Watch a video presentation of this article
Answer questions and earn CME https://www.wileyhealthlearning.com/Activity/4438365/Activity.aspx#lnk4438365
Abbreviations
- AHR
adjusted hazard ratio
- AKT
V‐AKT murine thymoma viral oncogene homolog 1
- AOR
adjusted odds ratio
- AMPK
adenosine monophosphate activated protein kinase
- ERK
extracellular signal‐regulated kinase
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HIF‐1
hypoxia‐inducible factor‐1α
- mTOR
mammalian target of rapamycin
- NR
not reported
- OR
odds ratio
- PI3K
phosphoinositide 3‐kinase
- RCT
randomized controlled trial
- RR
relative risk
- TSC
tumor suppressor complex
Hepatocellular carcinoma (HCC) is the sixth most common cancer and the second or third leading cause of death from cancer worldwide, with the uncertainty reflecting the paucity or absence of reliable and accurate estimates in high‐incidence countries.1, 2 Most HCC cases occur in hepatitis B virus (HBV) endemic areas such as East Asia and sub‐Saharan Africa.3 However, the incidence of HCC has been increasing in Western countries over the past several decades, mainly as a result of increasing incidences of chronic hepatitis C, nonalcoholic fatty liver disease, diabetes, and obesity, which are known risk factors for HCC.4 Although several curative treatment modalities, including surgical resection, radiofrequency ablation, and liver transplantation have been developed to improve the prognosis of HCC, because of the lack of effective surveillance systems for early diagnosis of HCC in both low‐ and high‐resource countries, the 5‐year survival rate of HCC is still poor. Because of the dismal prognosis of HCC, chemoprevention is an appealing approach but is unproven at the present time. In this review, we highlight two promising drugs—statins and metformin—for the chemoprevention of HCC.
Statins
Mechanisms
Statins, 3‐hydroxy‐3‐methyl‐glutaryl‐coenzyme A reductase inhibitors, are widely used for prevention of cardiovascular and cerebrovascular events because of their lipid‐lowering effects. Thus, statins are the second most common prescription drug in the United States, and one in four Americans aged 45 years or older are prescribed statins for cardiovascular disease.5 Statins have antitumor effects through the following mechanisms: (1) they down‐regulate the RAF/mitogen‐activated protein kinase 1/extracellular signal‐regulated kinase (ERK) pathway, thus reducing cell survival and contributing to antitumor apoptotic responses; (2) they limit the degradation of the cyclin‐dependent kinase inhibitors p21 and p27, which have growth‐inhibitory and tumor‐suppressor effects; (3) they prevent phosphorylation and activation of c‐Myc, which is a critical step in hepatocarcinogenesis; and (4) they have anti‐inflammatory and antioxidant effects mediated by effects on the phosphoinositide 3‐kinase (PI3K)/V‐AKT murine thymoma viral oncogene homolog 1 (AKT) pathway.
Evidence
A large, population‐based, observational study in HBV‐infected Taiwanese patients showed that statin users had a 53% risk reduction in HCC as compared with statin nonusers.6 Another population‐based observational study among hepatitis C virus (HCV)–infected patients showed that statin use was associated with a 47% reduction in HCC risk.7 In a meta‐analysis of 10 studies evaluating 4298 cases of HCC in 1,459,417 patients, statin use decreased the risk for HCC by 37% in both Asian and Western populations. Interestingly, the chemopreventive effect of statin was more pronounced in Asian populations [adjusted odds ratio (AOR) 0.52] as compared with Western populations (AOR, 0.67).8 However, two randomized controlled trials (RCTs) included in the earlier meta‐analysis did not show significant chemopreventive effects of statins, possibly because of the small number of HCC occurrences, short duration of follow‐up, and low risk for HCC development for enrolled patients. To date, no large RCTs have evaluated the chemopreventive effect of statins in patients who are at risk for HCC, such as those with liver cirrhosis or HBV or HCV infection with advanced fibrosis.
Limitations
Although favorable results have been shown in observational studies, suggesting a role for statins in chemoprevention, the strongest evidence of beneficial effect of statin for cancer prevention should be determined from RCT designs that minimize biases and confounders. Given the relatively low incidence of HCC in Western populations, it may be challenging to conduct an RCT of statins for HCC prevention. Singh et al.9 reported that, assuming a 4% annual rate of progression to HCC among patients with cirrhosis and a 50% decline in the risk for HCC with the use of statins, as compared with placebo, 2396 patients with cirrhosis would need to be followed for 1 year. Another limitation is that due to the propensity of statins to increase liver transaminases, physicians may have a high threshold for prescribing statin among patients with chronic liver disease, especially liver cirrhosis, which is one of the strongest risk factors for HCC. Consequently, the chemopreventive effect of statins has probably been overestimated in previous observational studies. Furthermore, previous observational studies failed to adjust for the concomitant use of aspirin, nonsteroidal anti‐inflammatory drugs, or antidiabetic medication (especially metformin), which may also be associated with decreases in risk for HCC, even though they adjusted for multiple other confounding factors in their analyses in an effort to minimize bias.
Metformin
Mechanisms
Metformin is one of the most commonly used drugs for the treatment of type 2 diabetes. In addition, metformin has been shown to be effective for prevention of progression of nonalcoholic fatty liver disease. Mounting evidence from both in vivo and in vitro studies suggests that metformin use is associated with a decreased risk for cancer in patients with diabetes. The postulated mechanisms are as follows: (1) inhibition of the mammalian target of rapamycin (mTOR) pathway through activation of adenosine monophosphate activated protein kinase (AMPK), (2) induction of apoptosis through either p53‐dependent or independent mechanisms, (3) inhibition of angiogenesis through negative regulation of hypoxia‐inducible factor‐1a and vascular endothelial growth factor, and (4) blocking the cell cycle partly by decreasing levels of cyclin D1 expression and by retinoblastoma‐like protein phosphorylation.10
Evidence
In a large, population‐based, case–control study, metformin showed a reduced risk for HCC in patients with diabetes, and each incremental year increase in metformin use resulted in a 7% reduction in the risk for HCC in patients with diabetes.11 In a meta‐analysis of four studies, metformin was associated with an estimated 70% reduction in the risk for liver cancer among patients with type 2 diabetes.12 In another meta‐analysis of 10 studies reporting 22,650 cases of HCC developing in 334,307 patients with type 2 diabetes, metformin use was associated with a 50% reduction in HCC incidence.13
Limitations
Previous studies should be interpreted with caution because of the inherent drawbacks of observational studies. Time‐related biases, including immortal time bias and time‐lagging bias, may allow metformin to seem effective against HCC, as has been shown for other cancers.14, 15 Another concern is that the chemopreventive effect of metformin may be overestimated by the use of other antidiabetic medications that might modify the risk for HCC, such as thiazolidinediones, sulfonylureas, and insulin, because diabetes medication regimens are frequently changed to optimize a patient's glucose control. As mentioned earlier, some previous studies did not take into account concomitant medication use, particularly statins, as a substantial portion of patients with diabetes are also taking statins for lipid control. Physicians are generally reluctant to prescribe metformin for patients with diabetes with chronic liver disease. However, a recent study of a large cohort of diabetic patients with cirrhosis showed that metformin is safe and may be potentially beneficial, improving overall survival.16
Conclusions
Evidence for the chemopreventive effect of statins and metformin is derived mainly from observational studies, and interpretation of these studies should be approached with caution. Considering their relatively low prices, satisfactory safety profiles, and the proven benefits for decreasing cardiovascular events for statins and controlling diabetes for metformin, the use of these two drugs/drug classes for chemoprevention against HCC could be an attractive option in patients at high risk for HCC. Additional well‐designed RCTs are warranted to definitively establish the role of these two drugs in chemoprevention of HCC.
Table 1.
Summary of Studies of Chemopreventive Effect of Statins Against Hepatocellular Carcinoma
| Study | Design | Location | Subjects (N) | HCC Cases (n) | Results |
|---|---|---|---|---|---|
| Friis et al. (2005)17 | Population‐based cohort | Denmark | 334,754 | 171 | AHR: 1.2 (0.5‐2.9) |
| Friedman et al. (2008)18 | Population‐based cohort | United States | 361,859 | 42 |
AHR for men: 0.5 (0.3–0.7) AHR for women: 0.4 (0.2‐0.8) |
| Marelli et al. (2011)19 | Population‐based cohort | United States | 91,714 | 105 | AOR: 0.9 (0.6‐1.2) |
| Tsan et al. (2012)6 | Population‐based cohort | Taiwan | 33,413 | 1021 | AHR: 0.5 (0.4‐0.6) |
| Tsan et al. (2013)7 | Population‐based cohort | Taiwan | 260,864 | 27,883 | AHR: 0.5 (0.5‐0.6) |
| El‐Serag et al. (2009)20 | Population‐based case–control | United States | 6515 | 1303 | AOR: 0.7 (0.6‐0.9) |
| Chiu et al. (2011)21 | Population‐based case–control | Taiwan | 2332 | 1166 | AOR: 0.6 (0.4‐0.9) |
| Sato et al. (2006)22 | RCT | Japan | 263 | 1 | RR: 0.6 (0.1‐3.5) |
| Matsushita et al. (2010)23 | RCT | Japan | 13,724 | 12 | RR: 0.6 (0.2‐1.9) |
| Cholesterol Treatment Trialists' (CTT) Collaborators et al. (2012)24 | RCT | International | 134,537 | 68 | RR: 1.1 (0.7‐1.7) |
Abbreviations: AHR, adjusted hazard ratio; RR, relative risk.
Table 2.
Summary of Studies of Chemopreventive Effect of Metformin Against Hepatocellular Carcinoma
| Study | Design | Location | Subjects | Metformin | HCC Cases | Results |
|---|---|---|---|---|---|---|
| Oliveria et al. (2008)25 | Population‐based cohort | United States | 191,223 | NR | 39 | AOR: 0.7 (0.3–1.6) |
| Ruiter et al. (2012)26 | Population‐based cohort | Netherlands | 85,289 | 61.8% | 31 | OR: 0.7 (0.5–0.9) |
| Chen et al. (2013)11 | Population‐based case–control | Taiwan | 47,820 | NR | 22,047 | AOR: 0.8 (0.7–0.8) |
| Kawaguchi et al. (2010)27 | Hospital‐based case–control | Japan | 241 | 3.7% | 138 | AOR: 0.6 (0.2–2.2) |
| Hassan et al. (2010)28 | Hospital‐based case–control | United States | 255 | 47.1% | 140 | AOR: 0.3 (0.2–0.6) |
| Donadon et al. (2010)29 | Hospital‐based case–control | Italy | 549 | 23.5% | 190 | AOR: 0.2 (0.1–0.4) |
| Nkontchou et al. (2011)30 | Hospital‐based cohort | France | 100 | 26.0% | 39 | AOR: 0.2 (0.04–0.8) |
| RECORD (2010)31 | RCT | International | 4447 | 75.2% | 4 | AOR: 3.0 (0.2–55.3) |
Abbreviations: NR, not reported; OR, odds ratio.
Figure 1.
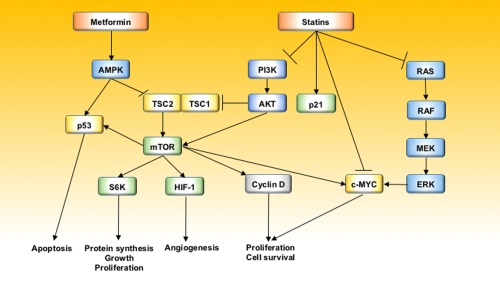
Antitumor mechanisms of statins and metformin. Abbreviations: MEK, ; RAS,.
Potential conflict of interest: Nothing to report.
REFERENCES
- 1. Sartorius K, Sartorius B, Aldous C, Govender PS, Madiba TE. Global and country underestimation of hepatocellular carcinoma (HCC) in 2012 and its implications. Cancer Epidemiol 2015;39:284–290. [DOI] [PubMed] [Google Scholar]
- 2. Global, regional, and national age‐sex specific all‐cause and cause‐specific mortality for 240 causes of death, 1990‐2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;385:117–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gomaa AI, Khan SA, Toledano MB, Waked I, Taylor‐Robinson SD. Hepatocellular carcinoma: epidemiology, risk factors and pathogenesis. World J Gastroenterol 2008;14:4300–4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. El‐Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med 1999;340:745–750. [DOI] [PubMed] [Google Scholar]
- 5. IMS Institute for Healthcare Informatics . The use of medicines in the United States: review of 2010. Published 2010.
- 6. Tsan YT, Lee CH, Wang JD, Chen PC. Statins and the risk of hepatocellular carcinoma in patients with hepatitis B virus infection. J Clin Oncol 2012;30:623–630. [DOI] [PubMed] [Google Scholar]
- 7. Tsan YT, Lee CH, Ho WC, Lin MH, Wang JD, Chen PC. Statins and the risk of hepatocellular carcinoma in patients with hepatitis C virus infection. J Clin Oncol 2013;31:1514–1521. [DOI] [PubMed] [Google Scholar]
- 8. Singh S, Singh PP, Singh AG, Murad MH, Sanchez W. Statins are associated with a reduced risk of hepatocellular cancer: a systematic review and meta‐analysis. Gastroenterology 2013;144:323–332. [DOI] [PubMed] [Google Scholar]
- 9. Singh S, Singh PP. Statins for prevention of hepatocellular cancer: one step closer? Hepatology 2014;59:724–726. [DOI] [PubMed] [Google Scholar]
- 10. Bhat A, Sebastiani G, Bhat M. Systematic review: preventive and therapeutic applications of metformin in liver disease. World J Hepatol 2015;7:1652–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen HP, Shieh JJ, Chang CC, Chen TT, Lin JT, Wu MS, et al. Metformin decreases hepatocellular carcinoma risk in a dose‐dependent manner: population‐based and in vitro studies. Gut 2013;62:606–615. [DOI] [PubMed] [Google Scholar]
- 12. Zhang ZJ, Zheng ZJ, Shi R, Su Q, Jiang Q, Kip KE. Metformin for liver cancer prevention in patients with type 2 diabetes: a systematic review and meta‐analysis. J Clin Endocrinol Metab 2012;97:2347–2353. [DOI] [PubMed] [Google Scholar]
- 13. Singh S, Singh PP, Singh AG, Murad MH, Sanchez W. Anti‐diabetic medications and the risk of hepatocellular cancer: a systematic review and meta‐analysis. Am J Gastroenterol 2013;108:881–891; quiz 892. [DOI] [PubMed] [Google Scholar]
- 14. Suissa S, Azoulay L. Metformin and the risk of cancer: time‐related biases in observational studies. Diabetes Care 2012;35:2665–2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chaiteerakij R, Petersen GM, Bamlet WR, Chaffee KG, Zhen DB, Burch PA, et al. Metformin use and survival of patients with pancreatic cancer: a cautionary lesson. J Clin Oncol; doi: 10.1200/JCO.2015.63.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang X, Harmsen WS, Mettler TA, Kim WR, Roberts RO, Therneau TM, et al. Continuation of metformin use after a diagnosis of cirrhosis significantly improves survival of patients with diabetes. Hepatology 2014;60:2008–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Friis S, Poulsen AH, Johnsen SP, McLaughlin JK, Fryzek JP, Dalton SO, et al. Cancer risk among statin users: a population‐based cohort study. Int J Cancer 2005;114:643–647. [DOI] [PubMed] [Google Scholar]
- 18. Friedman GD, Flick ED, Udaltsova N, Chan J, Quesenberry CP Jr, Habel LA. Screening statins for possible carcinogenic risk: up to 9 years of follow‐up of 361,859 recipients. Pharmacoepidemiol Drug Saf 2008;17:27–36. [DOI] [PubMed] [Google Scholar]
- 19. Marelli C, Gunnarsson C, Ross S, Haas S, Stroup DF, Cload P, et al. Statins and risk of cancer: a retrospective cohort analysis of 45,857 matched pairs from an electronic medical records database of 11 million adult Americans. J Am Coll Cardiol 2011;58:530–537. [DOI] [PubMed] [Google Scholar]
- 20. El‐Serag HB, Johnson ML, Hachem C, Morgana RO. Statins are associated with a reduced risk of hepatocellular carcinoma in a large cohort of patients with diabetes. Gastroenterology 2009;136:1601–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chiu HF, Ho SC, Chen CC, Yang CY. Statin use and the risk of liver cancer: a population‐based case‐control study. Am J Gastroenterol 2011;106:894–898. [DOI] [PubMed] [Google Scholar]
- 22. Sato S, Ajiki W, Kobayashi T, Awata N, Group PCS Study Group . Pravastatin use and the five‐year incidence of cancer in coronary heart disease patients: from the prevention of coronary sclerosis study. J Epidemiol 2006;16:201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Matsushita Y, Sugihara M, Kaburagi J, Ozawa M, Iwashita M, Yoshida S, et al. Pravastatin use and cancer risk: a meta‐analysis of individual patient data from long‐term prospective controlled trials in Japan. Pharmacoepidemiol Drug Saf 2010;19:196–202. [DOI] [PubMed] [Google Scholar]
- 24. Cholesterol Treatment Trialists' (CTT) Collaborators ; Emberson JR, Kearney PM, Blackwell L, Newman C, Reith C, et al. Lack of effect of lowering LDL cholesterol on cancer: meta‐analysis of individual data from 175,000 people in 27 randomised trials of statin therapy. PLoS One 2012;7:e29849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Oliveria SA, Koro CE, Yood MU, Sowell M. Cancer incidence among patients treated with antidiabetic pharmacotherapy. Diabetes Metab Syndr 2008;2:47–57. [Google Scholar]
- 26. Ruiter R, Visser LE, van Herk‐Sukel MP, Coebergh JW, Haak HR, Geelhoed‐Duijvestijn PH, et al. Lower risk of cancer in patients on metformin in comparison with those on sulfonylurea derivatives: results from a large population‐based follow‐up study. Diabetes Care 2012;35:119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kawaguchi T, Taniguchi E, Morita Y, Shirachi M, Tateishi I, Nagata E, Sata M. Association of exogenous insulin or sulphonylurea treatment with an increased incidence of hepatoma in patients with hepatitis C virus infection. Liver Int 2010;30:479–486. [DOI] [PubMed] [Google Scholar]
- 28. Hassan MM, Curley SA, Li D, Kaseb A, Davila M, Abdalla EK, et al. Association of diabetes duration and diabetes treatment with the risk of hepatocellular carcinoma. Cancer 2010;116:1938–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Donadon V, Balbi M, Mas MD, Casarin P, Zanette G. Metformin and reduced risk of hepatocellular carcinoma in diabetic patients with chronic liver disease. Liver Int 2010;30:750–758. [DOI] [PubMed] [Google Scholar]
- 30. Nkontchou G, Cosson E, Aout M, Mahmoudi A, Bourcier V, Charif I, et al. Impact of metformin on the prognosis of cirrhosis induced by viral hepatitis C in diabetic patients. J Clin Endocrinol Metab 2011;96:2601–2608. [DOI] [PubMed] [Google Scholar]
- 31. Home PD, Kahn SE, Jones NP, Noronha D, Beck‐Nielsen H, Viberti G, et al. Experience of malignancies with oral glucose‐lowering drugs in the randomised controlled ADOPT (A Diabetes Outcome Progression Trial) and RECORD (Rosiglitazone Evaluated for Cardiovascular Outcomes and Regulation of Glycaemia in Diabetes) clinical trials. Diabetologia 2010;53:1838–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]


