Summary
Aims
Streptococcus pneumoniae infection in acute bacterial meningitis can lead to widespread brain damage and mortality. Inflammatory responses by immune cells in the brain are thought to determine the degree of brain injury. Yet, the mechanisms underlying host responses to pneumococcal meningitis are largely unknown. To explore host responses as a potential therapeutic target for preventing brain injury after pneumococcal meningitis.
Methods
We evaluated signaling mechanisms that minimize neuronal damage caused by pneumococcal infection; specifically, we assessed pathways related to neuronal survival after enhancing estrogen receptor‐β (ER‐β) expression using a natural therapeutic substance known as ginsenoside Rb1 and Rg3 enhanced ginseng.
Results
Tissue damage caused by bacterial infection was reduced in Rb1/Rg3‐treated mice as a result of microglial activation and the inhibition of apoptosis. Furthermore, Rb1 upregulated the expression of brain‐derived neurotrophic factor (BDNF) as well as anti‐apoptotic factors including Bcl‐2 and Bcl‐xL. Using BV2 microglial cells in vitro, Rb1 treatment inhibited microglial apoptosis in a manner associated with JAK2/STAT5 phosphorylation.
Conclusion
After S. pneumoniae infection in mice, particularly in female mice, Rb1‐containing ginseng increased bacterial clearance and survival. These findings inform our understanding of the host immune response to pneumococcal meningitis.
Keywords: apoptosis; bacterial meningitis; estrogen receptor‐β; ginsenoside Rb1, Rg3; microglia; neuroinflammation
1. INTRODUCTION
Estrogen receptors (ERs) play several physiological roles including anti‐inflammatory functions. Among ER subtypes (ER‐α and ER‐β), ER‐β, but not ER‐α, is mainly expressed in central nervous system (CNS) microglia and regulates microglial activation.1 ER‐β activation induces the expression of brain‐derived neurotrophic factor (BDNF), a neuroprotective factor, to regulate the survival of local neurons against trauma.2 Although microglial survival after neuronal damage is beneficially associated with neuronal recovery through wound‐healing mechanisms, BDNF reduces mortality by inhibiting the release of pro‐inflammatory factors.3 Similarly, in a clinical study, serum BDNF levels were reduced in patients with increased levels of pro‐inflammatory factors such as IL‐6 and TNF‐α compared with normal subjects.4 The molecular mechanisms by which ER‐β associated repression of neuro‐inflammatory responses have not been established, and the potential impact of ER‐β function on the neuronal cell death has not been evaluated.5, 6
Ginseng is a traditional medicinal herb that has antioxidant, anti‐inflammatory, and thus neuroprotective properties. Consequently, ginseng has been proposed as useful for the prevention of bacterial meningitis by inhibiting neuroinflammation.7 Ginsenoside Rb1 (Rb1) is a bioactive component of ginseng that exerts neuropharmacological effects by BDNF.8 Rb1 attenuates microglial activation, reducing the expression of pro‐inflammatory cytokines associated with the immune response including tumor necrosis factor‐α (TNF‐α) and interleukin (IL)‐1β with lipopolysaccharides (LPS). However, the mechanism and significance of Rb1‐related neuroinflammation and cell death are largely obscure.9 Microglial activation and the associated production of pro‐inflammatory cytokines has been linked to neuronal death and several neurodegenerative disease states in clinical studies.10 Bacterial meningitis also produces microglial activation and the release of inflammatory cytokines as well as microglial cell death due to infection.11
Acute bacterial meningitis occurs due to bacterial infection of the meninges and produces significant neuronal inflammation and injury. Several reports indicate the rate of meningitis‐associated mortality in adults has remained the same despite the development of new antibiotics.12, 13 Streptococcus pneumoniae (S. pneumoniae) is a bacterium that causes bacterial pneumonia, otitis media, and sepsis, and the most important pathogenic cause of bacterial meningitis.14 Bacterial meningitis caused by S. pneumoniae has a high mortality rate; 30% of survivors experience significant brain damage, cognitive impairment, hearing loss, and sensorimotor deficits.15 While other therapies using corticosteroids (dexamethasone) have recently been developed, these approaches are costly and are still being validated for clinical use. Therefore, existing therapeutic strategies do not effectively prevent bacterial meningitis and are limited in its treatment.16 A better understanding of the mechanisms underlying the damaging effects of acute bacterial meningitis can facilitate the development of new prophylactic and therapeutic strategies.17
In this study, the therapeutic effects of Rb1/Rg3 in mice with S. pneumoniae bacterial meningitis were investigated. Specifically, the effects on ER‐β activation were evaluated and the hypothesis that Rb1/Rg3 induces BDNF expression via ER‐β and decreases microglial apoptosis was tested. Additionally, rodent survival and bacterial burden after treatment with Rb1/Rg3 were evaluated to determine the potential clinical utility of Rb1/Rg3 and ginseng as a cost‐effective option in pneumococcal meningitis.
2. METHODS
2.1. Mice
Male and female C57BL/6 mice (6‐8 weeks of age) were received from Orient Bio Inc. (Seoul, Korea) and acclimatized to a temperature‐ and humidity‐controlled environment under a 12‐hour light‐dark cycle with daily access to water and food. The care and use of animals as well as the study protocol adhered to the guidelines of the Korean Academy of Medical Sciences and was regularly reviewed by the Ethical Committee of Sungkyunkwan University.
2.2. Treatments
Ginsenoside Rb1 (ab142646, Abcam) was dissolved in 0.9% saline and administered intraperitoneally to the treatment group (50 mg/kg) for 7 days. The control group received vehicle treatment (0.9% saline). All injections were performed at the same time (10:00) each day.18 Furthermore, to examine the effects on survival, P. ginseng from the Korea Ginseng Corporation (Seoul, Korea) was steamed for 3 hours at 100°C and dried for extraction. The final product was verified using ultra‐performance liquid chromatography. Based on our previous study using a model of bacterial sepsis, ginseng extract was administered at 4 doses (0, 50, 100, and 200 mg/kg) with the following Korea Red Ginseng ingredients (mg/g ± standard deviation): Rb1 (4.62 ± 0.05), Rb2 (1.83 ± 0.02), Rc (2.41 ± 0.02), Rd (0.89 ± 0.02), Re (0.93 ± 0.01), Rf (1.21 ± 0.02), Rg1 (0.71 ± 0.01), Rg2(r) (1.29 ± 0.02), Rg2(s) (1.92 ± 0.03), Rg3(r) (0.91 ± 0.02), Rg3(s) (2.14 ± 0.01), and Rh1 (0.78 ± 0.00),19, 20 with the addition of Rg3 (17 mg/g) from the Korea Ginseng Corporation (Seoul, Korea) and dissolved in 0.9% saline. Extract dilutions were orally administered to mice at 10:00 and 16:00 every day for the duration of the survival study.
2.3. Bacteria and bacterial meningitis model
Streptococcus pneumoniae D39 (serotype 2) was obtained from Professor David E. Briles of the University of Alabama at Birmingham.21 Bacteria were suspended in a solution of 3% Todd‐Hewitt broth, 0.5% yeast extract, and 1.5% agar (selectively), and 2% of the solution was inoculated into a sterilized culture medium (15 minutes at 121°C) and cultured at 37°C in a humidified incubator. We used a well‐known pneumococcal meningitis model with some modifications; briefly, 1 × 105 S. pneumoniae dissolved in 10 μL 0.9% saline was injected intracerebroventricularly at the soft point of the cranium (1‐2 mm anterior to the coronal suture) in mice.22
2.4. Immunohistochemistry and immunofluorescence
Mice were euthanized in a CO2 chamber 16 hours after pneumococcal infection. Brains were harvested and fixed with formalin using a conventional method. Fixed tissues were stained with hematoxylin and eosin. Microglia were detected using an anti‐Iba1 antibody (ab5076, Abcam). Brain sections were analyzed using light microscopy (Olympus BX50) or confocal laser scanning microscopy (Zeiss, Seoul, Korea).
2.5. Immunoblotting
Mice brains were harvested as described above, and tissues were lysed with a lysis buffer (Abcam) containing protease and phosphatase inhibitors. Proteins were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to a nitrocellulose/polyvinylidene fluoride membrane using a Trans‐Blot® Turbo™ Transfer System (Bio‐Rad). Membranes were blocked in skim milk or BSA for 2 hours and subsequently incubated overnight with one of the following primary antibodies: anti‐ER‐β (gtx70174, Gentex), anti‐Bcl‐2 (gtx100064, Gentex), anti‐BDNF (sc‐546, Santa Cruz), anti‐Nurr1 (sc‐991, Santa Cruz), anti‐Bax (sc‐493, Santa Cruz), anti‐Bcl‐xL (sc‐8392, Santa Cruz), β‐actin (sc‐47778, Santa Cruz), TNF‐α (sc‐1350, Santa Cruz), phospho‐Jak2 (#3776, Cell Signaling), Jak2 (#3230, Cell Signaling), phospho‐Stat5 (#9359 Cell Signaling), or Stat5 (#94205, Cell Signaling). Then, blots were washed with Tris‐buffered saline containing Tween 20, treated with either anti‐mouse or anti‐rabbit secondary antibody as appropriate, and visualized with an electrochemiluminescence solution (GenDEPOT). Semi‐quantification of blot bands was performed using Image J 2.1.4.6 software (Wayne Rasband, National Institutes of Health, Bethesda, MD).
2.6. Cell culture
For in vitro studies, the mouse microglial BV‐2 cell line was obtained from Professor Choon‐gon Jang of the School of Pharmacy at Sungkyunkwan University.23 BV2 cells were cultured in Dulbecco's modified Eagle's medium (Gibco) containing 10% heat‐inactivated fetal bovine serum (30‐2020, ATCC) and antibiotics (100 μg/mL streptomycin and 100 IU/mL penicillin, Gibco). Cells were maintained in an incubator at 37°C under a humidified atmosphere of 5% CO2.
2.7. RNA extraction and siRNA
RNA was extracted from BV‐2 cells using an RNeasy Plus Mini Kit (74134, Qiagen), and cDNA was synthesized using the RNA to cDNA EcoDry Premix (639546, Takara). Ab Step One Plus Real‐time PCR (Abcam) was performed with the following primers: Bcl‐2 forward 5′‐ACG AGT GGG ATG GGG GAG ATG TG‐3′, BCL‐2 reverse 5′‐GCG GTA GCG GCG GGA GAA GTC‐3′, BDNF forward 5′‐TCA AGT TGG AAG CCT GAA TGA ATG‐3′, BDNF reverse 5′‐CTG ATG CTC AGG AAC CCA GGA‐3′, ER‐β forward 5′‐TGA GCC ACC CAA TGT GCT A‐3′, ER‐β reverse 5′‐AAC AGG CTG AGC TCC ACA AAG‐3′. Fold changes in mRNA expression were calculated.
siRNA suspensions were prepared with control (scrambled) siRNA or siRNAs targeting the mouse estrogen receptor beta (ESR2) (L‐065564‐01‐0005, Dharmacon) and a transfection reagent (mir 2700, Mirus) and used in accordance with SMARTpool siRNA reagent manufacturer's specifications.
2.8. Enzyme‐linked immunosorbent assay (ELISA)
Cytokines in brain homogenates were assayed using the mouse IL‐1β ELISA Kit (88‐7013‐76, Invitrogen) and mouse IL‐10 ELISA kit (88‐7105‐76, Invitrogen) in accordance with manufacturer specifications.
2.9. Statistical analysis
Differences between groups were analyzed using Mann‐Whitney rank sum tests or a 1‐way or 2‐way analysis of variance. Mouse survival was analyzed using log‐rank tests. All data were analyzed across a minimum of 3 trials. Statistical significance was set at P < 0.05. All analyses were conducted using GraphPad Prism software version 5.01.
3. RESULTS
3.1. Ginseng promotes survival after bacterial meningitis
As the effects of ginseng on bacteria‐induced neuroinflammation have not been previously reported, the therapeutic utility of ginseng supplementation in pneumococcal meningitis was evaluated utilizing the pathogenic S. pneumoniae as inflammation factors. Ginseng extract (100 mg/kg or 200 mg/kg) was orally administered to both sexes of a murine mice model of bacterial meningitis, and survival was evaluated. In male mice, the untreated group died within 140 hours of infection. Regarding ginseng treatment, the 100 mg/kg treatment group showed better survival than the 200 mg/kg treatment group (18% survival vs 9% survival, respectively) (Figure 1A). In female mice, ginseng administration not only increased survival in a dose‐dependent manner but also had a statistically significant effect on mortality (Figure 1B, left panel). Furthermore, administration of ginseng (200 mg/kg) supplemented with Rg3, a component of ginseng, to female mice increased the survival rate from 16% in the regular ginseng group to 33% (Figure 1B, right panel). The results show the effects of ginseng and Rg3 were therapeutically more useful in female mice than in male mice, thereby improving survival. Moreover, we performed colonization at 24 hours postinfection to measure changes in brain bacterial burden after treatment. The results showed that bacterial burden was slightly reduced in the ginseng group compared with the control group in male mice, whereas ginseng significantly increased bacterial clearance in female mice (Figure 1C). The ability of ginseng to limit bacterial burden was dose‐dependent in the early phase of infection in female mice (Figure 1C,D). As brain inflammation was caused by pro‐inflammatory IL‐1β expression,24 we investigated IL‐1β levels in mice brains. Ginseng treatment significantly impaired IL‐1β expression at 16 hours postinfection compared with wild type (WT; Figure 1E), demonstrating the increased survival rate due to ginseng might be associated with decreased IL‐1β levels. Moreover, when anti‐inflammatory cytokine IL‐10, which maintains homeostasis of the inflammatory response to pathogen invasion or neuronal damage,25, 26 was evaluated, ginseng treatment significantly increased IL‐10 levels at 16 hours postinfection in the brains of female mice (Figure 1F). Therefore, ginseng increased the survival of female mice infected with bacterial meningitis by diminishing the inflammatory response of a host defense.
Figure 1.
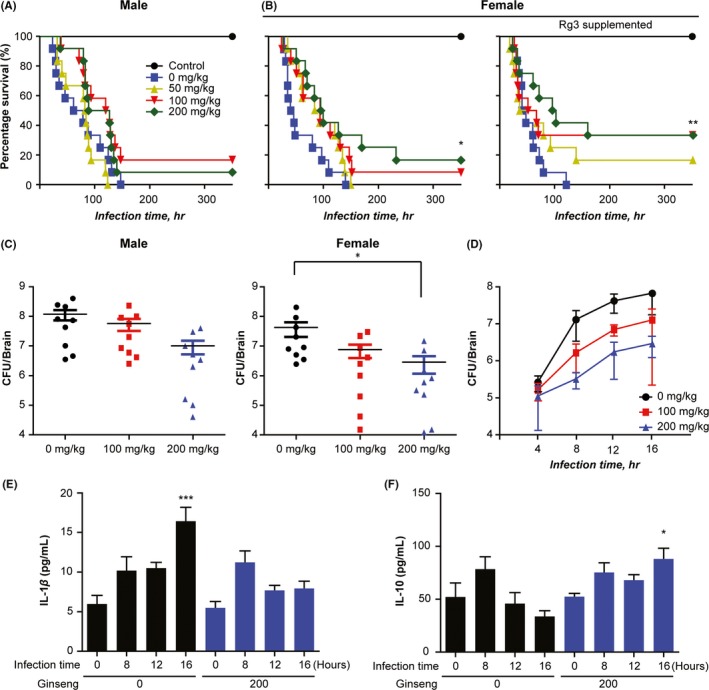
Ginseng extract promotes survival after pneumococcal meningitis in a mouse model. Survival was evaluated after oral administration of ginseng extract or Rg3‐supplemented ginseng extract (0, 100, or 200 mg/kg; n = 12) for 15 d and subsequent Streptococcus pneumoniae infection in (A) male and (B) female mice. (C) Colonization (n = 9) at 24 h postinfection of S. pneumoniae. (D) Colonization over time, (E) brain IL‐1β content, and (F) brain IL‐10 cytokine level content in the early phase of infection measured using ELISA (<16 h, n = 4). (E, F) Data are representative of 3 independent samples at each time point. Results are representative of 2 separate experiments. *P < 0.05 and **P < 0.01, 1‐way or 2‐way analysis of variance
3.2. Ginseng with supplemented Rg3, an Rb1‐derivative, also reduced apoptosis via the ER‐β
Because of its structural similarity to Rg3, Rb1 is easily converted to Rg3 by catalyzing the addition of glucose with a hydrolyzing enzyme (Figure 2A). Early studies indicated microglia play a causative pro‐inflammatory role in the early phase of infection; however, microglia are currently known to also exert neuroprotective actions by releasing growth factors such as BDNF after brain injury.27 Thus, we examined whether other ginsenosides also have anti‐apoptotic effects through ER‐β/BDNF signaling (Figure 2B). As expected, Rg3‐supplemented ginseng extract significantly increased the expression levels of BDNF, Bcl‐2, Bcl‐xL, and ER‐β in BV2 cells after S. pneumoniae infection compared with the control group (Figure 2C‐F). As the survival of microglia after neuronal damage promotes neuronal recovery,3 these findings confirmed Rb1 and its derivative Rg3 promote survival signaling in infection‐challenged microglial cells by upregulating ER‐β.
Figure 2.
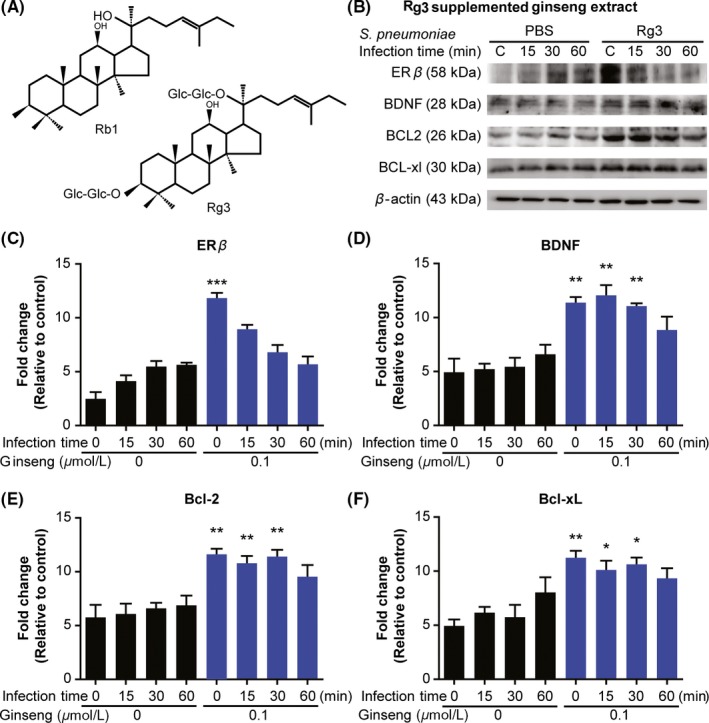
Rg3‐supplemented ginseng mimics the effects of Rb1 on BV2 microglia. BV2 cells were treated with Rg3‐supplemented ginseng extract (0.1 μmol/L) for 24 h and were subsequently infected with Streptococcus pneumoniae. Immunoblotting was performed on protein extracted at 0, 15, 30, and 60 min postinfection. (A) Similarity of the chemical structures of Rb1 and Rg3. (B) Western blot of ER‐β, BDNF, Bcl‐2, and Bcl‐xL. (C‐F) Quantification of bands in panel B. Data are representative of 3 independent samples. Results are representative of 3 separate experiments. *P < 0.05 and **P < 0.01, 2‐way analysis of variance. BDNF, brain‐derived neurotrophic factor; ER‐β, estrogen receptor beta; JAK2, Janus kinase 2; STAT5, signal transducer and activator of transcription 5
3.3. Rb1 promotes Bcl‐2/Bcl‐xL expression in microglia via the ER‐β
Microglial apoptosis is more detrimental than microglial activation in response to inflammatory challenge.28 The neuroprotective effects of Rb1 have been confirmed in several studies; however, the mechanism of Rb1 interacting with microglia is largely unknown.29, 30 Thus, we evaluated the ability of Rb1 to affect microglial apoptosis in cultured BV2 microglial cells infected with S. pneumoniae. Rb1 treatment significantly increased the expression of ER‐β, BDNF (Figure 3A,C,D), and the antiapoptosis pathways Bcl‐2 and Bcl‐xL (Figure 3A,E,F) after infection compared with vehicle treatment. Furthermore, several studies showed JAK2/STAT5 signaling controls cell survival by inhibiting apoptosis through Bcl‐2 and Bcl‐xL.31, 32 However, how JAK2/STAT5 signaling manipulates microglia against neuronal inflammation factors remains largely unknown. Thus, we examined whether ginseng/ginsenosides could mediate JAK2/STAT5 signaling in microglia. Results showed Rb1 treatment increased phosphorylation of JAK2 and STAT5 in response to infection compared with the control group (Figure 3B).
Figure 3.
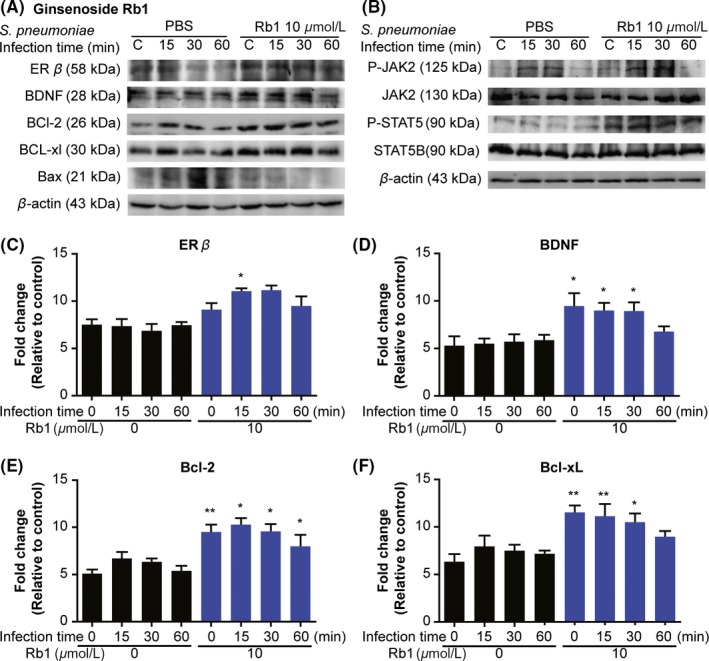
Rb1 inhibits apoptosis in BV2 microglial cells via ER‐β and JAK2/STAT5 pathway. BV2 cells were treated with Rb1 (10 μmol/L) for 24 h, and cells were subsequently infected with Streptococcus pneumoniae. Immunoblotting was performed on protein extracted at 0, 15, 30, and 60 min postinfection. (A) Western blot of ER‐β, BDNF, Bcl‐2, Bcl‐xL, and Bax. (B) Western blot of phospho‐JAK2, JAK2, phospho‐STAT5, and STAT5. (C‐F) Quantifications of bands in panel (A). (A‐F) Blots are representative of 3 independent samples at each time point. Results are representative of 2 separate experiments
Next, mRNA expression levels were analyzed to confirm the relationship among Rb1 treatment, ER‐β expression, and decreased apoptosis. Consistent with the protein data, Rb1 treatment significantly increased mRNA levels of ER‐β, BDNF, and Bcl‐2 after S. pneumoniae infection compared with the control group. (Figure 4A‐C); notably, these mRNA changes occurred prior to significant alterations in protein.
Figure 4.
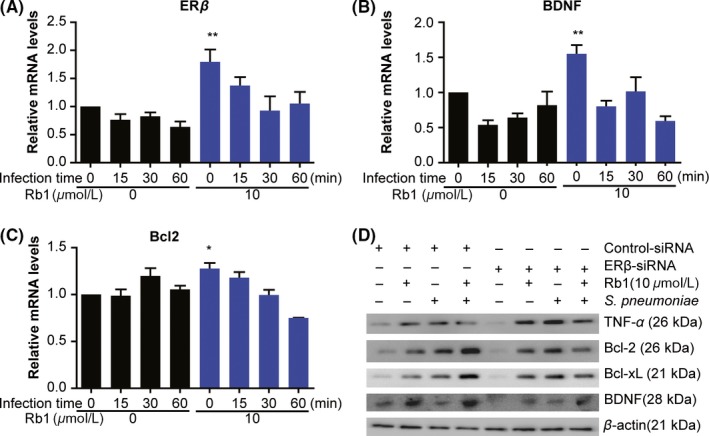
Rb1 promotes antiapoptosis activation by upregulating ER‐β‐dependent Bcl‐2 protein family. (A‐C) RT‐PCR was performed on RNA extracted at 0, 15, 30, and 60 min postinfection. mRNA expression levels of ER‐β, BDNF, and Bcl‐2. (D) Western blot of BDNF, Bcl‐2, Bcl‐xL, and TNF‐α after treatment with scrambled control siRNA or siRNA against ER‐β. Data are representative of 4 independent samples. Results are representative of 3 separate experiments. *P < 0.05 and **P < 0.01, 2‐way analysis of variance. BDNF, brain‐derived neurotrophic factor; ER‐β, estrogen receptor beta; JAK2, Janus kinase 2; STAT5, signal transducer and activator of transcription 5
To corroborate ER‐β‐dependent increase in cell survival, protein expression levels were evaluated after siRNA knockdown of ER‐β. Compared to BV2 cells treated with control scrambled siRNA, cells treated with ER‐β siRNA did not show elevated Bcl‐2 or Bcl‐xL, but exhibited increases in pro‐inflammatory cytokines including TNF‐α in response to Rb1 treatment (Figure 4D). Taken together, these results showed Rb1 treatment decreased microglial apoptosis via the ER‐β upregulation.
3.4. Rb1 exerts protective effects against bacterial meningitis
We evaluated hematoxylin and eosin‐stained brain sections from bacterial meningitis model mice treated with Rb1 or vehicle to visualize the protective effects of Rb1 in pneumococcal meningitis. The control group showed staining patterns consistent with acute meningitis, while the Rb1 group tended to show recovery from pneumococcal infection33 (Figure 5A). Compared with the control group, the Rb1 group showed decreased Bax expression and increased expression of ER‐β, BDNF, and Nurr1 in the early stages of infection (Figure 5B). In particular, ER‐β expression was significantly increased at 4, 8 hours postinfection in the Rb1 group compared with the control group (Figure 5C). These data showed Rb1 exerts protective effects in a murine model of pneumococcal meningitis through a mechanism involving ER‐β upregulation.
Figure 5.
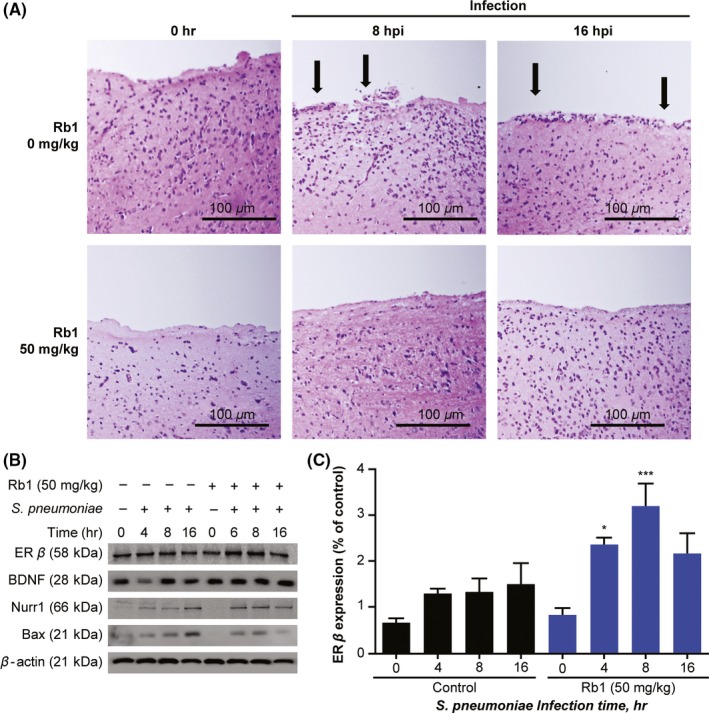
Rb1 mitigates the damaging effects of pneumococcal meningitis. (A) Rb1 (0 mg/kg or 50 mg/kg) was administered to female C57BL/6 mice for 7 d. Subsequently, mice were infected with Streptococcus pneumoniae to observe overall cells (hematoxylin and eosin staining) at 0, 8, and 16 h postinfection (n = 6; scale bars = 100 μm). Brain homogenate immunoblotting results for the same groups and time points are shown in (B) and (C). Data are presented as the mean ± standard deviation of triplicate samples. Results are representative of 3 separate experiments. *P < 0.05 and **P < 0.01, 2‐way analysis of variance. BDNF, brain‐derived neurotrophic factor; ER‐β, estrogen receptor beta; hpi, hours postinfection
4. DISCUSSION
In the present study, we demonstrated the potential therapeutic utility of Rb1, ginseng, and Rg3‐supplemented ginseng in S. pneumoniae bacterial meningitis. Rb1 and Rg3 enhanced the expression of ER‐β, anti‐apoptotic factors Bcl‐2 and Bcl‐xL, and BDNF in BV2 microglial cells and in a fatal murine model of S. pneumoniae bacterial meningitis. Moreover, these effects were associated with JAK2/STAT5 activation and required ER‐β expression in BV2 cultures.
Although sex has not been identified as a protective factor in bacteria‐induced neuroinflammation, previous animal and clinical studies have substantiated the hypothesis that females have increased neuroprotective capacity and often exhibit better survival compared with males in Alzheimer's disease, Parkinson's disease, and stroke.34, 35, 36, 37, 38, 39, 40 In our murine model, female mice responded more favorably to the administration of ginseng extract, exhibiting prolonged survival and decreased brain bacterial burden. Collectively, these results showed ginseng enhances an endogenous host defense mechanism involving the ER‐β expressed on microglia (Figure 6).
Figure 6.
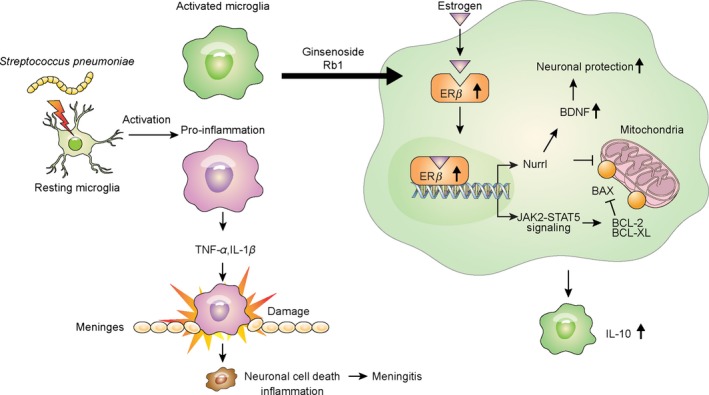
Pathway summary of a host defense mechanism response to bacterial meningitis caused by Streptococcus pneumoniae. Resting microglia are activated by S. pneumoniae. Rb1‐treated microglia express high levels of surface ER‐β to bind estrogen, which induces BDNF generation via Nurr1 as a pro‐survival signal for neurons. Microglial survival is increased through JAK2/STAT5 phosphorylation, and surviving microglia produce anti‐inflammatory mediators and facilitate bacterial clearance. In the absence of these survival signals, microglia promote neuronal death by creating harmful inflammation in the meninges
Ginsenoside Rb1 exerts neuroprotective effects against CNS injury. However, the underlying neuronal mechanism remains unclear.41 ER‐β activation is one mechanism that protects microglia by downregulating NF‐κB signaling.1 Increased ER‐β signaling leads to activation of the PI3K/AKT pathway, which in turn promotes BDNF release as part of a pro‐survival mechanism.42 Furthermore, ER‐β was shown to regulate apoptosis via Bcl‐2 family proteins.43 These reports are consistent with the present study results showing the ER‐β/BDNF/Bcl‐2 signaling pathway is an important pathway for promoting microglial survival and reducing pathological alterations associated with bacterial meningitis.
BDNF is a member of the neurotrophin family that generally promotes neuronal survival. Brains with reduced levels of BDNF become vulnerable to neurodegenerative disorders such as Alzheimer's disease, Huntington's disease, and Parkinson's disease.44, 45 Although previous reports indicated BDNF is an effective therapeutic substance against bacterial meningitis, the exact mechanism of its efficacy remains unclear.46 In a previous study, Rb1 activated BDNF through cAMP/protein kinase A (PKA) signaling in a model of Alzheimer's disease.47 Our study contributes to this knowledge by showing Rb1 increases brain BDNF expression as well as cell survival in a manner associated with ER‐β induction and reduced neuroinflammation in a model of infection. Treatment with ginseng extract increased IL‐10 levels in the brain. This anti‐inflammatory cytokine balances neuroimmune response against neurotoxicity.25, 26 Rb1 treatment similarly increased the expression of Nurr1 in meningitis model mice; Nurr1 not only showed anti‐inflammatory effects by inhibiting microglial IL‐1β and TNF‐α release, but also regulated the generation of ROS by microglia and astrocytes, increasing protection against neuronal cell death.24 Additionally, Nurr1 is an essential factor inhibiting microglial apoptosis, as evidenced by the increased expression of proapoptotic Bax protein in Nurr1‐KO mice.48 Notably, Nurr1 expression is dependent on PKA/cAMP signaling, consistent with the abovementioned result showing Rb1 regulates the PKA/cAMP pathway.49 BDNF is also known to regulate Nurr1 expression.50 Future studies should use multilateral approaches to investigate associations among ER‐β, Nurr1, and BDNF in neuroprotection.
In the present study, ginseng administration was significantly more effective for increasing survival in female mice than in male mice. Female mice exhibited dose‐dependent improvement in survival and decrease in brain bacterial burden with ginseng treatment. Sex differences in the efficacy of ginseng and Rb1/Rg3 may have been associated with the importance of ER‐β/BDNF/Bcl‐2 signaling as a primary mechanism of neuroprotection. While females can directly synthesize and utilize estrogen as a ligand for ER‐β, males must convert testosterone into estrogen; this potentially represents a sex difference in endogenous host defense mechanisms.51, 52 Therefore, the present study results showed a potential sex difference in the efficacy of Rb1 and associated effects mediated through the ER‐β in bacterial meningitis.
The phosphorylation of JAK2/STAT5 plays an important role in microglial activation.53, 54 In particular, STAT5 reportedly activates AKT phosphorylation, a key factor determining neuronal cell survival. Activation of STAT5 in neurons similarly promotes neuronal survival by interacting with the AKT pathway and facilitating anti‐apoptotic Bcl‐xL expression. Further, STAT5 upregulates BDNF by interacting with cAMP.55 Based on these results and because Rb1 increased JAK2/STAT5 phosphorylation, we hypothesize Rb1 increases AKT signaling as a pro‐survival signal synergizes with BDNF effects, and these mechanisms underlie the increased survival observed after ginseng treatment for bacterial meningitis. Future studies evaluating ER‐β signaling in this context should also examine relationships of Nurr1 and PKA/cAMP with BDNF, thus allowing identification of more detailed disease mechanisms and treatment targets.
In summary, we showed Rb1 reduces microglial cell death and promotes survival through ER‐β/BDNF signaling. We also confirmed the neuroprotective effects of Rb1/Rg3 as bioactive components of ginseng in a murine model of S. pneumoniae bacterial meningitis. Finally, we propose ER‐β/BDNF signaling represents an important endogenous host defense mechanism against bacterial meningitis, especially in females.
CONFLICT OF INTEREST
The authors declare no conflict of interests.
ACKNOWLEDGMENTS
The authors thank the Korea Ginseng Corporation for the supply of the KRG extract produced by a standardized process. This work was supported by a National Research Foundation of Korea (NRF‐2015R1 A2 A1 A10052511) grant and a grant from the Korean Society of Ginseng funded by the Korea Ginseng Cooperation (2015). The funding body played no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Lee S, Lee S‐O, Kim G‐L, Rhee D‐K. Estrogen receptor‐β of microglia underlies sexual differentiation of neuronal protection via ginsenosides in mice brain. CNS Neurosci Ther. 2018;24:930–939. 10.1111/cns.12842
REFERENCES
- 1. Wu WF, Tan XJ, Dai YB, Krishnan V, Warner M, Gustafsson JA. Targeting estrogen receptor beta in microglia and T cells to treat experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2013;110:3543‐3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Meltser I, Tahera Y, Simpson E, et al. Estrogen receptor beta protects against acoustic trauma in mice. J Clin Invest. 2008;118:1563‐1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Loane DJ, Byrnes KR. Role of microglia in neurotrauma. Neurotherapeutics. 2010;7:366‐377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mondelli V, Cattaneo A, Belvederi Murri M, et al. Stress and inflammation reduce brain‐derived neurotrophic factor expression in first‐episode psychosis: a pathway to smaller hippocampal volume. J Clin Psychiatry. 2011;72:1677‐1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nilsen J, Mor G, Naftolin F. Estrogen‐regulated developmental neuronal apoptosis is determined by estrogen receptor subtype and the Fas/Fas ligand system. J Neurobiol. 2000;43:64‐78. [DOI] [PubMed] [Google Scholar]
- 6. Calabrese F, Rossetti AC, Racagni G, Gass P, Riva MA, Molteni R. Brain‐derived neurotrophic factor: a bridge between inflammation and neuroplasticity. Front Cell Neurosci. 2014;8:430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee S, Rhee DK. Effects of ginseng on stress‐related depression, anxiety, and the hypothalamic‐pituitary‐adrenal axis. J Ginseng Res. 2017;41:589‐594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu Y, Huang XF, Bell C, Yu Y. Ginsenoside Rb1 improves leptin sensitivity in the prefrontal cortex in obese mice. CNS Neurosci Ther. 2018;24:98‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee JS, Song JH, Sohn NW, Shin JW. Inhibitory effects of ginsenoside Rb1 on neuroinflammation following systemic lipopolysaccharide treatment in mice. Phytother Res. 2013;27:1270‐1276. [DOI] [PubMed] [Google Scholar]
- 10. Liddelow SA, Guttenplan KA, Clarke LE, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481‐487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barichello T, Generoso JS, Simoes LR, et al. Role of Microglial Activation in the Pathophysiology of Bacterial Meningitis. Mol Neurobiol. 2016;53:1770‐1781. [DOI] [PubMed] [Google Scholar]
- 12. Durand ML, Calderwood SB, Weber DJ, et al. Acute bacterial meningitis in adults. A review of 493 episodes. N Engl J Med. 1993;328:21‐28. [DOI] [PubMed] [Google Scholar]
- 13. Kim KS. Pathogenesis of bacterial meningitis: from bacteraemia to neuronal injury. Nat Rev Neurosci. 2003;4:376‐385. [DOI] [PubMed] [Google Scholar]
- 14. O'Brien KL, Wolfson LJ, Watt JP, et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374:893‐902. [DOI] [PubMed] [Google Scholar]
- 15. Mook‐Kanamori BB, Geldhoff M, van der Poll T, van de Beek D. Pathogenesis and pathophysiology of pneumococcal meningitis. Clin Microbiol Rev. 2011;24:557‐591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bradley JS, Scheld WM. The challenge of penicillin‐resistant Streptococcus pneumoniae meningitis: current antibiotic therapy in the 1990s. Clin Infect Dis. 1997;24(Suppl 2):S213‐S221. [DOI] [PubMed] [Google Scholar]
- 17. van de Beek D, Brouwer M, Hasbun R, Koedel U, Whitney CG, Wijdicks E. Community‐acquired bacterial meningitis. Nat Rev Dis Primers. 2016;2:16074. [DOI] [PubMed] [Google Scholar]
- 18. Wang QO, Sun LH, Jia W, et al. Comparison of Ginsenosides Rg1 and Rb1 for Their Effects on Improving Scopolamine‐induced Learning and Memory Impairment in Mice. Phytother Res. 2010;24:1748‐1754. [DOI] [PubMed] [Google Scholar]
- 19. Kim EH, Kim IH, Lee MJ, et al. Anti‐oxidative stress effect of red ginseng in the brain is mediated by peptidyl arginine deiminase type IV (PADI4) repression via estrogen receptor (ER) beta up‐regulation. J Ethnopharmacol. 2013;148:474‐485. [DOI] [PubMed] [Google Scholar]
- 20. Nguyen CT, Luong TT, Lee SY, et al. Panax ginseng aqueous extract prevents pneumococcal sepsis in vivo by potentiating cell survival and diminishing inflammation. Phytomedicine. 2015;22:1055‐1061. [DOI] [PubMed] [Google Scholar]
- 21. Benton KA, Everson MP, Briles DE. Pneumolysin‐Negative Mutant of Streptococcus‐Pneumoniae Causes Chronic Bacteremia Rather Than Acute Sepsis in Mice. Infect Immun. 1995;63:448‐455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim JY, Paton JC, Briles DE, Rhee DK, Pyo S. Streptococcus pneumoniae induces pyroptosis through the regulation of autophagy in murine microglia. Oncotarget. 2015;6:44161‐44178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kwon SH, Ma SX, Hong SI, Lee SY, Jang CG. Lonicera japonica THUNB. Extract Inhibits Lipopolysaccharide‐Stimulated Inflammatory Responses by Suppressing NF‐kappa B Signaling in BV‐2 Microglial Cells. J Med Food. 2015;18:762‐775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Saijo K, Winner B, Carson CT, et al. A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation‐induced death. Cell. 2009;137:47‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhu Y, Chen X, Liu Z, Peng YP, Qiu YH. Interleukin‐10 Protection against Lipopolysaccharide‐Induced Neuro‐Inflammation and Neurotoxicity in Ventral Mesencephalic Cultures. Int J Mol Sci. 2015;17:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lobo‐Silva D, Carriche GM, Castro AG, Roque S, Saraiva M. Balancing the immune response in the brain: IL‐10 and its regulation. J Neuroinflammation. 2016;13:297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gomes C, Ferreira R, George J, et al. Activation of microglial cells triggers a release of brain‐derived neurotrophic factor (BDNF) inducing their proliferation in an adenosine A2A receptor‐dependent manner: A2A receptor blockade prevents BDNF release and proliferation of microglia. J Neuroinflammation. 2013;10:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sierra A, Encinas JM, Deudero JJ, et al. Microglia shape adult hippocampal neurogenesis through apoptosis‐coupled phagocytosis. Cell Stem Cell. 2010;7:483‐495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yuan QL, Yang CX, Xu P, et al. Neuroprotective effects of ginsenoside Rb1 on transient cerebral ischemia in rats. Brain Res. 2007;1167:1‐12. [DOI] [PubMed] [Google Scholar]
- 30. Yan X, Tian J, Wu H, et al. Ginsenoside rb1 protects neonatal rat cardiomyocytes from hypoxia/ischemia induced apoptosis and inhibits activation of the mitochondrial apoptotic pathway. Evid Based Complement Alternat Med. 2014;2014:149195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Malin S, McManus S, Cobaleda C, et al. Role of STAT5 in controlling cell survival and immunoglobulin gene recombination during pro‐B cell development. Nat Immunol. 2010;11:171‐179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Waibel M, Solomon VS, Knight DA, et al. Combined targeting of JAK2 and Bcl‐2/Bcl‐xL to cure mutant JAK2‐driven malignancies and overcome acquired resistance to JAK2 inhibitors. Cell Rep. 2013;5:1047‐1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mittal R, Krishnan S, Gonzalez‐Gomez I, Prasadarao NV. Deciphering the roles of outer membrane protein A extracellular loops in the pathogenesis of Escherichia coli K1 meningitis. J Biol Chem. 2011;286:2183‐2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bray GP, DeFrank RS, Wolfe TL. Sexual functioning in stroke survivors. Arch Phys Med Rehabil. 1981;62:286‐288. [PubMed] [Google Scholar]
- 35. Diamond SG, Markham CH, Hoehn MM, McDowell FH, Muenter MD. An examination of male‐female differences in progression and mortality of Parkinson's disease. Neurology. 1990;40:763‐766. [DOI] [PubMed] [Google Scholar]
- 36. Wooten GF, Currie LJ, Bovbjerg VE, Lee JK, Patrie J. Are men at greater risk for Parkinson's disease than women? J Neurol Neurosurg Psychiatry. 2004;75:637‐639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Olsen TS, Dehlendorff C, Andersen KK. Sex‐related time‐dependent variations in post‐stroke survival–evidence of a female stroke survival advantage. Neuroepidemiology. 2007;29:218‐225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Barone TA, Gorski JW, Greenberg SJ, Plunkett RJ. Estrogen increases survival in an orthotopic model of glioblastoma. J Neurooncol. 2009;95:37‐48. [DOI] [PubMed] [Google Scholar]
- 39. Correia SC, Santos RX, Cardoso S, et al. Effects of estrogen in the brain: is it a neuroprotective agent in Alzheimer's disease? Curr Aging Sci. 2010;3:113‐126. [DOI] [PubMed] [Google Scholar]
- 40. Kim TH, Vemuganti R. Effect of sex and age interactions on functional outcome after stroke. CNS Neurosci Ther. 2015;21:327‐336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ardah MT, Paleologou KE, Lv GH, et al. Ginsenoside Rb1 inhibits fibrillation and toxicity of alpha‐synuclein and disaggregates preformed fibrils. Neurobiol Dis. 2015;74:89‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kumar S, Patel R, Moore S, et al. Estrogen receptor beta ligand therapy activates PI3K/Akt/mTOR signaling in oligodendrocytes and promotes remyelination in a mouse model of multiple sclerosis. Neurobiol Dis. 2013;56:131‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ruddy SC, Lau R, Cabrita MA, et al. Preferential estrogen receptor beta ligands reduce Bcl‐2 expression in hormone‐resistant breast cancer cells to increase autophagy. Mol Cancer Ther. 2014;13:1882‐1893. [DOI] [PubMed] [Google Scholar]
- 44. Josephy‐Hernandez S, Jmaeff S, Pirvulescu I, Aboulkassim T, Saragovi HU. Neurotrophin receptor agonists and antagonists as therapeutic agents: an evolving paradigm. Neurobiol Dis. 2017;97(Pt B):139‐155. [DOI] [PubMed] [Google Scholar]
- 45. Mariga A, Mitre M, Chao MV. Consequences of brain‐derived neurotrophic factor withdrawal in CNS neurons and implications in disease. Neurobiol Dis. 2017;97(Pt B):73‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lian D, He D, Wu J, et al. Exogenous BDNF increases neurogenesis in the hippocampus in experimental Streptococcus pneumoniae meningitis. J Neuroimmunol. 2016;294:46‐55. [DOI] [PubMed] [Google Scholar]
- 47. Shi YQ, Huang TW, Chen LM, et al. Ginsenoside Rg1 attenuates amyloid‐beta content, regulates PKA/CREB activity, and improves cognitive performance in SAMP8 mice. J Alzheimers Dis. 2010;19:977‐989. [DOI] [PubMed] [Google Scholar]
- 48. Zhang T, Wang P, Ren H, Fan J, Wang G. NGFI‐B nuclear orphan receptor Nurr1 interacts with p53 and suppresses its transcriptional activity. Mol Cancer Res. 2009;7:1408‐1415. [DOI] [PubMed] [Google Scholar]
- 49. Kovalovsky D, Refojo D, Liberman AC, et al. Activation and induction of NUR77/NURR1 in corticotrophs by CRH/cAMP: involvement of calcium, protein kinase A, and MAPK pathways. Mol Endocrinol. 2002;16:1638‐1651. [DOI] [PubMed] [Google Scholar]
- 50. Koo JW, Mazei‐Robison MS, LaPlant Q, et al. Epigenetic basis of opiate suppression of Bdnf gene expression in the ventral tegmental area. Nat Neurosci. 2015;18:415‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Puts DA, Jordan CL, Breedlove SM. Defending the brain from estrogen. Nat Neurosci. 2006;9:155‐156. [DOI] [PubMed] [Google Scholar]
- 52. Radad K, Moldzio R, Rausch WD. Ginsenosides and their CNS targets. CNS Neurosci Ther. 2011;17:761‐768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kim OS, Park EJ, Joe EH, Jou I. JAK‐STAT signaling mediates gangliosides‐induced inflammatory responses in brain microglial cells. J Biol Chem. 2002;277:40594‐40601. [DOI] [PubMed] [Google Scholar]
- 54. Natarajan C, Sriram S, Muthian G, Bright JJ. Signaling through JAK2‐STAT5 pathway is essential for IL‐3‐induced activation of microglia. Glia. 2004;45:188‐196. [DOI] [PubMed] [Google Scholar]
- 55. Nyga R, Pecquet C, Harir N, et al. Activated STAT5 proteins induce activation of the PI 3‐kinase/Akt and Ras/MAPK pathways via the Gab2 scaffolding adapter. Biochem J. 2005;390:359‐366. [DOI] [PMC free article] [PubMed] [Google Scholar]


