Abstract
Purpose
Vascular endothelial growth factor (VEGF) inhibitors have produced demonstrable but limited benefit for various cancers. One mechanism of resistance includes revascularization, secondary to upregulation of alternative pro-angiogenic platelet-derived growth factor receptor (PDGFR) and fibroblast growth factor receptor (FGFR) pathways. Nintedanib is an oral, triple kinase inhibitor that blocks these pathways and may improve anti-tumor activity by overcoming resistance to anti-VEGF therapies. The primary objective of this first in-human study was to evaluate the safety and tolerability of nintedanib in combination with bevacizumab.
Methods
Patients were treated with escalating doses of nintedanib (150mg or 200mg oral twice daily) and bevacizumab (15 mg/kg once intravenously every 3weeks) until disease progression or unacceptable toxicity using standard 3 + 3 phase1 design. Plasma levels of angiogenic biomarkers were correlated with clinical outcomes.
Results
Eighteen patients with advanced tumors (lung (n=9), colon (n=8), and cervical (n=1)) previously treated with at least two lines of chemotherapy including bevacizumab (n=9, 50%) were enrolled. The highest dose of nintedanib was 200mg twice a day with no observed dose limiting toxicities (DLT). Common adverse events (AE) were fatigue (grade 1–3) and diarrhea (grade 1–2). Durable clinical response was observed in 55% patients pre-treated with bevacizumab (1 complete and 4 stable response). Better disease control was correlated with higher than median baseline values for VEFGR2 and E-selectin, and lower levels for SDF-1α.
Conclusion
Nintedanib was well-tolerated with bevacizumab with no DLT. Significant clinical activity was observed, including in bevacizumab pretreated patients, suggesting nintedanib can overcome bevacizumab resistance.
Keywords: Nintedanib, bevacizumab, metastasis, solid tumors, vascular endothelial growth factors
Introduction
Angiogenesis is a complex biologic process that plays a crucial role in tumor growth, progression, and metastasis. The vascular endothelial growth factor (VEGF) family (including ligands, VEGF-A to VEGF-D and receptors, VEGFR-1 to VEGFR-3) is considered to be one of the most important pathways involved in the regulation of tumor angiogenesis1. In addition, emerging evidence suggests that upregulation and activation of alternative pro-angiogenic pathways, such as platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), placental growth factor (PlGF), transforming growth factors (TGF-β), and angiopoietins, may be involved in the acquisition of resistance to anti-VEGF agents2–5. Therefore, their inhibition could be associated with improved anti-tumor activity. The simultaneous abrogation of these pathways may result in effective growth inhibition of both endothelial and perivascular cells, which may be more effective than inhibition of endothelial cell growth alone via the VEGF pathway.
Nintedanib (BIBF1120) is an oral, triple angiokinase, adenosine triphosphate (ATP) competitive inhibitor that targets VEGFRs (VEGFR-1,2,3), PDGFRs (PDGFR-α/β), and FGFR (FGFR-1,2,3) pathways. Preclinical models have demonstrated that nintedanib may have a direct anti-tumor effect on malignant cells that overexpress PDGFR and/or FGFR (e.g. H1703 NSCLC cells). In mouse xenograft models, nintedanib, as a single agent and in combination with standard chemotherapies, suppressed tumor growth of a broad range of various human tumor types, including renal cell, colorectal, ovarian, non-small cell lung, and prostate6, 7.
Earlier studies with nintedanib demonstrated favorable pharmacokinetic and excretion profiles with metabolic characteristics independent of cytochrome P450-catalyzed metabolic pathways8. Available pharmacokinetic data indicate that the systemic exposure needed for its biological activity can be achieved with starting doses of 100 mg once daily8. Phase I dose escalation studies revealed that nintedanib is generally well-tolerated with mild to moderate adverse effects, such as gastrointestinal symptoms (nausea, diarrhea, and vomiting) and reversible elevations of liver enzymes. Initial signs of clinical activity, including an encouraging rate of patients with tumor stabilization, have been observed in patients with various solid tumours9.
LUME-Lung 1 is a phase 3 trial that reported progression free survival (PFS) and overall survival (OS) benefit in combination with docetaxel in NSCLC10. LUME-Colon 1 showed only a marginal increase in PFS over placebo in refractory metastatic colorectal cancer (mCRC) patients, with a toxicity profile similar to other anti-angiogenic agents and no benefit in OS11. Nintedanib showed similar efficacy to sorafenib in hepatocellular carcinoma with a favorable and manageable adverse events (AE) profile12. In comparison to bevacizumab, nintedanib showed a similar level of safety and efficacy, along with a comparable exposure and dose intensity of mFOLFOX613.
Bevacizumab, a humanized monoclonal antibody against VEGF-A, is the first anti-angiogenic agent approved for mCRC and is associated with modest PFS and minimal OS benefit14,15, 16. It has shown clinical benefit in combination with chemotherapy in several advanced tumors. However, the clinical benefit (duration of response) is limited, most likely secondary to the fact that tumors develop salvage or alternative pathways of angiogenesis. Therefore, by targeting the most active salvage pathways of tumor angiogenesis with the addition of nintedanib to bevacizumab, a potential benefit may be expected via delayed tumor growth.
Based on the phase I dose escalation trials with Nintedanib monotherapy, the maximum tolerated dose was defined to be 250 mg for twice daily dosing 17. The maximum tolerated dose for combination therapy of nintedanib in combination with pemetrexed, docetaxel, paclitaxel/carboplatin and FOLFOX is 200mg bid. The predominant adverse events were nausea, diarrhea, vomiting, abdominal pain and fatigue of mostly low to moderate severity. Dose limiting toxicities were mainly confined to reversible hepatic enzyme elevations which increased dose-dependently10, 18, 19. Most cases occurring at doses of 250 mg and above, and a very low incidence at doses below 200 mg and were reversible after discontinuation of Nintedanib treatment. Therefore, the starting dose in this dose escalation study was determined to be 150mg oral twice a day.
In this phase 1 study, we sought to determine whether nintedanib could be safely combined with bevaciumab in patients with advanced solid tumors with an approved indication for bevacizumab. Furthermore, plasma levels of angiogenic markers were correlated with preliminary anti-tumor activity.
Patients and Methods
Study design and endpoints
This is an investigator initiated (IIS), phase I, open-label, single center, dose escalation trial of the combination of nintedanib plus bevacizumab in patients with advanced solid tumors. The criteria for dose escalation was based on a standard 3 + 3 design, for dose escalation of nintedanib up to 200mg twice daily in combination with fixed dose bevacizumab 15mg/kg. The primary endpoint was to determine the maximum tolerated dose (MTD) and the dose-limiting toxicity (DLT) of nintedanib combined with bevacizumab. Secondary endpoints included: 1) evaluation of anti-angiogenic biomarkers during treatment, and 2) determination of clinical efficacy as measured by PFS and response rate.
The protocol was approved by the University of Alabama at Birmingham (UAB) Institutional Review Board and followed the Declaration of Helsinki and International Conference on Harmonisation Good Clinical Practice guidelines. All patients signed informed consent forms, which fully disclosed the investigational nature of the trial, prior to enrollment. The study was supported by Boehringer Ingelheim and UAB Cancer Center core grant (CA 13148). This study was registered at (ClinicalTrials.gov (NCT02835833).
Patients
Adult patients (≥18 years of age) with a histologically-confirmed diagnosis of an advanced tumor for which bevacizumab has an indication (renal cell carcinoma, colorectal adenocarcinoma, non-squamous non-small cell lung cancer, platinum-refractory ovarian carcinoma, and cervical carcinoma) were eligible. Eligible patients had: 1) progressed on at least one line of standard systemic therapy; 2) Eastern Cooperative Oncology Group (ECOG) performance status 0–1; 3) adequate organ function as defined by adequate organ function as defined by normal serum bilirubin, AST/ALT ≤ 2.5x upper limit of normal (ULN), Serum creatinine < 1.5x ULN, absolute neutrophil count (ANC) > 1500,Platelets > 100k and hemoglobin >9.0 without transfusion support in the past 28 days, Urinalysis ≤ 1+ protein and 4) no contra-indications to anti-angiogenic therapy.
Patients were ineligible if they had: 1) previously experienced serious toxicities while on bevacizumab therapy; and 2) hypersensitivity to nintedanib. Patients with a history of prior brain metastasis were eligible provided the lesions were fully-treated, asymptomatic, and stable as evidenced by repeat MRI brain imaging within 2 weeks prior to starting study treatment. Patients were also excluded if they: 1) required therapeutic anti-coagulation; 2) had history of clinically-significant hemorrhagic or thromboembolic event(s) in the past 6 months and/or surgery within the past 4 weeks, prior to start of study treatment; 3) had significant cerebrovascular and cardiovascular events within the past 6 months; 4) had proteinuria of ≥ grade 2; and 5) had pulmonary hemorrhage or hemoptysis within 6 months of starting study treatment.
Treatment
The starting dose of nintedanib was 150mg, administered orally, twice every day with potential to escalate to a dose of 200mg twice daily or deescalate to 100mg twice daily. Bevacizumab was administered intravenously on day 1 of every 21 day cycle, at the dose of 15mg/kg. Nintedanib administration was started on day 2 of each cycle (Table 1). Premedication with a 5-HT3 antagonist was used if needed. Intrapatient dose escalation was not permitted. Treatment duration was through to disease progression, unacceptable toxicity, or patient refusal, whichever occurred first. Dose escalations were not allowed in patients requiring dose reductions due to toxicity. No dose adjustments for bevacizumab were allowed. If a patient experienced toxicities > grade 3, then drug was held for subsequent cycles until toxicities returned to ≤ grade 1. For treatment-related grade 2/3 toxicity, the treatment was interrupted until resolution of toxicity to grade ≤1. If nintedanib was held, then bevacizumab was also placed on hold during treatment-interrupted period.
Table 1.
Dose escalation table of nintedanib given orally in combination with fixed dose of bevacizumab given IV (21-day cycle); Total number of patients,18
| Dose level | Nintadenib (mg) | Bevacizumab (mg/kg) |
|---|---|---|
| 1 (n=3) | 150 | 15 |
| 2 (n=3) | 200 | 15 |
| 2a (n=12) | 200 | 15 |
2a expansion cohort
Study assessments
Safety
Once patients went off study, follow-up assessments with clinical examination and imaging were conducted every 2 months until 2 years after enrollment, in accordance with standard of care for treatment of patients with advanced solid tumors. Safety was evaluated at baseline, at regular intervals during treatment, and for 28 days after completing study therapy. Safety assessments included physical examination, hematologic parameters, serum chemistry, and urine analysis performed every 3 weeks. Toxicities were characterized by type, frequency, seriousness, and relationship to study drug, and were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE), version 4.03. DLTs were assessed during the first cycle. The following AEs were considered DLT if they were attributable to study drug: 1) any grade 3 or 4 non-hematologic toxicity, as defined by CTCAE 4.03, even if believed to be unrelated to the study medications (except transient electrolyte abnormality, alopecia, untreated vomiting or diarrhea, and isolated elevation of gamma glutamyl transpeptidase); and 2) any grade 4 hematologic toxicity lasting ≥ 7 days or longer or associated with bleeding or requiring transfusions.
Serum biomarker correlative analysis
Angiogenic biomarkers were assessed in patient plasma (n=18) at the following time points: baseline, at second and fourth cycle, and end of treatment. Biomarkers analyzed include pro-angiogenic factors VEGF, Interleukin-8 (IL-8), ICAM-1, Angiopoietin-1 & 2, KDR/VEGFR2, E-selectin, transforming growth factor-β (TGF-β), FGF-2, placental growth factor (PIGF), human stromal-cell derived factor-1α (SDF-1α), endocan, PDGF-AA, endoglin, and anti-angiogenic factors such as thrombospondin-1. Blood samples were collected in two EDTA tubes (2–4 mls each) and were sent to a UAB research biomarker core facility. The samples were immediately spun down and the separated plasma was stored in two aliquots at −80ºC. All samples were analyzed at study completion. Enzyme-linked immunosorbent assay (ELISA, R&D Systems, Minneapolis, MN) was used to analyze levels of PDGF-AA, thrombospondin-1, SDF-1α, and endoglin. The remaining biomarkers were analyzed using electrochemiluminescence (Meso Scale Discovery, Gaithersburg, MD).
Anti-tumor activity
Assessment of tumor response using the Response Evaluation Criteria in Solid Tumors (RECIST version 1.1) guidelines was performed at baseline, beginning of cycle three (6 weeks), and then every two cycles of therapy until progressive disease.
Statistics
An algorithm-based 3+3 dose escalation design was used to find the maximum tolerated dose (MDT) of combination therapy and to characterize the AEs and DLTs. Descriptive statistics (means, medians, standard deviations, and ranges for continuous data and percentages for categorical data) were used to summarize patient characteristics, treatment administration, safety, and efficacy. Response rates, along with corresponding 95% confidence intervals, were calculated, based on the exact binomial distribution. PFS was defined as the time from the start of study to the first documentation of tumor progression or death and censored on the last day of study. OS was defined as the time from the start of study to death and censored on the last day of study. PFS and OS were evaluated with Kaplan-Meier method. Biomarker differences between responders and non-responders were assessed by the Wilcoxon test. Based on medians at baseline, biomarkers were categorized as low and high levels. The association between PFS/OS and biomarker levels was evaluated using Cox proportional hazards model and the association between response status and biomarker levels was evaluated using Fisher’s exact test. All analysis was performed using SAS 9.4 (Cary, NC) and p-values smaller than 0.1 were considered significant.
Results
From June 2016 to June 2017, a total of 18 patients with advanced tumors [lung (n=9), colon (n=8), and cervical (n=1)] pretreated with at least two lines of chemotherapy were treated with nintedanib combined with bevacizumab at 15mg/kg in 2 dose escalation groups (dose levels 1 and 2): 150mg x 2 (n=3) and 200mg x 2 (n=3), respectively. Twelve patients were treated in dose expansion cohort (200mg x 2). Nine patients (50%) were pretreated with bevacizumab. Baseline patient characteristics are reported in Table 2. Median patient age was 59 years (range, 30–76). The majority of patients were male (67%) and white (72%). The most common tumor types were non-small cell lung (n=9, 50%) and colorectal carcinoma (n=8, 44%). Two patients (11%) had brain metastases. At baseline, patients had either ECOG 0 (n=6) or 1 (n=12). The majority of patients had received two or more lines of systemic anti-cancer therapy prior to the enrollment to this study. The median number of prior therapies was 3 (range 2–5). Median duration of treatment with this combination across all dose levels was 9.5 months (range, 4–19). All 18 patients who received treatment on protocol completed at least one cycle.
Table 2.
Patient demographics and baseline disease characteristics
| Variables | N=18 | % |
|---|---|---|
| Age in years (median and range) | 58.5 (30–76) | |
| Male | 12 | 66.7 |
| Race | ||
| Black | 5 | 27.8 |
| White | 13 | 72.2 |
| Smoking history | ||
| Current | 1 | 5.7 |
| Former | 14 | 77.8 |
| Never | 3 | 16.7 |
| Diagnosis | ||
| Lung | 9 | 50.0 |
| Colorectal | 8 | 44.4 |
| Cervical | 1 | 5.6 |
| Histology | ||
| Adenocarcinoma of Lung | 9 | 50.0 |
| Adenocarcinoma of colorectum | 8 | 44.4 |
| Adenocarcinoma Of The Cervix | 1 | 5.6 |
| Performance Status (ECOG) | ||
| ECOG 0 | 6 | 33.3 |
| ECOG 1 | 12 | 66.7 |
| Weight loss | 10 | 55.6 |
| LDH abnormal | 1 | 5.6 |
| Brain metastasis | 2 | 11.1 |
| Pre-treated with Bevacizumab | 9 | 50.0 |
During the dose escalation portion of the study, no DLTs were observed at nintedanib doses of 150mg and 200mg, twice a day. Therefore, dose level of 200mg twice a day was used in the expansion cohort.
Toxicity, as described in Table 3, was assessed in all treated patients (n=18). Overall, 16 patients (89%) had no toxicity greater than grade 1/2 by the end of first cycle. Grade 3/4 toxicity was observed in 5 patients (28%) by completion of the study. The most common non-hematologic grade 1/2 toxicities were fatigue (n=15, 83%) and diarrhea (n=11, 61%) by the completion of the study. Other adverse effects include nausea, proteinuria, elevated transaminases, hypertension, epistaxis and hypertension (Table 3). The grade 3 fatigue (n=3, 17%), occurred beyond cycle 1 of the study. The grade 3 elevation of transaminases was seen in one patient that was resolved with brief treatment interruption. No grade 2 to 4 hematologic or grade 4 non-hematological toxicity was seen in this study. Eight patients (44%) developed grade 1 anemia during treatment course and required no dose interruption or adjustments. During the first cycle of treatment, one patient developed grade 3 proteinuria and another patient had grade 3 nausea. Treatment was briefly interrupted to control proteinuria and nausea. Eventually, both patients continued the study until progression with dose modification (dose reduced to 100mg oral twice a day) of nintedanib. Grade 3 hypertension was observed in one patient that required brief interruption of treatment and management with anti-hypertensives. No patients required treatment with granulocyte colony-stimulating factors for management of neutropenia. Overall, no deaths due to toxicity were reported. At the time of last follow up, no patients remained on study. The patient majority (n=16, 89%) discontinued treatment as a result of disease progression and two patients (11%) voluntarily discontinued the study due to grade 2 diarrhea (after 10 cycles) and grade 2 fatigue (after 8 cycles). Dose reduction was required in three patients. (One in 150mg and two in 200mg dose level), due to grade 2 diarrhea and grade 3 emesis and elevation of transaminases, respectively.
Table 3.
Adverse events. Grade 3 and 4 adverse events possibly related to the therapy in all 18 evaluable patients.
| End of Cycle # 1 | n (%) | End of All Cycles | n (%) | |
|---|---|---|---|---|
| AE | Grade3/4 | All grade n (%) | Grade 3/4 n (%) | All grade n (%) |
| Hematological1 | 0 | 2(11) | 0 | 8 (44) |
| Nausea | 1 (5) | 9 ( 50) | 1 (5) | 10 (55) |
| Diarrhea | 0 | 6 (33) | 1 (5) | 11 (61) |
| Fatigue | 0 | 12 (67) | 3 (17) | 15 (83) |
| Hypertension | 0 | 2 (11) | 1 (5) | 4 (22) |
| Proteinuria | 1 (5) | 5 (1) | 1 (5) | 3 (17) |
| Epistaxis | 0 | 4 (22) | 0 | 5 (28) |
| Elevated Transaminase | 0 | 0 | 1 (6) | 1(6) |
Anemia, thrombocytopenia and neutropenia
Anti-tumor activity
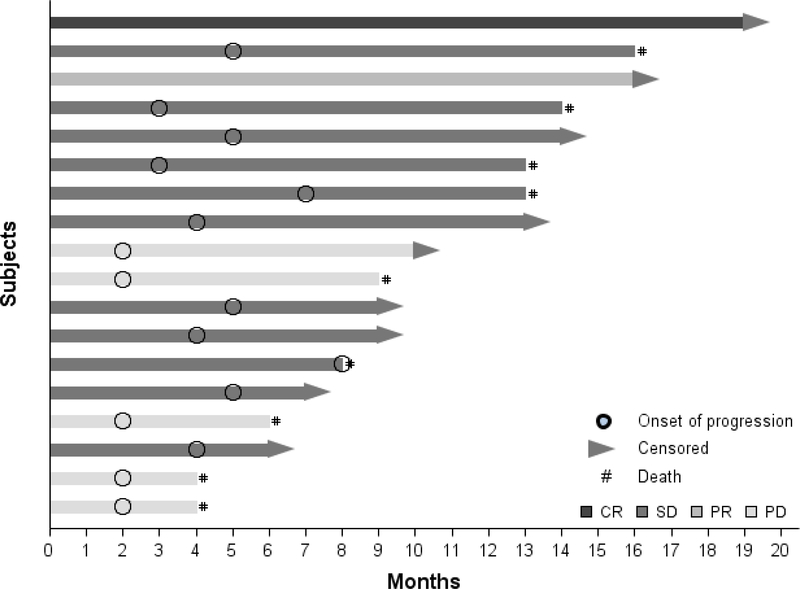
All 18 patients enrolled in this study were evaluable by RECIST response and included in the efficacy analyses. Figure 1 depicts patient duration of response. The overall response rate (ORR) was 11% [one complete response (CR) and one partial response (PR) (Table 4)]. The complete responder who had lung cancer was treated in the first cohort (150mg twice a day) and previously received 2 lines of chemotherapy (one with bevacizumab and nivolumab). This patient achieved CR by the third cycle and was maintained on study for 10 cycles before discontinuing the study due to grade 2 diarrhea. Another patient with colon cancer who had received prior surgery, chemotherapy, and radiation achieved PR with 200mg twice a day dose group by the fifth cycle. This patient was not pretreated with bevacizumab and was maintained on the study for seven cycles before discontinuing the study due to grade 2 fatigue.
Figure 1: DURATION OF RESPONSE.
CR: Complete response
PR: Partial Response
SD: Stable disease
PD: Progressive disease
Table 4.
Response Evaluation
| RESPONSE | FREQUENCY (%) |
|---|---|
| CR | 1 (5.6%) |
| PR | 1 (5.6%) |
| SD | 11 (61.1%) |
| PD | 5 (27.8%) |
| ORR (PR or CR) | 2 (11.1%) |
| DCR (PR, CR or SD) | 13 (72.2%) |
CR: Complete response
PR: Partial Response
SD: Stable disease
PD: Progressive disease
ORR: Overall response rate
DCR: Disease Control rate
Stable Disease (SD) was achieved by 11 patients (61%). Five of these cancer patients (n=1 cervical, n=2 colorectal, and n=2 lung) had SD for ≥5 months. Disease control rate (DCR) (combined 1 CR, 1 PR, and 11 SD) was achieved in 13 patients (72%). No responses were observed in 5 patients (28%). Patients who did not respond (n=5, 28%) included 2 patients with colorectal (pretreated with bevacizumab) and 3 with lung adenocarcinoma of which 2 were pretreated with bevacizumab. After a median follow-up of 9.5 months, the median PFS was 4 months (95%CI, 2–5) and median OS was 14 months (95% CI, 8-NR). The PFS rate at 6, 12, and 18 months was 22%, 11%, and 11%, respectively, and the OS rate at 6, 12, and 18 months was 83%, 70%, and 28%, respectively. Durable clinical response (DCR) was observed in pre-bevacizumab treated patients (1 CR, 4 SD). The median OS for colon (n=8) was not achieved, with 57% alive at 18 months; median OS for lung (n=9) was 13 months, with 13% alive at 18 months. Figure 1 depicts the duration of response for individual patients. Fifty percent of patients (n=3 lung, n=5 colon, and n=1 cervical) were pretreated with bevacizumab. One lung cancer patient achieved CR (33%), 3 colon cancer patients (60%), and the cervical cancer patient achieved SD. Overall, the disease control rate was 55.5% in patients previously treated with bevacizumab.
Exploratory Biomarker Analysis
The results of exploratory association analysis are shown in Table 5. In the analysis of 15 angiogenic markers, higher (relative to median) concentrations of VEGFR2 (p=0.0669) and E-selectin (p=0.0173) and lower levels (relative to median) of SDF-1α (p=0.017) were associated with better PFS. Also lower PIGF levels correlated with better DCR (p=0.0294). Percentage of change in plasma levels from baseline was assessed. Increased levels of ICAM (p=0.0837) and PIGF (0.005) and decreased levels of IL-8 (p=0.0565) were associated with better DCR (data not shown here).
Table 5:
Association of potential angiogeneic biomarkers with clinical response parameters
| OS |
PFS |
DCR |
||||
|---|---|---|---|---|---|---|
| Biomarkers (Low vs High) | Hazard Ratio (95%CI) | P value | Hazard Ratio (95%CI) | P value | Risk difference (95%CI) | P value |
| IL-8 | 1.57 (0.39, 6.35) | 0.5256 | 0.79 (0.29, 2.11) | 0.6314 | −0.11 (−0.52, 0.30) | 1.0000 |
| ICAM-1 | 0.22 (0.04, 1.11) | 0.0661 | 0.42 (0.15, 1.19) | 0.1025 | 0.33 (−0.05, 0.72) | 0.2941 |
| Angiopoietin-2 | 0.62 (0.17, 2.35) | 0.4843 | 0.62 (0.23, 1.67) | 0.3441 | 0.11 (−0.30, 0.52) | 1.0000 |
| KDR/VEGFR2 | 1.00 (0.22, 4.37) | 0.9992 | 2.71 (0.93, 7.84) | 0.0669 | −0.11 (−0.52, 0.30) | 1.0000 |
| E-selectin | 1.29 (0.26, 6.37) | 0.7588 | 4.67 (1.31, 16.63) | 0.0173 | −0.11 (−0.52, 0.30) | 1.0000 |
| Angiopoietin-1 | 1.20 (0.32, 4.50) | 0.7908 | 0.99 (0.37, 2.64) | 0.9756 | −0.11 (−0.52, 0.30) | 1.0000 |
| TGF-β | 0.82 (0.22, 3.14) | 0.7737 | 0.63 (0.23, 1.72) | 0.3667 | −0.11 (−0.52, 0.30) | 1.0000 |
| bFGF | 0.31 (0.06, 1.54) | 0.1516 | 0.60 (0.21, 1.70) | 0.3385 | 0.11 (−0.30, 0.52) | 1.0000 |
| PIGF | 0.37 (0.09, 1.51) | 0.1671 | 0.77 (0.29, 2.06) | 0.6017 | 0.56 (0.23, 0.88) | 0.0294 |
| VEGF | 0.70 (0.19, 2.61) | 0.5901 | 0.90 (0.34, 2.41) | 0.8319 | 0.11 (−0.30, 0.52) | 1.0000 |
| SDF-1α | 0.40 (0.08, 2.03) | 0.2677 | 0.21 (0.06, 0.76) | 0.0173 | 0.33 (−0.05, 0.72) | 0.2941 |
| Endocan | 0.55 (0.13, 2.42) | 0.4289 | 0.61 (0.21, 1.78) | 0.3643 | −0.11 (−0.52, 0.30) | 1.0000 |
| PDGF-AA | 1.56 (0.38, 6.36) | 0.5384 | 1.77 (0.63, 4.98) | 0.2769 | 0.05 (−0.36, 0.46) | 1.0000 |
| Endoglin | 0.40 (0.10, 1.63) | 0.203 | 0.80 (0.30, 2.13) | 0.6473 | 0.33 (−0.05, 0.72) | 0.2941 |
| Thrombospondin | 0.62 (0.16, 2.33) | 0.4788 | 0.79 (0.29, 2.11) | 0.6314 | 0.11 (−0.30, 0.52) | 1.0000 |
Discussion
Tumor angiogenesis results from the interplay of overlapping signaling pathways, thus the inhibition of one pathway could possibly lead to compensatory mechanisms of the others that could promote resistance. Recent strategies have focused on developing new multi-targeted drugs with the ability to simultaneously block several angiogenic signaling pathways, while maintaining an acceptable safety and tolerability profile20. Nintedanib is a next generation tyrosine kinase inhibitor that targets three receptor pathways (VEGF, FGFR, and PDGFR). This unique targeting profile has the potential to effectively control tumor growth and dissemination, while also avoiding intrinsic and/or acquired resistance to VEGF inhibition alone. Upregulation of PDGFR and FGFR signaling has been validated as one of the common tumor escape mechanisms to a sustained VEGF/VEGFR blockade 21, 22
This is the first in human study that evaluates the combination of nintedanib and bevacizumab in heavily pretreated, solid tumor patients. It is based on the rationale that the combination can improve anti-tumor activity by complementing VEGF inhibition and simultaneously overcoming resistance to VEGF inhibition. This study demonstrates that the combination of nintedanib and bevacizumab was safe and well-tolerated with grade 1/2 fatigue and diarrhea, which emerged as the most common adverse effects. As there were no DLTs, the last dose level tested in the escalation phase (nintedanib 200 mg twice a day and bevacizumab 15 mg/kg intravenously every 3 weeks) was chosen as the expansion phase dose. Only 3 patients (17%) experienced grade 3 treatment-related fatigue, which resolved after temporary hold of treatment. Three patients (17%) had transient grade 1 transaminase elevations during the course of treatment requiring no dose interruptions. The DLT in an earlier study with dosing of 250mg twice daily was reversible for liver enzyme elevations9. Therefore, 200mg twice daily administrations in our study allowed an increase in total daily exposure without additional toxicity. The most common non-hematologic toxicities (observed in ≥20% of patients) were fatigue, nausea, and diarrhea. The majority of non-hematologic AEs were reversible. There was no incidence of hematologic toxicity > grade 1 in this study. Eight patients developed grade 1 anemia while on study and none required blood transfusion. One patient developed sepsis, but it was not attributed to study medications. One patient developed proteinuria, grade 3, after first cycle; the onset of proteinuria was possibly related to bevacizumab.
This study was not powered to assess efficacy; however, the combination of nintedanib with bevacizumab suggests anti-tumor activity in terms of disease control at 72% for whole cohort and 55% for patients previously treated with bevacizumab. Two of the 18 patients (11%) achieved confirmed responses (one CR in 300mg/day dosing cohort and one PR in 400mg/day dosing cohort). Of the two responders, one received bevacizumab prior to the study (CR patient) and the other was bevacizumab naïve (PR patient). The responses were durable for at least 5 months in 8 patients (44%). These results appear promising with favorable side effect profiles in these pretreated patients. Therefore, it signals that the anti-VEGF activity in bevacizumab-naïve and bevacizumab-pretreated patients. Patients, who did not respond or progressed on this study, subsequently received other treatments as tolerated.
Plasma levels of angiogenic biomarkers were correlated with clinical outcomes. Tissue biopsy at the time of study entry or up on progression was not required per protocol. We therefore chose blood based biomarker analysis where serial samples was collected at pre-specified intervals as it could assess the changes in molecular phenotype and also possible inter and intra-tumoral heterogeneity. Previous work demonstrated prognostic significance of variations in plasma angiogenic biomarkers relative to median values17,23, 24. Better DCR was correlated with lower baseline PIGF levels, as well as increased level from baseline. Longer PFS was associated with higher than median baseline values for VEFGR2 and E-selectin and lower values for SDF-1α.
Our study has several notable strengths. It represents the first time that a study with the combination of anti-VEGF and a triple angiokinase inhibitor have shown to be safe, tolerable, and associated with clinical activity. Also we identified several plasma biomarkers that could potentially prognosticate responders to this dual combination. However, our study was limited by enrolling relatively few patients from a single academic center; therefore, our results require validation in larger multi-center studies.
In conclusion, nintedanib 200mg twice a day combined with bevacizumab shows a favorable safety profile with preliminary clinical efficacy. Nintedanib has the potential to overcome acquired resistance to anti-angiogenic drugs. Thus, this combination is a viable treatment option which warrants further investigation in larger studies.
Acknowlegements
The authors thank the patients and their caregivers, in addition to the research nurses, study coordinators, and operations staff who contributed to this study
Funding
The study was supported by Cancer Center core grant (CA 13148) and partially by Boehringer Ingelheim. This study was registered at ClinicalTrials.gov (NCT02835833). The funding sources provided financial resources only with no involvement in the study design, data collection, analysis and interpretation of data, and in the decision to submit the article for publication.
Footnotes
Disclosure of Potential Conflicts of Interest
Dr. Fransisco Robert: He is a member of speaker bureau for Boehringer Ingelheim. He is not involved with any of the drugs in this clinical study
NO affiliations with or involvement in any organization or entity with any financial interest.
All the authors declare no potential conflicts of interest
References
- 1.Ferrara N Vascular Endothelial Growth Factor: Basic Science and Clinical Progress. Endocrine Reviews 2004; 25(4): 581–611. doi: 10.1210/er.2003-0027 [DOI] [PubMed] [Google Scholar]
- 2.Erber R, Thurnher A, Katsen AD, Groth G, Kerger H, Hammes H-P et al. Combined inhibition of VEGF and PDGF signaling enforces tumor vessel regression by interfering with pericyte-mediated endothelial cell survival mechanisms. The FASEB Journal 2004; 18(2): 338–340. doi: 10.1096/fj.03-0271fje [DOI] [PubMed] [Google Scholar]
- 3.Presta M, Dell’Era P, Mitola S, Moroni E, Ronca R, Rusnati M. Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine & growth factor reviews 2005; 16(2): 159–178. doi: 10.1016/j.cytogfr.2005.01.004 [DOI] [PubMed] [Google Scholar]
- 4.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nature Reviews Cancer 2008; 8(8): 592–603. doi: 10.1038/nrc2442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bottsford-Miller JN, Coleman RL, Sood AK. Resistance and Escape From Antiangiogenesis Therapy: Clinical Implications and Future Strategies. Journal of Clinical Oncology 2012; 30(32): 4026–4034. doi: 10.1200/jco.2012.41.9242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kutluk Cenik B, Ostapoff KT, Gerber DE, Brekken RA. BIBF 1120 (Nintedanib), a Triple Angiokinase Inhibitor, Induces Hypoxia but not EMT and Blocks Progression of Preclinical Models of Lung and Pancreatic Cancer. Molecular Cancer Therapeutics 2013; 12(6): 992–1001. doi: 10.1158/1535-7163.mct-12-0995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hilberg F, Roth GJ, Krssak M, Kautschitsch S, Sommergruber W, Tontsch-Grunt U et al. BIBF 1120: Triple Angiokinase Inhibitor with Sustained Receptor Blockade and Good Antitumor Efficacy. Cancer Research 2008; 68(12): 4774–4782. doi: 10.1158/0008-5472.can-07-6307 [DOI] [PubMed] [Google Scholar]
- 8.Stopfer P, Rathgen K, Bischoff D, Lüdtke S, Marzin K, Kaiser R et al. Pharmacokinetics and metabolism of BIBF 1120 after oral dosing to healthy male volunteers. Xenobiotica 2011; 41(4): 297–311. doi: 10.3109/00498254.2010.545452 [DOI] [PubMed] [Google Scholar]
- 9.Mross K, Stefanic M, Gmehling D, Frost A, Baas F, Unger C et al. Phase I Study of the Angiogenesis Inhibitor BIBF 1120 in Patients with Advanced Solid Tumors. Clinical Cancer Research 2009; 16(1): 311–319. doi: 10.1158/1078-0432.ccr-09-0694 [DOI] [PubMed] [Google Scholar]
- 10.Reck M, Kaiser R, Mellemgaard A, Douillard J-Y, Orlov S, Krzakowski M et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial. The lancet oncology 2014; 15(2): 143–155. doi: 10.1016/s1470-2045(13)70586-2 [DOI] [PubMed] [Google Scholar]
- 11.Lenz H-J, Yoshino T, Argiles G, Iveson T, Sastre J, Harrison M et al. Nintedanib (N) plus best supportive care (BSC) versus placebo (P) plus BSC for the treatment of patients (pts) with colorectal cancer (CRC) refractory to standard therapies: Subanalysis of the phase III LUME-colon 1 study in pts by prior regorafenib (R) treatment. Journal of Clinical Oncology 2017; 35(4_suppl): 660–660. doi: 10.1200/jco.2017.35.4_suppl.66028045622 [DOI] [Google Scholar]
- 12.Cheng A-L, Yen C-J, Kim T-Y, Feng Y-H, Chao Y, Lin D-Y et al. Efficacy and safety of nintedanib versus sorafenib in Asian patients with advanced hepatocellular carcinoma (HCC): A randomized phase II trial. Journal of Clinical Oncology 2015; 33(3_suppl): 339–339. doi: 10.1200/jco.2015.33.3_suppl.339 [DOI] [Google Scholar]
- 13.Van Cutsem E, Prenen H, Guillen-Ponce C, Bennouna J, Di Benedetto M, Bouche O et al. A Phase l/lI, Open-label, Randomised Study of BIBF 1120 Plus mFOLFOX6 Compared to Bevacizumab Plus mFOLFOX6 in Patients with Metastatic Colorectal Cancer. European Journal of Cancer 2011; 47: 8–9. doi: 10.1016/s0959-8049(11)70113-721095116 [DOI] [Google Scholar]
- 14.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W et al. Bevacizumab plus Irinotecan, Fluorouracil, and Leucovorin for Metastatic Colorectal Cancer. New England Journal of Medicine 2004; 350(23): 2335–2342. doi: 10.1056/nejmoa032691 [DOI] [PubMed] [Google Scholar]
- 15.Bevacizumab in Combination With Oxaliplatin-Based Chemotherapy As First-Line Therapy in Metastatic Colorectal Cancer: A Randomized Phase III Study. Journal of Clinical Oncology 2008; 26(12): 2013–2019. doi: 10.1200/jco.2007.14.9930 [DOI] [PubMed] [Google Scholar]
- 16.Giantonio BJ, Catalano PJ, Meropol NJ, O’Dwyer PJ, Mitchell EP, Alberts SR et al. Bevacizumab in Combination With Oxaliplatin, Fluorouracil, and Leucovorin (FOLFOX4) for Previously Treated Metastatic Colorectal Cancer: Results From the Eastern Cooperative Oncology Group Study E3200. Journal of Clinical Oncology 2007; 25(12): 1539–1544. doi: 10.1200/jco.2006.09.6305 [DOI] [PubMed] [Google Scholar]
- 17.Mross K, Stefanic M, Gmehling D, Frost A, Baas F, Unger C et al. Phase I study of the angiogenesis inhibitor BIBF 1120 in patients with advanced solid tumors. Clinical cancer research : an official journal of the American Association for Cancer Research 2010; 16(1): 311–319. e-pub ahead of print 2009/12/24; doi: 10.1158/1078-0432.ccr-09-0694 [DOI] [PubMed] [Google Scholar]
- 18.Hanna NH, Kaiser R, Sullivan RN, Aren OR, Ahn M-J, Tiangco B et al. Nintedanib plus pemetrexed versus placebo plus pemetrexed in patients with relapsed or refractory, advanced non-small cell lung cancer (LUME-Lung 2): A randomized, double-blind, phase III trial. Lung Cancer 2016; 102: 65–73. doi: 10.1016/j.lungcan.2016.10.011 [DOI] [PubMed] [Google Scholar]
- 19.Ledermann JA, Hackshaw A, Kaye S, Jayson G, Gabra H, McNeish I et al. Randomized Phase II Placebo-Controlled Trial of Maintenance Therapy Using the Oral Triple Angiokinase Inhibitor BIBF 1120 After Chemotherapy for Relapsed Ovarian Cancer. Journal of Clinical Oncology 2011; 29(28): 3798–3804. doi: 10.1200/jco.2010.33.5208 [DOI] [PubMed] [Google Scholar]
- 20.Fernando NT, Koch M, Rothrock C, Gollogly LK, D’Amore PA, Ryeom S et al. Tumor Escape from Endogenous, Extracellular Matrix-Associated Angiogenesis Inhibitors by Up-Regulation of Multiple Proangiogenic Factors. Clinical Cancer Research 2008; 14(5): 1529–1539. doi: 10.1158/1078-0432.ccr-07-4126 [DOI] [PubMed] [Google Scholar]
- 21.Kopetz S, Hoff PM, Morris JS, Wolff RA, Eng C, Glover KY et al. Phase II Trial of Infusional Fluorouracil, Irinotecan, and Bevacizumab for Metastatic Colorectal Cancer: Efficacy and Circulating Angiogenic Biomarkers Associated With Therapeutic Resistance. Journal of Clinical Oncology 2010; 28(3): 453–459. doi: 10.1200/jco.2009.24.8252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okamoto I, Kaneda H, Satoh T, Okamoto W, Miyazaki M, Morinaga R et al. Phase I Safety, Pharmacokinetic, and Biomarker Study of BIBF 1120, an Oral Triple Tyrosine Kinase Inhibitor in Patients with Advanced Solid Tumors. Molecular Cancer Therapeutics 2010; 9(10): 2825–2833. doi: 10.1158/1535-7163.mct-10-0379 [DOI] [PubMed] [Google Scholar]
- 23.Jones BS, Jerome MS, Miley D, Jackson BE, DeShazo MR, Reddy VVB et al. Pilot phase II study of metronomic chemotherapy in combination with bevacizumab in patients with advanced non-squamous non-small cell lung cancer. Lung Cancer 2017; 106: 125–130. doi: 10.1016/j.lungcan.2017.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tran HT, Liu Y, Zurita AJ, Lin Y, Baker-Neblett KL, Martin A-M et al. Prognostic or predictive plasma cytokines and angiogenic factors for patients treated with pazopanib for metastatic renal-cell cancer: a retrospective analysis of phase 2 and phase 3 trials. The lancet oncology 2012; 13(8): 827–837. doi: 10.1016/s1470-2045(12)70241-3 [DOI] [PubMed] [Google Scholar]