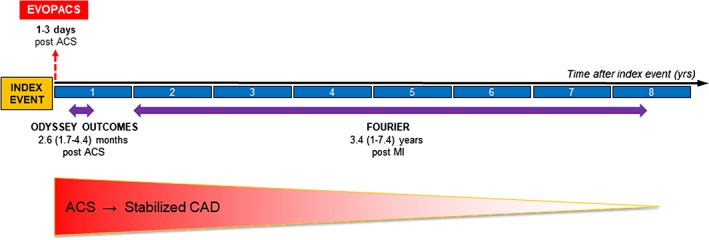
Timing of patient enrollment following the index acute coronary event in EVOPACS (red dotted line) vs the FOURIER and ODYSSEY OUTCOMES trials. ACS indicates acute coronary syndrome; CAD, coronary artery disease; and MI, myocardial infarction
For patients with inadequate reduction in LDL‐C levels despite statin treatment, currently recommended approaches include combination with nonstatin medications.6 Ezetimibe, when added to statins, results in 20% incremental LDL‐C lowering and a small reduction in cardiovascular morbidity.24 In patients requiring a greater magnitude of LDL‐C lowering to achieve recommended targets, PCSK9 inhibitors are a reasonable therapeutic option as they can lower LDL‐C by >50%. Initiation of PCKS9 inhibitor treatment in the acute phase of ACS has not been studied previously, and the EVOPACS trial was designed to fill this gap in evidence by addressing the safety and LDL‐C‐lowering efficacy of evolocumab in this setting. While centrally adjudicated cardiovascular outcomes are a prespecified, exploratory endpoint in EVOPACS, the study is not powered to assess clinical outcomes. Larger studies will be required to evaluate whether the impact of PCK9 inhibitors on cardiovascular morbidity, as reported in patients with more stable manifestations of atherosclerotic CVD,7, 8 holds true in the acute phase of ACS.
In animal studies, hepatic mRNA expression, and plasma levels of PCSK9 increase over time following acute MI.25 Consistently, plasma levels of PCSK9 are increased in patients with MI compared with stable CAD.26 Pharmacokinetic studies with PCSK9 inhibitors have shown rapid reductions in PCSK9 levels, immediately followed by consistent reductions in LDL‐C levels.27 If administered during the acute phase of ACS, as in the present study, the effect of PCSK9 inhibitors would be expected to occur at the time point of peak levels of the PCSK9 enzyme, thereby potentially enabling early‐onset and sustained reduction of LDL‐C levels.
Previous studies with PCSK9 inhibitors included stable, primary‐ or secondary‐prevention patients and typically applied a run‐in period for up‐titration of statins, to ensure the inclusion of patients with elevated LDL‐C levels despite maximally tolerated statin therapy. EVOPACS will assess PCSK9‐inhibitor treatment initiated in the acute phase of ACS; based on the study design, a run‐in period is not possible. However, the LDL‐C cut‐offs defining eligibility for study participation are based on robust evidence regarding the LDL‐C‐lowering potential of atorvastatin 40 mg,6 either as a newly initiated treatment (in statin‐naïve patients) or in the context of treatment escalation (in patients treated with lower‐intensity statin regimens prior to enrolment). Despite inherent limitations of these assumptions, our approach will ensure with reasonable certainty that patients with LDL‐C levels above currently recommended targets despite intensive (pre‐existing, or newly established) statin treatment are enrolled. Regarding tolerability of high‐dose atorvastatin, although subjects with reported statin intolerance are excluded from the study, it is possible that some of the enrolled patients (especially those never exposed to a statin prior to enrollment) may develop statin‐related side effects. However, considering the short duration of the study and the usually more delayed pattern of onset of reported side effects with statins,28 it is reasonable to expect that statin intolerance (possibly requiring de‐escalation or cessation of the background statin treatment) will not occur frequently and thus will not have a substantive confounding effect on the study outcomes.
Statins and ezetimibe reduce levels of inflammatory biomarkers,10, 29 and several studies have reported improved cardiovascular outcomes in the presence of hs‐CRP reduction.30 In experimental atherosclerosis, alirocumab reduces monocyte recruitment and inflammatory infiltrations.31 In humans, plasma levels of PCSK9 correlate with levels of CRP, and knockdown of PCSK9 attenuates the expression of proinflammatory genes in macrophages.32 In the context of ACS, in particular, there appears to be a cross‐reaction between PCSK9 and oxidized LDL‐C that leads to a mutually amplified proinflammatory response.33 Although previous studies showed no significant effect of PCSK9 inhibitors on CRP levels,34 these results need to be interpreted in view of previous study populations including patients with familial hypercholesterolemia (in whom markedly increased LDL‐C levels are not coupled with systemic inflammatory activation35) or primary‐prevention patients without CAD and without elevated baseline levels of CRP. Along these lines, studies of statins have reported modest CRP lowering in primary‐prevention or stable CAD patients (between 14% and 36%)36 vs substantial (8‐ to 10‐fold) reductions in patients with ACS.10, 29 Against this background, the EVOPACS study will test the hypothesis that evolocumab suppresses inflammation and reduce levels of inflammatory biomarkers in patients with ACS—an acute setting characterized by marked inflammatory activation.
Despite potent antiplatelet therapies, residual platelet reactivity may occur in a proportion of treated ACS patients, thus increasing the risk of recurrent ischemic events.37 Multiple pathways are involved in platelet activation and fibrin synthesis in this setting. High levels of LDL‐C (particularly oxidized LDL) correlate with plasma fibrinogen levels and platelet activation.38 Statins inhibit platelet aggregation, reduce platelet‐mediated thrombus formation,39 augment the pharmacodynamic response to antiplatelet agents, and attenuate platelet reactivity in patients undergoing PCI.13 Notably, the PCSK9 enzyme is associated with upregulation of tissue factor expression and increased lipoprotein(a) levels,40 thus promoting a prothrombotic milieu, and plasma levels of PCSK9 correlate with increased platelet count and activation41 in ACS patients treated with ticagrelor or prasugrel. By increasing LDL‐C clearance from the circulation and reducing lipoprotein(a), one can speculate that PCSK9 inhibitors might attenuate platelet reactivity induced by native and oxidized LDL‐C.19 This putative platelet‐inhibitory effect of PCSK9 inhibitors has not been previously explored and will be tested in this study.
Patients with ACS undergoing coronary angiography are at high risk of developing CI‐AKI, a relevant complication that adversely affects clinical prognosis. Statins reduce the risk CI‐AKI, and this protective effect appears to be in part unrelated to their lipid‐lowering potential. PCSK9 inhibitors exert antioxidant, anti‐inflammatory,17 and antiapoptotic properties42 similar to statins according to preclinical investigations. The EVOPACS study will explore, in a modestly sized ACS population, whether these mechanistic effects translate into prevention of renal damage in patients exposed to iodinated contrast medium.
4.1. Conclusion
Patients with ACS are at particularly high cardiovascular risk and require aggressive risk factor management. Because many ACS patients cannot achieve adequate LDL‐C lowering with statins, substantial residual risk remains. Moreover, in view of the delayed onset of action of statins and the early risk of recurrent events following an ACS, early initiation of PCSK9‐inhibitors (ie, rapidly acting, highly potent LDL‐lowering agents) may be of clinical value in this setting. The EVOPACS trial will provide new insights into the safety and efficacy of evolocumab in the acute setting of ACS, and thus improve our understanding of the potential role of PCSK9 inhibition in the management of these patients.43 In addition, the study will explore mechanistic evidence regarding potential beneficial effects of PCSK9 inhibition beyond the primary impact on LDL‐C in the ACS setting.
CONFLICTS OF INTEREST
F.M. has received (through the Geneva University Hospital) research grants from Amgen, Sanofi, and MSD for his work in the lipid field. S.W. reports research grants to the institution from Abbott, Amgen, Bayer, Boston Scientific, Biotronik, Medtronic, Edwards Lifesciences, St Jude, Terumo. C.M. has received research grants to institution from Abbott, Beckman Coulter, Biomerieux, Brahms, Roche, Siemens, Singulex, Sphingotec, and speakers/consulting honoraria from Amgen, Astra Zeneca, Boehringer Ingelheim, Duke Research Institute, Roche, Sanofi, Siemens, and Singulex. L.R. received research grants from Sanofi/Regeneron and speaker fees from Sanofi/Regeneron and Amgen. The other authors have no conflict of interest to declare.
Supporting information
Table S1 Additional inclusion criteria for the intracoronary imaging substudy.
Table S2. Additional exclusion criteria for the intracoronary imaging substudy.
Koskinas KC, Windecker S, Buhayer A, et al. Design of the randomized, placebo‐controlled evolocumab for early reduction of LDL‐cholesterol levels in patients with acute coronary syndromes (EVOPACS) trial. Clin Cardiol. 2018;41:1513–1520. 10.1002/clc.23112
Funding information Amgen
REFERENCES
- 1. Roffi M, Patrono C, Collet JP, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation: task force for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:267‐315. [DOI] [PubMed] [Google Scholar]
- 2. Koskinas KC, Siontis GCM, Piccolo R, et al. Effect of statins and non‐statin LDL‐lowering medications on cardiovascular outcomes in secondary prevention: a meta‐analysis of randomized trials. Eur Heart J. 2018;39:1172‐1180. [DOI] [PubMed] [Google Scholar]
- 3. Ray KK, Cannon CP, McCabe CH, et al. Early and late benefits of high‐dose atorvastatin in patients with acute coronary syndromes. Results from the PROVE IT‐TIMI 22 trial. J Am Coll Cardiol. 2005;46:1405‐1410. [DOI] [PubMed] [Google Scholar]
- 4. Schwartz GG, Olsson AG, Ezekowitz MD, et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes. JAMA. 2001;285:1711‐1718. [DOI] [PubMed] [Google Scholar]
- 5. Berwanger O, Santucci EV, de Barros E, et al. Effect of loading dose of atorvastatin prior to planned percutaneous coronary intervention on major adverse cardiovascular events in acute coronary syndrome: the SECURE‐PCI randomized clinical trial. JAMA. 2018;319:1331‐1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Catapano AL, Graham I, De Backer G, et al. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J. 2016;37:2999‐3058. [DOI] [PubMed] [Google Scholar]
- 7. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713‐1722. [DOI] [PubMed] [Google Scholar]
- 8. Schwartz GG, Szarek M, Bhatt DL, et al. The ODYSSEY OUTCOMES trial: Topline results. Alirocumab in patients after acute coronary syndrome. Presented at the American College of Cardiology 67th Scientific Sessions, March 10, 2018.
- 9. Baum SJ, Toth PP, Underberg JA, et al. PCSK9 inhibitor access barriers‐issues and recommendations: improving the access process for patients, clinicians and payers. Clin Cardiol. 2017;40:243‐254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ridker PM, Morrow DA, Rose LM, et al. Relative efficacy of atorvastatin 80 mg and pravastatin 40 mg in achieving the dual goals of low‐density lipoprotein cholesterol <70 mg/dl and C‐reactive protein <2 mg/l: an analysis of the PROVE‐IT TIMI‐22 trial. J Am Coll Cardiol. 2005;45:1644‐1648. [DOI] [PubMed] [Google Scholar]
- 11. Kini AS, Baber U, Kovacic JC, et al. Changes in plaque lipid content after short‐term intensive versus standard statin therapy: the YELLOW trial (reduction in yellow plaque by aggressive lipid‐lowering therapy). J Am Coll Cardiol. 2013;62:21‐29. [DOI] [PubMed] [Google Scholar]
- 12. Komukai K, Kubo T, Kitabata H, et al. Effect of atorvastatin therapy on fibrous cap thickness in coronary atherosclerotic plaque as assessed by optical coherence tomography: the EASY‐FIT study. J Am Coll Cardiol. 2014;64:2207‐2217. [DOI] [PubMed] [Google Scholar]
- 13. Leoncini M, Toso A, Maioli M, et al. High‐dose atorvastatin on the pharmacodynamic effects of double‐dose clopidogrel in patients undergoing percutaneous coronary interventions: The ACHIDO (Atorvastatin and Clopidogrel HIgh DOse in stable patients with residual high platelet activity) study. JACC Cardiovasc Interv. 2013;6:169‐179. [DOI] [PubMed] [Google Scholar]
- 14. Zhang F, Dong L, Ge J. Effect of statins pretreatment on periprocedural myocardial infarction in patients undergoing percutaneous coronary intervention: a meta‐analysis. Ann Med. 2010;42:171‐177. [DOI] [PubMed] [Google Scholar]
- 15. Quintavalle C, Fiore D, De Micco F, et al. Impact of a high loading dose of atorvastatin on contrast‐induced acute kidney injury. Circulation. 2012;126:3008‐3016. [DOI] [PubMed] [Google Scholar]
- 16. Ferri N, Tibolla G, Pirillo A, et al. Proprotein convertase subtilisin kexin type 9 (PCSK9) secreted by cultured smooth muscle cells reduces macrophages LDLR levels. Atherosclerosis. 2012;220:381‐386. [DOI] [PubMed] [Google Scholar]
- 17. Bergeron N, Phan BA, Ding Y, et al. Proprotein convertase subtilisin/kexin type 9 inhibition: a new therapeutic mechanism for reducing cardiovascular disease risk. Circulation. 2015;132:1648‐1666. [DOI] [PubMed] [Google Scholar]
- 18. Navarese EP, Kolodziejczak M, Kereiakes DJ, et al. Proprotein convertase subtilisin/kexin type 9 monoclonal antibodies for acute coronary syndrome: a narrative review. Ann Intern Med. 2016;164:600‐607. [DOI] [PubMed] [Google Scholar]
- 19. Koskinas KC, Ughi GJ, Windecker S, et al. Intracoronary imaging of coronary atherosclerosis: validation for diagnosis, prognosis and treatment. Eur Heart J. 2016;37:524‐535. [DOI] [PubMed] [Google Scholar]
- 20. Windecker S, Kolh P, Alfonso F, et al. 2014 ESC/EACTS guidelines on myocardial revascularization: the task force on myocardial revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio‐Thoracic Surgery (EACTS). Eur Heart J. 2014;35:2541‐2619. [DOI] [PubMed] [Google Scholar]
- 21. Stone GW, Maehara A, Lansky AJ, et al. A prospective natural‐history study of coronary atherosclerosis. N Engl J Med. 2011;364:226‐235. [DOI] [PubMed] [Google Scholar]
- 22. Libby P. Mechanisms of the acute coronary syndromes and their implications for therapy. N Engl J Med. 2013;368:2004‐2013. [DOI] [PubMed] [Google Scholar]
- 23. Reiner Ž, De Backer G, Fras Z, et al. Lipid lowering drug therapy in patients with coronary heart disease from 24 European countries – findings from the EUROASPIRE IV survey. Atherosclerosis. 2016;246:243‐250. [DOI] [PubMed] [Google Scholar]
- 24. Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387‐2397. [DOI] [PubMed] [Google Scholar]
- 25. Zhang Y, Liu J, Li S, et al. Proprotein convertase subtilisin/kexin type 9 expression is transiently upregulated in the acute period of myocardial infarction in rat. BMC Cardiovasc Disord. 2014;14:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Almontashiri NA, Vilmundarson RO, Ghasemzadeh N, et al. Plasma PCSK9 levels are elevated with acute myocardial infarction in two independent retrospective angiographic studies. PLoS One. 2014;9:e106294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dias CS, Shaywitz AJ, Wasserman SM, et al. Effects of AMG 145 on low‐density lipoprotein cholesterol levels: results from 2 randomized, double‐blind, placebo‐controlled, ascending‐dose phase 1 studies in healthy volunteers and hypercholesterolemic subjects on statins. J Am Coll Cardiol. 2012;60:1888‐1898. [DOI] [PubMed] [Google Scholar]
- 28. Bruckert E, Hayem G, Dejager S, et al. Mild to moderate muscular symptoms with high‐dosage statin therapy in hyperlipidemic patients—the PRIMO study. Cardiovasc Drugs Ther. 2005;19:403‐414. [DOI] [PubMed] [Google Scholar]
- 29. Bohula EA, Giugliano RP, Cannon CP, et al. Achievement of dual low‐density lipoprotein cholesterol and high‐sensitivity C‐reactive protein targets more frequent with the addition of ezetimibe to simvastatin and associated with better outcomes in IMPROVE‐IT. Circulation. 2015;132:1224‐1233. [DOI] [PubMed] [Google Scholar]
- 30. Ridker PM, Cannon CP, Morrow D, et al. C‐reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20‐28. [DOI] [PubMed] [Google Scholar]
- 31. Kühnast S, van der Hoorn JW, Pieterman EJ, et al. Alirocumab inhibits atherosclerosis, improves the plaque morphology, and enhances the effects of a statin. J Lipid Res. 2014;55:2103‐2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tang Z, Jiang L, Peng J, et al. PCSK9 siRNA suppresses the inflammatory response induced by oxLDL through inhibition of NF‐κB activation in THP‐1‐derived macrophages. Int J Mol Med. 2012;30:931‐938. [DOI] [PubMed] [Google Scholar]
- 33. Ding Z, Liu S, Wang X, Deng X, et al. Cross‐talk between LOX‐1 and PCSK9 in vascular tissues. Cardiovasc Res. 2015;107:556‐567. [DOI] [PubMed] [Google Scholar]
- 34. Sahebkar A, Di Giosia P, Stamerra CA, et al. Effect of monoclonal antibodies to PCSK9 on high‐sensitivity C‐reactive protein levels: a meta‐analysis of 16 randomized controlled treatment arms. Br J Clin Pharmacol. 2016;81:1175‐1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Varbo A, Benn M, Tybjærg‐Hansen A, Nordestgaard BG. Elevated remnant cholesterol causes both low‐grade inflammation and ischemic heart disease, whereas elevated low‐density lipoprotein cholesterol causes ischemic heart disease without inflammation. Circulation. 2013;128:1298‐1309. [DOI] [PubMed] [Google Scholar]
- 36. Albert MA, Danielson E, Rifai N, Ridker PM. Effect of statin therapy on C‐reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA. 2001;286:64‐70. [DOI] [PubMed] [Google Scholar]
- 37. Tantry US, Bonello L, Aradi D, et al. Consensus and update on the definition of on‐treatment platelet reactivity to adenosine diphosphate associated with ischemia and bleeding. J Am Coll Cardiol. 2013;62:2261‐2273. [DOI] [PubMed] [Google Scholar]
- 38. Podrez EA, Byzova TV, Febbraio M, et al. Platelet CD36 links hyperlipidemia, oxidant stress and a prothrombotic phenotype. Nat Med. 2007;13:1086‐1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Piorkowski M, Fischer S, Stellbaum C, et al. Treatment with ezetimibe plus low‐dose atorvastatin compared with higher‐dose atorvastatin alone: is sufficient cholesterol‐lowering enough to inhibit platelets? J Am Coll Cardiol. 2007;49:1035‐1042. [DOI] [PubMed] [Google Scholar]
- 40. Romagnuolo R, Scipione CA, Boffa MB, et al. Lipoprotein(a) catabolism is regulated by proprotein convertase subtilisin/kexin type 9 through the low density lipoprotein receptor. J Biol Chem. 2015;290:11649‐11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Navarese EP, Kolodziejczak M, Winter MP, et al. Association of PCSK9 with platelet reactivity in patients with acute coronary syndrome treated with prasugrel or ticagrelor: the PCSK9‐REACT study. Int J Cardiol. 2017;227:644‐649. [DOI] [PubMed] [Google Scholar]
- 42. Wu CY, Tang ZH, Jiang L, et al. PCSK9 siRNA inhibits HUVEC apoptosis induced by ox‐LDL via Bcl/Bax‐caspase9‐caspase3 pathway. Mol Cell Biochem. 2012;359:347‐358. [DOI] [PubMed] [Google Scholar]
- 43. Baum SJ, Cannon CP. PCSK9 inhibitor valuation: a science‐based review of the two recent models. Clin Cardiol. 2018;41:544‐550. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Additional inclusion criteria for the intracoronary imaging substudy.
Table S2. Additional exclusion criteria for the intracoronary imaging substudy.


