Summary
Aims
Despite therapeutic advances in glioma management including surgery, radiation, and chemotherapy, the improvement of patient outcome is far from satisfactory. Nucleolar and spindle‐associated protein 1 (NUSAP1) is an important functional protein during mitosis, and its abnormal expression is implicated in progression of different types of tumors. However, the role of NUSAP1 in gliomas remains unclear.
Methods
NUSAP1 expression in gliomas with different grades was investigated based on GEO glioma datasets. Kaplan‐Meier survival analysis was used to evaluate its prognostic significance. In vitro assays were also performed to evaluate effects of NUSAP1 on malignant phenotypes of glioma cells by silencing NUSAP1.
Results
NUSAP1 expression was correlated not only with glioma grade but also with prognosis of glioma patients. NUSAP1 depletion suppressed proliferation of U251 cells by inducing cell cycle arrest at G2/M phase and apoptosis. NUSAP1 depletion rendered U251 cells impaired migratory ability as well.
Conclusion
NUSAP1 is a potential prognosis marker for glioma patients and therapeutic strategies targeting NUSAP1 might hold promise in improving glioma treatment.
Keywords: gene expression profile, Glioma, NUSAP1, pathological grade, prognosis
1. INTRODUCTION
Gliomas are the most prevalent primary tumors of the central nervous system (CNS) with certain glial cell‐like characteristics.1, 2 According to pathologic evaluation, gliomas are classified into four grades from Grade I to IV, depending on which the survival time of glioma patients varies strikingly.3 For example, patients with pilocytic astrocytoma (Grade I) had a five‐year survival rate of 94.4%, much higher than 4.7%, the five‐year survival rate of patients with glioblastoma multiforme (GBM, Grade IV).4 Despite therapeutic advances in surgery, radiation, and pharmacotherapy, the median survival for GBM patients is only 14 months.5, 6, 7 With the identification of specific genetic mutations as “driver factors” of gliomagenesis, molecular parameters have been introduced to refine the diagnosis of gliomas such as IDH mutation, 1p/19q codeletion, and H3 K27M mutation, along with pathological parameters.1, 8 However, current glioma management is unsatisfactory in terms of survival gain. Therefore, it is critical to investigate mechanisms underlying glioma initiation and malignant evolution and to dig out biomarkers with targetable potential for attenuating the lethality of glioma and improving patients' prognosis.
Nucleolar and spindle‐associated protein 1 (NUSAP1) is involved in regulating the assembly of spindle and maintaining normal cytokinesis by virtue of its microtubule‐binding and DNA‐binding domains, which renders it an important regulator in mitosis and cell proliferation.9, 10, 11 The cellular expression and distribution of NUSAP1 is under rigorous control. Dysregulation of NUSAP1 has been identified in different tumors including hepatocellular carcinoma, lung adenocarcinoma, and renal cell carcinoma.12, 13, 14 NUSAP1 upregulation is associated with malignant progression of melanoma and oral squamous cell carcinoma.15, 16 Moreover, NUSAP1 may confer resistance to DNA‐damaging drugs due to its participation in DNA double‐strand break repair.17 However, the role of NUSAP1 in glioma development and its association with glioma grade remains unclear.
In this study, we evaluated the expression of NUSAP1 in gliomas with varying grades using microarray data, immunohistochemistry data, and performing reverse transcription‐PCR (RT‐PCR) analysis. The prognostic significance of NUSAP1 in gliomas was investigated. We also conducted in vitro loss of function experiments to explore the effects of NUSAP1 on phenotypes of glioma cells. Our data showed that NUSAP1 expression was associated not only with glioma grade but also with prognosis of glioma patients, and that NUSAP1 depletion would suppress proliferation of glioma cells by inducing cell cycle arrest and apoptosis.
2. MATERIALS AND METHODS
2.1. Transcriptome data
Three glioma microarray datasets GSE4290, GSE4412, and GSE43378 were downloaded from the GEO database. The Cancer Genome Atlas (TCGA) RNA‐seq data of GBM and corresponding clinical data were downloaded from UCSC Cancer Browser (https://genome-cancer.ucsc.edu/). Samples without complete clinical information were excluded.
2.2. Patients and tissue specimens
Tumor samples, including 3 oligodendrogliomas, 41 astrocytomas, and 10 GBMs, were collected from glioma patients who underwent surgical resection between 2014 and 2016 at Xiangya Hospital of Central South University. All tissue samples were immediately snap‐frozen and stored in liquid nitrogen after resection until further use. This study was approved by the Institutional Review Board.
2.3. Cell culture
U251 and U87 cells were cultured in Dulbecco's modified Eagle's medium (Gibco, USA) supplemented with 10% (v/v) fetal bovine serum (Gibco) at 37°C in a humidified incubator with 5% CO2. When reaching approximate 70% confluence, cells were digested and seeded in six‐well plates for siRNA transfection at a density of ~2 × 105 cells per well.
2.4. siRNA transfection
Cells at ~70% confluence were transfected with siRNAs targeting NUSAP1 (Ribobio, China) or negative control siRNAs (Santa Cruz, USA) at a final concentration of 10 nmol L−1 using Lipofectamine 2000 (Invitrogen, USA) as per the manufacturer's instructions. The siRNA sequences for NUSAP1 were listed in Table S1.18 Twenty‐four hours or forty‐eight hours after transfection, cells were subjected to either RNA extraction or further assays, or protein extraction, respectively.
2.5. Real‐Time RT‐PCR
Total RNA of glioma cell lines or tissues was isolated with RNAiso Plus (Takara, China) and reverse‐transcribed into cDNA by PrimeScript™ RT reagent Kit (Takara). The PCR amplification procedure using the LightCycler® 480 Instrument (Roche, Switzerland) was described previously.19 Briefly, 2 μL cDNA template was amplified in a 20‐μL reaction mix system of SYBR Green (Takara) under the following conditions: 30 s at 95°C, followed by 40 cycles of three reaction steps with different temperatures (5 s at 95°C, 30 s at 55°C, and 30 s at 72°C). The mRNA level of NUSAP1 was normalized to that of β‐actin using the 2−ΔΔCt method unless otherwise indicated. Sequences of primers (synthesized by Biosune, China) used in this study were listed as follows: NUSAP1, Forward: 5′‐AGCCCATCAATAAGGGAGGG‐3′, Reverse: 5′‐ACCTGAC ACCCGTTTTAGCTG‐3′; β‐actin, Forward: 5′‐TGCGTGACATTAAGGAGAA‐3′, Reverse: 5′‐AAGGAAGGCTGGAAGAGT‐3′.
2.6. Western blotting
Cells were lysed for protein extraction using PMSF‐containing RIPA lysis solution (Beyotime, China). Protein extracts were electrophoresed on a 10% SDS‐PAGE gel and transferred onto a nitrocellulose membrane. The blotted membrane was then blocked in 5% skimmed milk and incubated with corresponding primary antibodies overnight at 4°C. The primary antibodies against NUSAP1 (Proteintech Group, USA) and GAPDH (Sigma‐Aldrich, USA) were diluted and used following the manufactures' recommendations. The protein bands were visualized via the ChemiDoc XRS+ image analyzer (Bio‐Rad, USA) shortly after soaked with dilutions of the enhanced chemiluminescence reagent (GE Healthcare, UK).
2.7. MTS and colony‐formation assays for cell proliferation
U251 cells transfected with siRNA were seeded in 96‐well plates at a density of 1000 cells/well and cultured for from 24 to 96 h. At an interval of 24 h, cells in each well were incubated with 100 μL 10% MTS (MTS: DMEM, v/v) for 1 h, and the absorbance at 490 nm was measured by the BioTek®Eon (Synergy™, USA). For colony‐formation assay, U251 cells were plated in 6‐well plates at a density of 500 cells/well and cultured for 15 days with culture media changed every 3 days. At the indicated end of culture, cells were fixed for 30 min with 4% paraformaldehyde and stained for 20 min with crystal violet solution (Beyotime), followed by washing with ddH2O to enable cells to be clearly photographed and counted.
2.8. Cell cycle analysis
U251 cells were harvested and fixed in precooled 70% ethanol for 12 h at 4°C. After fixation, cells were centrifuged at 1000 rpm for 5 min and resuspended in staining solution (500 μL staining buffer solution, 25 μL propidium iodide, and 10 μL ribonuclease A for each sample; Beyotime), followed by incubation for 30 min at 37°C in the dark. A total of 10 000 cells for each sample were acquired, and their DNA contents were analyzed using Cytomics™ FC500 Flow Cytometer (Beckman Coulter, USA) under 488 nm excitation wavelength.
2.9. Wound‐healing assay
Cells transfected with siRNA were seeded into a 6‐well plates at a density of 1 × 106 cells per well. After cells were grown to confluence, wounds were introduced by scraping the monolayer cells using 100‐μL pipette tips. Prior to adding DMEM supplemented with 2% FBS, cells were washed with PBS to remove floating cells. The process of wound healing was monitored at indicated time points using a photographable microscope (Leica, German). The areas of scratches were quantified using ImageJ (Bethesda, USA).
2.10. Cell apoptosis assay
Apoptotic cells were detected under a fluorescence microscope (Leica) using the Hoechst Staining Kit (Beyotime) according to the manufacture's instruction. In brief, cells were fixed overnight at 4°C and washed with PBS, followed by Hoechst 33258 staining for 5 min in the dark.
2.11. Statistical analysis
Differences in experimental data between groups were evaluated using unpaired Student's t tests or one‐way ANOVA. To compare overall survival times of patients in GSE4412 and GSE43378, the Kaplan‐Meier method and the log‐rank test were used.20 The Cox proportional hazards regression model was used to assess the effects of clinical variables on patients' overall survival. All statistical analyses were performed by SPSS 22.0 or GraphPad Prism 5. Differences were considered statistically significant when P < 0.05.
3. RESULTS
3.1. NUSAP1 expression in different grades of glioma
To investigate the transcriptional level of NUSAP1 in glioma, we downloaded and analyzed three microarray datasets (GSE4290, GSE4412, and GSE43378) and the TCGA RNA‐seq dataset of GBM, with a total of 487 samples included. Basic clinical features of each dataset were listed in Table 1. As compared with normal brain tissues, NUSAP1 was consistently upregulated in glioma tissues in GSE4290 and TCGA datasets which contained normal specimens (Figure 1A,B). Furthermore, patients with distinct glioma grades had different NUSAP1 levels, showing a tendency that NUSAP1 expression was significantly increased with the malignant degree of glioma rising (Figure 2A‐C). To validate these different expressing patterns, we measured NUSAP1 mRNA levels by RT‐PCR in another cohort consisted of 54 glioma samples, clinical information of which was listed in Table 2. As shown in Figure 2D, the average level of NUSAP1 was higher in patients with high‐grade glioma (Grade III and IV) than in those with low‐grade glioma (Grade I and II; P = 0.019).
Table 1.
Baseline clinical characteristics of the analyzed datasets
| Characteristic | GSE4290 | GSE4412 | GSE43378 | TCGA Glioblastoma |
|---|---|---|---|---|
| No. of cases | 180 | 85 | 50 | 172 |
| Age (years) | ||||
| ≥55 | NA | 19 | 26 | 110 |
| <55 | NA | 66 | 24 | 56 |
| Undefined | NA | 0 | 0 | 6 |
| Gender | ||||
| Male | NA | 32 | 34 | 107 |
| Female | NA | 53 | 16 | 59 |
| Undefined | NA | 0 | 0 | 6 |
| WHO grade | ||||
| Nontumor | 23 | 0 | 0 | 5 |
| II | 45 | 0 | 5 | 0 |
| III | 31 | 26 | 13 | 0 |
| IV | 77 | 59 | 32 | 167 |
| Undefined | 4 | 0 | 0 | 0 |
NA, not available.
Figure 1.
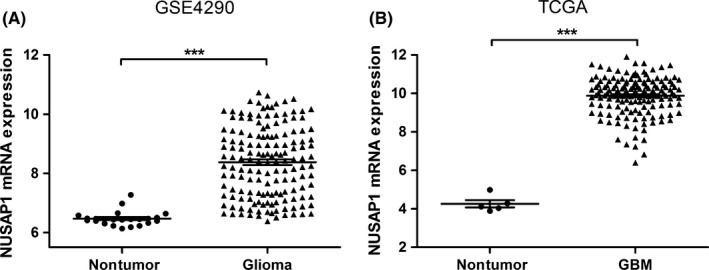
NUSAP1 is overexpressed in gliomas. (A, B) Scatter plots of NUSAP1 expression based on data from GSE4290 (A) and TCGA GBM dataset (B). ***P < 0.001
Figure 2.
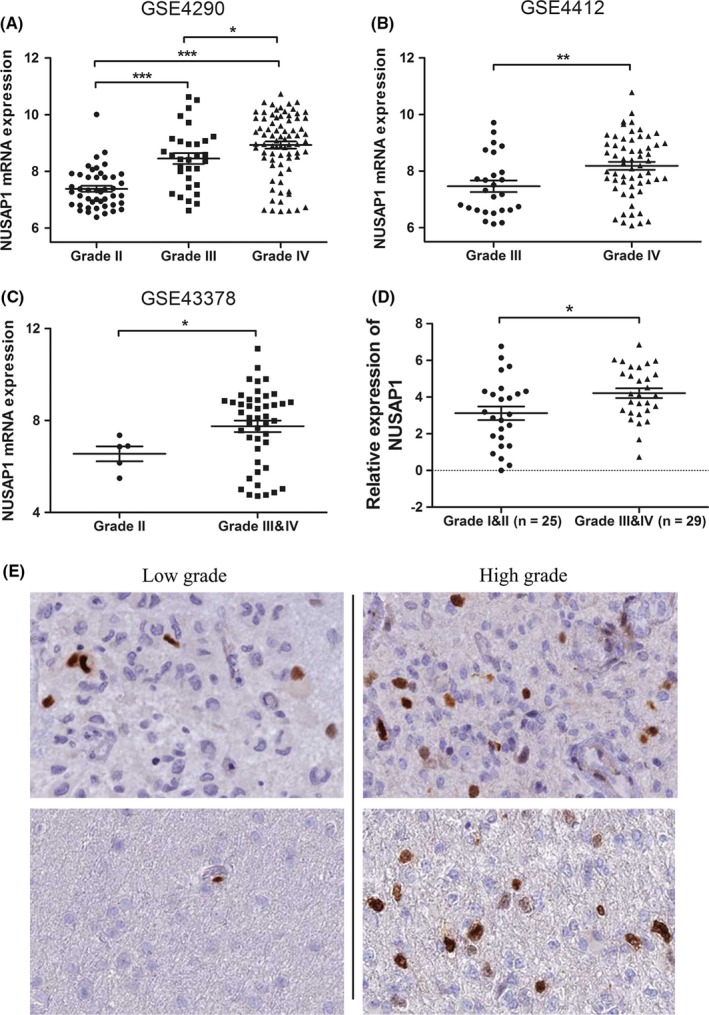
NUSAP1 expression is correlated with glioma grade. A‐C, Increased expression of NUSAP1 was detected with pathological grade rising, based on analyses of data from GSE4290 (A), GSE4412 (B), and GSE43378 (C). (D) Quantitative RT‐PCR analysis of NUSAP1 mRNA expression in 25 low‐grade and 29 high‐grade glioma tissues. NUSAP1 expression was normalized to the level of β‐actin. Data are presented as ‐∆∆Ct. (E) NUSAP1 protein expression in low‐grade and high‐grade glioma. Images were taken from the human protein atlas (http://www.proteinatlas.org). *P < 0.05, **P < 0.01, ***P < 0.001
Table 2.
Baseline clinical characteristics of patients in the validation cohort
| Characteristic | Oligodendroglioma | Astrocytoma | Glioblastoma |
|---|---|---|---|
| No. of cases | 3 | 41 | 10 |
| Age (years): Mean ± SD | 39 ± 11 | 39 ± 19 | 47 ± 22 |
| Gender | |||
| Male | 1 | 25 | 6 |
| Female | 2 | 16 | 4 |
| WHO grade | |||
| I | – | 3 | – |
| II | 3 | 19 | – |
| III | 0 | 19 | – |
| IV | – | – | 10 |
We next asked if the protein level of NUSAP1 exhibited a similar expression trend. To this end, we analyzed immunohistochemistry data of glioma tissues and found that NUSAP1 was positively stained in a few cells in low‐grade tumors, while in high‐grade tumors, the proportion of NUSAP1‐ positively stained cells was elevated (Figure 2E). These results consistently indicated that NUSAP1 expression was positively correlated with pathological grade of glioma.
3.2. NUSAP1 expression is correlated with poor prognosis of glioma patients
Survival time data of patients in GSE4412 and GSE43378 enabled us to evaluate the prognostic significance of NUSAP1 in glioma patients, although we had not obtained survival data of all the 54 patients mentioned above. As shown in Figure 3A,B, in both datasets, patients with high NUSAP1 expression showed significantly reduced overall survival (OS), as compared with those with low NUSAP1 expression (GSE4412: P = 0.0001, HR = 2.872, 95% CI: 1.667‐4.948; GSE43378: P = 0.01, HR = 2.325, 95% CI: 1.229‐4.399). For patients in GSE4412, the median OS of those with low NUSAP1 expression was 28.3 months, while those with high NUSAP1 expression had median OS of 7.43 months. For patients in GSE43378, the median OS of those with low and high NUSAP1 expression was 23.3 months and 13.9 months, respectively.
Figure 3.
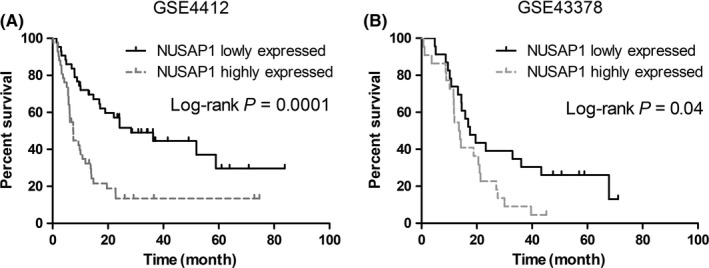
NUSAP1 expression is positively correlated with overall survival of glioma patients. (A, B) Kaplan‐Meier analysis of overall survival based on expression of NUSAP1. Patients in GSE4412 (A) and GSE43378 (B) were divided into NUSAP1 highly and lowly expressed groups with the median level of NUSAP1 used as the cutoff. Only patients with Grade III and IV tumors were included for analysis
To rule out the possibility that the poorer prognosis of patients with high NUSAP1 expression was a result of higher pathological grades, we further performed multivariate Cox proportional hazards regression analyses, introducing other clinical variables. As shown in Table 3, NUSAP1 level and WHO grade were independent prognosis factors. In addition, age and the Karnofsky performance score (KPS) were also independent prediction factors for OS (Table 4).
Table 3.
Cox proportional hazards regression analysis of patients in GSE4412
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| P value | HR (95% CI) | P value | HR (95% CI) | |
| Age | 0.008 | 2.323 (1.246‐4.333) | 0.291 | 1.407 (0.746‐2.654) |
| Gender | 0.311 | 1.315 (0.775‐2.231) | ||
| NUSAP1 level | 0.000 | 2.702 (1.587‐4.601) | 0.006 | 2.152 (1.247‐3.712) |
| WHO grade | 0.000 | 3.843 (1.960‐7.536) | 0.002 | 3.021 (1.486‐6.141) |
HR, hazard ratios; CI, confidence interval.
Table 4.
Cox proportional hazards regression analysis of patients in GSE43378
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| P value | HR (95% CI) | P value | HR (95% CI) | |
| Age | 0.001 | 3.073 (1.578‐5.984) | 0.009 | 2.570 (1.263‐5.227) |
| Gender | 0.855 | 0.941 (0.488‐1.813) | ||
| NUSAP1 level | 0.011 | 2.257 (1.202‐4.240) | 0.044 | 1.965 (1.019‐3.789) |
| KPS | 0.000 | 0.314 (0.164‐0.599) | 0.034 | 0.478 (0.242‐0.946) |
HR, hazard ratios; CI, confidence interval; KPS, Karnofsky performance score.
3.3. Knockdown of NUSAP1 suppresses proliferation and migration of glioma cells
To investigate the role of NUSAP1 in glioma progression, we performed in vitro assays evaluating phenotypic alterations of glioma cells after siRNA‐induced NUSAP1 depletion. The interference efficacy of two different NUSAP1 siRNAs was evaluated at both the mRNA and protein levels. As shown in Figure 4A,B, NUSAP1 siRNAs significantly inhibited expression of their target. NUSAP1 depletion rendered U251 cells impaired proliferation capacity, with significantly reduced absorbance 72 h after cells were seeded and thereafter, compared with U251 cells transfected with control siRNA, as indicated by the proliferation curves (Figure 5A). To further support this finding, we performed colony‐formation assay. In line with the above result, loss of NUSAP1 in U251 cells led to significantly decreased colony‐forming ability (Figure 5B). Taken together, these data demonstrate that NUSAP1 plays a positive role in facilitating glioma growth.
Figure 4.
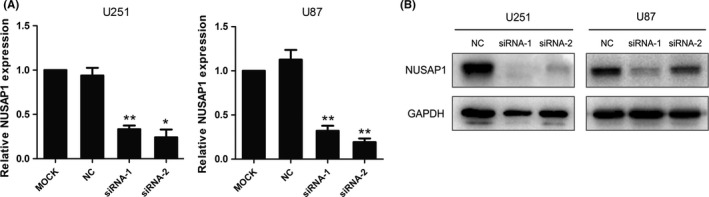
NUSAP1 silencing was achieved using specific siRNAs. (A) Quantitative RT‐PCR analysis of NUSAP1 mRNA expression in U251 and U87 cells 24 h after siRNA transfection. (B) Western blot analysis of NUSAP1 protein expression 48 h after siRNA transfection. *P < 0.05,**P < 0.01
Figure 5.
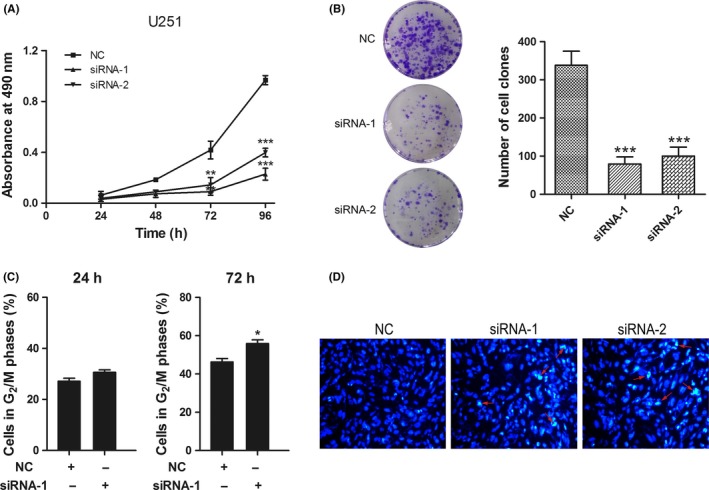
Knockdown of NUSAP1 suppresses proliferation and induces cell cycle arrest and apoptosis of glioma cells. (A) Proliferation of U251 cells after NUSAP1 depletion was evaluated by MTS assay. (B) Colony‐formation assay of U251 cells after a culture period of 15 days. Left panel: representative staining images; right panel: quantitative colony numbers. (C) Cell cycle analysis of U251 cells 24 h and 72 h after NUSAP1 siRNAs transfection. (D) Hoechst staining of apoptotic U251 cells after NUSAP1 depletion. Representative densely stained cells are indicated by red arrows. *P < 0.05, **P < 0.01, ***P < 0.001
3.4. Knockdown of NUSAP1 induces cell cycle arrest and apoptosis of glioma cells
U251 cells showed restrained proliferation ability after NUSAP1 ablation, which we hypothesized was a result, at least in part, from disorganized cell cycle progression considering the microtubule‐binding capacity of NUSAP1. We then detected the cell cycle distribution of U251 cells at multiple time points after NUSAP1 depletion using flow cytometry. U251 cells transfected with NUSAP1 siRNA were accumulated in G2/M phase 72 h, but not 24 h, after transfection, as compared with cells transfected with negative control siRNA (Figure 5C).
A decreased proliferation rate could be also due to increased cell death. For this reason, we next investigated the effects of NUSAP1 loss on apoptosis of U251 cells using Hoechst 33258 which enables us to distinguish apoptotic cells from nonapoptotic cells based on the staining intensity of cell nuclei. As shown in Figure 5D, the proportion of cells with densely stained nuclei was higher in NUSAP1‐depleted cells than in control cells, which indicated that NUSAP1 ablation would induce apoptosis in glioma cells. Despite elevated apoptotic cells after NUSAP1 knock down, no significant alterations of caspase 3 and cleaved caspase 3 levels were observed according to the immunoblotting assay (Figure S1). It is possible that NUSAP1 depletion‐induced cell apoptosis is the result of involvement of other apoptosis proteins. Collectively, our investigations demonstrate that NUSAP1 is implicated in maintaining growth of glioma cells by regulating cell cycle progression and influencing the occurrence of cell apoptosis.
3.5. NUSAP1 depletion renders glioma cells impaired migratory ability
High‐grade gliomas are characterized by high invasiveness which is a challenge for complete surgical resection of tumors. To explore whether NUSAP1 was implicated in glioma migration, we next tested the migrating ability of NUSAP1‐depleted U251 cells by calculating the ratios of the residual areas of scratches 24 h after the introduction of wounds to the initial areas of scratches. As shown in Figure 6A‐B, the unaffected areas were significantly larger for U251 cells with NUSAP1 depleted, indicating that NUSAP1 contributes to glioma migration.
Figure 6.
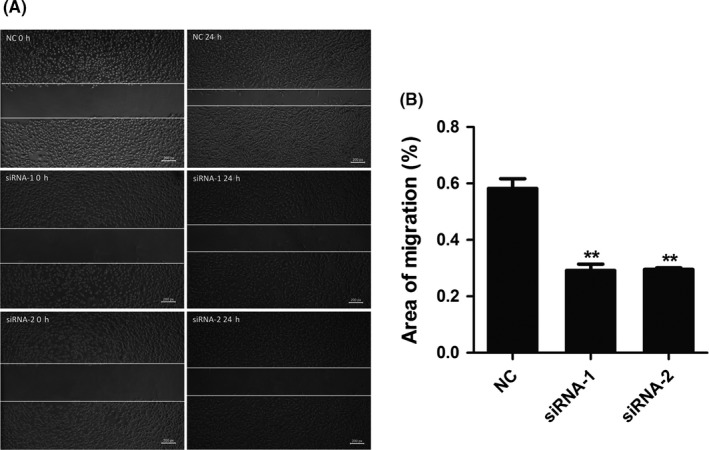
Knockdown of NUSAP1 impairs migration of glioma cells. (A) Wound‐healing assay of U251 cells 24 h after NUSAP1 siRNAs transfection. (B) Quantitative data of migrating areas 24 h after introduction of wounds. **P < 0.01
4. DISCUSSION
Treatment of gliomas is still a challenge in that, on one hand, population‐based prevention and early screening are beyond the reach of current strategies21, 22 and, on the other, the brain‐locating characteristic of gliomas and the blood‐brain barrier (BBB) undermine the efficacy of surgical resection, radiotherapy, and chemotherapy.23 Identification of oncogenic markers and design of druggable compounds with an enhanced BBB‐penetrating property can offer an avenue for treatment of this malignancy. In this study, we investigated NUSAP1 expression in different grades of gliomas and its effects on biologicals behaviors of glioma cells. We found that NUSAP1 expression was increased with the pathological grade of gliomas rising, which indicates a positive association between the NUSAP1 level and the grade of gliomas. Moreover, NUSAP1 could serve as a prognosis marker for patients' overall survival and a proliferation marker for glioma cells.
Accumulating evidence has shown that low‐grade gliomas have the potential to evolve into high‐grade gliomas, and this process can be triggered by factors such as inflammation in the tumor microenvironment and somatic mutation.24, 25, 26 Malignant transformation of low‐grade gliomas threatens the prognosis of patients seriously. According to a previous study, the overall survival was not significantly different between patients with secondary GBM (transformed from a lower grade glioma) and those with primary GBM.27 Our data showed that knockdown of NUSAP1 suppressed proliferation of U251 cells by inducing apoptosis and cell cycle arrest at G2/M phase. In addition, NUSAP1 depletion rendered U251 cells impaired migratory ability. In zebrafish embryos, decreased migration of neural crest cells was also observed when NUSAP1 was depleted.28 These results imply that targeting NUSAP1 may attenuate malignancy of high‐grade gliomas which are characterized by rapid growth and aggressiveness.29, 30 And, this was supported by the finding that glioma patients with high NUSAP1 expression exhibited significantly reduced overall survival than those with low expression of NUSAP1, according to Kaplan‐Meier analysis. Considering that the poorer prognosis of glioma patients was likely due to high tumor grades but not due to high NUSAP1 expression, we performed multivariate Cox regression analyses and found that NUSAP1 was an independent prognostic factor. However, in vivo experiments are warranted to test effects of NUSAP1 under conditions resembling the physiological environment. In addition, NUSAP1 expression was correlated with retinal neovascularization in mouse models of oxygen‐induced retinopathy.31 As angiogenesis is a rate‐limiting step which guarantees sufficient nutrients and oxygen supply in tumor growth,32, 33 targeting NUSAP1 may hold promise in preventing tumor progression in this respect. However, similar results remain to be replicated in human gliomas.
Distinguishing cancer cells from normal cells and thereby killing tumors selectively is a desired strategy for oncotherapy.34 Neural stem cell (NSC)‐based therapy is such a tumor‐targeting approach owing to the glioma tropism of NSCs.23, 35 Given that NUSAP1 is proliferation related and that brain cells are of nonproliferative nature,9, 36 a strategy targeting NUSAP1 has an advantage that traditional chemotherapeutics do not have: It may bring minor damage to normal brain cells, even if it is unable to target cancer cells specifically. Moreover, NUSAP1‐targeting therapies have the potential to resensitize tumor cells to DNA‐damaging drugs, as NUSAP1 is involved in double‐strand DNA break repair.18
Taken together, our study suggests that NUSAP1 might be involved in glioma progression and can serve as a prognosis biomarker for glioma patients. NUSAP1 silencing suppresses malignant phenotypes of glioma cells, which offer a theoretical foundation for further investigation of NUSAP1 as a possible target with advantages for glioma treatment.
DISCLOSURE
The authors declare no conflict of interest.
Supporting information
ACKNOWLEDGMENTS
This work was supported by grants from the National Key Research and Development Programs (2016YFC1306900, 2016YFC0905000), the National Natural Science Foundation of China (81373490, 81573508), and The Strategy‐Oriented Special Project of Central South University in China (ZLXD2017003). We are grateful for their financial support.
Zhu T, Xie P, Gao Y‐F, et al. Nucleolar and spindle‐associated protein 1 is a tumor grade correlated prognosis marker for glioma patients. CNS Neurosci Ther. 2018;24:178–186. 10.1111/cns.12803
REFERENCES
- 1. Chen R, Smith‐Cohn M, Cohen AL, Colman H. Glioma subclassifications and their clinical significance. Neurotherapeutics. 2017;14:284‐297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Reifenberger G, Wirsching HG, Knobbe‐Thomsen CB, Weller M. Advances in the molecular genetics of gliomas ‐ implications for classification and therapy. Nat Rev Clin Oncol. 2017;14:434‐452. [DOI] [PubMed] [Google Scholar]
- 3. Ostrom QT, Bauchet L, Davis FG, et al. The epidemiology of glioma in adults: a “state of the science” review. Neuro Oncol. 2014;16:896‐913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ostrom QT, Gittleman H, Farah P, et al. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006‐2010. Neuro Oncol. 2013;15(Suppl 2):ii1‐ii56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Howard SP, Krauze A, Chan MD, Tsien C, Tome WA. The evolving role for re‐irradiation in the management of recurrent grade 4 glioma. J Neurooncol. 2017;134:523‐530. [DOI] [PubMed] [Google Scholar]
- 6. Kegelman TP, Wu B, Das SK, et al. Inhibition of radiation‐induced glioblastoma invasion by genetic and pharmacological targeting of MDA‐9/Syntenin. Proc Natl Acad Sci USA. 2017;114:370‐375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yan D, Kowal J, Akkari L, et al. Inhibition of colony stimulating factor‐1 receptor abrogates microenvironment‐mediated therapeutic resistance in gliomas. Oncogene 2017;36:6049‐6058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Louis DN, Perry A, Reifenberger G, et al. The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131:803‐820. [DOI] [PubMed] [Google Scholar]
- 9. Raemaekers T, Ribbeck K, Beaudouin J, et al. NuSAP, a novel microtubule‐associated protein involved in mitotic spindle organization. J Cell Biol. 2003;162:1017‐1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vanden Bosch A, Raemaekers T, Denayer S, et al. NuSAP is essential for chromatin‐induced spindle formation during early embryogenesis. J Cell Sci. 2010;123:3244‐3255. [DOI] [PubMed] [Google Scholar]
- 11. Iyer J, Moghe S, Furukawa M, Tsai MY. What's Nu(SAP) in mitosis and cancer? Cell Signal. 2011;23:991‐998. [DOI] [PubMed] [Google Scholar]
- 12. Satow R, Shitashige M, Kanai Y, et al. Combined functional genome survey of therapeutic targets for hepatocellular carcinoma. Clin Cancer Res. 2010;16:2518‐2528. [DOI] [PubMed] [Google Scholar]
- 13. Bidkhori G, Narimani Z, Hosseini Ashtiani S, Moeini A, Nowzari‐Dalini A, Masoudi‐Nejad A. Reconstruction of an integrated genome‐scale co‐expression network reveals key modules involved in lung adenocarcinoma. PLoS ONE. 2013;8:e67552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fang L, Zhang M, Chen L, et al. Downregulation of nucleolar and spindle‐associated protein 1 expression suppresses cell migration, proliferation and invasion in renal cell carcinoma. Oncol Rep. 2016;36:1506‐1516. [DOI] [PubMed] [Google Scholar]
- 15. Ryu B, Kim DS, Deluca AM, Alani RM. Comprehensive expression profiling of tumor cell lines identifies molecular signatures of melanoma progression. PLoS ONE. 2007;2:e594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Okamoto A, Higo M, Shiiba M, et al. Down‐regulation of nucleolar and spindle‐associated protein 1 (NUSAP1) expression suppresses tumor and cell proliferation and enhances anti‐tumor effect of paclitaxel in oral squamous cell carcinoma. PLoS ONE. 2015;10:e0142252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li L, Zhou Y, Sun L, et al. NuSAP is degraded by APC/C‐Cdh1 and its overexpression results in mitotic arrest dependent of its microtubules' affinity. Cell Signal. 2007;19:2046‐2055. [DOI] [PubMed] [Google Scholar]
- 18. Kotian S, Banerjee T, Lockhart A, Huang K, Catalyurek UV, Parvin JD. NUSAP1 influences the DNA damage response by controlling BRCA1 protein levels. Cancer Biol Ther. 2014;15:533‐543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gao YF, Zhu T, Mao XY, et al. Silencing of Forkhead box D1 inhibits proliferation and migration in glioma cells. Oncol Rep. 2017;37:1196‐1202. [DOI] [PubMed] [Google Scholar]
- 20. Zhu T, Gao YF, Chen YX, et al. Genome‐scale analysis identifies GJB2 and ERO1LB as prognosis markers in patients with pancreatic cancer. Oncotarget. 2017;8:21281‐21289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rice T, Lachance DH, Molinaro AM, et al. Understanding inherited genetic risk of adult glioma ‐ a review. Neurooncol Pract. 2016;3:10‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Weller M, van den Bent M, Tonn JC, et al. European Association for Neuro‐Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol. 2017;18:e315‐e329. [DOI] [PubMed] [Google Scholar]
- 23. Bovenberg MS, Degeling MH, Tannous BA. Advances in stem cell therapy against gliomas. Trends Mol Med. 2013;19:281‐291. [DOI] [PubMed] [Google Scholar]
- 24. Michelson N, Rincon‐Torroella J, Quinones‐Hinojosa A, Greenfield JP. Exploring the role of inflammation in the malignant transformation of low‐grade gliomas. J Neuroimmunol. 2016;297:132‐140. [DOI] [PubMed] [Google Scholar]
- 25. Leu S, von Felten S, Frank S, Boulay JL, Mariani L. IDH mutation is associated with higher risk of malignant transformation in low‐grade glioma. J Neurooncol. 2016;127:363‐372. [DOI] [PubMed] [Google Scholar]
- 26. Kanamori M, Suzuki H, Takei H, Sonoda Y, Uenohara H, Tominaga T. Malignant transformation of diffuse astrocytoma to glioblastoma associated with newly developed BRAF V600E mutation. Brain Tumor Pathol. 2016;33:50‐56. [DOI] [PubMed] [Google Scholar]
- 27. Jaeckle KA, Decker PA, Ballman KV, et al. Transformation of low grade glioma and correlation with outcome: an NCCTG database analysis. J Neurooncol. 2011;104:253‐259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nie J, Wang H, He F, Huang H. Nusap1 is essential for neural crest cell migration in zebrafish. Protein Cell. 2010;1:259‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cordier D, Krolicki L, Morgenstern A, Merlo A. Targeted Radiolabeled Compounds in Glioma Therapy. Semin Nucl Med. 2016;46:243‐249. [DOI] [PubMed] [Google Scholar]
- 30. Emdad L, Hu B, Das SK, Sarkar D, Fisher PB. AEG‐1‐AKT2: A novel complex controlling the aggressiveness of glioblastoma. Mol Cell Oncol. 2015;2:e995008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Recchia FM, Xu L, Penn JS, Boone B, Dexheimer PJ. Identification of genes and pathways involved in retinal neovascularization by microarray analysis of two animal models of retinal angiogenesis. Invest Ophthalmol Vis Sci. 2010;51:1098‐1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wong PP, Bodrug N, Hodivala‐Dilke KM. Exploring novel methods for modulating tumor blood vessels in cancer treatment. Curr Biol. 2016;26:R1161‐R1166. [DOI] [PubMed] [Google Scholar]
- 33. Jimenez‐Valerio G, Casanovas O. Angiogenesis and metabolism: entwined for therapy resistance. Trends Cancer. 2017;3:10‐18. [DOI] [PubMed] [Google Scholar]
- 34. Anastasiou D. Tumour microenvironment factors shaping the cancer metabolism landscape. Br J Cancer. 2017;116:277‐286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Aboody KS, Brown A, Rainov NG, et al. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci USA. 2000;97:12846‐12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rass U, Ahel I, West SC. Defective DNA repair and neurodegenerative disease. Cell. 2007;130:991‐1004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


