Abstract
Background
The perception of cardiovascular (CV) risk is essential for adoption of healthy behaviors. However, subjects underestimate their own risk.
Hypothesis
Clinical characteristics might be associated with self‐underestimation of CV risk.
Methods
This is a retrospective, cross‐sectional study of individuals submitted to routine health evaluation between 2006 and 2012, with calculated lifetime risk score (LRS) indicating intermediate or high risk for CV disease (CVD). Self‐perception of risk was compared with LRS. Logistic regression analysis was performed to test the association between clinical characteristics and subjective underestimation of CV risk.
Results
Data from 5863 subjects (age 49.4 ± 7.1 years; 19.9% female) were collected for analysis. The LRS indicated an intermediate risk for CVD in 45.7% and a high risk in 54.3% of individuals. The self‐perception of CV risk was underestimated compared with the LRS in 4918 (83.9%) subjects. In the adjusted logistic regression model, age (odds ratio [OR]: 1.28, 95% confidence interval [CI]: 1.10‐1.47 per 10 years, P = 0.001), smoking (OR: 1.99, 95% CI: 1.40‐2.83, P < 0.001), dyslipidemia (OR: 1.21, 95% CI: 1.01‐1.46, P = 0.045), physical activity (OR: 1.66, 95% CI: 1.36‐2.02, P < 0.001), and use of antihypertensive (OR: 1.49, 95% CI: 1.15‐1.92, P = 0.002) and lipid‐lowering medications (OR: 2.13, 95% CI: 1.56‐2.91, P < 0.001) were associated with higher chance of risk underestimation, whereas higher body mass index (OR: 0.92, 95% CI: 0.90‐0.94, P < 0.001), depressive symptoms (OR: 0.46, 95% CI: 0.37‐0.57, P < 0.001), and stress (OR: 0.41, 95% CI: 0.33‐0.50, P < 0.001) decreased the chance.
Conclusions
Among individuals submitted to routine medical evaluation, aging, smoking, dyslipidemia, physical activity, and use of antihypertensive and lipid‐lowering medications were associated with higher chance of CV risk underestimation. Subjects with these characteristics may benefit from a more careful risk orientation.
Keywords: Cardiovascular Diseases, Risk Factors, Self‐Concept, Self‐Report
1. INTRODUCTION
Despite efforts in prevention and treatment, cardiovascular disease (CVD) remains the leading cause of death worldwide and the main cause of years of life lost due to premature death.1, 2 International guidelines recommend lifestyle modifications to prevent CVD,3 but usually patients have low adherence to these recommendations, which is associated with worse clinical outcomes and higher healthcare costs.4 Because previous evidence indicates that the adoption of healthy behaviors could prevent ≥80% of CVD,5 new approaches on prevention are desired, with emphasis on healthy lifestyle and medical treatment adherence.6 Treatment adherence improvement depends on a change in patient mindset and behavior. Importantly, risk self‐perception is a pivotal step on the process of behavior change. Previous evidence suggests that adherence to medical treatment and lifestyle modifications are highly associated with self‐perceived risk.7, 8, 9, 10, 11 In addition, individual characteristics and external motivators such as family and social relationships, media, and local culture could influence risk perception.8, 11, 12
Interestingly, several studies have demonstrated that very often patients have an optimistic bias and underestimate their personal risks,10, 13, 14, 15, 16, 17 but only 1 previous study (Dallas Heart Study), a community‐based study conducted in Texas, evaluated the association of clinical characteristics with self‐underestimation of cardiovascular (CV) risk considering the lifetime risk score (LRS).15 Therefore, the aim of this study was to identify clinical characteristics associated with subjective self‐underestimation of CV risk according to the LRS in a large sample of primary‐prevention individuals submitted to a routine health evaluation solicited by their employers.
2. METHODS
This was a retrospective cross‐sectional study based on a large database of individuals who underwent a routine health evaluation, supported by their employers, between 2006 and 2012 at the preventive medicine center at the Hospital Israelita Albert Einstein in São Paulo, Brazil.
The included subjects were age ≥ 40 years and reported no previous clinical manifestation of serious CV events such as stroke, myocardial infarction, and heart failure. The checkup protocol has been previously described and included clinical, laboratory, ultrasound, and treadmill‐test evaluation.13, 18 The clinical evaluation comprised a medical interview and blood pressure (mm Hg), height (m), and weight (kg) measures.
Diabetes mellitus (DM), hypertension (HTN), and dyslipidemia were identified by use of medications for these conditions or self‐reported. Smoking status was self‐reported as current smoker or nonsmoker. The Beck Depression Inventory (BDI), with a cutoff point of 11, ascertained depressive symptoms. Subjects were considered to have depressive symptoms if they scored ≥12 on BDI.19, 20, 21 Stress was subjectively defined by the participant as present or absent. Physical activity was ascertained by the International Physical Activity Questionnaire (IPAQ) questionnaire22 and individuals were classified as physically active (moderate and high level of physical activity) or sedentary (no activity or low level of physical activity). Alcohol consumption was assessed by the Alcohol Use Disorders Identification Test (AUDIT)23 and classified as low‐, intermediate‐, or high‐risk consumption.
During physical examination, blood pressure was measured according to the method recommended by the American Heart Association.24 The body mass index (BMI; kg/m2) was calculated as weight/height2 and individuals were classified as low weight (BMI <18.5), normal weight (BMI 18.5–24.9), overweight (BMI 25.0–29.9), and obese (BMI ≥30). Blood samples were collected after an overnight fast of ≥12 hours, and plasma lipid and glucose levels were determined using standard laboratory techniques (Vitros system; Ortho Clinical Diagnostics, Raritan, NJ).
CV risk was objectively evaluated by the LRS according to the Lloyd‐Jones/Framingham risk algorithm.25 LRS is a long‐term estimator of CV risk throughout the individual's life expectancy and is calculated according to criteria outlined in Table 1.
Table 1.
LRS according to Lloyd‐Jones/Framingham risk algorithm25
| Variable | Risk Factors | ||
|---|---|---|---|
| Minor | Moderate | Major | |
| Total cholesterol | 180–199 mg/dL | 200–239 mg/dL | ≥240 mg/dL |
| SBP | 120–139 mmHg | 140–159 mmHg | ≥160 mmHg or use of medications |
| DM | Absent | Absent | Present |
| Smoking | Absent | Absent | Present |
Abbreviations: DM, diabetes mellitus; LRS, lifetime risk score; SBP, systolic blood pressure.
LRS is considered low if subjects have no risk factors, intermediate if subjects have ≥1 minor or moderate risk factor and no major risk factor, and high if they have ≥1 major risk factor.
Before individuals were aware of their calculated CV risk, each participant answered a questionnaire that included the question “How do you consider your CV risk during the next years: low, intermediate, or high?” Self‐perception was compared with the calculated LRS, and participants were then classified as normo‐perceivers (ie, perceived risk is coincident with actual estimated risk), hypo‐perceivers (ie, perceived risk underestimates actual risk), and hyper‐perceivers (ie, perceived risk overestimates actual risk).13
Because the objective of the present study was to evaluate factors affecting underestimation of risk, low‐risk subjects according to LRS and hyper‐perceivers were excluded from the analysis.
The study protocol was conducted in accordance with the Declaration of Helsinki and was approved by the institution's ethics committee under protocol number 32933214.1.0000.0071. The study was granted a waiver for informed consent.
2.1. Statistical analysis
Baseline characteristics are summarized by normo‐perceiver or hypo‐perceiver individual status based on LRS. Continuous variables are reported as mean ± SD or median with interquartile range and compared using t tests or Mann–Whitney tests. Categorical variables are reported as frequencies and percentages and compared using the Pearson χ2 test. A logistic regression model was used to test the association between clinical factors and CV risk underestimation. The model used the backward stepwise approach, with entry and removal criteria of P < 0.05 and P < 0.10, respectively, and was adjusted for sex; age; BMI; smoking; use of medications for HTN, DM, and/or dyslipidemia; referred DM; referred HTN; referred dyslipidemia; physical activity; depression; stress; and alcohol consumption. All statistical tests were 2‐sided, and the criterion for statistical significance was P < 0.05. All statistical analyses were performed using SPSS software version 20.0 (IBM Corp., Armonk, NY).
3. RESULTS
After excluding low‐risk subjects and hyper‐perceivers, from 6544 consecutive individuals evaluated, ultimately 5863 (mean age, 49.4 ± 7.1 years; 19.9% female) were included in this study (Figure 1).
Figure 1.
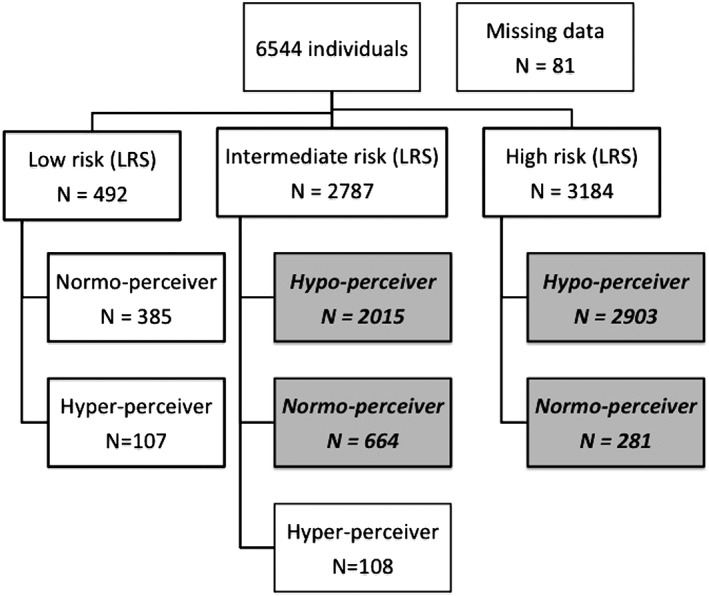
Flowchart of the inclusion procedure. In grey, bold, and italic, individuals who were considered for the analysis. Abbreviations: LRS, lifetime risk score
The calculated CV risk, estimated by LRS, was intermediate in 45.7% (n = 2679) and high in 54.3% (n = 3184) of the study population. From the 5863 subjects included in the study, 4918 (83.9%) were hypo‐perceivers, whereas 945 (16.1%) were normo‐perceivers. High‐risk subjects were more frequently hypo‐perceivers than individuals at intermediate risk (91.2% vs 75.2%; P < 0.001, respectively).
Table 2 presents the baseline characteristics according to the self‐perception of CV risk. Overall, hypo‐perceivers were older and more frequently had systemic HTN, dyslipidemia, and smoking. On the other hand, they had a lower BMI, were more physically active, and were less frequently depressed or stressed.
Table 2.
Baseline characteristics according to the self‐perception of CV risk
| Variable | Normo‐perceiver, n = 945 | Hypo‐perceiver, n = 4918 | Total, N = 5863 | P Valuea |
|---|---|---|---|---|
| Age, y | 47.9 ± 6.2 | 49.7 ± 7.2 | 49.4 ± 7.1 | <0.001 |
| Age group, y | <0.001 | |||
| 40–49 | 618 (65.4) | 2669 (54.3) | 3287 (56.1) | |
| 50–59 | 289 (30.6) | 1788 (36.4) | 2077 (35.4) | |
| 60–69 | 29 (3.1) | 386 (7.8) | 415 (7.1) | |
| ≥70 | 9 (0.9) | 75 (1.5) | 84 (1.4) | |
| Female sex | 209 (22.1) | 957 (19.5) | 1166 (19.9) | 0.061 |
| BMI, kg/m2 | 28.3 ± 4.5 | 26.9 ± 3.9 | 27.2 ± 4.1 | <0.001 |
| BMI | <0.001 | |||
| Underweight | 3 (0.3) | 49 (1.0) | 52 (0.9) | |
| Normal weight | 221 (23.5) | 1632 (33.3) | 1853 (31.8) | |
| Overweight | 426 (45.3) | 2317 (47.3) | 2743 (47.0) | |
| Obese | 290 (30.9) | 898 (18.3) | 1188 (20.4) | |
| Smoking | 56 (5.9) | 465 (9.5) | 521 (8.9) | <0.001 |
| DMb | 54 (5.7) | 339 (6.9) | 393 (6.7) | 0.184 |
| On treatment | 45/54 (83.3) | 304/339 (89.7) | 349/393 (88.8) | 0.170 |
| Blood glucose, mg/dL | 91.4 ± 17.1 | 91.5 ± 19.5 | 91.5 ± 19.1 | 0.892 |
| HTNc | 200 (21.2) | 1207 (24.5) | 1407 (24.0) | 0.026 |
| On treatment | 166/200 (83.0) | 1085/1207 (89.9) | 1251/1407 (88.9) | 0.004 |
| SBP, mm Hg | 120.8 ± 12.6 | 119.7 ± 12.5 | 119.9 ± 12.5 | 0.015 |
| DBP, mm Hg | 78.6 ± 8.2 | 77.8 ± 7.7 | 78.0 ± 7.8 | 0.008 |
| Dyslipidemiad | 444 (47.0) | 2684 (54.6) | 3128 (53.4) | <0.001 |
| On treatment | 97/444 (21.8) | 999/2684 (37.2) | 1096/3128 (35.0) | <0.001 |
| LDL‐C, mg/dL | 129.4 ± 29.1 | 131.8 ± 36.2 | 131.4 ± 35.1 | 0.028 |
| HDL‐C, mg/dL | 47.0 ± 12.2 | 49.3 ± 12.9 | 48.9 ± 12.8 | <0.001 |
| TG, mg/dL | 133 (97–191) | 125 (90–176) | 126 (91–179) | 0.011 |
| CRP, mg/dL | 1.4 (0.7–3.0) | 1.3 (0.6–2.7) | 1.3 (0.6–2.7) | 0.905 |
| Cr, mg/dL | 0.84 ± 0.18 | 0.87 ± 0.29 | 0.87 ± 0.27 | 0.003 |
| Medications | ||||
| Antihypertensive | 166 (17.6) | 1085 (22.1) | 1251 (21.3) | 0.002 |
| Lipid‐lowering | 97 (10.3) | 999 (20.3) | 1096 (18.7) | <0.001 |
| Glucose‐lowering | 45 (4.8) | 304 (6.2) | 349 (6.0) | 0.091 |
| Use of alcohole | 0.134 | |||
| Low | 785 (84.4) | 4177 (86.2) | 4962 (85.9) | |
| Intermediate | 122 (13.1) | 589 (12.2) | 711 (12.3) | |
| High | 23 (2.5) | 79 (1.6) | 102 (1.8) | |
| Physical activityf | 257 (29.3) | 2043 (44.8) | 2300 (42.3) | <0.001 |
| Depressiong | 266 (28.7) | 575 (12.0) | 841 (14.7) | <0.001 |
| Stressh | 352 (50.4) | 886 (23.8) | 1238 (28.0) | <0.001 |
| Lifetime risk | <0.001 | |||
| Intermediate | 664 (70.3) | 2015 (41.0) | 2679 (45.7) | |
| High | 281 (29.7) | 2903 (59.0) | 3184 (54.3) |
Abbreviations: AUDIT, Alcohol Use Disorders Identification Test; BDI, Beck Depression Inventory; BMI, body mass index; BP, blood pressure; Cr, creatinine; CRP, C‐reactive protein; CV, cardiovascular; DBP, diastolic blood pressure; DM, diabetes mellitus; HDL‐C, high‐density lipoprotein cholesterol; HTN, hypertension; IPAQ, International Physical Activity Questionnaire; IQR, interquartile range; LDL‐C, low‐density lipoprotein cholesterol; SBP, systolic blood pressure; SD, standard deviation; TG, triglycerides.
Data are presented as n (%), mean ± SD, or median (IQR).
P values refer to the comparison between normo‐ and hypo‐perceivers.
DM self‐reported or using glucose‐lowering medications.
HTN self‐reported or using antihypertensive medications.
Dyslipidemia self‐reported or using lipid‐lowering medications.
Use of alcohol estimated by the AUDIT test.
Physical activity moderate or high level of physical activity according to IPAQ.
Depressive symptoms according to BDI (cutoff point of 11).
Stress referred by the participant.
Table 3 shows the adjusted logistic regression model testing the association between clinical factors and CV risk underestimation. As shown by the adjusted model, age, smoking, dyslipidemia, physical activity, and use of antihypertensive and lipid‐lowering medications were associated with a higher chance of risk underestimation, whereas higher BMI, depressive symptoms, and stress decreased the chance of risk underestimation. DM, sex, referred HTN, and alcohol consumption were not associated with individuals' perception of risk.
Table 3.
Adjusted logistic regression analysisa testing the association of clinical factors with the underestimation of CV risk
| Variable | OR | 95% CI | P Valueb |
|---|---|---|---|
| Age (by 10 years) | 1.28 | 1.10‐1.47 | 0.001 |
| BMI | 0.92 | 0.90‐0.94 | <0.001 |
| Smoking | 1.99 | 1.40‐2.83 | <0.001 |
| Antihypertensive medications | 1.49 | 1.15‐1.92 | 0.002 |
| Lipid‐lowering medications | 2.13 | 1.56‐2.91 | <0.001 |
| Dyslipidemia | 1.21 | 1.01‐1.46 | 0.045 |
| DM | 1.57 | 0.93‐2.66 | 0.095 |
| Physical activity | 1.66 | 1.36‐2.02 | <0.001 |
| Depressive symptoms | 0.46 | 0.37‐0.57 | <0.001 |
| Stress | 0.41 | 0.33‐0.50 | <0.001 |
Abbreviations: BDI, Beck Depression Inventory; BMI, body mass index; CI, confidence interval; CV, cardiovascular; DM, diabetes mellitus; HTN, hypertension; OR, odds ratio.
Logistic regression analysis adjusted for sex, age, BMI, smoking (self‐reported, current smoker), use of antihypertensive medications, use of lipid‐lowering medications, use of glucose‐lowering medications, referred DM, referred HTN, referred dyslipidemia, physical activity (moderate and high level of physical activity), depressive symptoms (BDI ≥12 points), stress (self‐reported), and alcohol consumption (moderate‐ and high‐risk consumption).
Final model after a backward stepwise approach, with entry and removal criteria of P < 0.05 and P < 0.10, respectively.
4. DISCUSSION
In a previous study, we demonstrated the high prevalence of CV risk underestimation among individuals submitted to a routine health evaluation and that high‐risk subjects were more frequently hypo‐perceivers than individuals at intermediate risk.13 Building on this prior knowledge, we have now explored the clinical factors associated with the subjective underestimation of CV risk, and we found that age, smoking, dyslipidemia, physical activity, and use of antihypertensive and lipid‐lowering medications were associated with a higher chance of risk underestimation, whereas a higher BMI, depressive symptoms, and stress decreased the chance of risk underestimation.
Patient adherence to medical recommendations depends on several factors, but accurate perception of one's own risks plays a central role for patient engagement.4 From this perspective, our study demonstrated that clinical characteristics might affect self‐perception of risk, contributing to the identification of subgroups that deserve more attention.
In our study, with a relatively young population, aging was associated with a higher chance of risk underestimation. Although previous studies showed conflicting results,7, 15, 16, 17, 26 the optimistic bias theory10 might have influenced the association of aging and risk hypo‐perception. According to this theory, individuals believe that if a disease has not occurred yet, there is a low chance of it appearing in the future.10
The association of smoking with a higher chance for risk hypo‐perception was an unexpected finding because previously it has been shown to positively affect risk perception.7, 14, 16, 26 Marteau et al14 used a British regional heart study score to estimate the 10‐ to 15‐year CV risk, whereas van der Weijden et al16 used the Dutch risk score to estimate the absolute 10‐year risk of CVD. Frijling et al26 calculated the 10‐year risk using the Framingham risk functions, and Avis et al7 estimated CV risk using health risk‐appraisal instruments. Importantly, none of these studies used the LRS to estimate CV risk. Perhaps in our study, smokers' subjective self‐perception of risk did not accurately reflect the huge impact smoking has on LRS (ie, once a patient is a smoker, he/she is classified as high risk for CVD, independently of all other risk variables).
Physical activity and use of antihypertensive and lipid‐lowering medications were associated with higher chance of risk underestimation. The literature is scarce regarding the impact of the use of medications such as antihypertensive or lipid‐lowering drugs, or even physical activity, on individuals' self‐perception of risk. Monsuez et al27 evaluated self‐perceived risk relative to actual CV risk according to the Framingham score in 5240 young and middle‐aged women. Those who were engaged in any leisure‐time physical activity or sport were more likely to consider their risk lower than those who did not. In our study, it is possible that the wellness promoted by physical exercise affected the perception of risk. In another retrospective cohort study that included 3150 participants and evaluated the impact of traditional checkup appointments on the progression of CV risk over time, most CV risk factors worsened over time, meaning that the clinical evaluation, all medical education, and all pharmacologic and nonpharmacologic interventions had little effect on patient behavior.28 Extrapolating these findings to our study, it might be possible that the use of medications also had minimal effect on individuals' perception of risk. Hence, our exploratory results inform clinicians that even patients under medical treatment may have misperceptions about their own risks.
Dyslipidemia was also associated with higher chance of risk underestimation. In a previous qualitative, focus‐group interview study, many participants had inadequate knowledge about hypercholesterolemia and CV risk, and few knew their cholesterol numbers.29 In our study, it is possible that the lack of knowledge by the patient about the impact of dyslipidemia on CVD was associated with underestimation of CV risk.
BMI, depressive symptoms, and stress were related to a decreased chance of risk underestimation. Personal characteristics that directly affect self‐image, such as obesity, usually are associated with higher chance of interfering with an accurate self‐perception of risk.14, 16 Regarding depressive symptoms and stress, we speculate that anxiety disorders, stress, and even depression could amplify the impact of clinical symptoms and risks on personal beliefs, contributing to a subjective perception of risk greater than objective risk estimation, therefore decreasing the chance of subjective risk underestimation. To our knowledge, this is the first study to demonstrate an association between mental disorders such as stress and depressive symptoms and a decreased chance of risk underestimation, using the LRS. Considering the exploratory nature of our findings, further studies are needed to confirm this association.
4.1. Study limitations
Our study has some relevant limitations. First, the cross‐sectional nature of our study design does not allow inferring causality. Second, data on family history of early CVD and socioeconomic status were not available and could not be included in the logistic regression model. Finally, this is a relatively young population on primary prevention, and therefore our findings should be extrapolated with caution to older individuals or those with established CVD.
5. CONCLUSION
Among individuals submitted to a routine medical evaluation, aging, smoking, dyslipidemia, physical activity, and use of antihypertensive and lipid‐lowering medications were associated with a higher chance of CV risk underestimation. From a clinical point of view, underestimating one's own risks could be associated with lower adherence to treatment recommendations. Thus, patients in these conditions may benefit from a more focused risk orientation on routine health checkups.
Conflicts of interest
Raul D. Santos has received honoraria for consulting, speaker activities, and research from Amgen, AstraZeneca, Biolab, Boehringer‐Ingelheim, Eli Lilly, Kowa, Merck, Novo Nordisk, Pfizer, Sanofi/Regeneron, and Procaps. Antonio G. Laurinavicius is an employee at Sanofi. The authors declare no other potential conflicts of interest.
Helou TN, Santos RD, Laurinavicius AG, et al. Association between clinical factors and self‐underestimation of cardiovascular risk in subjects submitted to a routine health evaluation. Clin Cardiol. 2018;41:28–33. 10.1002/clc.22841
Partial results of this study were presented as a poster at the American Heart Association EPI/Lifestyle 2015 Scientific Sessions and published as an abstract in Circulation (Helou et al. Factors affecting cardiovascular risk perception in subjects submitted to a routine health evaluation. Circulation. 2015;131:AP060).
REFERENCES
- 1. Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association [published corrections appear in Circulation. 2015;131:e535 and 2016;133:e417]. Circulation. 2015;131:e29–e322. [DOI] [PubMed] [Google Scholar]
- 3. Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published correction appears in J Am Coll Cardiol. 2014;63(25 part B):3027–3028]. J Am Coll Cardiol. 2014;63(25 part B):2960–2984. [DOI] [PubMed] [Google Scholar]
- 4. Bosworth HB, Granger BB, Mendys P, et al. Medication adherence: a call for action. Am Heart J. 2011;162:412–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ford ES, Greenlund KJ, Hong Y. Ideal cardiovascular health and mortality from all causes and diseases of the circulatory system among adults in the United States. Circulation. 2012;125:987–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Spring B, Ockene JK, Gidding SS, et al; American Heart Association Behavior Change Committee of the Council on Epidemiology and Prevention, Council on Lifestyle and Cardiometabolic Health, Council for High Blood Pressure Research, and Council on Cardiovascular and Stroke Nursing . Better population health through behavior change in adults: a call to action. Circulation. 2013;128:2169–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Avis NE, Smith KW, McKinlay JB. Accuracy of perceptions of heart attack risk: what influences perceptions and can they be changed? Am J Public Health. 1989;79:1608–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Glanz K, Bishop DB. The role of behavioral science theory in development and implementation of public health interventions. Annu Rev Public Health. 2010;31:399–418. [DOI] [PubMed] [Google Scholar]
- 9. Webster R, Heeley E. Perceptions of risk: understanding cardiovascular disease. Risk Manag Healthc Policy. 2010;3:49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Weinstein ND. Unrealistic optimism about susceptibility to health problems: conclusions from a community‐wide sample. J Behav Med. 1987;10:481–500. [DOI] [PubMed] [Google Scholar]
- 11. Janz NK, Becker MH. The Health Belief Model: a decade later. Health Educ Q. 1984;11:1–47. [DOI] [PubMed] [Google Scholar]
- 12. Hunt K, Davison C, Emslie C, et al. Are perceptions of a family history of heart disease related to health‐related attitudes and behaviour? Health Educ Res. 2000;15:131–143. [DOI] [PubMed] [Google Scholar]
- 13. Katz M, Laurinavicius AG, Franco FG, et al. Calculated and perceived cardiovascular risk in asymptomatic subjects submitted to a routine medical evaluation: the perception gap. Eur J Prev Cardiol. 2015;22:1076–1082. [DOI] [PubMed] [Google Scholar]
- 14. Marteau TM, Kinmonth AL, Pyke S, et al; Family Heart Study Group . Readiness for lifestyle advice: self‐assessments of coronary risk prior to screening in the British Family Heart Study. Br J Gen Pract. 1995;45:5–8. [PMC free article] [PubMed] [Google Scholar]
- 15. Petr EJ, Ayers CR, Pandey A, et al. Perceived lifetime risk for cardiovascular disease (from the Dallas Heart Study). Am J Cardiol. 2014;114:53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van der Weijden T, van Steenkiste B, Stoffers HE, et al. Primary prevention of cardiovascular diseases in general practice: mismatch between cardiovascular risk and patients' risk perceptions. Med Decis Making. 2007;27:754–761. [DOI] [PubMed] [Google Scholar]
- 17. Niknian M, McKinlay SM, Rakowski W, et al. A comparison of perceived and objective CVD risk in a general population. Am J Public Health. 1989;79:1653–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ndumele CE, Nasir K, Conceição RD, et al. Hepatic steatosis, obesity, and the metabolic syndrome are independently and additively associated with increased systemic inflammation. Arterioscler Thromb Vasc Biol. 2011;31:1927–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. [DOI] [PubMed] [Google Scholar]
- 20. Richter P, Werner J, Heerlein A, et al. On the validity of the Beck Depression Inventory: a review. Psychopathology. 1998;31:160–168. [DOI] [PubMed] [Google Scholar]
- 21. Gomes‐Oliveira MH, Gorenstein C, Lotufo Neto F, et al. Validation of the Brazilian Portuguese version of the Beck Depression Inventory‐II in a community sample. Rev Bras Psiquiatr. 2012;34:389–394. [DOI] [PubMed] [Google Scholar]
- 22. Craig CL, Marshall AL, Sjöström M, et al. International Physical Activity Questionnaire: 12‐country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–1395. [DOI] [PubMed] [Google Scholar]
- 23. Saunders JB, Aasland OG, Babor TF, et al. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption–II. Addiction. 1993;88:791–804. [DOI] [PubMed] [Google Scholar]
- 24. Perloff D, Grim C, Flack J, et al. Human blood pressure determination by sphygmomanometry. Circulation. 1993;88(5 part 1):2460–2470. [DOI] [PubMed] [Google Scholar]
- 25. Lloyd‐Jones DM, Leip EP, Larson MG, et al. Prediction of lifetime risk for cardiovascular disease by risk factor burden at 50 years of age. Circulation. 2006;113:791–798. [DOI] [PubMed] [Google Scholar]
- 26. Frijling BD, Lobo CM, Keus IM, et al. Perceptions of cardiovascular risk among patients with hypertension or diabetes. Patient Educ Couns. 2004;52:47–53. [DOI] [PubMed] [Google Scholar]
- 27. Monsuez JJ, Pham T, Karam N, et al. Awareness of individual cardiovascular risk factors and self‐perception of cardiovascular risk in women. Am J Med Sci. 2017;354:240–245. [DOI] [PubMed] [Google Scholar]
- 28. Conceição RD, Laurinavicius AG, Kashiwagi NM, et al. Check‐up and cardiovascular risk progression: is there room for innovation? Einstein (São Paulo). 2015;13:196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Goldman RE, Parker DR, Eaton CB, et al. Patients' perceptions of cholesterol, cardiovascular disease risk, and risk communication strategies [published correction appears in Ann Fam Med. 2006;4:371]. Ann Fam Med. 2006;4:205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]


