Abstract
Background
Due to chronic inflammatory status, rheumatoid arthritis (RA) patients are exposed to changes in left ventricular (LV) geometry and function. We assessed prevalence, factors associated with, and prognostic role of concentric LV geometry and systolic dysfunction (LVSD) detected by echocardiography in a large cohort of patients with RA and normal blood pressure.
Hypothesis
Changes in LV geometry and function are widely detectable in normotensive patients with RA analyzed in primary prevention.
Methods
We prospectively analyzed 194 normotensive RA patients without overt cardiac disease recruited between March 2014 and May 2016, compared with 194 non‐RA matched controls. Relative wall thickness >0.43 defined concentric LV geometry. LVSD was defined as impaired global longitudinal strain (GLS). The prespecified study endpoints were all‐cause hospitalization and hospitalization for cardiovascular cause.
Results
The 194 normotensive subjects (mean age, 54 years; 63% female; RA duration 13 years) had a prevalence of LV concentric geometry 5‐fold higher and LVSD 5‐fold higher than non‐RA matched controls. Body mass index, LVSD, and diastolic dysfunction were associated with concentric LV geometry, while worsening renal function and older age were associated with LVSD. LVSD was independently related to the study endpoints (HR 2.37 [1.24‐4.53], p = 0.009, for all‐causes hospitalization and HR 6.60 [1.47‐29.72], p = 0.01 for cardiovascular hospitalization).
Conclusions
Despite normotensive status, a consistent proportion of RA patients analyzed in primary prevention have cardiac abnormalities detectable by echocardiography. LVSD is a strong prognosticator of adverse outcome at midterm period in these patients.
Keywords: Cardiovascular Risk, Global Longitudinal Strain, Hypertension, Left Ventricular Function, Prognosis, Rheumatoid Arthritis
1. INTRODUCTION
Patients with rheumatoid arthritis (RA) consistently develop in the early phase of disease some maladaptive changes in the cardiac phenotype, mostly described as left ventricular (LV) concentric geometry, LV hypertrophy (LVH), and/or subclinical LV dysfunction.1, 2, 3, 4, 5, 6 These cardiac abnormalities, which are closely associated with poor clinical outcomes in several settings of patients at increased risk for adverse cardiovascular (CV) events,7, 8, 9, 10, 11 including RA patients themselves,12 progress for a long time in an asymptomatic mode and can be detected by echocardiography. Arterial hypertension (HTN) is the main hemodynamic cause of these changes, together with other unfavorable hemodynamic conditions such as aortic stenosis,7, 8 diabetes mellitus (DM),9, 10 chronic kidney disease,13 and obstructive sleep apnea.14
In clinical practice, however, the detection of these cardiac abnormalities in normotensive patients with RA is not infrequent. This condition could be attributable to several nonhemodynamic mechanisms potentially affecting the hearts of those with RA, including increased serum levels of several pro‐inflammatory, immunomodulatory, and hyperactivated neurohormonal pathways.15, 16, 17, 18, 19 Although normotensive as well as hypertensive RA patients are greatly exposed to the development of cardiac abnormalities and CV events, the cardiac phenotype of normotensive RA patients has never been analyzed in detail.
Accordingly, this study was designed to assess prevalence and factors associated with LV concentric geometry and/or LV systolic dysfunction (LVSD) in a large cohort of normotensive patients with RA analyzed in primary prevention. Furthermore, we tested the hypothesis that LV concentric geometry and/or LVSD is associated in these subjects with increased hospitalizations, either total or CV related.
2. METHODS
2.1. Study population
The initial study population comprised 380 noninstitutionalized subjects age > 18 years with RA diagnosed by clinical and laboratory examination according to the American College of Rheumatology (ACR) criteria.20 Among these 380 patients, 194 (51%) had normal blood pressure (BP) at baseline evaluation, were not receiving any antihypertensive medication, and formed the final study population of the present study. For evaluating the BP values in all patients we applied the standard office BP‐measurement technique, according to the recommendations of the current international clinical guidelines for the management of arterial HTN. For this purpose, validated oscillometric or auscultatory semiautomatic sphygmomanometers were used, with all patients kept at 5‐minute rest in a sitting position.21
Participants were consecutively recruited from March 2014 to May 2016 at the Division of Rheumatology, Department of Medicine, University and Azienda Ospedaliera Universitaria Integrata of Verona (Italy) with fully accessible cardiac units provided in which patients underwent echocardiographic, clinical, and laboratory evaluations. All subjects were free of symptoms/signs of cardiac disease. Exclusion criteria were a history of myocardial infarction, myocarditis, or heart failure; coronary heart disease diagnosed by clinical electrocardiographic evaluation at rest and by the results of exercise/scintigraphy/echocardiographic stress test; primary hypertrophic cardiomyopathy; asymptomatic known LVSD; prior myocardial revascularization; significant valvular heart disease; and atrial fibrillation.
2.2. Non‐RA matched controls
The 194 normotensive patients with RA were compared with 194 patients without RA but with some CV risk factors, matched for age, sex, body mass index (BMI), and prevalence of type 2 DM. These subjects were selected by a pool of 490 patients in whom an assessment of CV risk in primary prevention was performed by clinical evaluation, laboratory tests, and echocardiography during the same period of RA patients' enrollment according to the following procedure: a Gower generalized distance from each of the 490 non‐RA individuals was computed and ranked in ascending order. The distance was calculated using these variables ordered as follows: age, sex, BMI, and DM. The 194 non‐RA patients used as controls were then defined by taking for every patient with RA the closest non‐RA matched control.
All patients gave written informed consent. The study was approved by ethics committees in all participating centers and conforms to the ethical guidelines of the Declaration of Helsinki as revised in 2000.
2.3. Definitions
HTN was defined as systolic BP of ≥140 mm Hg and/or a diastolic BP of ≥90 mm Hg and/or pharmacologically treated BP of unknown etiology. Obesity was recognized at BMI ≥30 kg/m2. Dyslipidemia was defined as levels of total serum cholesterol >190 mg/dL and or triglycerides >150 mg/dL, or pharmacologically treated high lipid serum levels. To assess renal function, we considered the glomerular filtration rate (GFR) estimated with the CKD‐EPI equation.
2.4. Endpoints and follow‐up
The prespecified primary endpoint of the study was defined as all‐cause hospitalization. We also considered a co–primary endpoint defined as hospitalization for CV causes. The follow‐up information was obtained every 6 months by visiting or interviewing the patients and/or their relatives. Follow‐up lasted from March 1, 2014, to May 31, 2017. All clinical events were examined by an independent endpoint classification committee formed by 3 expert clinicians.
2.5. Echocardiography
All Doppler echocardiographic studies were performed using the MyLab Alpha machine (Esaote Biomedical, Florence, Italy) following a standardized protocol by experienced cardiologists. Images were stored on compact disc or magneto‐optical disc and forwarded for final interpretation at the echocardiography core laboratory at Villa Bianca Hospital, Trento, Italy. LV chamber dimensions and wall thicknesses were measured by the American Society of Echocardiography (ASE) guidelines and LV mass was calculated using a validated formula.22 LV mass was normalized for height to the 2.7 power and LVH was defined as LV mass ≥ 49.2 g/m2.7 for men and ≥ 46.7 g/m2.7 for women.23 Relative wall thickness was calculated as the ratio 2 × end‐diastolic posterior wall thickness/LV diameter and indicated concentric LV geometry if ≥0.43 (the 97.5th percentile in a normal population).24
LV end‐diastolic and end‐systolic volumes were measured by the biplane method of disks from 2D apical 4‐ and 2‐chamber view and used to calculate LV ejection fraction (LVEF). Thus, the circumferential and longitudinal components of LV systolic function were also separately assessed. The circumferential systolic component was assessed by calculating the LV midwall shortening, as previously described.25 Midwall end‐systolic circumferential stress was calculated and related to midwall shortening to assess afterload‐independent LV systolic function.25 Thus, stress‐corrected midwall shortening (sc‐MS) refers to the ratio observed/predicted midwall shortening for a given end‐systolic stress and corrects the LV systolic function for this variable. An sc‐MS <87% was considered indicative of impairment of the circumferential component of LV systolic function (circumferential LVSD).14 The longitudinal systolic component was assessed offline by a single researcher cardiologist experienced in echocardiography by XStrain 2D speckle‐tracking technology software (Esaote Biomedica) equipped using a 3.5‐MHz annular‐array transducer; the researcher was blinded to patient data at the time of the analysis. Apical 2D views were recorded and analyzed for global longitudinal strain (GLS), including the 4‐chamber, 2‐chamber, and apical long‐axis views. GLS was calculated as the average of 16 myocardial segments, as previously reported.12 The cutoff value for low GLS (suggesting impairment of longitudinal component of LV systolic function) was a priori identified as −16.0%.12 Manual endocardial border tracing of a single frame at end‐systole was performed. Thus, the software automatically tracked the complete displacement of the tracking taking place in successive frames. Myocardial deformation was assessed by the software taking into account the shift of the points divided by time between 2D color scale frames. Finally, the software automatically calculated peak systolic segmental strain and GLS from the velocity of the myocardial tissue deformation. The quality of myocardial images and tracking was checked visually, and the tracking was manually corrected if poor or erroneous. If 1 segment could not be successfully tracked, it was excluded from the analysis (10 patients). If >1 segment could not be successfully tracked, the patient was not enrolled into the study (1 patient).
Transmitral and pulmonary vein pulsed‐wave Doppler curves and early diastolic tissue Doppler velocity of mitral annulus (E') were assessed according to the ASE recommendations.26 Early diastolic velocity of transmitral flow (E) was divided by E' and used to classify LV diastolic function together with other diastolic parameters in 4 degrees, as proposed by Redfield et al.,27 of normal, mild dysfunction, moderate dysfunction, and severe dysfunction. Maximal left atrial volume was also computed from 2D apical 4‐chamber view using the area length method and was normalized for body surface area.
2.6. Statistical analysis
Data are reported as mean ±SD (medians and interquartile ranges for variables deviating from normality) or percentages. Unpaired Student t test and χ2 statistics were used for descriptive statistics. Between‐group comparisons of categorical and continuous variables were performed by χ2 test and analysis of variance (ANOVA), with comparison between each group by Scheffé test for unequal sample, as appropriate. The study population was stratified by status of normotensive at baseline; thus, the 194 normotensive RA patients were compared with 194 non‐RA matched controls. The prevalence of patients with concentric LV geometry, circumferential LVSD, and longitudinal LVSD (analyzed separately by sc‐MS and GLS, respectively) was calculated. Specific multivariable logistic regression analyses were performed to identify the factors independently related to these 3 conditions. Variables that were significantly related to concentric LV geometry, circumferential LVSD, or longitudinal LVSD in univariate tests (P <0.05) were included in the multivariate models.
Log cumulative hazard functions were computed by univariate and multivariate Cox proportional hazards analyses to identify the factors independently associated with the study endpoints. Variables that were significantly related to the study endpoints in univariate tests (P <0.01) were included in the multivariate models, and probabilities of event‐free survival and Kaplan–Meier survival curves were obtained (differences between the curves were tested for significance by the log‐rank test). All analyses were performed using SPSS 19.0 (IBM Corp., Armonk, NY), and statistical significance was identified by 2‐tailed P <0.05.
3. RESULTS
3.1. Study population
The study population initially consisted of 380 patients with RA (mean age, 58 ±12 years; 64% female) whose baseline clinical and echocardiographic characteristics are shown in Table 1. They had prevalence of obesity and dyslipidemia of 15% and 59%, respectively; the prevalence of DM was 10%; the mean duration of RA was 13 ±10 years; and disease activity was high in 19% of them. Regarding echocardiographic features, 29% had LVH and 63% had concentric LV geometry; circumferential LVSD or longitudinal LVSD was diagnosed in 53% and 24% of cases, respectively.
Table 1.
Main clinical characteristics of the 380 patients with RA divided according to the presence of arterial HTN
| Variables | No Arterial HTN, n = 194 | Arterial HTN, n = 186 | P Value | Total Study Population, N = 380 |
|---|---|---|---|---|
| Age, y | 54 ±11 | 62 ±11 | <0.001 | 58 ±12 |
| Female sex | 63 | 66 | 0.49 | 64 |
| BMI, kg/m2 | 24.4 ± 4.1 | 27.6 ±4.4 | <0.001 | 25.8 ±4.6 |
| Waist circumference, cm | 88 ±13 | 99 ±11 | <0.001 | 93 ±13 |
| Obesity | 5 | 26 | <0.001 | 15 |
| SBP, mm Hg | 126 ±14 | 136 ±18 | <0.001 | 131 ±18 |
| DBP, mm Hg | 81 ±7 | 84 ±10 | 0.002 | 82 ±9 |
| Dyslipidemia | 52 | 66 | 0.005 | 59 |
| Active smoker | 40 | 34 | 0.09 | 37 |
| DM | 3 | 17 | <0.001 | 10 |
| GFR, mL/min/1.73m2 | 97 ± 21 | 91 ±25 | 0.01 | 94 ±23 |
| LDL‐C, mg/dL | 121 (98–139) | 119 (100–135) | 0.65 | 120 (94–142) |
| TG, mg/dL | 104 (74–136) | 134 (83–170) | 0.006 | 120 (80–162) |
| CRP, mg/L | 3.3 (1.5–5.8) | 5.4 (2.2–7.9) | 0.02 | 4.4 (1.8–6.8) |
| RA status | ||||
| RF positive | 47 | 49 | 0.83 | 38 |
| CCP positive | 53 | 46 | 0.41 | 38 |
| Duration of RA, y | 12 ± 9 | 14 ±11 | 0.07 | 13 ±10 |
| Clinical disease activity index | 8 ± 7 | 14 ±10 | <0.001 | 11 ±10 |
| High activity of disease | 9 | 29 | <0.001 | 19 |
| Pharmacological treatment | ||||
| β‐Blockers | 2 | 33 | <0.001 | 17 |
| ACEIs/ARBs | — | 63 | <0.001 | 30 |
| Diuretics | — | 20 | <0.001 | 10 |
| Calcium antagonists | — | 12 | <0.001 | 6 |
| Antiplatelet agents | 6 | 24 | <0.001 | 14 |
| Statins | 13 | 33 | <0.001 | 23 |
| NSAIDs | 35 | 33 | 0.76 | 34 |
| Methotrexate | 42 | 47 | 0.34 | 44 |
| Hydroxychloroquine | 9 | 10 | 0.78 | 11 |
| Immunomodulatory/anticytotoxic agents | 69 | 66 | 0.47 | 68 |
| Corticosteroids | 34 | 44 | 0.06 | 39 |
| Echocardiography | ||||
| LVEDV, mL/m2 | 50 ± 11 | 48 ± 11 | 0.03 | 49 ± 11 |
| LV relative wall thickness | 0.43 ±0.07 | 0.47 ± 0.07 | <0.001 | 0.45 ± 0.07 |
| Concentric LV geometry | 47 | 78 | <0.001 | 63 |
| LVMI, g/m2.7 | 41 ± 11 | 48 ±11 | <0.001 | 45 ±11 |
| LVH | 20 | 48 | <0.001 | 29 |
| LVEF, % | 65 ± 6 | 66 ± 6 | 0.21 | 65 ± 6 |
| LV stress‐corrected midwall shortening | 87 ± 15 | 83 ±14 | 0.006 | 85 ±15 |
| Low LV stress‐corrected midwall shortening | 49 | 57 | 0.14 | 53 |
| LV GLS, % | −18.6 ± −3.7 | −18.2 ± 3.3 | 0.38 | −18.4 ± −3.5 |
| Low LV GLS (<16%) | 20 | 29 | 0.16 | 24 |
| E/A ratio transmitral flow | 1.05 ± 0.31 | 0.86 ±0.36 | <0.001 | 0.95 ±0.35 |
| E/E' ratio | 6.0 ± 1.4 | 6.7 ±1.9 | <0.001 | 6.3 ±1.7 |
| LV diastolic dysfunction | 16 | 39 | <0.001 | 28 |
| Maximal LA volume, mL/m2 | 18 ± 6 | 20 ± 7 | 0.008 | 19 ± 7 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; CCP, cyclic citrullinated peptide; CRP, C‐reactive protein; DBP, diastolic blood pressure; DM, diabetes mellitus; GFR, glomerular filtration rate; GLS, global longitudinal strain; HTN, hypertension; IQR, interquartile range; LA, left atrial; LDL‐C, low‐density lipoprotein cholesterol; LV, left ventricular; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; LVH, left ventricular hypertrophy; LVMI, left ventricular mass index; NSAID, nonsteroidal anti‐inflammatory drug; RA, rheumatoid arthritis; RF, rheumatoid factor; SBP, systolic blood pressure; SD, standard deviation; TG, triglycerides. Data are presented as %, mean ± SD, or median (IQR) unless otherwise noted.
3.2. Normotensive vs hypertensive RA patients
The 194 normotensive RA patients epitomized the definite population of the study. They represented the 51% of the whole RA population analyzed in the present study. As expected, they were younger; had lower prevalence of obesity, dyslipidemia, and DM; and had better renal function and lower clinical disease activity index than the 186 RA counterparts with HTN. Therefore, the former had lower prevalence of concentric LV geometry and LVH, better diastolic LV function, and smaller left atrial volume. All parameters of LV systolic function were similar between the 2 groups (Table 1).
3.3. Normotensive RA vs non‐RA matched controls
Clinical and echocardiographic variables of normotensive RA patients and non‐RA matched controls are shown and compared in Table 2. The 2 groups had similar clinical features, whereas they had noticeable differences in LV geometry and function. Considering the echocardiographic variables, indeed, normotensive RA patients had significantly higher LV relative wall thickness, as well as a prevalence of LV concentric geometry (47%) and LVH (20%) that was near 5‐fold and 2‐fold higher, respectively, than non‐RA matched controls. Furthermore, circumferential LVSD or longitudinal LVSD were detected more frequently in normotensive RA patients than in non‐RA matched controls (49% vs 10% and 20% vs 5%, respectively; all P <0.001). LVEF was similar between the 2 groups.
Table 2.
Comparison between clinical features of 194 normotensive patients with RA and 194 non‐RA matched controls
| Variables | Normotensive RA Patients, n = 194 | Normotensive Non‐RA Matched Controls, n = 194 | P Value |
|---|---|---|---|
| Clinical and laboratory parameters | |||
| Age, y | 54 ±11 | 56 ±13 | 0.12 |
| Female sex | 63 | 60 | 0.22 |
| BMI, kg/m2 | 24.4 ± 4.1 | 24.6 ± 3.6 | 0.10 |
| Waist circumference, cm | 88 ± 13 | 90 ±10 | 0.09 |
| Obesity | 5 | 8 | 0.18 |
| SBP, mm Hg | 126 ± 14 | 127 ±15 | 0.37 |
| DBP, mm Hg | 81 ± 7 | 80 ±7 | 0.39 |
| Dyslipidemia | 52 | 54 | 0.43 |
| Active smoker | 40 | 38 | 0.18 |
| DM | 3 | 2 | 0.62 |
| GFR, mL/min/1.73m2 | 97 ± 21 | 94 ± 21 | 0.60 |
| LDL‐C, mg/dL | 121 (98–139) | 148 (121–177) | 0.02 |
| TG, mg/dL | 104 (74–136) | 123 (92–160) | 0.54 |
| CRP, mg/L | 3.3 (1.5–5.8) | 2.9 (0.8–6.1) | 0.86 |
| Pharmacological treatment | |||
| β‐Blockers | 2 | 3 | 0.40 |
| Antiplatelet agents | 6 | 8 | 0.22 |
| Statins | 13 | 14 | 0.35 |
| Echocardiography | |||
| LVEDV, mL/m2 | 50 ± 11 | 51 ± 12 | 0.22 |
| LV relative wall thickness | 0.43 ± 0.07 | 0.37 ± 0.05 | <0.001 |
| Concentric LV geometry | 47 | 10 | <0.001 |
| LVMI, g/m2.7 | 41 ± 11 | 38 ± 7 | <0.001 |
| LVH | 20 | 10 | <0.001 |
| LVEF, % | 65 ± 6 | 64 ± 6 | 0.06 |
| LV sc‐MS, % | 87 ± 15 | 101 ±13 | <0.001 |
| Low LV sc‐MS (<87%) | 49 | 10 | <0.001 |
| LV GLS, % | −18.6 ± −3.7 | −20.1 ± 2.6 | 0.02 |
| Low LV GLS (<16%) | 20 | 5 | <0.001 |
| E/A ratio transmitral flow | 1.05 ± 0.31 | 0.99 ±0.30 | 0.06 |
| E/E' ratio | 6.0 ± 1.4 | 6.0 ±1.5 | 0.97 |
| LV diastolic dysfunction | 16 | 16 | 0.95 |
| Maximal LA volume, mL/m2 | 18 ± 6 | 19 ± 5 | 0.58 |
Abbreviations: BMI, body mass index; CRP, C‐reactive protein; DBP, diastolic blood pressure; DM, diabetes mellitus; GFR, glomerular filtration rate; GLS, global longitudinal strain; IQR, interquartile range; LA, left atrial; LDL‐C, low‐density lipoprotein cholesterol; LV, left ventricular; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; LVH, left ventricular hypertrophy; LVMI, left ventricular mass index; RA, rheumatoid arthritis; SBP, systolic blood pressure; sc‐MS, stress‐corrected midwall shortening; SD, standard deviation; TG, triglycerides. Data are presented as %, mean ± SD, or median (IQR) unless otherwise noted.
3.4. Covariates of concentric LV geometry
Concentric LV geometry was found in 92 of 194 normotensive patients with RA (47%). At univariate analysis, older age, higher BMI, higher serum levels of C‐reactive protein (CRP), lower sc‐MS, and worse LV diastolic function (measured as higher E/E') were the variables related to this condition. Multivariate analysis showed that lower sc‐MS, worse LV diastolic function, and higher BMI were the independent variables associated with concentric LV geometry (Table 3).
Table 3.
Variables significantly related to concentric LV geometry and impairment of circumferential and longitudinal components of LVSD in the 194 normotensive patients with RA
| 194 Normotensive Patients With RA | OR | 95% CI | P Value |
|---|---|---|---|
| Concentric LV geometry | |||
| sc‐MS | 0.91 | 0.88–0.94 | <0.001 |
| E/e' | 1.39 | 1.02–1.91 | 0.04 |
| BMI | 1.10 | 1.00–1.21 | 0.04 |
| CRP | 1.00 | 0.94–1.08 | 0.79 |
| Age | 1.02 | 0.98–1.06 | 0.40 |
| Impaired circumferential component of LV systolic function (low sc‐MS) | |||
| Male sex | 2.45 | 1.23–4.80 | 0.01 |
| CRP | 1.08 | 1.00–1.17 | 0.04 |
| E/e' | 0.83 | 0.64–1.07 | 0.16 |
| LV mass | 1.03 | 0.99–1.06 | 0.13 |
| Impaired longitudinal component of LV systolic function (low GLS) | |||
| GFR | 1.04 | 1.01–1.07 | 0.01 |
| Age | 1.07 | 1.00–1.14 | 0.03 |
| CRP | 0.76 | 0.56–1.04 | 0.09 |
Abbreviations: BMI, body mass index; CI, confidence interval; CRP, C‐reactive protein; GFR, glomerular filtration rate; GLS, global longitudinal strain; LV, left ventricular; LVSD, left ventricular systolic dysfunction; OR, odds ratio; RA, rheumatoid arthritis; sc‐MS, stress‐corrected midwall shortening.
3.5. Covariates of LVSD
The circumferential LVSD was impaired in 95 of 194 normotensive patients with RA (49%). This condition was related to male sex, higher serum levels of CRP, and higher E/E' and LV mass at univariate analysis (Table 3). Male sex and higher CRP serum levels were the 2 independent variables associated with circumferential LVSD at multivariate analysis.
The longitudinal LVSD was impaired in 39 normotensive patients with RA (20%). This condition was related to lower GFR, older age, and higher CRP serum levels at univariate analysis. Lower GFR and older age were the 2 independent variables associated longitudinal LVSD at multivariate analysis (Table 3).
3.6. Clinical events
During a median follow‐up of 24 months (interquartile range, 16–30 months), a primary endpoint occurred in 28 patients (14%). No death occurred during the follow‐up. Twelve patients (6% of the total study population) had a CV event requiring hospitalization: 2 congestive heart failure, 2 thromboembolism, and 8 persistent and symptomatic atrial fibrillation associated with reduced work capacity and hospitalized for early cardioversion. Hospitalizations unrelated to CV causes were 16 (10 for acute orthopedic disease or surgery, 2 for lung infection, 2 for symptoms/signs related to a new diagnosis of cancer [1 colorectal and 1 pancreas], and 2 for severe uveitis).
3.7. LV concentric geometry, LVSD, and outcome
At univariate Cox regression analyses, longitudinal LVSD measured as low GLS was associated with the primary study endpoint (all‐cause hospitalization; hazard ratio [HR]: 1.86, 95% confidence interval [CI]: 1.07–3.24, P =0.02), whereas circumferential LVSD and LV concentric geometry did not. Thus, a first multivariate Cox regression analysis was performed. Age, sex, E/E', GFR, and CRP were the variables associated with primary endpoint at univariate analysis, which were considered together with longitudinal LVSD as covariates in the multivariate model (circumferential LVSD and concentric LV geometry were forced into the model). Longitudinal LVSD (HR: 2.37, 95% CI: 1.24–4.53, P =0.009) was the only variable independently related to the primary endpoint.
Furthermore, the same analyses were performed to individuate the covariate of co–primary endpoint (CV hospitalization). At univariate analysis, LV concentric geometry (HR: 3.52, 95% CI: 1.04–11.90, P =0.04), circumferential LVSD (HR: 3.42, 95% CI: 1.15–10.1, P =0.02), and longitudinal LVSD (HR: 3.61, 95% CI: 1.39–9.42, P =0.009) were all associated with the co–primary endpoint. Once again, multiple Cox regression analyses revealed that only longitudinal LVSD (HR: 6.60, 95% CI: 1.47–29.72, P =0.01) was the independent predictor of CV hospitalization. The Figure 1 shows all‐cause hospitalization–free survival curves (left panel) and CV hospitalization–free survival curves (right panel) in groups of patients with and without longitudinal LVSD.
Figure 1.
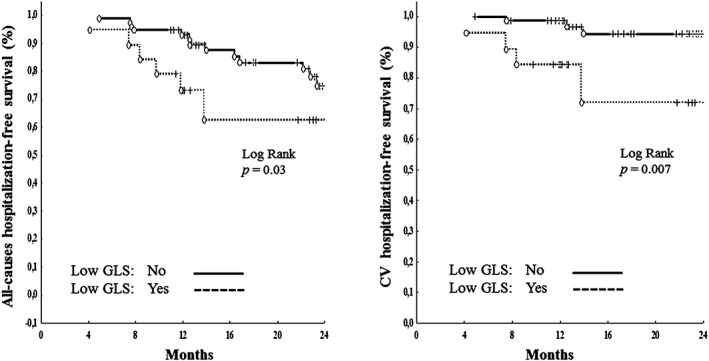
All‐cause hospitalization–free survival curves (left panel) and CV hospitalization–free survival curves (right panel) in groups of normotensive patients with and without low GLS. Abbreviations: CV, cardiovascular; GLS, global longitudinal strain
4. DISCUSSION
Our study showed several findings in patients with RA: (1) about half of subjects with long‐standing RA without overt cardiac disease keep normal BP values at long‐term follow‐up; (2) these patients have a risk profile for adverse CV events much lower than their RA counterparts with HTN, and they have an exceedingly higher prevalence of maladaptive changes in LV geometry and systolic function in comparison with non‐RA matched controls; (3) subclinical LVSD and diastolic dysfunction together with higher BMI are the independent variables associated with concentric LV geometry, male sex and higher CRP serum levels are the independent variables associated with circumferential LVSD, whereas lower GFR and older age were the 2 independent variables associated with longitudinal LVSD at multivariate analysis; and (4) longitudinal LVSD was the only condition independently related to the clinical endpoints in the normotensive RA population.
The data available in the literature support the hypothesis that chronic inflammation due to RA is associated with reduced arterial elasticity, which may precede atherosclerosis and HTN.16, 28 Our findings, however, demonstrate that arterial HTN is not the mandatory final consequence of these pathophysiological processes, because nearly half of RA patients are in the normotensive status after about 13 years from onset of RA diagnosis and treatment.
Although HTN largely influences LV geometry and function in RA patients (our hypertensive patients had higher LV mass, more frequently concentric LV geometry and LVH, and worse LV systolic and diastolic function than normotensive ones), it does not represent the needed condition promoting these important LV changes. In our normotensive RA patients, indeed, the prevalence of LV concentric geometry and LVH was 5‐fold and 2‐fold higher, respectively, than in non‐RA matched controls, as well as the prevalence of circumferential LVSD or longitudinal LVSD, which was 5‐fold and 4‐fold higher in the former than in the latter. Because our clinical model was adjusted for age, sex, BMI, and prevalence of DM, and patients with HTN were not considered, it appears evident that some nonhemodynamic mechanisms have to play a key role in generating the subclinical cardiac abnormalities found in our normotensive RA patients. Mitbø et al. recently demonstrated that the moderate to high RA disease activity was associated with concentric LV geometry and lower LV myocardial systolic function independent of the traditional CV risk factors.29, 30 Furthermore, several researchers have demonstrated that the pharmacological inhibition of interleukin‐1 or anti–tumor necrosis factor‐α therapy improves vascular and LV systolic and diastolic function in patients with RA,31, 32, 33 with an effect that was independent of changes in arterial BP. Such evidences circuitously (but reasonably) suggest that increased serum levels of several pro‐inflammatory and immunomodulatory agents that characterize RA may have a straight cardiotoxic effect and/or lead to modifications in the cardiac phenotype.
Furthermore, the close association between adverse CV events and the status of persistent chronic inflammation leading diffuse arterial atherosclerosis by nonhemodynamic pathways in patients with RA has been clearly demonstrated.19, 34, 35, 36 Our clinical and prognostic findings complete this assumption, showing that higher CRP serum levels are related to LVSD, and this condition emerged by multivariate analysis as a powerful prognosticator of poorer outcome at midterm follow‐up in normotensive patients with RA.
4.1. Study limitations
Several limitations have to be underlined. First, data exposed in the present study assessed the prognostic role of LVSD in normotensive patients with RA at 24‐month follow‐up (no longer time), and the number of CV events was relatively small. Second, the biochemical processes leading to LVSD (which are not known) were not specifically searched for and clarified in our study. Third, our findings could be prejudiced by some pharmacological or nonpharmacological treatment for RA whose effect on cardiac geometry and function would be really difficult to quantify. Finally, the primary endpoint was dominated by non‐CV causes. The analysis of a relationship between LVSD and orthopedic disease or surgery is not immediately self‐evident. An explanation could be found in the increased serum levels of several pro‐inflammatory and cytotoxic molecules, which impact both on LV systolic function and the joints of RA patients. The strengths of our study include its prospective nature and design, a large number of participants of both sexes recruited consecutively, the reliable methods for the assessment of LV geometry and systolic function, and the complete nature of the dataset.
5. CONCLUSION
The present findings may add useful information to better comprehend the pathophysiologic processes existing between chronic inflammation, cardiac abnormalities, and the development of CV events in patients with RA. Our results, which should be confirmed by larger studies with longer follow‐up and a higher number of adverse clinical events, suggest that LVSD may represent a prognostically relevant condition that can be recognized at an early stage of RA. This has to be interpreted as the start for finding clinical answers to that problem for patients with RA who frequently experience CV adverse events at a rate which does not seem to change over time.37, 38
Author contributions
Conception and design, Giovanni Cioffi, Ombretta Viapiana, Federica Ognibeni, Maurizio Rossini; generation of clinical data, Giovanni Cioffi, Ombretta Viapiana, Federica Ognibeni, Andrea Dalbeni, Alessandro Giollo, Giovanni Orsolini; analysis and interpretation of data, or both: Giovanni Cioffi, Ombretta Viapiana, Federica Ognibeni, Giovanni Orsolini, Maurizio Rossini; drafting of the manuscript or revising it critically for important intellectual content, Giovanni Cioffi, Ombretta Viapiana, Federica Ognibeni, Andrea Dalbeni, Alessandro Giollo, Davide Gatti, Maurizio Rossini; final approval of the manuscript submitted, Giovanni Cioffi, Ombretta Viapiana, Federica Ognibeni, Andrea Dalbeni, Alessandro Giollo, Giovanni Orsolini, Davide Gatti, Maurizio Rossini.
Conflicts of interest
The authors declare no potential conflicts of interest.
Cioffi G, Ognibeni F, Dalbeni A, et al. High prevalence of occult heart disease in normotensive patients with rheumatoid arthritis. Clin Cardiol. 2018;41:736–743. 10.1002/clc.22926
REFERENCES
- 1. Myasoedova E, Davis JM 3rd, Crowson CS, et al. Brief report: rheumatoid arthritis is associated with left ventricular concentric remodeling: results of a population‐based cross‐sectional study. Arthritis Rheum. 2013;65:1713–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rudominer RL, Roman MJ, Devereux RB, et al. Independent association of rheumatoid arthritis with increased left ventricular mass but not with reduced ejection fraction. Arthritis Rheum. 2009;60:22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Giles JT, Malayeri AA, Fernandes V, et al. Left ventricular structure and function in patients with rheumatoid arthritis, as assessed by cardiac magnetic resonance imaging. Arthritis Rheum. 2010;62:940–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sitia S, Tomasoni L, Cicala S, et al. Detection of preclinical impairment of myocardial function in rheumatoid arthritis patients with short disease duration by speckle tracking echocardiography. Int J Cardiol. 2012;160:8–14. [DOI] [PubMed] [Google Scholar]
- 5. Fine NM, Crowson CS, Lin G, et al. Evaluation of myocardial function in patients with rheumatoid arthritis using strain imaging by speckle‐tracking echocardiography. Ann Rheum Dis. 2014;73:1833–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cioffi G, Viapiana O, Ognibeni F, et al. Prevalence and factors related to left ventricular systolic dysfunction in asymptomatic patients with rheumatoid arthritis: a prospective tissue‐Doppler echocardiography study. Herz. 2015;40:989–996. [DOI] [PubMed] [Google Scholar]
- 7. Cioffi G, Faggiano P, Vizzardi E, et al. Prognostic effect of inappropriate left ventricular mass in asymptomatic severe aortic stenosis. Heart. 2011;97:301–307. [DOI] [PubMed] [Google Scholar]
- 8. Kearney LG, Lu K, Ord M, et al. Global longitudinal strain is a strong independent predictor of all‐cause mortality in patients with aortic stenosis. Eur Heart J Cardiovasc Imaging. 2012;13:827–833. [DOI] [PubMed] [Google Scholar]
- 9. Cioffi G, Rossi A, Zoppini G, et al. Inappropriate left ventricular mass independently predicts cardiovascular mortality in patients with type 2 diabetes. Int J Cardiol. 2013;168:4953–4956. [DOI] [PubMed] [Google Scholar]
- 10. Cioffi G, Rossi A, Targher G, et al. Usefulness of subclinical left ventricular midwall dysfunction to predict cardiovascular mortality in patients with type 2 diabetes mellitus. Am J Cardiol. 2014;113:1409–1414. [DOI] [PubMed] [Google Scholar]
- 11. de Simone G, Devereux RB, Koren MJ, et al. Midwall left ventricular mechanics: an independent predictor of cardiovascular risk in arterial hypertension. Circulation. 1996;93:259–265. [DOI] [PubMed] [Google Scholar]
- 12. Cioffi G, Viapiana O, Ognibeni F, et al. Prognostic Role of subclinical left ventricular systolic dysfunction evaluated by speckle‐tracking echocardiography in rheumatoid arthritis. J Am Soc Echocardiogr. 2017;30:602–611. [DOI] [PubMed] [Google Scholar]
- 13. Cioffi G, Tarantini L, Frizzi R, et al. Chronic kidney disease elicits excessive increase in left ventricular mass growth in patients at increased risk for cardiovascular events. J Hypertens. 2011;29:565–573. [DOI] [PubMed] [Google Scholar]
- 14. Cioffi G, Russo TE, Selmi A, et al. Analysis of left ventricular systolic function by midwall mechanics in patients with obstructive sleep apnoea. Eur J Echocardiogr. 2011;12:61–68. [DOI] [PubMed] [Google Scholar]
- 15. Solomon DH, Goodson NJ, Katz JN, et al. Patterns of cardiovascular risk in rheumatoid arthritis. Ann Rheum Dis. 2006;65:1608–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wong M, Toh L, Wilson A, et al. Reduced arterial elasticity in rheumatoid arthritis and the relationship to vascular disease risk factors and inflammation. Arthritis Rheum. 2003;48:81–89. [DOI] [PubMed] [Google Scholar]
- 17. Nagata‐Sakurai M, Inaba M, Goto H, et al. Inflammation and bone resorption as independent factors of accelerated arterial wall thickening in patients with rheumatoid arthritis. Arthritis Rheum. 2003;48:3061–3067. [DOI] [PubMed] [Google Scholar]
- 18. Maradit‐Kremers H, Nicola PJ, Crowson CS, et al. Cardiovascular death in rheumatoid arthritis: a population‐based study. Arthritis Rheum. 2005;52:722–732. [DOI] [PubMed] [Google Scholar]
- 19. Gonzalez‐Gay MA, Gonzalez‐Juanatey C, Lopez‐Diaz MJ, et al. HLA‐DRB1 and persistent chronic inflammation contribute to cardiovascular events and cardiovascular mortality in patients with rheumatoid arthritis. Arthritis Rheum. 2007;57:125–132. [DOI] [PubMed] [Google Scholar]
- 20. Mancia G, Fagard R, Narkiewicz K, Redo'n J, et al. The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) . 2013 ESH/ESC Guidelines for the management of arterial hypertension. J Hypertens. 2013;31:1281–1357. [DOI] [PubMed] [Google Scholar]
- 21. Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. [DOI] [PubMed] [Google Scholar]
- 22. Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. [DOI] [PubMed] [Google Scholar]
- 23. de Simone G, Devereux RB, Daniels SR, et al. Effect of growth on variability of left ventricular mass: assessment of allometric signals in adults and children and their capacity to predict cardiovascular risk. J Am Coll Cardiol. 1995;25:1056–1062. [DOI] [PubMed] [Google Scholar]
- 24. de Simone G, Daniels SR, Kimball TR, et al. Evaluation of concentric left ventricular geometry in humans: evidence for age‐related systematic underestimation. Hypertension. 2005;45:64–68. [DOI] [PubMed] [Google Scholar]
- 25. de Simone G, Devereux RB, Roman MJ, et al. Assessment of left ventricular function by the midwall fractional shortening/end systolic stress relation in human hypertension. J Am Coll Cardiol. 1994;23:1444–1451. [DOI] [PubMed] [Google Scholar]
- 26. Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;10:165–193. [DOI] [PubMed] [Google Scholar]
- 27. Redfield MM, Jacobsen SJ, Burnett JC Jr, et al. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. [DOI] [PubMed] [Google Scholar]
- 28. Cioffi G, Viapiana O, Ognibeni F, et al. Clinical profile and outcome of patients with rheumatoid arthritis and abnormally high aortic stiffness. Eur J Prev Cardiol. 2016;23:1848–1859. [DOI] [PubMed] [Google Scholar]
- 29. Mitbø H, Gerdts E, Kvien TK, et al. Disease activity and left ventricular structure in patients with rheumatoid arthritis. Rheumatology. 2015;54;511–519. [DOI] [PubMed] [Google Scholar]
- 30. Mitbø H, Semb AG, Matre K, et al. Disease activity is associated with reduced left ventricular systolic myocardial function in patients with rheumatoid arthritis. Ann Rheum Dis. 2017;76:371–376. [DOI] [PubMed] [Google Scholar]
- 31. Ikonomidis I, Lekakis JP, Nikolaou M, et al. Inhibition of interleukin‐1 by anakinra improves vascular and left ventricular function in patients with rheumatoid arthritis. Circulation. 2008;117:2662–2669. [DOI] [PubMed] [Google Scholar]
- 32. Kobayashi H, Kobayashi Y, Giles JT, et al. Tocilizumab treatment increases left ventricular ejection fraction and decreases left ventricular mass index in patients with rheumatoid arthritis without cardiac symptoms: assessed using 3.0 Tesla cardiac magnetic resonance imaging. J Rheumatol. 2014;41:1916–1921. [DOI] [PubMed] [Google Scholar]
- 33. Mäki‐Petäjä KM, Hall FC, Booth AD, et al. Rheumatoid arthritis is associated with increased aortic pulse‐wave velocity, which is reduced by anti‐tumor necrosis factor‐alpha therapy. Circulation. 2006;114:1185–1192. [DOI] [PubMed] [Google Scholar]
- 34. Nagata‐Sakurai M, Inaba M, Goto H, et al. Inflammation and bone resorption as independent factors of accelerated arterial wall thickening in patients with rheumatoid arthritis. Arthritis Rheum. 2003;48:3061–3067. [DOI] [PubMed] [Google Scholar]
- 35. Maradit‐Kremers H, Crowson CS, Nicola PJ, et al. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: a population‐based cohort study. Arthritis Rheum. 2005;52:402–411. [DOI] [PubMed] [Google Scholar]
- 36. Wallberg‐Jonsson S, Johansson H, Ohman ML, et al. Extent of inflammation predicts cardiovascular disease and overall mortality in seropositive rheumatoid arthritis: a retrospective cohort study from disease onset. J Rheumatol. 1999;26:2562–2571. [PubMed] [Google Scholar]
- 37. Avina‐Zubieta JA, Choi HK, Sadatsafavi M, et al. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta‐analysis of observational studies. Arthritis Rheum. 2008;59:1690–1697. [DOI] [PubMed] [Google Scholar]
- 38. Gonzales A, Maradit Kremers H, Crowson CS, et al. The widening mortality gap between rheumatoid arthritis patients and the general population. Arthritis Rheum. 2007;56:3583–3587. [DOI] [PubMed] [Google Scholar]


